The receptor for abscisic acid, which regulates seed dormancy and stomatal closure, mediates desiccation tolerance and sugar responses in liverworts that represent early diverging lineages of embryophytes.
Abstract
Abscisic acid (ABA) controls seed dormancy and stomatal closure through binding to the intracellular receptor Pyrabactin resistance1 (Pyr1)/Pyr1-like/regulatory components of ABA receptors (PYR/PYL/RCAR) in angiosperms. Genes encoding PYR/PYL/RCAR are thought to have arisen in the ancestor of embryophytes, but the roles of the genes in nonvascular plants have not been determined. In the liverwort Marchantia polymorpha, ABA reduces growth and enhances desiccation tolerance through increasing accumulation of intracellular sugars and various transcripts such as those of Late Embryogenesis Abundant (LEA)-like genes. In this study, we analyzed a gene designated MpPYL1, which is closely related to PYR/PYL/RCAR of angiosperms, in transgenic liverworts. Transgenic lines overexpressing MpPYL1-GFP showed ABA-hypersensitive growth with enhanced desiccation tolerance, whereas Mppyl1 generated by CRISPR-Cas9-mediated genome editing showed ABA-insensitive growth with reduced desiccation tolerance. Transcriptome analysis indicated that MpPYL1 is a major regulator of abiotic stress-associated genes, including all 35 ABA-induced LEA-like genes. Furthermore, these transgenic plants showed altered responses to extracellular Suc, suggesting that ABA and PYR/PYL/RCAR function in sugar responses. The results presented here reveal an important role of PYR/PYL/RCAR in the ABA response, which was likely acquired in the common ancestor of land plants. The results also indicate the archetypal role of ABA and its receptor in sugar response and accumulation processes for vegetative desiccation tolerance in bryophytes.
Abscisic acid (ABA) is a phytohormone that mediates developmental and physiological processes in vegetative and reproductive organs in plants. These processes include seed maturation and dormancy, bud dormancy, closure of leaf stomata, and acquisition of tolerance to various environmental stresses, such as drought and desiccation (Milborrow, 1974; Vishwakarma et al., 2017). ABA triggers the expression of stress-associated transcripts such as those encoding Late Embryogenesis Abundant (LEA)-like proteins (Cuming and Lane, 1979; Chandler and Robertson, 1994). ABA elicits the plant responses mentioned through binding to intracellular receptors known as Pyrabactin resistance1 (Pyr1)/Pyr1-like/regulatory components of ABA receptors (PYR/PYL/RCAR). All of the 14 PYR/PYL/RCARs in Arabidopsis (Arabidopsis thaliana) have been shown either by mutant analysis or transient gene expression assays to function as ABA receptors (Ma et al., 2009; Park et al., 2009; Fuchs et al., 2014). The pyr1pyl1pyl2pyl4 quadruple mutant shows reduced sensitivity to ABA in seed germination and stomatal closure (Park et al., 2009; Nishimura et al., 2010), and the pyr1pyl1pyl2pyl4pyl5pyl8 sextuple mutant shows strong ABA insensitivity with little inhibition even by 50 µM ABA (González-Guzmán et al., 2012). In the presence of ABA, the ABA-receptor complex binds to group A protein phosphatase 2C (PP2C), which is a negative regulator of ABA signaling, and inhibits its phosphatase activity. Inhibition of the activity of PP2C causes activation of subclass III Suc nonfermenting1 (SNF1)-related protein kinase2 (SnRK2), a central regulatory kinase that phosphorylates and activates various key cellular molecules necessary for ABA responses (Umezawa et al., 2009; Vlad et al., 2009; Cutler et al., 2010). Among the SnRK2 substrates are the bZIP transcription factors that recognize the ABA-responsive elements (ABREs) conserved in promoters of ABA-induced genes (Marcotte et al., 1989; Uno et al., 2000). With other promoter elements, such as CE3 and Sph/RY, ABREs facilitate a tissue-specific ABA response (Hattori et al., 1992).
Functions of ABA as a phytohormone have been shown not only in angiosperms but also in nonvascular basal land plants such as mosses. ABA treatment increases tolerance to rapid drying in the moss Funaria hygrometrica (Werner et al., 1991) and freezing tolerance in the moss Physcomitrella patens (Minami et al., 2003). In P. patens protonemata, treatment with ABA induces accumulation of stress-associated transcripts, including those of LEA-like genes, and low-molecular-weight soluble sugars, such as Suc (Knight et al., 1995; Nagao et al., 2005). Completed sequencing of the P. patens genome has revealed four genes for putative PYR/PYL/RCAR, two genes for group A PP2C, and four genes for subclass III SnRK2 (Rensing et al., 2008; Sakata et al., 2014). Disruption of both group A PP2C genes, PpABI1A and PpABI1B, results in a constitutive ABA response and desiccation tolerance (Komatsu et al., 2013). Furthermore, failure in SnRK2 activation in the P. patens mutant line AR7 causes loss of both ABA sensitivity and abiotic stress tolerance (Saruhashi et al., 2015). These results indicate that core signaling mechanisms for ABA signaling are common in embryophytes, although the physiological function of PYR/PYL/RCAR receptors in basal land plants has not been demonstrated.
A role of ABA in stress signaling has also been demonstrated in liverworts, comprising another class of basal land plants. A rise in the level of endogenous ABA is associated with the development of desiccation tolerance in thalli of the liverworts Exormotheca holstii and Riccia fluitans (Hellwege et al., 1994, 1996). We previously reported that gemma, a dormant form for asexual reproduction in the liverwort Marchantia polymorpha, shows a sensitive response to ABA (Tougane et al., 2010). ABA induces accumulation of LEA-like transcripts and soluble sugars, mainly Suc, in M. polymorpha, with enhancement of tolerance to freezing and desiccation (Akter et al., 2014). Exogenously applied Suc with ABA inhibited growth of gemmae and further enhanced the desiccation tolerance, and therefore, Suc may affect the ABA signaling pathway and accelerate the accumulation of LEA-like transcripts and further accumulation of Suc (Akter et al., 2014).
To understand the mechanisms underlying ABA responses in liverworts, we conducted a search for genes for the PYR/PYL/RCAR-like receptor in M. polymorpha. We analyzed the gene designated M. polymorpha PYR/PYL/RCAR-like1 (MpPYL1) by transient gene expression assays and in transgenic liverworts to determine its roles in ABA response, desiccation tolerance, and sugar response.
RESULTS
Identification of PYR/PYL/RCAR-Related Genes in M. polymorpha
By phylogenetic analysis based upon a sequence analysis of the M. polymorpha genome (Bowman et al., 2017; Sussmilch et al., 2017), we identified five genes as putative orthologs of PYR/PYL/RCAR of other land plant groups (Fig. 1A). These genes are designated as MpPYL1 (Mapoly0030s0080), MpPYL2 (Mapoly0313s0001), MpPYL3 (Mapoly0508s0001), MpPYL4 (Mapoly0030s0140), and MpPYL5 (Mapoly0145s0004). Of these, the polypeptide encoded by MpPYL1 had the highest sequence similarity to the products of 14 PYR/PYL/RCARs of Arabidopsis and four related genes of P. patens. Structural modeling of MpPYL1 indicated that three-dimensional features of the polypeptide resemble those of Arabidopsis PYR/PYL/RCAR with the gate and latch structures (Supplemental Fig. S1). Furthermore, amino acids for interaction with ABA and group A PP2C (Mosquna et al., 2011) are conserved in the MpPYL1-encoded polypeptide (Supplemental Fig. S2). To determine the role of MpPYL1 in ABA response, its cDNA was used in transient assays of P. patens with that of Arabidopsis PYL6 for comparison. Protonemal cells of P. patens have been used for assays of ABA-induced gene expression with the wheat (Triticum aestivum) Em promoter fused to the GUS reporter gene (proEm-GUS) in bryophytes (Knight et al., 1995; Marella et al., 2006). When we introduced MpPYL1 cDNA into the protonemal cells with proEm-GUS as a reporter, GUS activity significantly increased without ABA treatment (Fig. 1B), and the activity was similar to that achieved by introduction of Arabidopsis PYL6.
Figure 1.
PYR/PYL/RCAR-Like Genes in Marchantia polymorpha. A, Phylogenetic analysis of PYR/PYL/RCAR-related proteins in Arabidopsis, Amborella trichopoda, Picea abies, Selaginella moellendorffii, Physcomitrella patens, and M. polymorpha. Amino acid sequences were aligned with MUSCLE. Phylogenetic relationship among PYR/PYL/RCAR-like proteins was inferred using the neighbor-joining method. Numbers on the branches indicate bootstrap values (100 replicates). The bar represents the number of amino acid changes per branch length. Phylogenetic analyses were conducted in MEGA7. B, Effect of overexpression of MpPYL1 on ABA-induced gene expression. The cDNA of MpPYL1 fused with the rice actin promoter was introduced into the P. patens protonemata cells by particle bombardment. The ABA-inducible Em promoter fused with the GUS reporter gene was used as a reporter construct. The tissues were incubated with or without ABA (10 µM) and used for fluorometric GUS assays. GUS activity was normalized by expression of the cointroduced luciferase (LUC) reporter gene. Results of PYL6 of Arabidopsis (AtPYL6, At2g40330) are shown for comparison. *P < 0.05 and ***P < 0.001 by the t test.
To determine whether MpPYL1 activates ABA-induced gene expression via an ABA-specific pathway, we used promoters with mutations in conserved cis-regulatory elements for transient gene expression assays. The wheat Em promoter contains RY (CATGCATG) and ABRE (ABRE1a: GACACGTGGC) motifs. Disruption of either of these motifs results in reduction of ABA-induced gene expression in P. patens protonemal cells (Sakata et al., 2010). To determine the role of RY and ABRE motifs in MpPYL1-induced activation of proEm-GUS, we conducted transient assays using mutant promoters. We introduced Em promoters with mutations in ABRE (mABRE), RY element (mRY), and both elements (mABRE/mRY) into P. patens protonemal cells with or without the MpPYL1 construct to analyze GUS expression. GUS activity was increased by MpPYL1 in cells cointroduced with nonmutated proEm-GUS and proEm(mRY)-GUS. But this increase effect was reduced in cells in which proEm(mABRE)-GUS and proEm(mABRE/mRY)-GUS were introduced, indicating that the effect of MpPYL1 requires ABRE but not RY (Fig. 2A). To confirm the roles of ABRE in MpPYL1-activated gene expression, we used the pro4xABRE-GUS construct with quadruple repeats of ABRE fused for assays. Overexpression of MpPYL1 increased pro4xABRE-GUS expression without ABA (Fig. 2B).
Figure 2.
MpPYL1-Enhanced Gene Expression Is Mediated by ABRE. A, Transient assays of Physcomitrella patens protonemata cells using the GUS reporter gene fused to the nonmutated Em promoter and the promoters with mutations in ABRE (mABRE), RY (mRY), or both (mABRE/mRY). B, Enhanced expression by MpPYL1 of GUS fused with the 4xABRE promoter (pro4xABRE-GUS). The GUS construct without the promoter (GUS) was used as a control. GUS expression was normalized by the cointroduced LUC gene. Error bars indicate se (n = 3). *P < 0.05 by the t test.
Similar assays conducted using Marchantia callus cells (Ghosh et al., 2016a) indicated that overexpression of MpPYL1 cDNA enhanced proEm-GUS and pro4xABRE-GUS expression (Supplemental Fig. S3, A and B). Furthermore, disruption of ABRE resulted in reduction of the MpPYL1-enhanced gene expression (Supplemental Fig. S3A).
Generation of Transgenic Liverwort Lines Overexpressing MpPYL1-GFP and Genome-Editing Lines with Mutated MpPYL1
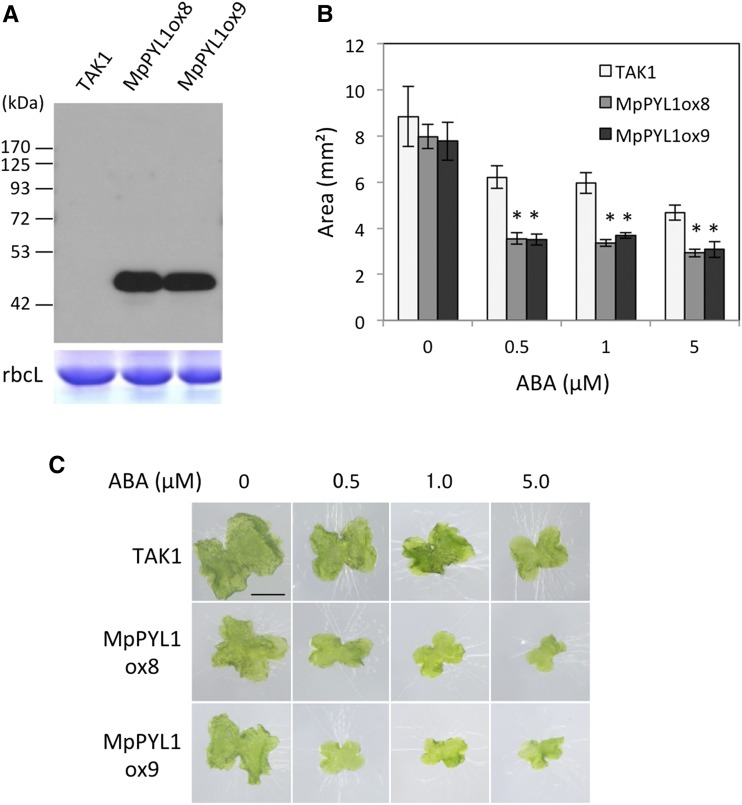
To analyze the ABA response in intact liverwort plants, we generated transgenic M. polymorpha carrying MpPYL1 cDNA fused in frame to GFP (Niwa, 2003) driven by the M. polymorpha EF1 promoter (proMpEF1:MpPYL1-GFP) for overexpression. Among several transgenic lines generated, we obtained two lines, MpPYL1ox8 and MpPYL1ox9, expressing high levels of the MpPYL1-GFP protein as shown by immunoblot analysis (Fig. 3A). To test the sensitivity to ABA of the Takaragaike1 (TAK1) wild type and transgenic lines, we grew gemmae of these plants on a medium containing ABA, which inhibits growth (Tougane et al., 2010). Growth of both MpPYL1ox8 and MpPYL1ox9 was more severely inhibited by ABA than was the growth of TAK1, indicating that the MpPYL1-GFP overexpression lines are hypersensitive to ABA (Fig. 3, B and C; Supplemental Fig. S4).
Figure 3.
Enhanced ABA Sensitivity in MpPYL1-GFP Overexpression Lines of Transgenic Marchantia polymorpha. A, Two transgenic lines, MpPYL1ox8 and MpPYL1ox9, were subjected to immunoblot analysis using an anti-GFP antibody. Molecular size markers are shown in kilodalton (kDa). B and C, Growth of TAK1 (the wild type) and the transgenic lines on a medium containing 0 to 5 µM ABA are shown by area (B) and appearance (C) of the thalli. Error bars indicate se (n = 3). *P < 0.05 compared with TAK1 of the same treatment by the t test. Long-term effect of ABA on growth of the thalli is shown in Supplemental Fig. S4.
We also generated MpPYL1-deficient lines (Mppyl1) by CRISPR-Cas9-mediated genome editing (Sugano et al., 2018. We designed and expressed two target sequences of 18 nucleotides (tg1 and tg2; Fig. 4A) within the MpPYL1 gene in transgenic M. polymorpha with the Cas9 gene. We then analyzed the nucleotide sequence of the MpPYL1 locus of the generated transgenic lines for each construct. All of the generated lines, Mppyl1ge1a, Mppyl1ge1b, Mppyl1ge1c, Mppyl1ge2a, Mppyl1ge2b, and Mppyl1ge2c, had either addition or deletion of a single nucleotide causing a frame shift within the tg1 and tg2 regions (Fig. 4A). When we grew gemmalings of these lines on a medium containing ABA, the plants of all of the genome-edited lines tested grew similarly in the presence or absence of ABA in the medium, indicating that they were insensitive to ABA (Fig. 4, B and C; Supplemental Fig. S5).
Figure 4.
Generation and Growth Analysis of MpPYL1 Genome-Edited Lines. A, Nucleotide sequences of the MpPYL1 genome region of TAK1 (the wild type) and genome-edited lines Mppyl1ge1b, Mppyl1ge1c, Mppyl1ge2b, and Mppyl1ge2c. A hyphen represents a deletion of a nucleotide in the genome-edited lines. B, Fresh weight of gemmalings cultured on medium containing 10 µM ABA for two weeks. Error bars indicate the se (n = 3). *P < 0.05 compared with the minus ABA control by the t test. C, Appearance of the representative gemmalings shown in (B). Effects of higher concentration of ABA on growth are shown in Supplemental Fig. S5.
Transcriptome Analysis of Transgenic Lines with Altered MpPYL1
To determine changes in ABA-induced gene expression by manipulation of MpPYL1, we subjected ABA-responsive transcriptomes in representative transgenic lines, MpPYL1ox8 and Mppyl1ge2b, and TAK1 to RNA-sequencing (seq) analysis. Analysis of 19,287 expressed genes of gemmalings cultured with or without 1 µM ABA for 6 h revealed that of 567 ABA-induced genes in TAK1, expression of 455 genes (80%) was decreased in Mppyl1ge2b. Expression of 236 genes (42%) was increased in MpPYL1ox8 (Fig. 5A; Supplemental Table S1). Furthermore, among 378 ABA-repressed genes in TAK1, expression of 235 genes (62%) was increased in Mppyl1ge2b. Hierarchical clustering and heatmap analysis also indicated that ABA-responsive expression of the majority of transcripts was affected in Mppyl1ge2b (Fig. 5B). Gene ontology analysis using 455 genes (which were induced by ABA in TAK1 but reduced in Mppyl1ge2b) revealed that transcripts of genes involved in abiotic stress response and seed development (Supplemental Fig. S6), including genes encoding various LEA-like proteins, were under the control of MpPYL1. Of 35 LEA-like transcripts induced by ABA in TAK1, expression of all transcripts was reduced in Mppyl1ge2b. Expression of 32 transcripts was increased in MpPYL1ox8 (Fig. 6A; Supplemental Table S2). We previously showed that expression of several MpLEAL genes encoding LEA-like proteins was strongly induced by ABA (Ghosh et al., 2016b). Both RT-PCR (Fig. 6B) and RNA gel-blot analysis (Supplemental Fig. S7) indicated that accumulation of MpLEAL transcripts was enhanced in MpPYL1ox8 and reduced in Mppyl1ge2b, being consistent with the results of RNA-seq analysis.
Figure 5.
Transcriptome Analysis of MpPYL1-Dependent Genes. A, Venn diagrams showing the number of MpPYL1-regulated genes revealed by RNA-seq analysis. The upper diagram shows relationships between 567 genes induced by ABA in TAK1 (the wild type), 397 genes for which expression in ABA-treated MpPYL1ox8 was more than 2-fold of that in ABA-treated TAK1 and 1013 genes for which expression in ABA-treated Mppyl1ge2b was less than 2-fold of that in ABA-treated TAK1. The lower diagram shows relationships between 378 genes repressed by ABA in TAK1, 182 genes for which expression in ABA-treated MpPYL1ox8 was less than 2-fold of that in ABA-treated TAK1, and 423 genes for which expression in ABA-treated Mppyl1ge2b was more than 2-fold of that in ABA-treated TAK1. B, A heatmap shows hierarchical clustering of transcripts. Gene expression values are represented as relative to the mean of all samples; blue color represents lower expression, and red color represents higher expression.
Figure 6.
ABA-Induced Expression of LEA-Like Genes in TAK1, MpPYL1ox8, and Mppyl1ge2b. A, Venn diagram showing expression of LEA-like genes in TAK1 (the wild type) and expression of 38 genes in MpPYL1ox8 and Mppyl1ge2b. The diagram shows the relationship between 35 LEA-like genes for which expression was induced by ABA in TAK1, 33 genes for which expression was increased in MpPYL1ox8, and 38 genes for which expression was decreased in Mppyl1ge2b. B, RT-PCR analysis of RNA isolated from gemmalings treated with 1 µM ABA for 6 h. Primers for MpLEAL1 (Mapoly0112s0030), MpLEAL2 (Mapoly0119s0008), MpLEAL3 (Mapoly0035s0082), MpLEAL4 (Mapoly0016s0148), MpLEAL5 (Mapoly0087s0015), MpLEAL6 (Mapoly0027s0114), and MpLEAL7 (Mapoly0025s0042) were used for amplification. Results for MpEF1 (Mapoly0024s0116) unchanged by ABA and ethidium bromide-stained rRNA bands are shown for comparison.
Sugar Responses in Transgenic Lines
In our previous study, both ABA treatment and sugar treatment caused inhibition of gemma growth and enhancement of desiccation tolerance with increased expression of LEA-like transcripts (Akter et al., 2014). Here, we grew gemmae of TAK1, MpPYL1ox8, and Mppyl1ge2b on media containing 0, 0.1 and 0.2 m Suc. Growth of MpPYL1ox8 gemmae was more severely inhibited by 0.1 m Suc than was growth of TAK1 gemmae (Fig. 7). Such Suc hypersensitivity was also observed in MpPYL1ox9 (Supplemental Fig. S8). These results indicated that enhancement of ABA signals by overexpression of MpPYL1-GFP might have increased sensitivity to sugars in the gemmalings. Thus, we tested how externally applied ABA and sugar coordinately affect accumulation of representative MpLEAL transcripts in MpPYL1ox8 and Mppyl1ge2b. Accumulation of the MpLEAL transcripts was induced by 0.1 m Suc only slightly after 6-h treatment in TAK1, whereas they were more abundantly induced by Suc in MpPYL1ox8. When the medium contained both ABA and Suc, expression of the MpLEAL genes were strongly induced in TAK1, and the difference in abundance of the transcripts between TAK1 and MpPYL1ox8 was small. In contrast, there was little accumulation of MpLEAL transcripts in Mppyl1ge2b with or without ABA and Suc (Fig. 8A; Supplemental Figs. S9 and S10).
Figure 7.
Sensitivity of Growth to Suc in Gemmalings of TAK1, MpPYL1ox8, and Mppyl1ge2b lines. A, Growth appearance on media containing 0, 0.1, and 0.2 m Suc after 2 weeks of culture. B, Fresh weight measurement of plants shown in (A). Error bars indicate se (n = 3). *P < 0.05 by the t test.
Figure 8.
Effects of ABA and Suc on Gene Expression and Sugar Accumulation. A, ABA- and Suc-induced gene expression in gemmalings of TAK1, MpPYL1ox8, and Mppyl1ge2b. Gemmalings were cultured for 6 h in a medium with or without 0.1 m Suc, and 1 µM ABA were used for RNA extraction. The extracted RNA was used for RT-PCR analysis using primers of LEA-like genes, MpLEAL1 (Mapoly0112s0030), MpLEAL2 (Mapoly0119s0008), and MpLEAL3 (Mapoly0035s0082). Results for MpEF1 (Mapoly0024s0116) and ethidium bromide-stained rRNA bands are shown for comparison. B and C, ABA-induced sugar accumulation is shown in gemmalings of TAK1, MpPYL1ox8, and Mppyl1ge2b. Gemmalings were cultured for one day with or without 1 µM ABA in a half-strength B5 medium containing no sugar (B) or 0.1 m Suc (C). The extracted sugars were used for quantitation by anthrone assays with Glc as a standard. Error bars indicate se (n = 3). *P < 0.05 by the t test.
In addition to MpLEAL transcripts, we analyzed ABA- and Suc-increased accumulation of low-molecular-weight soluble sugars associated with desiccation tolerance (Akter et al., 2014) in gemmalings. We determined levels of soluble sugars in tissues of TAK1, MpPYL1ox8, and Mppyl1ge2b with or without ABA and 0.1 m Suc in the medium. Without external Suc or ABA, levels of soluble sugars in tissues were similar in all three lines. Treatment with ABA but without Suc induced sugar accumulation in tissues of MpPYL1ox8 and TAK1 but not in Mppyl1ge2b (Fig. 8B). In the presence of 0.1 m Suc but without ABA in the medium, sugar contents in tissues were similar in TAK1 and MpPYL1ox8, but sugar content was significantly less than TAK1 in Mppyl1ge2b. ABA treatment with 0.1 m Suc in the medium increased sugar levels in tissues of all three lines, but the sugar levels were less in Mppyl1ge2b than in TAK1 and MpPYL1ox8 (Fig. 8C). These results indicated that MpPYL1 is required for ABA- and Suc-induced sugar accumulation. We also analyzed desiccation tolerance in TAK1, MpPYL1ox8, and Mppyl1ge2b. Gemmalings of these plants were incubated with or without ABA and Suc and then dried in a chamber containing silica gel. We found that treatment with both ABA and Suc increased desiccation tolerance in TAK1. Treatment with ABA alone could induce desiccation tolerance in MpPYL1ox8. Either of these treatments (and the treatment with both ABA and Suc) did not increase desiccation tolerance in Mppyl1ge2b (Fig. 9).
Figure 9.
Desiccation Tolerance of Gemmalings of TAK1, MpPYL1ox8, and Mppyl1ge2b. Gemmalings cultured with or without 10 µM ABA (+ABA) and 0.1 m Suc (+Suc) were desiccated in a container with silica gel for two days in the dark. The rehydrated gemmalings were transferred onto agar medium and cultured for two weeks.
DISCUSSION
Important Role of PYR/PYL/RCAR Receptors in ABA Signaling in Embryophytes
Among several candidates for the ABA receptors, PYR/PYL/RCARs identified in Arabidopsis have been recognized as bona fide ABA receptor family proteins. Overexpression of PYR/PYL/RCAR orthologs in tomato (Solanum lycopersicum) and rice (Oryza sativa) results in enhanced tolerance to drought, indicating that PYR/PYL/RCAR-mediated stress signaling is common in angiosperms (Kim et al., 2012; González-Guzmán et al., 2014). Phylogenetic analysis indicates that PYR/PYL/RCAR-like genes are conserved in various embryophyte groups (Sakata et al., 2014; Sussmilch et al., 2017). However, there has also been controversy on whether ABA receptors function in liverworts, representing one of the earliest land plant lineages (Chen et al., 2017), and physiological roles of PYR/PYL/RCAR-like genes in liverworts have not been demonstrated. Of five putative PYR/PYL/RCAR orthologs identified in the M. polymorpha genome, MpPYL1 appears to be the only PYR/PYL/RCAR-like gene strongly expressed in gametophytes (gemma and thalli), while four other paralogs (MpPYL2, MpPYL3, MpPYL4, and MpPYL5) are preferentially expressed in sporophytes (Bowman et al., 2017). We showed that overexpression of MpPYL1-GFP in the pyr1/pyl1/pyl2/pyl4 quadruple mutant of Arabidopsis could partially complement ABA-insensitivity of the mutant (Bowman et al., 2017). In this study, we show that MpPYL1 plays a primary role in ABA signaling in the liverwort gametophyte by analysis of transgenic M. polymorpha. Preferential expression in the sporophyte of four other PYR/PYL/RCAR-like genes of M. polymorpha does not necessarily indicate these genes are nonfunctional. Transient gene expression assays indicated that overexpression of MpPYL2 and MpPYL3 can enhance expression of proEm-GUS, indicating that these paralogs might also encode functional ABA receptors (Supplemental Fig. S11). In contrast, overexpression of MpPYL4 with an extra N-terminal amino acid stretch and MpPYL5 lacking several conserved amino acids (Supplemental Fig. S1) did not enhance the proEm-GUS expression.
Transcriptome analysis with gene ontology enrichment indicated that MpPYL1 controls the expression of genes involved in abiotic stress tolerance, including the majority of ABA-induced LEA-like transcripts (Fig. 6; Supplemental Fig. S6; Supplemental Table S2). Results also indicated that MpPYL1 regulates the expression of Mapoly0072s0050 encoding an ABA-responsive element-binding protein (AREB)-like transcription factor (Supplemental Table S1), which activates ABA-induced genes by binding to an ABRE (Uno et al., 2000; Fujita et al., 2005). We previously showed that ABA-induced gene expression in M. polymorpha is dependent on an ABRE as shown for both the wheat Em promoter and endogenous MpDHN1 promoter (Ghosh et al., 2016a). ABA-induced gene expression by both promoters was inhibited by overexpression of M. polymorpha ABI1A (MpABI1A) encoding group A PP2C, a negative regulator of ABA signaling (Tougane et al., 2010; Ghosh et al., 2016a). Yeast two-hybrid assays indicated that MpPYL1 interacted with MpABI1A (Supplemental Fig. S12). With MpPYL1-dependent gene activation mediated by ABRE (Fig. 2), results of our study indicate that the core signaling pathway leading to ABA-induced gene expression is common in nonvascular plants, such as liverworts, and in vascular plants. Phylogenetic analysis of PYR/PYL/RCAR indicated that its orthologs are also present in the moss P. patens but not in the Charophyte Klebsormidium flaccidum (Klebsormidium nitens), implicating that PYR/PYL/RCAR had emerged in the common ancestor of land plants (Sakata et al., 2014). The genes for other elements required for the ABA response, namely, group A PP2C, SnRK2, and clade A bZIP, are identified not only in embryophytes but also in Charophytes (Corrêa et al., 2008; Fuchs et al., 2013; Hori et al., 2014; Bowman et al., 2017). The results of our study therefore suggest that acquisition of PYR/PYL/RCAR by the common ancestor of embryophytes is associated with establishment of ABA as a phytohormone during the process of adaptation to a terrestrial environment with limited water availability (Ligrone et al., 2012; Sakata et al., 2014).
Possible Roles of ABA and MpPYL1 in Sugar Response for Desiccation Tolerance
We previously reported that ABA induces accumulation of low-molecular-weight soluble sugars, especially Suc, and that ABA and Suc synergistically inhibit growth and enhance desiccation tolerance of gemmalings of M. polymorpha (Akter et al., 2014). Results of expression studies indicated that the Suc-induced gene expression is mediated by PYR/PYL/RCAR (Fig. 8A; Supplemental Fig. S10), suggesting signal crosstalk between ABA and Suc in liverworts. Crosstalk between ABA and sugar signaling processes is well documented in angiosperms through identification of ABA-biosynthetic and ABA-insensitive mutants in sugar response mutant screens of Arabidopsis seeds (Arenas-Huertero et al., 2000; Laby et al., 2000; Rolland et al., 2002). However, the physiological significance of the relationship between ABA and sugar responses is not clearly understood. Sugars are among the essential metabolic components as an energy source, structural components, and osmolytes for maintenance of cell turgor (Koch, 2004). Sugars also play a role in desiccation tolerance by protecting cellular membranes and proteins from dehydration-induced damage (Hoekstra et al., 2001). It has been suggested that Suc serves as a signaling molecule that plays a distinct role in metabolic and developmental responses in plants by affecting gene expression (Chiou and Bush, 1998). Through crosstalk between other environmental signals such as changes in light, water potential, and temperatures or levels of phytohormones, it is thought that plants control carbohydrate metabolism for growth, storage, or environmental stress tolerance (Smeekens, 1998; Tognetti et al., 2013).
Signal transduction for sugars is known to be dependent on the activity of Suc non-fermenting-related kinase 1 (SnRK1), by which the cell senses low Suc levels under a stressful condition (Baena-González and Sheen, 2008). SnRK1 likely mediates ABA signaling, because transgenic plants overexpressing SnRK1.1 show hypersensitivity to Glc and ABA (Jossier et al., 2009). Two group A PP2Cs, ABSCISIC ACID INSENSITIVE1 and PP2CA, interact with SnRK1 and act as negative regulators (Rodrigues et al., 2013). It is thus likely that SnRK1 is under the control of ABA and PYR/PYL/RCAR. In addition to SnRK1, SnRK2 might also participate in ABA-mediated metabolic regulation of sugars, because overexpression of SnRK2.6 increases Suc accumulation in Arabidopsis (Zheng et al., 2010). M. polymorpha has genes for proteins with high similarity to SnRK1 (Mapoly0160s0010) and SnRK2 (Mapoly0061s0075 and Mapoly0011s0096), although their functions in ABA and sugar responses have not been determined.
Our results also indicate that MpPYL1 is involved in accumulation of soluble sugars in tissues (Fig. 8, B and C). This is consistent with the results of gene ontology analysis of the transcriptome showing that the expression of genes involved in polysaccharide catabolism, possibly contributing to degradation of starch to produce low-molecular-weight soluble sugars, is MpPYL1 dependent (Supplemental Fig. S4). The analysis also revealed that the expression of genes for putative sugar transporters (Mapoly0009s0099, Mapoly0050s0068, and Mapoly0175s0014) for sugar uptake can be controlled by MpPYL1 (Supplemental Table S1). We previously reported that accumulation of soluble sugars is increased by exogenous ABA, which enhances freezing and desiccation tolerance in P. patens (Nagao et al., 2005), indicating that there might be a common mechanism for ABA-induced accumulation of sugars in bryophytes. It is likely that the accumulated sugars contribute to protecting cells from damage caused by cellular dehydration during freezing and desiccation with assistance of various species of LEA-like proteins induced by ABA.
Conclusion
PYR/PYL/RCAR plays a role in ABA-induced accumulation of stress-associated transcripts through conserved promoter elements in liverworts and angiosperms. Our results not only clarify the role of the ABA receptor in gene expression but also indicate that the ABA signaling pathway mediates sugar response and accumulation for vegetative desiccation tolerance. These responses represent the archetypal function of ABA, which was likely acquired before establishment of the ABA-mediated regulation of stomata (Chen et al., 2017) or determination of sex (McAdam et al., 2016) in vascular plants. Our future works will focus on identification of key signaling molecules regulated by Suc and ABA through transcriptome and proteome analyses of transgenic plants, in which levels of MpPYL1 have been manipulated, along with analysis of soluble sugars and their intermediate metabolites.
MATERIALS AND METHODS
Chemicals and Plant Materials
Chemicals were purchased from Wako Jun-yaku (Osaka, Japan) unless otherwise stated. Abscisic acid (ABA) was from Sigma (A4906, St. Louis, MO). Gametophytes of Marchantia polymorpha were cultured at 22°C on half-strength Gamborg’s B5 agar medium containing 1% (w/v) Suc under continuous light (Kubota et al., 2013). Protonemata of Physcomitrella patens and Marchantia callus cells were cultured as described previously (Tougane et al., 2010).
Reporter Assays by Particle Bombardment
P. patens protonemata cultured on cellophane-overlaid BCDAT agar medium for 5 d were used for particle bombardment (Tougane et al., 2010). Marchantia callus cells were cultured in 1M51C liquid medium for 2 to 3 d and collected on a filter paper for bombardment (Ghosh et al., 2016a). The plasmids with cDNA of Mapoly0030s0080 (MpPYL1), Mapoly0313s0001 (MpPYL2), Mapoly0508s0001 (MpPYL3), Mapoly0030s0140 (MpPYL4), Mapoly0145s0004 (MpPYL5), or PYL6 (AT2G40330) fused to the rice (Oryza sativa) actin promoter were used as the effector constructs. The reporter construct proEm-GUS and its mutation constructs, proEm(mABRE)-GUS, proEm(mRY)-GUS, and proEm(mABRE/mRY)-GUS, were described in detail previously (Sakata et al., 2010). The 4xABRE-GUS construct has four ACGT-core repeats, AGCTCCACGTGTCGGCA, upstream of the minimal promoter of PpLEA1 (Yotsui et al., 2013). The cells were bombarded with DNAs of the effector and reporter constructs and the Ubi-LUC reference constructs coated on 1-µM gold particles using the PDS-1000He particle delivery system (Bio-Rad, Hercules, CA). The bombarded cells were incubated in a medium with or without ABA and were used for GUS and LUC assays (Jefferson et al., 1987; Tougane et al., 2010).
Generation of Transgenic M. polymorpha
The MpPYL1-GFP construct was fused with the MpEF1 promoter for overexpression (Althoff et al., 2014) using pCambia1300. For the MpPYL1 genome-editing construct, double-strand oligonucleotides containing the target guide RNA seq were fused downstream to the U6 promoter in the pMpGE010 vector containing the Cas9 gene cassette. Agrobacterium tumefaciens (Rhizobium radiobacter) C58 strain harboring the above constructs was used for infection of two-week-cultured gemmalings of M. polymorpha as described by Kubota et al. (2013). After selection on a medium containing 10 mg L−1 hygromycin and 100 mg L−1 cefotaxime, the generated transgenic lines were individually cultured on a fresh selection medium for further propagation.
Protein Gel Electrophoresis and Immunoblot Analysis
Tissues of gemmalings were homogenized using 50 mm Tris-Cl (pH 7.5), 2 mm EDTA, 100 mm NaCl, 2 mm DTT, and a 1:100 volume of the proteinase inhibitor cocktail (Sigma, St. Louis, MO). After centrifugation, the supernatant was subjected to SDS-PAGE. For immunoblot analysis, the electrophoresed proteins were transferred onto a polyvinylidene fluoride membrane. After blocking with 3% skim milk diluted in TBS-T (25 mm Tris-Cl, pH 7.5, 150 mm NaCl, and 0.05% (w/v) Tween 20), the proteins were reacted with a 1:1000 dilution of an anti-GFP antibody (MBL, Nagoya, Japan) and then with a 1:10000 dilution of a horseradish peroxidase-conjugated secondary antibody (170-6515, Bio-Rad, Hercules, CA). The membrane was reacted with chemiluminescence reagent (Chemi-Lumi One, Nacalai Tesque, Kyoto, Japan) and exposed to x-ray film for signal detection.
Extraction of Total RNA, Reverse Transcription (RT)-PCR, and RNA Gel-Blot Analysis
Gemmalings of M. polymorpha cultured for four days in a liquid medium were used for ABA and sugar treatment. RNA extraction, first-strand cDNA synthesis, and PCR reactions were conducted according to Ghosh et al. (2016b). For RNA gel-blot analysis, total RNA electrophoresed in a formaldehyde agarose gel was blotted on a nylon membrane and hybridized with 32P-labeled cDNA probes of M. polymorpha LEA-like (MpLEAL) genes (Akter et al., 2014). Hybridization was conducted using Ultrahyb hybridization buffer (Ambion, Austin, TX) at 42°C overnight, and washing was conducted at 65°C for one hour in a buffer containing 0.2 X SSC (30 mm NaCl and 3 mm Na citrate) and 0.2% (w/v) SDS. The membrane was then exposed to x-ray film to detect radioactive signals.
RNA-Seq Library Construction and Sequencing
RNA-seq libraries were prepared using a TruSeq RNA sample prep kit v2 (Illumina, San Diego, CA), and library quality control was performed with Bioanalyzer 2100 (Agilent, Santa Clara, CA). The libraries had inserts with a size range of approximately 200–500 bp. Library quantification was conducted using RT-quantitative PCR (RT-qPCR), and the concentration of each library was adjusted to 10 nM. The prepared libraries were sequenced using the Illumina HiSeq 2500 system. The raw reads for each library were deposited into DDBJ and are available through Sequence Read Archive with the accession number DRA005869.
RNA-Seq Alignment and Data Analysis
Single reads from each library were processed using CASAVA version 1.8.2 in FASTQ format. The FASTQ files were imported to CLC Genomics Workbench (QIAGEN, USA) for subsequent analysis. The Trim sequences tool in the suite was used to remove adapters and to filter out low-quality bases (<Q13), and only the reads that showed a quality score of 13 or greater were retained. The filtered sequence reads were mapped onto the M. polymorpha genome v3.1 (Phytozome 12; https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Mpolymorpha) using the CLC assembler with default parameters. Expression values were reported as reads per kilobase per million (RPKM) values. Differentially expressed genes were detected by the Empirical Analysis of Digital Gene Expression algorithm in the CLC software. The results were filtered on the basis of a False Discovery Rate (FDR), with P-values less than 0.05 and a corrected fold change greater than 2. Hierarchical clustering and heatmap analysis were performed on the basis of Euclidian distance using the CLC software. RPKM values were used for the hierarchical clustering analysis. Gene ontology enrichment analysis was performed using BiNGO (Biological Network Gene Ontology) software, a Cytoscape plug-in (available at www.cytoscape.org) for putative Arabidopsis (Arabidopsis thaliana) orthologs of MpPYL1-dependent ABA-induced genes of M. polymorpha. These putative Arabidopsis orthologs were identified by a BLASTP search against the TAIR10 protein database. The degree of functional enrichment for a given cluster was quantitatively assessed by a hypergeometric test, and a multiple test correction was applied using the FDR algorithm implemented in the BiNGO plug-in. Overrepresented GO terms were generated after FDR correction, with a significance level of 0.05.
Sugar Extraction and Quantitation
Gemmalings were weighed, frozen, and crushed using a mortar and a pestle in 80% (v/v) ethanol. After removal of insoluble materials by centrifugation at 14,000 g for 10 min at 4°C, the supernatant was dried and suspended in water. After removal of water-insoluble material by centrifugation, the supernatant was used as a sugar sample. The amounts of sugars were determined by the anthrone-sulfuric acid assay, with Glc as a standard (Yemm and Willis, 1954).
Tests for Desiccation Tolerance
Gemmae were cultured for three days in 1/2 B5 liquid medium with or without 10 µM ABA or 0.1 m Suc and transferred to a sheet of cellophane set on wet filter paper in a petri dish. The dish was then placed in a container containing silica gel and kept at 23°C for one day in the dark for desiccation treatment. The dried gemmae on the cellophane were rehydrated by adding 1/2 B5 liquid medium. The gemmae with the cellophane were then transferred to a fresh 1/2 B5 agar medium and cultured for 2 weeks to determine survival.
Accession Numbers
Accession numbers of genes mentioned in this study are listed in Supplemental Table S3.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Structural Models of MpPYL1.
Supplemental Figure S2. Comparison of the Amino Acid Sequences of PYR/PYL/RCAR-Like Proteins.
Supplemental Figure S3. Transient Assays of Marchantia callus Cells for Analysis of MpPYL1 Functions.
Supplemental Figure S4. Long-Term Effect of ABA Treatment on TAK1, MpPYL1ox8, and Mppyl1ge2b.
Supplemental Figure S5. Growth Response to a 50 µM ABA in TAK1 and Mppyl1ge2b.
Supplemental Figure S6. GO Term Network Analysis on 455 MpPYL1-Regulated Genes.
Supplemental Figure S7. RNA Gel-Blot Analysis of TAK1, MpPYL1ox8, and Mppyl1ge2b.
Supplemental Figure S8. Growth Response to Suc in TAK1, MpPYL1ox8, and MpPYL1ox9.
Supplemental Figure S9. Analysis of ABA- and Suc-Induced Expression of LEA-Like Genes.
Supplemental Figure S10. Effects of Suc on MpLEAL1 Gene Expression in TAK1 and Mppyl1ge2b.
Supplemental Figure S11. Transient Assays of P. patens protonemata and Marchantia callus Cells for Analysis of PYR/PYL/RCAR Paralogs.
Supplemental Figure S12. Interaction of MpPYL1 with MpABI1A in Yeast Cells.
Supplemental Table S1. ABA-Responsive Genes and Those Altered in MpPYL1ox8 and Mppyl1ge2b.
Supplemental Table S2. ABA-Induced LEA-Like Genes in M. polymorpha.
Supplemental Table S3. List of Accession Numbers of Genes Mentioned in This Study.
Acknowledgments
We thank Ryuichi Nishihama (Kyoto University), Takayuki Kohchi (Kyoto University), and Kimitsune Ishizaki (Kobe University) for providing the pMpGE10 vector and for critical discussion. We also thank Yasuo Niwa for GFP(S65T), Ralph Quatrano for proEm-GUS, Tuan-hua David Ho for Ubi-LUC constructs, and Totan Kumar Ghosh and Shuuhei Murai for technical assistance.
Footnotes
This work was supported by the Program for the Strategic Research Foundation at Private Universities (S1311017), JST PRESTO (13413773 to T.U.), Grant-in-Aid for Scientific Research (15H04383 to T.U.), and MEXT of Japan (18H04774 to D.T.).
References
- Akter K, Kato M, Sato Y, Kaneko Y, Takezawa D (2014) Abscisic acid-induced rearrangement of intracellular structures associated with freezing and desiccation stress tolerance in the liverwort Marchantia polymorpha. J Plant Physiol 171: 1334–1343 [DOI] [PubMed] [Google Scholar]
- Althoff F, Kopischke S, Zobell O, Ide K, Ishizaki K, Kohchi T, Zachgo S (2014) Comparison of the MpEF1α and CaMV35 promoters for application in Marchantia polymorpha overexpression studies. Transgenic Res 23: 235–244 [DOI] [PubMed] [Google Scholar]
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, León P (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14: 2085–2096 [PMC free article] [PubMed] [Google Scholar]
- Baena-González E, Sheen J (2008) Convergent energy and stress signaling. Trends Plant Sci 13: 474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y, Berger F, et al. (2017) Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–304.e15 [DOI] [PubMed] [Google Scholar]
- Chandler PM, Robertson M (1994) Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol 45: 113–141 [Google Scholar]
- Chen ZH, Chen G, Dai F, Wang Y, Hills A, Ruan YL, Zhang G, Franks PJ, Nevo E, Blatt MR (2017) Molecular evolution of grass stomata. Trends Plant Sci 22: 124–139 [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95: 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa LG, Riaño-Pachón DM, Schrago CG, dos Santos RV, Mueller-Roeber B, Vincentz M (2008) The role of bZIP transcription factors in green plant evolution: Adaptive features emerging from four founder genes. PLoS One 3: e2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuming AC, Lane BG (1979) Protein synthesis in imbibing wheat embryos. Eur J Biochem 99: 217–224 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Fuchs S, Grill E, Meskiene I, Schweighofer A (2013) Type 2C protein phosphatases in plants. FEBS J 280: 681–693 [DOI] [PubMed] [Google Scholar]
- Fuchs S, Tischer SV, Wunschel C, Christmann A, Grill E (2014) Abscisic acid sensor RCAR7/PYL13, specific regulator of protein phosphatase coreceptors. Proc Natl Acad Sci USA 111: 5741–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17: 3470–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh TK, Kaneko M, Akter K, Murai S, Komatsu K, Ishizaki K, Yamato KT, Kohchi T, Takezawa D (2016a) Abscisic acid-induced gene expression in the liverwort Marchantia polymorpha is mediated by evolutionarily conserved promoter elements. Physiol Plant 156: 407–420 [DOI] [PubMed] [Google Scholar]
- Ghosh TK, Kaneko M, Takezawa D (2016b) Transient assays of gemmalings of the liverwort Marchantia polymorpha for studies of abscisic acid-induced gene expression. Japanese Society for Cryobiology and Cryotechnology 62: 57–60 [Google Scholar]
- González-Guzmán M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW, Fernández MA, Holdsworth MJ, Perez-Amador MA, Kollist H,et al. (2012) Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24: 2483–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guzmán M, Rodríguez L, Lorenzo-Orts L, Pons C, Sarrión-Perdigones A, Fernández MA, Peirats-Llobet M, Forment J, Moreno-Alvero M, Cutler SR, et al. (2014) Tomato PYR/PYL/RCAR abscisic acid receptors show high expression in root, differential sensitivity to the abscisic acid agonist quinabactin, and the capability to enhance plant drought resistance. J Exp Bot 65: 4451–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Vasil V, Rosenkrans L, Hannah LC, McCarty DR, Vasil IK (1992) The Viviparous-1 gene and abscisic acid activate the C1 regulatory gene for anthocyanin biosynthesis during seed maturation in maize. Genes Dev 6: 609–618 [DOI] [PubMed] [Google Scholar]
- Hellwege EM, Dietz KJ, Volk OH, Hartung W (1994) Abscisic acid and the induction of desiccation tolerance in the extremely xerophilic liverwort Exormotheca holstii. Planta 194: 525–531 [Google Scholar]
- Hellwege EM, Dietz KJ, Hartung W (1996) Abscisic acid causes changes in gene expression involved in the induction of the landform of the liverwort Riccia fluitans L. Planta 198: 423–432 [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6: 431–438 [DOI] [PubMed] [Google Scholar]
- Hori K, Maruyama F, Fujisawa T, Togashi T, Yamamoto N, Seo M, Sato S, Yamada T, Mori H, Tajima N, et al. (2014) Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat Commun 5: 3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossier M, Bouly JP, Meimoun P, Arjmand A, Lessard P, Hawley S, Grahame Hardie D, Thomas M (2009) SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant J 59: 316–328 [DOI] [PubMed] [Google Scholar]
- Kim H, Hwang H, Hong JW, Lee YN, Ahn IP, Yoon IS, Yoo SD, Lee S, Lee SC, Kim BG (2012) A rice orthologue of the ABA receptor, OsPYL/RCAR5, is a positive regulator of the ABA signal transduction pathway in seed germination and early seedling growth. J Exp Bot 63: 1013–1024 [DOI] [PubMed] [Google Scholar]
- Knight CD, Sehgal A, Atwal K, Wallace JC, Cove DJ, Coates D, Quatrano RS, Bahadur S, Stockley PG, Cuming AC (1995) Molecular responses to abscisic acid and stress are conserved between moss and cereals. Plant Cell 7: 499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. (2004) Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7: 235–246 [DOI] [PubMed] [Google Scholar]
- Komatsu K, Suzuki N, Kuwamura M, Nishikawa Y, Nakatani M, Ohtawa H, Takezawa D, Seki M, Tanaka M, Taji T, et al. (2013) Group A PP2Cs evolved in land plants as key regulators of intrinsic desiccation tolerance. Nat Commun 4: 2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota A, Ishizaki K, Hosaka M, Kohchi T (2013) Efficient Agrobacterium-mediated transformation of the liverwort Marchantia polymorpha using regenerating thalli. Biosci Biotechnol Biochem 77: 167–172 [DOI] [PubMed] [Google Scholar]
- Laby RJ, Kincaid MS, Kim D, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23: 587–596 [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS (2012) Major transitions in the evolution of early land plants: A bryological perspective. Ann Bot 109: 851–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Marcotte WR Jr., Russell SH, Quatrano RS (1989) Abscisic acid-responsive sequences from the em gene of wheat. Plant Cell 1: 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella HH, Sakata Y, Quatrano RS (2006) Characterization and functional analysis of ABSCISIC ACID INSENSITIVE3-like genes from Physcomitrella patens. Plant J 46: 1032–1044 [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ, Banks JA, Hedrich R, Atallah NM, Cai C, Geringer MA, Lind C, Nichols DS, Stachowski K, et al. (2016) Abscisic acid controlled sex before transpiration in vascular plants. Proc Natl Acad Sci USA 113: 12862–12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milborrow BV. (1974) The chemistry and physiology of abscisic acid. Annu Rev Plant Physiol 25: 259–307 [Google Scholar]
- Minami A, Nagao M, Arakawa K, Fujikawa S, Takezawa D (2003) Abscisic acid-induced freezing tolerance in the moss Physcomitrella patens is accompanied by increased expression of stress-related genes. J Plant Physiol 160: 475–483 [DOI] [PubMed] [Google Scholar]
- Mosquna A, Peterson FC, Park S-Y, Lozano-Juste J, Volkman BF, Cutler SR (2011) Potent and selective activation of abscisic acid receptors in vivo by mutational stabilization of their agonist-bound conformation. Proc Natl Acad Sci USA 108: 20838–20843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Minami A, Arakawa K, Fujikawa S, Takezawa D (2005) Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens. J Plant Physiol 162: 169–180 [DOI] [PubMed] [Google Scholar]
- Nishimura N, Sarkeshik A, Nito K, Park SY, Wang A, Carvalho PC, Lee S, Caddell DF, Cutler SR, Chory J, et al. (2010) PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J 61: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y. (2003) A synthetic green fluorescent protein gene for plant biotechnology. Plant Biotechnology 20: 1–11 [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y, et al. (2008) The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319: 64–69 [DOI] [PubMed] [Google Scholar]
- Rodrigues A, Adamo M, Crozet P, Margalha L, Confraria A, Martinho C, Elias A, Rabissi A, Lumbreras V, González-Guzmán M, et al. (2013) ABI1 and PP2CA phosphatases are negative regulators of Snf1-related protein kinase1 signaling in Arabidopsis. Plant Cell 25: 3871–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14: S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y, Nakamura I, Taji T, Tanaka S, Quatrano RS (2010) Regulation of the ABA-responsive Em promoter by ABI3 in the moss Physcomitrella patens: Role of the ABA response element and the RY element. Plant Signal Behav 5: 1061–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y, Komatsu K, Takezawa D (2014) ABA as a universal plant hormone. Prog Bot 75: 57–96 [Google Scholar]
- Saruhashi M, Kumar Ghosh T, Arai K, Ishizaki Y, Hagiwara K, Komatsu K, Shiwa Y, Izumikawa K, Yoshikawa H, Umezawa T, et al. (2015) Plant Raf-like kinase integrates abscisic acid and hyperosmotic stress signaling upstream of SNF1-related protein kinase2. Proc Natl Acad Sci USA 112: E6388–E6396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. (1998) Sugar regulation of gene expression in plants. Curr Opin Plant Biol 1: 230–234 [DOI] [PubMed] [Google Scholar]
- Sugano SS, Nishihama R, Shirakawa M, Takagi J, Matsuda Y, Ishida S, Shimada T, Hara-Nishimura I, Osakabe K, Kohchi T (2018) Efficient CRISPR/Cas9-based genome editing and its application to conditional genetic analysis in Marchantia polymorpha. PLoS One 13: e0205117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmilch FC, Atallah NM, Brodribb TJ, Banks JA, McAdam SAM (2017) Abscisic acid (ABA) and key proteins in its perception and signaling pathways are ancient, but their roles have changed through time. Plant Signal Behav 12: e1365210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti JA, Pontis HG, Martínez-Noël GM (2013) Sucrose signaling in plants: A world yet to be explored. Plant Signal Behav 8: e23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tougane K, Komatsu K, Bhyan SB, Sakata Y, Ishizaki K, Yamato KT, Kohchi T, Takezawa D (2010) Evolutionarily conserved regulatory mechanisms of abscisic acid signaling in land plants: Characterization of ABSCISIC ACID INSENSITIVE1-like type 2C protein phosphatase in the liverwort Marchantia polymorpha. Plant Physiol 152: 1529–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106: 17588–17593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97: 11632–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, et al. (2017) Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front Plant Sci 8: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Laurière C, Merlot S (2009) Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21: 3170–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner O, Ros Espín RM, Bopp M, Atzorn R (1991) Abscisic-acid-induced drought tolerance in Funaria hygrometrica Hedw. Planta 186: 99–103 [DOI] [PubMed] [Google Scholar]
- Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57: 508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsui I, Saruhashi M, Kawato T, Taji T, Hayashi T, Quatrano RS, Sakata Y (2013) ABSCISIC ACID INSENSITIVE3 regulates abscisic acid-responsive gene expression with the nuclear factor Y complex through the ACTT-core element in Physcomitrella patens. New Phytol 199: 101–109 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Xu X, Crosley RA, Greenwalt SA, Sun Y, Blakeslee B, Wang L, Ni W, Sopko MS, Yao C, et al. (2010) The protein kinase SnRK2.6 mediates the regulation of Suc metabolism and plant growth in Arabidopsis. Plant Physiology 153: 99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]