The early bud outgrowth stimulated by decapitation or cytokinin occurs independent of auxin flow from axillary buds.
Abstract
Apical dominance is the process whereby the shoot tip inhibits the growth of axillary buds along the stem. It has been proposed that the shoot tip, which is the predominant source of the plant hormone auxin, prevents bud outgrowth by suppressing auxin canalization and export from axillary buds into the main stem. In this theory, auxin flow out of axillary buds is a prerequisite for bud outgrowth, and buds are triggered to grow by an enhanced proportional flow of auxin from the buds. A major challenge of directly testing this model is in being able to create a bud- or stem-specific change in auxin transport. Here we evaluate the relationship between specific changes in auxin efflux from axillary buds and bud outgrowth after shoot tip removal (decapitation) in the pea (Pisum sativum). The auxin transport inhibitor 1-N-naphthylphthalamic acid (NPA) and to a lesser extent, the auxin perception inhibitor p-chlorophenoxyisobutyric acid (PCIB), effectively blocked auxin efflux from axillary buds of intact and decapitated plants without affecting auxin flow in the main stem. Gene expression analyses indicate that NPA and PCIB regulate auxin-inducible, and biosynthesis and transport genes, in axillary buds within 3 h after application. These inhibitors had no effect on initial bud outgrowth after decapitation or cytokinin (benzyladenine; BA) treatment. Inhibitory effects of PCIB and NPA on axillary bud outgrowth only became apparent from 48 h after treatment. These findings demonstrate that the initiation of decapitation- and cytokinin-induced axillary bud outgrowth is independent of auxin canalization and export from the bud.
Shoot branching is a major determinant of plant architecture, influencing plant success in natural and agricultural systems. Lateral branches arise from the initiation and growth of axillary meristems, which are essentially embryonic shoots that develop into axillary buds and potentially into branches (Tantikanjana et al., 2001; Tian and Jiao, 2015). The control of axillary bud growth is also interesting from a developmental point of view because buds can be maintained in an inhibited or slow-growing state throughout the plants’ lifecycle or can be stimulated to grow out. This suppression/trigger system is a good model for stem cell arrest in plants because it operates without greatly affecting the growth of the main stem and roots. Axillary buds have the same developmental potential as the shoot apical bud. However in several plant species, once initiated, the growth of axillary buds is arrested, a condition referred to as “dormancy” (Domagalska and Leyser, 2011). Dormancy is not definitive, and the bud can often resume its growth if the shoot tip is damaged (Shimizu-Sato and Mori, 2001; Beveridge et al., 2003; Rameau et al., 2015). The mechanism by which the shoot tip inhibits axillary bud outgrowth is under debate (Rameau et al., 2015; Xu et al., 2015; Yuan et al., 2015).
The shoot tip consists of an apical bud and young expanding leaves, which regulate apical dominance through their capacity to produce auxin and/or through voracious consumption of plant resources (Phillips, 1975; Hosokawa et al., 1990; Cline, 1991; Girault et al., 2010; Henry et al., 2011; Rabot et al., 2012; Mason et al., 2014; Barbier et al., 2015a). Apically produced auxin is actively transported in the polar auxin transport (PAT) stream down the stem toward the roots (Ljung et al., 2001; Müller and Leyser, 2011; Shinohara et al., 2013). Classic experiments by Thimann and Skoog (1933) established that if auxin is applied to the stump of decapitated plants, axillary bud outgrowth is halted, suggesting that auxin originating from the shoot tip inhibits axillary bud outgrowth at nodes below. However, auxin is reportedly ineffective at inhibiting bud outgrowth after decapitation in some plant species, including Arabidopsis (Arabidopsis thaliana) and garden pea (Pisum sativum) for almost 24 h after treatment and under well-lit conditions (Cline, 1996; Cline et al., 2001; Morris et al., 2005). It is widely proposed that shoot tip decapitation stimulates axillary bud outgrowth through auxin depletion in the main stem (Prusinkiewicz et al., 2009; Teichmann and Muhr, 2015; Balla et al., 2016).
Studies have demonstrated that auxin depletion in the main stem is not correlated with initial bud outgrowth after shoot tip removal. Using 20-cm tall pea plants with a considerable distance between the shoot tip and basal buds, researchers showed that decapitation-induced bud outgrowth occurred outside the zone of auxin depletion (Morris et al., 2005; Renton et al., 2012; Mason et al., 2014). Recent evidence suggests that the initial trigger of bud outgrowth after removing the shoot tip could be the diversion of increasingly available photosynthates, mainly sugars, to dormant axillary buds (Mason et al., 2014; Fichtner et al., 2017). Further independent studies confirmed that the rate and extent of branching in rose (Rosa hybrida) and sorghum (Sorghum bicolor) is correlated with the availability of Suc and/or photosynthetic leaf area (Kim et al., 2010 Alam et al., 2014; Barbier et al., 2015b; Kebrom and Mullet, 2015). It is likely that the source/sink dynamics after decapitation are signaled to the bud through changes in the levels of trehalose-6-phosphate, a sugar signal of emerging importance (Fichtner et al., 2017). It is therefore plausible to hypothesize that the early stages of bud outgrowth occur independent of auxin dynamics in the stem after decapitation.
Auxin production increases in axillary buds after decapitation. A 5-fold increase in auxin content occurs in common bean (Phaseolus vulgaris L.) axillary buds 4 h after decapitation (Gocal et al., 1991). The main pathway for auxin biosynthesis in Arabidopsis involves enzymes related to TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA). These TAA enzymes convert tryptophan to indole-3-pyruvic acid; and YUCCA (YUC) flavin monooxygenase-like-related enzymes convert indole-3-pyruvic acid to the main auxin, indole-3-acetic acid (IAA) (Mashiguchi et al., 2011; Won et al., 2011). Genetic evidence and models of auxin transport in plants support the hypothesis that the flow or efflux of auxin from axillary buds is important for regulating bud outgrowth (Prusinkiewicz et al., 2009; Balla et al., 2011, 2016; Bennett et al., 2016). This model, referred to as the “auxin transport/canalization hypothesis,” is based on the principle that dormant buds must establish their own PAT stream out into the main stem as a prerequisite for bud outgrowth (Sachs, 1968, 1969; Li and Bangerth, 1999; Prusinkiewicz et al., 2009; Domagalska and Leyser, 2011; de Jong et al., 2014). Although the primary vasculature to axillary buds is formed and presumably functional, auxin canalization in xylem parenchyma cells is thought to be a requirement for bud outgrowth (Balla et al., 2011, 2016; Brewer et al., 2015; Bennett et al., 2016). Auxin flow out of axillary buds may also be important for contributing to bud sink strength (Brewer et al., 2009; Bennett et al., 2016), which might form a basis for triggering bud outgrowth. After decapitation, auxin depletion in the stem leads to the formation of a sink for auxin in the stem, which stimulates auxin flow/canalization out of the bud (Müller and Leyser, 2011). Alternatively, auxin canalization from buds would also be promoted if the axillary bud were somehow induced to export additional auxin into the PAT stream, even without auxin depletion in the stem. For example, sugars can induce the expression of auxin biosynthesis genes such as YUC8 and YUC9 and increase auxin levels in Arabidopsis seedlings (Sairanen et al., 2012).
Polar auxin transport is the directional cell-to-cell movement of auxin, which occurs through active transport mediated by specialized influx and efflux carrier proteins (Rubery and Sheldrake, 1974; Adamowski and Friml, 2015). Three main classes of auxin transporters have been identified: PIN-FORMED (PIN) exporters, the ATP-binding cassette (ABC)-B/multidrug resistance/P-glycoprotein (ABCB/MDR/PGP) exporters, and the AUXIN/LIKEAUXIN (AUX/LAX) importers (Balzan et al., 2014; Adamowski and Friml, 2015). AUX/LAX proteins actively import auxin into the cell (Péret et al., 2012; Swarup and Péret, 2012). PIN family proteins are widely studied auxin efflux carriers (Adamowski and Friml, 2015). The polar/asymmetric subcellular localization of PIN proteins at the plasma membrane determines the direction of auxin transport in plants (Adamowski and Friml, 2015). At least five members of the ABCB transporter family proteins have been reported to mediate cellular auxin export (Cho and Cho, 2013). Like PIN proteins, ABCB proteins contribute significantly to directional auxin transport in cells (Balla et al., 2011; Cho and Cho, 2013).
According to Balla et al. (2011), the observed early auxin export from axillary buds after decapitation could be a diffusion-driven flux or polar auxin transport mediated by PIN and ABCB transporters. While these auxin transporters have been shown to play a role in activating dormant buds, Balla et al. (2011) demonstrated that the polarization of PIN1 proteins to establish directional auxin export from activated axillary buds is a gradual process initially observable 6 h (and more pronounced 48 h) after decapitation in pea. After decapitation, bud outgrowth at nodes outside the zone of auxin depletion can occur within 2.5 h (Mason et al., 2014; Fichtner et al., 2017), thus bringing into question the role of auxin transporters during initial bud outgrowth. Certainly for buds that start growth from nodes within the zone of auxin depletion, reduced auxin content in the main stem is associated with enhanced auxin export from buds and a transient increase of bud auxin content (Gocal et al., 1991; Balla et al., 2002; Shimizu-Sato et al., 2009). It is possible that auxin canalization from buds could be the trigger for bud outgrowth after decapitation even at nodes outside the zone of auxin depletion (i.e. prior to auxin depletion in the adjacent stem). For example, if decapitation enhances sugar levels in axillary buds, as reported by Mason et al. (2014), this could enhance auxin biosynthesis (Lilley et al., 2012; Sairanen et al., 2012), which would likely increase auxin flow from buds through canalization.
We have previously shown that a large and widespread reduction in auxin efflux from pea axillary buds after decapitation has little effect on bud outgrowth over several days (Brewer et al., 2009, 2015). Initial bud outgrowth is not affected, whereas a 57% reduction in branch growth was observed 3 d after treatment (Brewer et al., 2009). However, to directly test the auxin canalization model, we need to create a situation where auxin transport from axillary buds can be modulated independently from the adjacent stem, rather than being altered in both stem and axillary bud. Unfortunately, bud-specific promoters have not been identified in any species for testing bud-specific inhibition of auxin pathway gene. However, localized treatment with auxin inhibitors can be useful in testing local PAT effects (e.g. Reinhardt et al., 2000; Brewer et al., 2009). Moreover, a range of auxin inhibitors that act at specific points in the pathway are available. For instance, 1-N-naphthylphthalamic acid (NPA) specifically inhibits PIN and ABCB cellular auxin transporters (Kleine-Vehn et al., 2006; Petrásek et al., 2006; Sauer et al., 2006; Blakeslee et al., 2007). On the other hand, PCIB is a putative auxin signaling inhibitor that has been reported to reduce polar auxin transport in diverse species by competing for auxin binding sites (Rose and Bopp, 1983; Oono et al., 2003; Hayashi et al., 2008; Belz and Piepho, 2013; Di et al., 2015). PCIB strongly inhibits auxin transport, probably because auxin signaling is required to promote PIN auxin transport (Sauer et al., 2006). Alternatively, l-Kynurenine (Kyn) selectively and competitively inhibits TAA-related proteins, which are key auxin biosynthesis enzymes (He et al., 2011).
We therefore enhanced our pharmacological approach to specifically alter auxin transport from axillary buds without affecting auxin transport in the main stem. We tested whether increased auxin transport from axillary buds occurs after decapitation in pea. We then focused on NPA and PCIB to specifically inhibit auxin transport from axillary buds of garden pea, and tests used digital time-lapse photography to accurately measure axillary bud growth in response to simultaneous decapitation and treatments of NPA or PCIB.
RESULTS
We focused on the axillary bud at node five of pea plants with seven fully expanded leaves (∼12-d-old plants) because this node is more than 10 cm below the shoot tip and is above a long internode that allows for quantification of auxin flow out of the bud into the stem. As auxin depletion in the stem occurs at a rate of about 1 cm per hour after decapitation (Morris et al., 2005; Renton et al., 2012), this method enabled tests of bud outgrowth within and outside the zone of auxin depletion after decapitation at different positions along the stem. This experimental design also enabled measurements of auxin transport from the bud and into the long internode of plants in vivo.
Dormant Buds Export Auxin, Which Increases after Decapitating the Shoot Tip
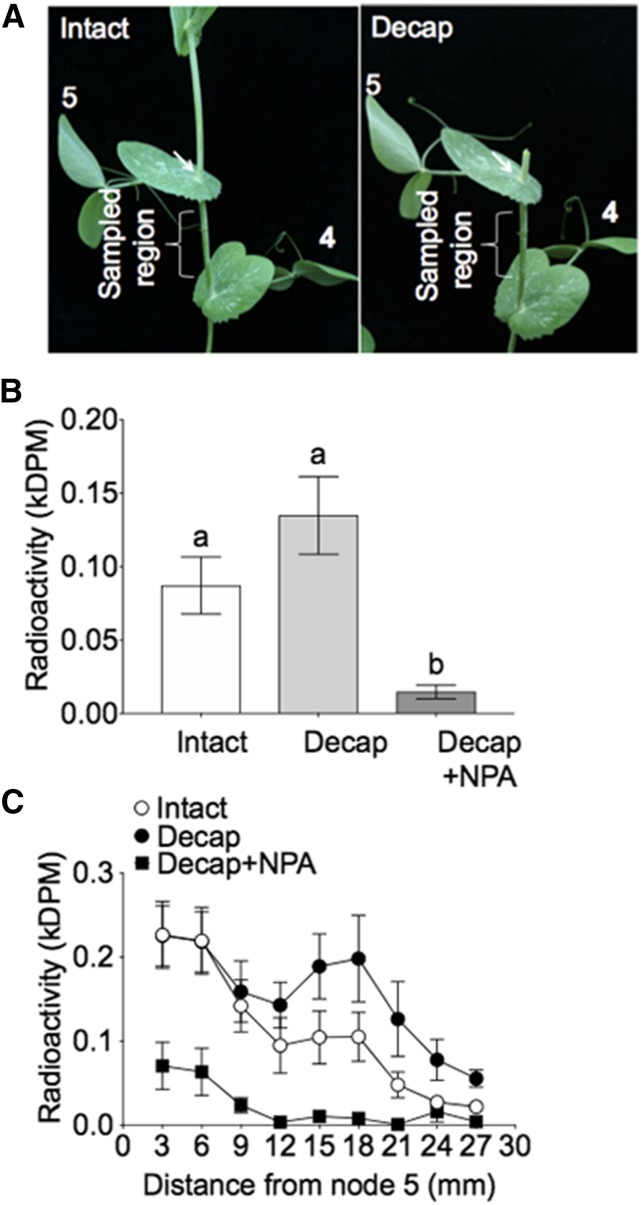
Dormant axillary buds export auxin ([3H]IAA) into the main stem in appreciable amounts (Fig. 1, A and B). Cotreatment with the auxin transport inhibitor 1-N-naphthylphthalamic acid (NPA) effectively blocked auxin efflux from the bud to levels lower than auxin efflux from intact buds (Fig. 1), confirming that the radioactive substance flowing out of the bud is predominantly [3H]IAA in the polar auxin transport pathway (Rashotte et al., 2000; Morris et al., 2005). Decapitating the shoot tip 1 cm above the node caused a slight but not statistically significant increase in auxin efflux from the bud into the main stem, as measured at 5 h after decapitation (Fig. 1B; segments 12 to 27 mm from the bud). This result is consistent with previous research, which found that auxin export from axillary buds might be enhanced after decapitation (Balla et al., 2011).
Figure 1.
Effects of decapitation and NPA on auxin efflux from node 5 axillary buds into the main stem. A, Pea plants with seven leaves fully expanded were decapitated (decap) 10 mm above node 5 (A, right). Treatment site and region were sampled for [3H]IAA quantification. Three hours after decapitation, node 5 axillary buds were treated with 0.25 μL of [3H]IAA alone or in solution with 1 mm NPA. Two hours after treatment, internode tissue 3 to 27 mm below node 5 was sampled in 3-mm segments and quantified for radioactivity. B, Total [3H]IAA exported out of buds; different letters (a, b) on the top of the columns indicate statistically significant differences (P < 0.05; one-way ANOVA with Tukey’s correction). C, [3H]IAA exported out the buds as quantified in adjacent 3-mm stem segments. Data are means ± se (n = 3 to 6).
Auxin Transport and Perception Inhibitors Block Auxin Export Out of Dormant and Activated Axillary Buds
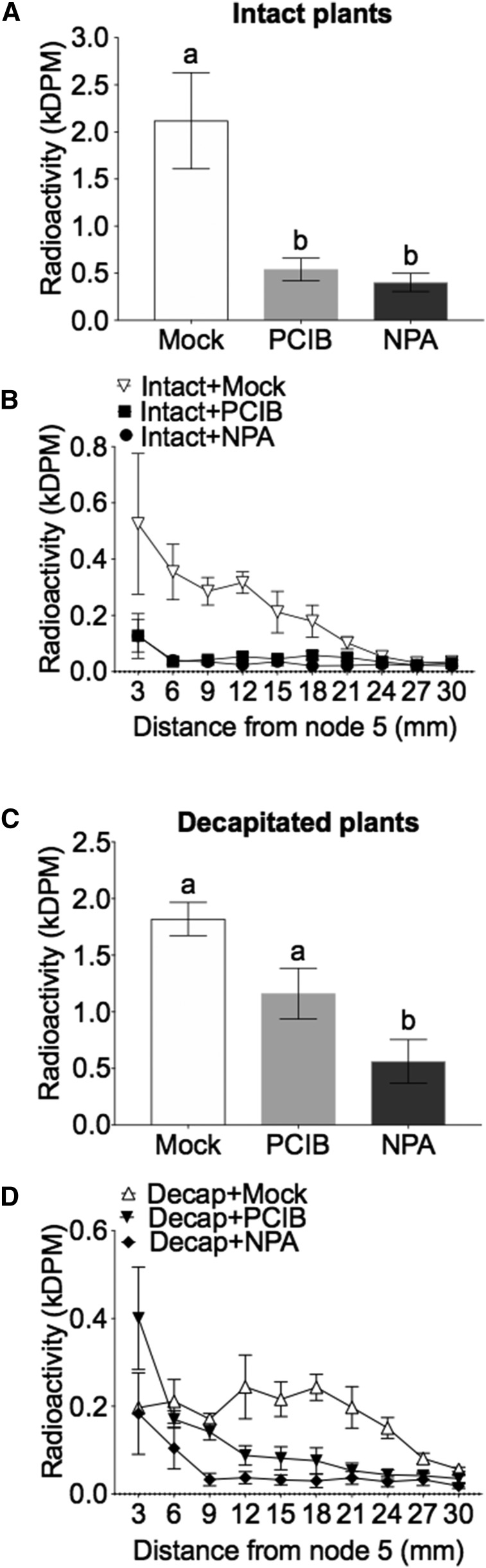
[3H]IAA efflux from axillary buds on intact and decapitated plants was inhibited when coapplied with NPA (Figs. 1 and 2) or p-chlorophenoxyisobutyric acid (PCIB), an auxin perception inhibitor (Fig. 2, A–C). Both NPA and PCIB significantly reduced auxin efflux from axillary buds in intact plants (Fig. 2A). Decapitation did not abolish the effect of PCIB and NPA on auxin transport, but PCIB was less effective in decapitated plants (Fig. 2, A and B).
Figure 2.
Reduced auxin signaling (1 mm PCIB) and auxin transport (1 mm NPA) reduces auxin efflux from axillary buds on intact plants (A and B) and in decapitated plants (C and D). A and C, Total [3H]IAA recovered in the stem after 3 h; different letters (a, b) on the top of the columns indicate statistically significant differences (P < 0.05; one-way ANOVA with Tukey’s correction). Data are means ± se (n = 4). B and D, [3H]IAA exported out the buds as quantified in adjacent 3-mm stem segments.
Auxin-Related Gene Expression Is Rapidly Altered by PCIB and NPA in Pea Axillary Buds
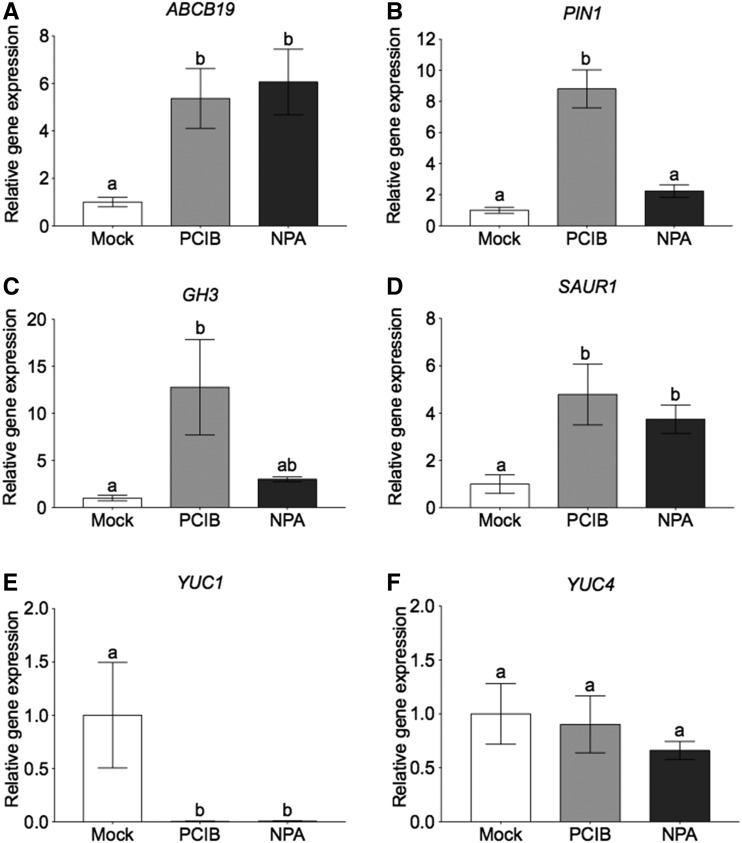
We conducted gene expression analysis to determine whether PCIB and NPA rapidly enter axillary buds and regulate auxin transport, auxin-inducible genes, and auxin biosynthesis genes. Auxin transport genes ABCB19 and PIN1 were up regulated in axillary buds 3 h after treatment with PCIB or NPA. PCIB and NPA block auxin transport out of buds (Fig. 2), which could lead to a build-up of auxin in the bud and result in the induction of these auxin transport genes as well as auxin-inducible GRETCHEN HAGEN 3 (GH3) and SMALL AUXIN-UP RNA 1 (SAUR1) genes (Fig. 3, A–D). The GH3 enzyme catalyzes the conjugation of indole acetic acid to amino acids, a detoxification reaction initiated by high concentrations of auxin (Hagen and Guilfoyle, 2002; Růžička and Hejátko, 2017). SAURs are the largest family of early auxin response genes (McClure and Guilfoyle, 1987; Ren and Gray, 2015). YUC1 and 4 genes were rapidly repressed by PCIB and NPA applications (Fig. 3, E and F). YUC proteins were identified as key enzymes catalyzing the rate-limiting step in tryptophan-dependent auxin biosynthesis in Arabidopsis (Zhao et al., 2001). Because NPA and PCIB block auxin transport from the buds (Fig. 2), the accumulation of auxin in the buds could result in the negative feedback inhibition of these auxin biosynthesis genes (Suzuki et al., 2015). YUC genes encode flavin monooxygenase-like enzymes; overexpression of YUC genes leads to auxin overproduction, while their inactivation causes developmental defects that can be recovered by exogenous auxin applications (Cheng et al., 2006; Zhao, 2010).
Figure 3.
PCIB and NPA enter axillary buds and rapidly regulate auxin marker genes. Node 5 buds of L107 pea plants with seven leaves fully unfolded were treated for 3 h with 1 mm PCIB or 1 mm NPA. Expression of auxin marker genes in the bud is represented relative to the mock (100% ethanol). A and B, Auxin transport genes ABCB19 and PIN1. C and D, Auxin-inducible genes GH3 and SAUR1. E and F, Auxin biosynthesis genes YUC1 and YUC4. EF1α and GADPH were used as the internal reference genes. Data are means ± se (n = 5 pools of 4 plants).
NPA and PCIB Applied on the Bud Do Not Block Polar Auxin Transport in the Main Stem
Previous studies show that NPA applied in a lanolin ring around the stem below the shoot tip depletes the endogenous auxin content of the stem and reduces auxin transport from the shoot tip above, thereby acting systemically (Morris et al., 2005; Brewer et al., 2009, 2015). This finding diminishes the value of using auxin transport inhibitors to study shoot branching because auxin transport in the main stem and axillary buds may be affected. To determine if NPA and PCIB, applied on the axillary bud, move out into the stem to antagonize the stem polar auxin transport stream, we applied [3H]IAA on the apical bud and attempted to intercept its transport down the stem by applying NPA and PCIB on the axillary bud at node 5 (Fig. 4A). Yet there was no effect of bud-applied NPA and PCIB on auxin flow in the main stem (Fig. 4, B and C), suggesting that as a developing sink, the bud might not be exporting NPA and PCIB into the main stem within the time frame in which we observe these inhibitors to block auxin efflux out of axillary buds (Figs. 1 and 2). Therefore, applying auxin transport inhibitors directly on buds enables a difference in auxin transport from the buds specifically—compared to the stem, which remains unaffected during the experimental period.
Figure 4.
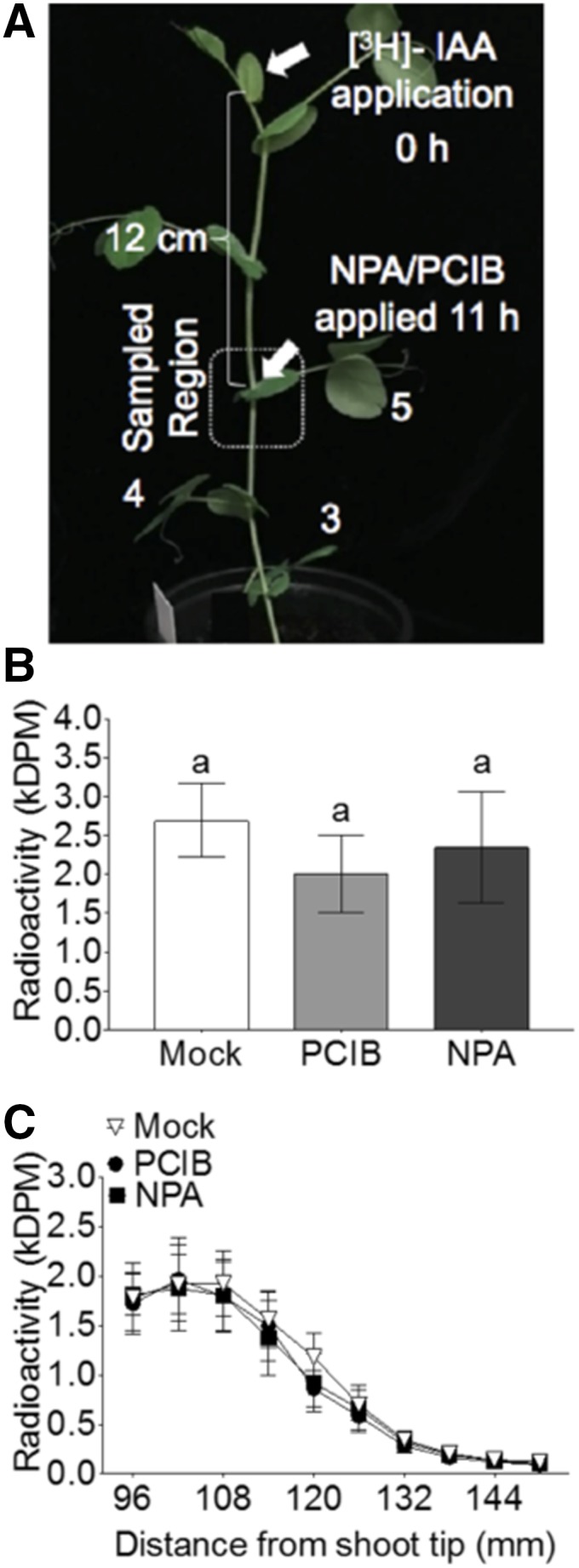
PCIB or NPA treatment to axillary buds has no effect on polar auxin transport (PATS) in the main stem. A, 0.25 μL of [3H]IAA was applied to the shoot tip of pea plants with seven leaves fully expanded and 120-mm distance between node 5 and the shoot tip. B, eleven hours after [3H]IAA application, 0.25 μL of either mock solution (100% ethanol), 1 mm NPA, or 1 mm PCIB was supplied to axillary bud on node 5. B and C, Two hours after NPA or PCIB application, internode tissue 30 mm above and below node 5 was sampled in 6-mm segments and quantified for radioactivity. B, Total [3H]IAA in the sampled stem segments; the letter “a” on the top of the columns indicates statistically significant differences (P < 0.05; one-way ANOVA with Tukey’s correction). Data are means ± se (n = 4). C, [3H]IAA in the sampled stem sections as quantified in continuous 3-mm stem segments.
NPA and PCIB Do Not Inhibit Early Bud Outgrowth after Decapitating the Shoot Tip
Having demonstrated that NPA (and to a lesser extent, PCIB) inhibit auxin efflux from axillary buds after decapitation (Fig. 2, A and B), we used time-lapse photography to measure the effect of these inhibitors on decapitation-induced bud growth in pea plants. Applying either NPA or PCIB directly on node 5 axillary buds does not inhibit their outgrowth within the first 20 h after decapitation (Fig. 5, A and B).
Figure 5.
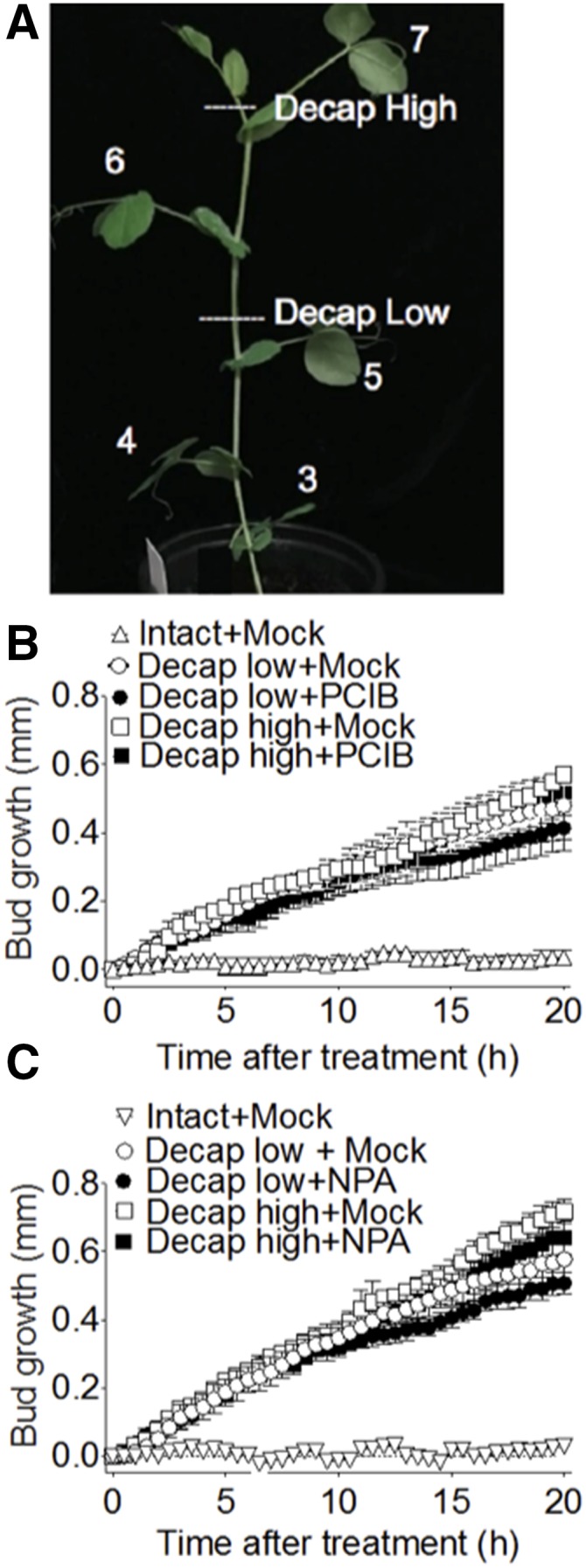
PCIB, NPA, and the distance at which plants were decapitated above node 5 had no effect on early bud growth after decapitation. Illustrated are the effects of 1 mm of either NPA or PCIB on the node 5 axillary bud outgrowth in pea plants. A, The position is shown at which the plants were decapitated, 1 cm (low) or 10 cm (high) above node 5. B and C, Bud growth responses are shown to 2 μL of treatments to the node 5 bud, including 1 mm PCIB (B) and 1 mm NPA (C).
Our experimental results indicate that initial bud growth occurs independent of changes in auxin content in the adjacent stem. It is well established that auxin transport—and inversely, its depletion in plant stems after decapitation—occurs at a rate of 1 cm per hour (Goldsmith et al., 1981; Morris et al., 2005; Renton et al., 2012; Mitchison, 2015). Decapitating the plants low (1 cm above the node; Fig. 5A) facilitates auxin depletion in the stem adjacent to the bud within 1 h. On the other hand, decapitating high (10 cm above the node; Fig. 5A) delays auxin depletion in the stem adjacent to the bud for up to 10 h. If auxin depletion in the main stem were the main facilitator of bud outgrowth after decapitation (Müller and Leyser, 2011; Balla et al., 2016), we would expect a delay in bud outgrowth in the plants decapitated high relative to those decapitated low. However, this was not the case (Fig. 5, A–C). This result is consistent with physiological and computational analysis of auxin transport dynamics in pea where auxin depletion in the stem after decapitation is not correlated to initial bud outgrowth (Morris et al., 2005; Renton et al., 2012; Mason et al., 2014).
NPA and PCIB Reduce Sustained Axillary Bud Growth
NPA and PCIB reduce bud outgrowth 48 to 72 h after decapitation (Fig. 6A), which is consistent with studies in which NPA inhibited sustained and not early bud growth (Brewer et al., 2009). NPA inhibition was more pronounced compared to that of PCIB, with a >3-fold repression in branch growth observed 3 d after decapitation (Fig. 6A). Similarly, NPA inhibited BA-induced bud growth in the long-term but not over the first two days (Fig. 6B), which is consistent with earlier reports (Brewer et al., 2009). PCIB was applied on buds together with the auxin biosynthesis inhibitor l-Kynurenine (Kyn), a potent inhibitor of TAA auxin biosynthesis enzymes (He et al., 2011). This application on axillary buds of decapitated plants could not inhibit early bud growth, even at a high dose of 10 mM, with inhibition becoming apparent from 3 d after treatment (Supplemental Fig. S1). Kyn should reduce any residual auxin that might not be blocked by PCIB. However, based on these results, we propose that sustained bud outgrowth requires the released bud to maintain auxin production and polar auxin transport.
Figure 6.
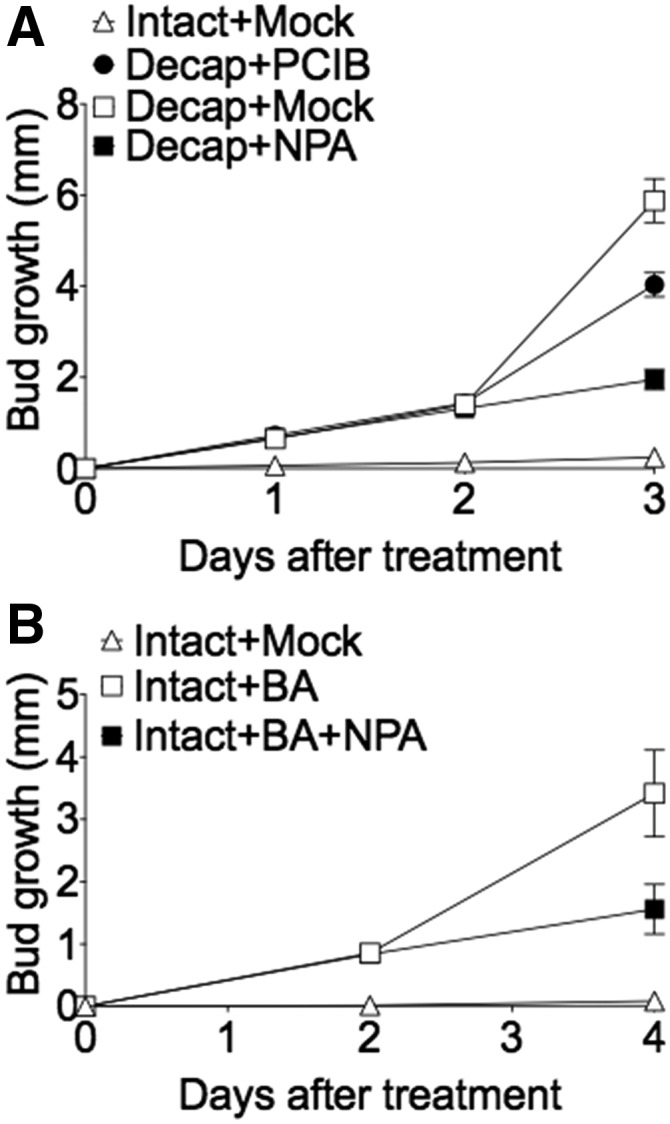
Effects of PCIB and NPA on initial and sustained axillary bud outgrowth. A, Pea plants with seven leaves fully expanded were decapitated 10mm above node 5 and treated with 2 μL of either a mock solution (100% ethanol), 1 mm NPA, or 1 mm PCIB. Bud lengths were measured daily over a period of 3 d after treatment. Data are means ± se (n = 12). B, Axillary buds at node 5 of pea plants with seven leaves fully expanded were treated with 2 μL of 50 μm BA alone (or in solution with 1 mm NPA). Bud lengths were measured 2 and 4 d after treatment. Data are means ± se (n = 4).
DISCUSSION
This study has overcome two problems in studying the auxin canalization theory concerning bud outgrowth. One problem was avoiding the use of mutants that have long-term effects on auxin transport or hormone signaling. These effects include irreversible changes in vascular development that may predispose plants to altered physiological responses. The second problem was the difficulty in obtaining temporal and tissue-specific changes in auxin transport. Such changes were needed to enable a direct test of the theory that bud dormancy and release are caused by the relative difference in auxin transport in the stem and axillary bud. By using a minuscule amount of chemicals supplied to the axillary bud, we suppressed auxin transport specifically from axillary buds without affecting auxin transport in the stem. Despite the plants exhibiting vastly different amounts of [3H]IAA transport from the axillary buds after decapitation, there was no difference in bud outgrowth.
We initially demonstrated that dormant buds export auxin into the main stem (Fig. 1, A and B; 2A). Stafstrom and Sussex (1988) showed that dormant buds of pea are metabolically active because they incorporate radiolabeled amino acids into proteins at rates equivalent to or greater than growing buds. These buds are also photosynthetically active and contain several primordia and vasculature (Stafstrom and Sarup, 2000; Kang et al., 2003). Removing the shoot tip did not consistently lead to an increase in auxin export from the bud (Figs. 1 and 2), indicating that the amount of auxin exported by a bud might not be a reliable marker of dormancy or bud activation. However, this finding is not, by itself, inconsistent with the auxin transport/canalization model (Bennett et al., 2016), which proposes that changes in the relative auxin transport from buds (compared with stem) influence axillary bud outgrowth.
Treatments that affect auxin transport primarily from axillary buds, without affecting auxin transport in the main stem, indicate that bud release is not dependent on the relative amount of auxin transport from the bud compared with the main stem (Fig. 4). NPA and PCIB have different modes of action that lead to altered auxin transport. NPA inhibits both PIN and ABCB auxin efflux activities in a noncompetitive manner (Kleine-Vehn et al., 2006; Petrásek et al., 2006; Sauer et al., 2006; Blakeslee et al., 2007), while PCIB blocks auxin receptor function (Hertel et al., 1969; Oono et al., 2003). Both NPA and PCIB rapidly inhibit auxin transport from axillary buds, NPA presumably acting directly and PCIB acting indirectly. PCIB can be applied in combination with l-Kynurenine (Kyn), a tryptophan (an auxin precursor) metabolite in animal systems; this is a competitive inhibitor of plant TAA enzymes (He et al., 2011; Gao et al., 2016; Tsugafune et al., 2017). Yet the combination also failed to not inhibit early bud outgrowth after decapitation (Supplemental Fig. S1). However, Kyn has been shown to enhance auxin biosynthesis in Arabidopsis plants overexpressing the bacterial tryptophan oxidase, RebO (35S::RebO). This enhancement could be because unlike plant TAAs, RebO is capable of converting Kyn to indole-3-propionic acid (IPA), which is then converted to auxin by YUCCA enzymes (Mashiguchi et al., 2011; Won et al., 2011; Tsugafune et al., 2017). PCIB and NPA rapidly enter the buds and regulate auxin-inducible, auxin biosynthesis and transport genes in axillary buds 3 h after application (Fig. 3). Importantly, NPA and PCIB applied to axillary buds of pea have a localized action because neither of these inhibitors had a measurable effect on auxin transport in the main stem within the 3-h time frame during which the inhibition of auxin transport from buds is observed (Figs. 1, 2, and 4). Blocking auxin export from buds with NPA or PCIB did not result in a corresponding decrease in timing of the onset of bud outgrowth after decapitation or in the amount of bud growth over 20 h (Fig. 5, C and D). These results separate the timing of early bud outgrowth with any relative changes in auxin transport in bud and stem. We therefore propose that initial bud outgrowth after removing the shoot tip occurs independently of auxin canalization/transport out of the buds.
These effects on auxin transport without an effect on growth are observed regardless of whether there is a decrease in auxin content in the adjacent stem (Fig. 4). According to the auxin canalization theory, auxin depletion in the stem should enhance auxin flow from the bud and lead to earlier growth. Decapitation 1 cm from the bud would cause a drop in auxin content in the adjacent stem up to 9 hours earlier than for buds 10 cm further down the stem. However, at these different positions, there was no obvious difference in the timing of bud growth. Furthermore, despite the earlier auxin depletion in the plants that were decapitated closer to the node, there was still no significant effect of the NPA or PCIB treatments during the 20-h period (Fig. 5). There is strong evidence suggesting that removing the shoot tip rapidly enhances sugar availability to axillary buds and triggers their growth (Mason et al., 2014; Fichtner et al., 2017). The diversion of sugar to the axillary buds after shoot tip removal is much faster than the auxin depletion that is required for the auxin canalization model (Morris et al., 2005; Renton et al., 2012; Mason et al., 2014). We would therefore predict that buds at different positions along the stem would have a similar timing of initial response due to the rapid redistribution of available sugars, and this timing is indeed observed (Fig. 5).
Sugars have been shown to induce RhTAR1 and RhYUC1 expression and increase auxin levels in axillary buds on rose (Rosa hybrida) stem sections grown in vitro (Barbier et al., 2015b), which could increase auxin efflux out of the buds. We show here that such an increase (Gocal et al., 1991; Balla et al., 2002), which we did not repeatedly observe, is not required for bud growth over 1 to 2 d (Fig. 6; Brewer et al., 2009). In the longer term, it is possible that auxin transport is important for continued bud growth in pea (Fig. 6; Supplemental Fig. 1; Brewer et al., 2009, 2015). After 48 h, auxin canalization/transport out of the bud and associated vasculature development may be required to support the continued growth and development of buds. This idea is consistent with the widely accepted role of auxin signaling and transport on organ development as well as vascular differentiation (Sachs, 2000; Gallavotti, 2013). Inhibiting polar auxin transport in pea bud and stem reduces the number of vascular connections that form in buds, even though their initial growth is not affected (Brewer et al., 2015). This effect is consistent with the role of auxin influx (AUX1/LAX) and efflux (PIN) proteins in regulating vascular patterning and xylem differentiation in plants (Scarpella and Meijer, 2004; Fàbregas et al., 2015). Interestingly, while the plant hormone strigolactone rapidly affects PIN protein localization (Crawford et al., 2010), it can still reduce bud growth in NPA-treated plants that have greatly reduced auxin transport (Brewer et al., 2015). This action suggests that strigolactones may have at least one mode of action that is independent of auxin-transport. Remarkably, the most striking effects on auxin transport for pea implicate correlative inhibition, which involves competition between well-established shoots (Morris, 1977).
The model supported by these findings is that initial bud outgrowth after removing the shoot tip or directly applying cytokinins (benzyladenine; BA) to axillary buds occurs independent of auxin canalization/transport out of the buds. This independence may be mediated, at least partly, by an increase in trehalose-6-phosphate levels in axillary buds (Fichtner et al., 2017), which is thought to be activated by enhanced Suc availability (Schluepmann et al., 2004; Lunn et al., 2006). That is, the initial growth of buds during the 24 h after activation is likely to be related to the diversion of sugars after removing the shoot tip or after directly applying cytokinin to the axillary bud (Dun et al., 2012; Mason et al., 2014; Roman et al., 2016; Wang et al., 2016; Fichtner et al., 2017). While auxin transport may still play a role in this process, we have demonstrated that decapitation does not require auxin transport to trigger bud outgrowth.
The development of these tools to measure early bud growth and the characterization of the physiological mediators will allow us to investigate this area further. Intriguingly, pea axillary buds are arrested in the G1 phase of the cell cycle (Devitt and Stafstrom, 1995; Shimizu and Mori, 1998). After decapitation, the initial cell divisions, i.e. G1 to S-phase transition (Horvath et al., 2003; Berckmans and De Veylder, 2009), occur from 6 h after decapitation in axillary buds (Devitt and Stafstrom, 1995), an action that is coupled with cell expansion as evidenced by expression of EXPANSIN genes (Roman et al., 2016, 2017). Results presented in this study indicate these molecular mechanisms driving the activation of dormant axillary buds by either decapitation or cytokinin applications occur independent to auxin signaling and auxin transport.
Conclusions
Our findings propose an alternative hypothesis to the long-standing hypothesis that bud outgrowth is dependent on the axillary bud establishing its own polar auxin transport stream into the main stem. Rather, initial bud outgrowth occurs independently of auxin flow out of the bud and without any changes in auxin transport in the adjacent stem. Future studies on apical dominance and the effects of decapitation should explore other candidates for the initial triggers of bud outgrowth—including cytokinins, strigolactones, and sugars (Dun et al., 2012; Mason et al., 2014; Roman et al., 2016; Fichtner et al., 2017). Moreover, the cellular events enabling growth after decapitation are still unknown, particularly the extent to which cell cycle regulation and/or cell expansion (Devitt and Stafstrom, 1995; Roman et al., 2016, 2017) are important in early bud growth. The role of auxin transport in the continued growth of shoots into branches needs further investigation. For example, it would be good to define changes in auxin transport in shoots. Are changes causal of different growth rates, and of branching patterns of plants with different shoot architecture?
MATERIALS AND METHODS
Plant Material and Growth Conditions
Torsdag L107 pea (Pisum sativum) plants were grown at two per two-liter pot using the potting mix described by Mason et al. (2014), in a temperature-controlled room with a 23°C day/18°C night, 18-h photoperiod with an irradiance of 150 to 200 μM m−2 s−1 provided by white fluorescent tubes. Nutritional supplements were administered as described by Brewer et al. (2015). Plants with seven leaves fully unfolded were used. Nodes were numbered acropetally from the first scale leaf as node 1. Node 5 buds were used.
Radiolabeled Assays
Decapitation Experiments
Plants were decapitated 1 cm above node 5 (Fig. 1) except for the upper decapitation shown in Figure 5. Three hours after decapitation, 0.25 μL of tritiated auxin (370 kBq [3H]IAA; American Radiolabeled Chemicals, Inc., St Louis, MO) in ethanol with 1 mm NPA, 1 mm PCIB, or 100% ethanol was applied directly to node 5 buds and allowed to translocate down the stem for a period of two hours. Two hours after the [3H]IAA ± NPA/PCIB application, and as in all transport experiments, the internode/ stem segment below node 5 was sampled in adjacent, 3-mm sections. Radioactivity was then quantified from the stem segments as dpm (DPM) in accordance with Brewer et al. (2009). The total radioactivity was the sum of all segments and was measured excluding the first four/five segments, which included the node itself and 3 mm below it.
Stem Auxin Transport Experiment
Plants with precisely 12-cm distance between node 5 and the shoot tip were used for this experiment (Fig. 4). 0.25-μL 3H-IAA was applied to the apical bud in the shoot tip and left to translocate down the stem for a period of eleven hours. As auxin travels at a rate of 1 cm/hour (Brewer et al., 2009), the peak front of [3H]IAA was estimated to be 1 cm above node 5 in the stem eleven hours after application. After the eleven-hour period, 0.25 μL of 1 mm NPA, 1 mm PCIB, or 100% ethanol was applied to the node 5 axillary bud; the stem was then sampled 2 h later to capture the auxin wave.
Bud Growth Measurements
Initial Bud Outgrowth
Bud growth within the first 20 h after decapitation was measured using time-lapse photography as per Mason et al. (2014).
Sustained Bud Outgrowth
Bud and branch lengths were measured with digital calipers.
BA Treatment
Two microliters of 50 μm BA in 0.01% dimethyl sulfoxide, 0.1 Tween-20, and 8% PEG 1450 solution ± NPA was treated to node 5 axillary buds. Buds were measured with digital calipers daily, two and four days after treatment.
Kyn and PCIB Treatment
The L107 pea plants were grown in conditions described by Brewer et al. (2015). Plants were decapitated 1 cm above node 4. Node 2 buds were treated with 2 μL of 1 mm Kyn + 1 mm PCIB, or 10 mm Kyn + 10 mm PCIB. The inhibitors were dissolved in 0.01% dimethyl sulfoxide, 0.1 Tween-20, and 8% PEG 1450 solution. Buds were measured with digital calipers daily, for four days.
Quantitative Gene Expression Analysis
For gene expression studies, node 5 buds of L107 pea plants having seven fully expanded leaves were treated with 1 μM of 100% ethanol, 1 mM PCIB, or 1 mM NPA. Three hours later, the buds were harvested and immediately frozen in liquid nitrogen and stored at −80°C. Total RNA was isolated using the cetyltrimethylammonium bromide (CTAB) protocol for RNA extraction (adapted from MacRae, 2007 with modifications) and quantified using the NanoVue spectrophotometer. cDNA synthesis, RT-qPCR, and data analysis were performed as described by Mason et al. (2014). Primer sequences used for the experiment are described in the supplementary data (Supplemental Table S1). ABCB19, GH3, PIN1, SAUR1, YUC1, and YUC4 expression was measured against that of TRANSLATION ELONGATION FACTOR 1-ALPHA (EF1α) and GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE (GADPH). For all experiments, five biological replicates (each consisting of four plants) were used, with error bars in the figures representing different biological replicates.
Statistical Analysis
For statistical analyses, one-way ANOVA was performed using GraphPad Prism version 7.03 (GraphPad Software; www.graphpad.com).
Supplemental Data
The following supplementary materials are available in the online version of this article.
Supplemental Figure 1. Combining L-Kynurenine (Kyn) and PCIB Failed to Inhibit Early Decapitation-Induced Bud Outgrowth but Antagonized Sustained Bud Growth.
Supplemental Table 1. List of Primers Used in this Study.
Acknowledgments
The authors would like to thank Dr. Elizabeth A Dun and Dr. François Barbier for their worthwhile comments on the manuscript.
Footnotes
This research was supported by the University of Queensland grant (DP110100997 to C.A.B.) and Australian Research Council Fellowship (FT100100806 to C.A.B.). Funding sources were not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Articles can be viewed without a subscription.
Senior author.
References
- Adamowski M, Friml J (2015) PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 27: 20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MM, Hammer GL, van Oosterom EJ, Cruickshank AW, Hunt CH, Jordan DR (2014) A physiological framework to explain genetic and environmental regulation of tillering in sorghum. New Phytol 203: 155–167 [DOI] [PubMed] [Google Scholar]
- Balla J, Blazkova J, Reinohl V, Prochazka S (2002) Involvement of auxin and cytokinins in initiation of growth of isolated pea buds. Plant Growth Regul 38: 149–156 [Google Scholar]
- Balla J, Kalousek P, Reinöhl V, Friml J, Procházka S (2011) Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J 65: 571–577 [DOI] [PubMed] [Google Scholar]
- Balla J, Medveďová Z, Kalousek P, Matiješčuková N, Friml J, Reinöhl V, Procházka S (2016) Auxin flow-mediated competition between axillary buds to restore apical dominance. Sci Rep 6: 35955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzan S, Johal GS, Carraro N (2014) The role of auxin transporters in monocots development. Front Plant Sci 5: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier FF, Lunn JE, Beveridge CA (2015a) Ready, steady, go! A sugar hit starts the race to shoot branching. Curr Opin Plant Biol 25: 39–45 [DOI] [PubMed] [Google Scholar]
- Barbier F, Péron T, Lecerf M, Perez-Garcia MD, Barrière Q, Rolčík J, Boutet-Mercey S, Citerne S, Lemoine R, Porcheron B, Roman H, Leduc N, et al. (2015b) Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot 66: 2569–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz RG, Piepho HP (2013) Variability of hormetic dose responses of the antiauxin PCIB on Lactuca sativa in a plant bioassay. Weed Res 53: 418–428 [Google Scholar]
- Bennett T, Hines G, van Rongen M, Waldie T, Sawchuk MG, Scarpella E, Ljung K, Leyser O (2016) Connective auxin transport in the shoot facilitates communication between shoot apices. PLoS Biol 14: e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berckmans B, De Veylder L (2009) Transcriptional control of the cell cycle. Curr Opin Plant Biol 12: 599–605 [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Weller JL, Singer SR, Hofer JMI (2003) Axillary meristem development. Budding relationships between networks controlling flowering, branching, and photoperiod responsiveness. Plant Physiol 131: 927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J, et al. (2007) Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19: 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150: 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Gui R, Mason MG, Beveridge CA (2015) Strigolactone inhibition of branching independent of polar auxin transport. Plant Physiol 168: 1820–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y (2006) Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20: 1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M, Cho HT (2013) The function of ABCB transporters in auxin transport. Plant Signal Behav 8: e22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MG. (1991) Apical dominance. Bot Rev 57: 318–358 [Google Scholar]
- Cline MG. (1996) Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot 78: 255–266 [Google Scholar]
- Cline MG, Chatfield SP, Leyser O (2001) NAA restores apical dominance in the axr3-1 mutant of Arabidopsis thaliana. Ann Bot 87: 61–65 [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913 [DOI] [PubMed] [Google Scholar]
- de Jong M, George G, Ongaro V, Williamson L, Willetts B, Ljung K, McCulloch H, Leyser O (2014) Auxin and strigolactone signaling are required for modulation of Arabidopsis shoot branching by nitrogen supply. Plant Physiol 166: 384–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt ML, Stafstrom JP (1995) Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol Biol 29: 255–265 [DOI] [PubMed] [Google Scholar]
- Di DW, Zhang C, Guo GQ (2015) Involvement of secondary messengers and small organic molecules in auxin perception and signaling. Plant Cell Rep 34: 895–904 [DOI] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12: 211–221 [DOI] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158: 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fàbregas N, Formosa-Jordan P, Confraria A, Siligato R, Alonso JM, Swarup R, Bennett MJ, Mähönen AP, Cano-Delgado AI, Ibanes M; PLOS Genetics Staff (2015) Auxin influx carriers control vascular patterning and xylem differentiation in Arabidopsis thaliana. PLoS Genet 11: e1005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner F, Barbier FF, Feil R, Watanabe M, Annunziata MG, Chabikwa TG, Höfgen R, Stitt M, Beveridge CA, Lunn JE (2017) Trehalose 6-phosphate is involved in triggering axillary bud outgrowth in garden pea (Pisum sativum L.). Plant J 92: 611–623 [DOI] [PubMed] [Google Scholar]
- Gallavotti A. (2013) The role of auxin in shaping shoot architecture. J Exp Bot 64: 2593–2608 [DOI] [PubMed] [Google Scholar]
- Gao Y, Dai X, Zheng Z, Kasahara H, Kamiya Y, Chory J, Ballou D, Zhao Y (2016) Overexpression of the bacterial tryptophan oxidase RebO affects auxin biosynthesis and Arabidopsis development. Sci Bull 61: 859–867 [Google Scholar]
- Girault T, Abidi F, Sigogne M, Pelleschi-Travier S, Boumaza R, Sakr S, Leduc N (2010) Sugars are under light control during bud burst in Rosa sp. Plant Cell Environ 33: 1339–1350 [DOI] [PubMed] [Google Scholar]
- Gocal GFW, Pharis RP, Yeung EC, Pearce D (1991) Changes after decapitation in concentrations of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv Tender Green. Plant Physiol 95: 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith MHM, Goldsmith TH, Martin MH (1981) Mathematical analysis of the chemosmotic polar diffusion of auxin through plant tissues. Proc Natl Acad Sci USA 78: 976–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle T (2002) Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol Biol 49: 373–385 [PubMed] [Google Scholar]
- Hayashi K, Tan X, Zheng N, Hatate T, Kimura Y, Kepinski S, Nozaki H (2008) Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proc Natl Acad Sci USA 105: 5632–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Brumos J, Li H, Ji Y, Ke M, Gong X, Zeng Q, Li W, Zhang X, An F, et al. (2011) A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23: 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Rabot A, Laloi M, Mortreau E, Sigogne M, Leduc N, Lemoine R, Sakr S, Vian A, Pelleschi-Travier S (2011) Regulation of RhSUC2, a sucrose transporter, is correlated with the light control of bud burst in Rosa sp. Plant Cell Environ 34: 1776–1789 [DOI] [PubMed] [Google Scholar]
- Hertel R, de la Fuente RK, Leopold AC (1969) Geotropism and the lateral transport of auxin in the corn mutant amylomaize. Planta 88: 204–214 [DOI] [PubMed] [Google Scholar]
- Horvath DP, Anderson JV, Chao WS, Foley ME (2003) Knowing when to grow: Signals regulating bud dormancy. Trends Plant Sci 8: 534–540 [DOI] [PubMed] [Google Scholar]
- Hosokawa Z, Shi L, Prasad TK, Cline MG (1990) Apical dominance control in Ipomea nil: The influence of the shoot apex, leaves and stem. Ann Bot 65: 547–556 [Google Scholar]
- Kang J, Tang J, Donnelly P, Dengler N (2003) Primary vascular pattern and expression of ATHB-8 in shoots of Arabidopsis. New Phytol 158: 443–454 [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Mullet JE (2015) Photosynthetic leaf area modulates tiller bud outgrowth in sorghum. Plant Cell Environ 38: 1471–1478 [DOI] [PubMed] [Google Scholar]
- Kim HK, van Oosterom E, Dingkuhn M, Luquet D, Hammer G (2010) Regulation of tillering in sorghum: Environmental effects. Ann Bot 106: 57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J (2006) Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 18: 3171–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Bangerth F (1999) Autoinhibition of indoleacetic acid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol Plant 106: 415–420 [Google Scholar]
- Lilley JL, Gee CW, Sairanen I, Ljung K, Nemhauser JL (2012) An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol 160: 2261–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28: 465–474 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae E. (2007) Extraction of plant RNA. Methods Mol Biol 353: 15–24 [DOI] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, et al. (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA 108: 18512–18517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci USA 111: 6092–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure BA, Guilfoyle T (1987) Characterization of a class of small auxin-inducible soybean polyadenylated RNAs. Plant Mol Biol 9: 611–623 [DOI] [PubMed] [Google Scholar]
- Mitchison G. (2015) The shape of an auxin pulse, and what it tells us about the transport mechanism. PLOS Comput Biol 11: e1004487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DA. (1977) Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.): Some implications for polarity and apical dominance. Planta 136: 91–96 [DOI] [PubMed] [Google Scholar]
- Morris SE, Cox MC, Ross JJ, Krisantini S, Beveridge CA (2005) Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol 138: 1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D, Leyser O (2011) Auxin, cytokinin and the control of shoot branching. Ann Bot 107: 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oono Y, Ooura C, Rahman A, Aspuria ET, Hayashi K, Tanaka A, Uchimiya H (2003) p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiol 133: 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Swarup K, Ferguson A, Seth M, Yang Y, Dhondt S, James N, Casimiro I, Perry P, Syed A, et al. (2012) AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell 24: 2874–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, Wisniewska J, Tadele Z, Kubes M, Covanová M, et al. (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918 [DOI] [PubMed] [Google Scholar]
- Phillips IDJ. (1975) Apical dominance. Annu Rev Plant Physiol 26: 341–367 [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA 106: 17431–17436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabot A, Henry C, Ben Baaziz K, Mortreau E, Azri W, Lothier J, Hamama L, Boummaza R, Leduc N, Pelleschi-Travier S, et al. (2012) Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol 53: 1068–1082 [DOI] [PubMed] [Google Scholar]
- Rameau C, Bertheloot J, Leduc N, Andrieu B, Foucher F, Sakr S (2015) Multiple pathways regulate shoot branching. Front Plant Sci 5: 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12: 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Gray WM (2015) SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol Plant 8: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton M, Hanan J, Ferguson BJ, Beveridge CA (2012) Models of long-distance transport: How is carrier-dependent auxin transport regulated in the stem? New Phytol 194: 704–715 [DOI] [PubMed] [Google Scholar]
- Roman H, Girault T, Barbier F, Péron T, Brouard N, Pěnčík A, Novák O, Vian A, Sakr S, Lothier J, et al. (2016) Cytokinins are initial targets of light in the control of bud outgrowth. Plant Physiol 172: 489–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H, Girault T, Le Gourrierec J, Leduc N (2017) In silico analysis of 3 expansin gene promoters reveals 2 hubs controlling light and cytokinins response during bud outgrowth. Plant Signal Behav 12: e1284725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Bopp M (1983) Uptake and polar transport of indoleacetic acid in moss rhizoids. Physiol Plant 58: 57–61 [Google Scholar]
- Rubery PH, Sheldrake AR (1974) Carrier-mediated auxin transport. Planta 118: 101–121 [DOI] [PubMed] [Google Scholar]
- Růžička K, Hejátko J (2017) Auxin transport and conjugation caught together. J Exp Bot 68: 4409–4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. (1968) On the determination of the pattern of vascular tissue in peas. Ann Bot 32: 781–790 [Google Scholar]
- Sachs T. (1969) Polarity and the induction of organised vascular tissues. Ann Bot 33: 263–275 [Google Scholar]
- Sachs T. (2000) Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol 41: 649–656 [DOI] [PubMed] [Google Scholar]
- Sairanen I, Novák O, Pěnčík A, Ikeda Y, Jones B, Sandberg G, Ljung K (2012) Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 24: 4907–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wiśniewska J, Reinöhl V, Friml J, Benková E (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20: 2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Meijer AH (2004) Pattern formation in the vascular system of monocot and dicot plant species. New Phytol 164: 209–242 [DOI] [PubMed] [Google Scholar]
- Schluepmann H, van Dijken A, Aghdasi M, Wobbes B, Paul M, Smeekens S (2004) Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiol 135: 879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Mori H (1998) Changes in protein interactions of cell cycle-related genes during the dormancy-to-growth transition in pea axillary buds. Plant Cell Physiol 39: 1073–1079 [DOI] [PubMed] [Google Scholar]
- Shimizu-Sato S, Mori H (2001) Control of outgrowth and dormancy in axillary buds. Plant Physiol 127: 1405–1413 [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Sato S, Tanaka M, Mori H (2009) Auxin-cytokinin interactions in the control of shoot branching. Plant Mol Biol 69: 429–435 [DOI] [PubMed] [Google Scholar]
- Shinohara N, Taylor C, Leyser O (2013) Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 11: e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom JP, Sarup VB (2000) Development of supernumerary buds from the axillary meristem of pea, Pisum sativum (Fabaceae). Australian J Bot 48: 271–278 [Google Scholar]
- Stafstrom JP, Sussex IM (1988) Patterns of protein synthesis in dormant and growing vegetative buds of pea. Planta 176: 497–505 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yamazaki C, Mitsui M, Kakei Y, Mitani Y, Nakamura A, Ishii T, Soeno K, Shimada Y (2015) Transcriptional feedback regulation of YUCCA genes in response to auxin levels in Arabidopsis. Plant Cell Rep 34: 1343–1352 [DOI] [PubMed] [Google Scholar]
- Swarup R, Péret B (2012) AUX/LAX family of auxin influx carriers—An overview. Front Plant Sci 3: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantikanjana T, Yong JWH, Letham DS, Griffith M, Hussain M, Ljung K, Sandberg G, Sundaresan V (2001) Control of axillary bud initiation and shoot architecture in Arabidopsis through the SUPERSHOOT gene. Genes Dev 15: 1577–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann T, Muhr M (2015) Shaping plant architecture. Front Plant Sci 6: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV, Skoog F (1933) Studies on the growth hormone of plants. III. The inhibiting action of growth substance on bud development. Proc Natl Acad Sci USA 19: 714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Jiao Y (2015) A systems approach to understand shoot branching. Curr Plant Biol 3-4: 13–19 [Google Scholar]
- Tsugafune S, Mashiguchi K, Fukui K, Takebayashi Y, Nishimura T, Sakai T, Shimada Y, Kasahara H, Koshiba T, Hayashi KI (2017) Yucasin DF, a potent and persistent inhibitor of auxin biosynthesis in plants. Sci Rep 7: 13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hao Q, Tian F, Li Q, Wang W (2016) Cytokinin-regulated sucrose metabolism in stay-green wheat phenotype. PLoS One 11: e0161351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y (2011) Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci USA 108: 18518–18523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zha M, Li Y, Ding Y, Chen L, Ding C, Wang S (2015) The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.). Plant Cell Rep 34: 1647–1662 [DOI] [PubMed] [Google Scholar]
- Yuan C, Xi L, Kou Y, Zhao Y, Zhao L (2015) Current perspectives on shoot branching regulation. Frontiers of Agricultural Science and Engineering 2: 38–52 [Google Scholar]
- Zhao Y. (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61: 49–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309 [DOI] [PubMed] [Google Scholar]