The tetraploid genomes of horseradish and watercress are almost identical structurally, differentiated only by a 2.4-Mb chromosome translocation, and both presumably originated by autopolyploidization.
Abstract
Horseradish (Armoracia rusticana) and watercress (Nasturtium officinale) are economically important cruciferous vegetable species with limited genomic resources. We used comparative chromosome painting to identify the extent of chromosomal collinearity between horseradish and watercress, and to reconstruct the origin and evolution of the two tetraploid genomes (2n = 4x = 32). Our results show that horseradish and watercress genomes originated from a common ancestral (n = 8) genome, structurally resembling the Ancestral Crucifer Karyotype (n = 8), which, however, contained two unique translocation chromosomes (AK6/8 and AK8/6). Except for a 2.4-Mb unequal chromosome translocation in watercress, both genomes are structurally identical. The structural similarity of the two parental subgenomes might suggest an autotetraploid origin of horseradish and watercress genomes. The subgenome stasis, apart from the single-chromosome translocation, indicates that homeologous recombination played a limited role in postpolyploid evolution in both tetraploid genomes. The octoploid genome of one-rowed watercress (N. microphyllum, 2n = 8x = 64), structurally mirroring the tetraploid horseradish and watercress genomes, originated via autopolyploidization from the immediate tetraploid predecessor of watercress or hybridization between this and another now-extinct tetraploid Nasturtium species. These comparative cytogenomic maps in horseradish and watercress represent a first stepping stone for future whole-genome sequencing efforts and genetic improvement of both crop species.
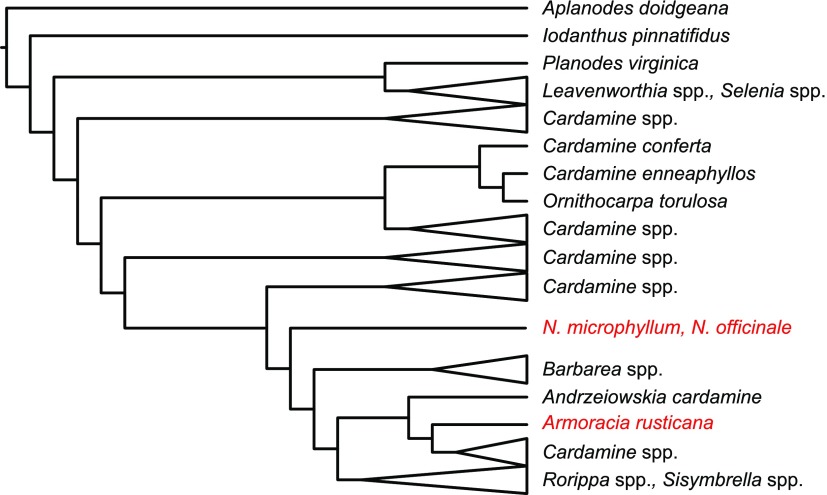
Tribe Cardamineae in the mustard family (Brassicaceae) contains some 340 species across 13 genera (Al-Shehbaz, 2012; BrassiBase, https://brassibase.cos.uni-heidelberg.de; Fig. 1). Although many Cardamineae species are edible, two tetraploid species (2n = 4x = 32) stand out from all the remaining ones for their economic importance—horseradish (Armoracia rusticana) and watercress (Nasturtium officinale).
Figure 1.
The phylogenetic position of genera Armoracia and Nasturtium in the tribe Cardamineae. The modified phylogenetic scheme, based on the ribosomal DNA sequences, was adopted from BrassiBase (https://brassibase.cos.uni-heidelberg.de/).
Horseradish is cultivated for its roots, which are used as a condiment after grating, either on their own or in combination with apples, cream, vinegar, and so forth. Horseradish cells contain the glucosinolate sinigrin. When the cells are burst open due to chewing insect damage or grating, the enzyme myrosinase hydrolyzes sinigrin into allyl isothiocyanate, which is responsible for the characteristic pungency of horseradish (Agneta et al., 2013; Kroener and Buettner, 2018). Horseradish has also a special place in the history of biochemistry as its roots are used to produce the enzyme horseradish peroxidase, which catalyzes the oxidation of organic and inorganic compounds by hydrogen peroxide. Horseradish peroxidase is used widely in diagnostics and experimental research as a detection reagent in enzyme immunoassays and immunohistochemistry techniques (Veitch, 2004; Krainer and Glieder, 2015).
Watercress is a perennial and usually aquatic herb occurring in the wild as well as being cultivated for its leaves. Watercress was traditionally used as a medicinal and leafy crop plant for many centuries. As in the case of horseradish and other cruciferous vegetable crops, the health benefits and peppery taste of watercress are associated with several types of glucosinolates and their metabolites, which are the isothiocyanates. Although watercress is typically grown for its leaves, which are used mainly in salads, the species has become increasing popular also for its anticancer, anti-inflammatory, and antiaging effects (e.g., Voutsina et al., 2016; Klimek-Szczykutowicz et al., 2018 and references therein; http://www.watercress.co.uk).
Notwithstanding their economic importance, genomic resources for horseradish and watercress are limited, as is the knowledge of the genome structure and evolution of these two crop species. The genomes of only two genera in tribe Cardamineae have been analyzed in some detail so far. Cardamine, as the most diverse cardaminoid genus comprising more than 230 species (Al-Shehbaz, 2012; Heenan, 2017), has been the prime target of whole-genome analyses. Comparative chromosome painting (CCP) and whole-genome sequencing of the C. hirsuta genome (n = 8) demonstrated that this model species shares six structurally conserved chromosomes with the Ancestral Crucifer Karyotype (ACK, n = 8; Schranz et al., 2006), whereas two chromosomes were rearranged to form translocation chromosomes AK6/8 and AK8/6 (Hay et al., 2014; Gan et al., 2016). The six ancestral and two translocation chromosomes were identified in other Cardamine species, such as C. amara, C. rivularis, their auto- and allotetraploid derivatives (Mandáková et al., 2013, 2014), and the diploidized tetraploid genome (2n = 4x = 24) of C. cordifolia (Mandáková et al., 2016). Chromosomes AK6/8 and AK8/6 were also identified in the second cardaminoid genus analyzed—the North American Leavenworthia (Haudry et al., 2013). Collectively, these results point to the existence of an ancestral cardaminoid n = 8 genome having two diagnostic translocation chromosomes and to its long-term evolutionary stasis during Cardamine speciation.
To gain a deeper insight into genome evolution in the Cardamineae and to lay the foundation for evolutionary genomics of horseradish and watercress, we exploited Arabidopsis thaliana (Arabidopsis) chromosome-specific BAC contigs to construct their comparative cytomolecular maps. Additionally, we analyzed the origin and genome structure of the octoploid one-rowed watercress, N. microphyllum (Boenn. ex Rchb.) Rchb., a species whose origin has been discussed repeatedly over decades (Manton, 1935; Howard and Manton, 1946; Bleeker et al., 1999). The established interspecies genome collinearity, based on mapping ancestral genomic blocks of crucifer genomes to horseradish and watercress chromosomes, will facilitate and expedite future genomic studies.
RESULTS
Genomes of Horseradish and Watercress
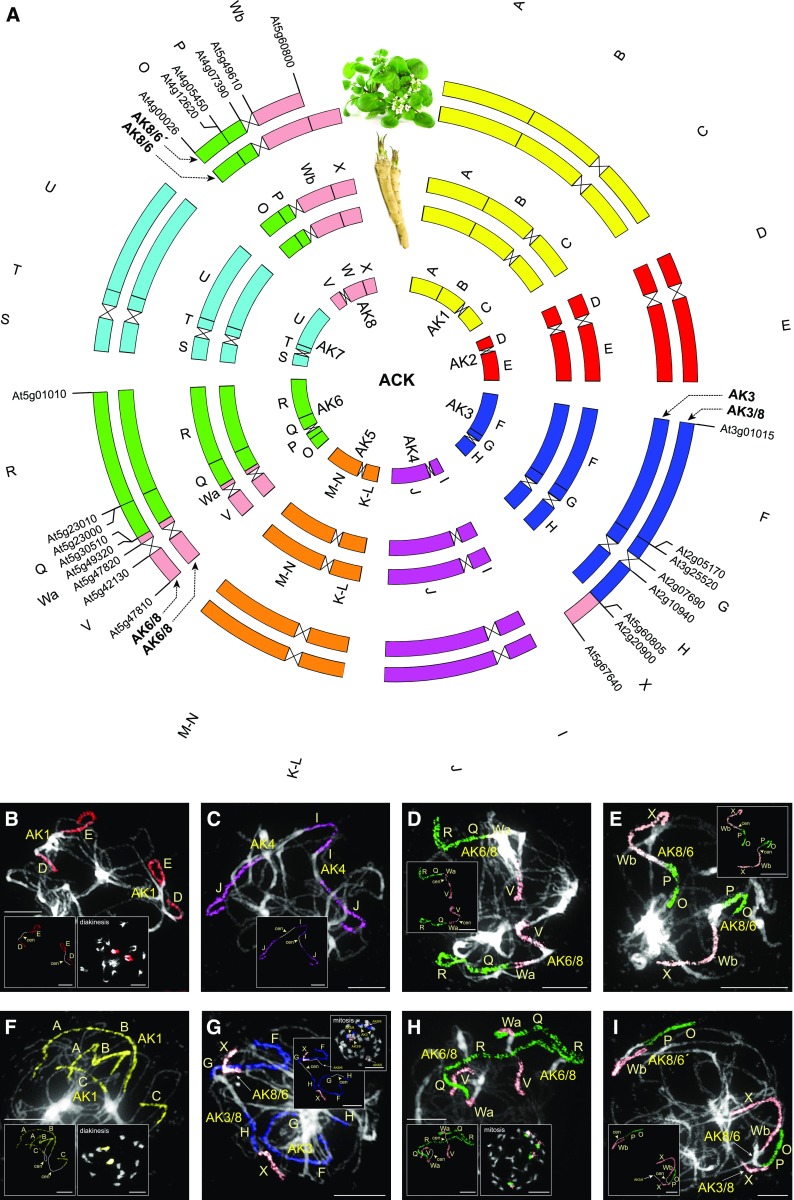
The analyzed accessions of both A. rusticana and N. officinale were tetraploid, with 16 chromosome pairs (2n = 4x = 32; Fig. 2A). To analyze genome structure of both species, we employed CCP with Arabidopsis BAC contigs, representing 22 conserved genomic blocks of ACK (n = 8; Fig. 2A) (Lysak et al., 2016), on mitoses and meiotic chromosomes at pachytene and diakinesis. The unambiguous identification of all 22 genomic blocks in two genomic copies on meiotic chromosomes (Fig. 2, B–I) confirmed the expected tetraploid origin of both genomes.
Figure 2.
Comparative structure of tetraploid horseradish and watercress genomes. A, Circos graphic displaying chromosomal collinearity between 16 chromosomes of watercress (outer circle) and horseradish (middle circle), and eight chromosomes and 22 genomic blocks (A–X) of ACK (inner circle). Color coding and capital letters correspond to eight chromosomes and 22 genomic blocks of the ACK, respectively. Chromosomal rearrangements specific to horseradish and watercress genomes are described by genes defining genomic blocks in the Arabidopsis thaliana genome (TAIR, www.arabidopsis.org), see Supplemental Figure S1 and Supplemental Table S1 for boundaries of the remaining genomic blocks. B–I, Homeologous chromosomes in pachytene complements of horseradish (B–E) and watercress (F–I) revealed by CCP using Arabidopsis BAC contigs as painting probes. The fluorescence of painting probes was captured as black and white photographs and pseudocolored to match the eight chromosomes in ACK. cen, centromere. Scale bars, 10 μm.
In horseradish, six out of eight chromosome pairs retained the ancestral structure of ACK. The two remaining chromosomes originated by a reciprocal translocation involving the long arm of AK6 and the major part (approximately 6 Mb; NB this and the following chromosome segment sizes relate to the sizes of homeologous regions in the Arabidopsis genome; TAIR, www.arabidopsis.org) of the long arm of chromosome AK8 (approximately 6.6 Mb). The breakpoints occurred at the (peri)centromere region of AK6 and within genomic block W on AK8 (between BAC clones K21P3 [At5g49320] and K6M13 [At5g49610]; Fig. 2, A, D–E; Supplemental Fig. S1). The resulting translocation chromosomes AK6/8 and AK8/6 consist of genomic blocks V+Wa+Q+X and O+P+Wb+X, respectively.
The watercress genome almost completely resembles that of horseradish, including translocation chromosomes AK6/8 and AK8/6 (Fig. 2, A, H–I; Supplemental Fig. S2C). The watercress genome differs from that of horseradish by an unequal reciprocal translocation between chromosomes AK3 and AK8/6, with breakpoints at the (sub)telomeric region of the AK3 short arm and between blocks Wb and X, respectively. Although this translocation moved an approximately 2.4-Mb segment of block X from one pair of AK8/6 to the short arm of chromosome AK3 to form chromosome AK3/8, the exchanged very short terminal segment of AK3 cannot be detected on chromosome AK8/6′ by CCP (Fig. 2, G and I). The identical rearrangement identified in two different watercress accessions (see “Materials and Methods”) suggests that the translocation is species-specific.
In horseradish, four 35S rDNA (nucleolar organizer region, NOR) loci were found on four different chromosomes, whereby homologs of these chromosomes did not bear a 35S rDNA locus. 5S rDNA loci were localized on two chromosome pairs (Supplemental Fig. S2, A and B). One chromosome pair with terminal NORs and two pairs with 5S rDNA were identified in watercress (Supplemental Fig. S2, C and D). Interstitial telomeric repeats (ITRs) were observed at (peri)centromere regions of approximately half of watercress chromosomes (Supplemental Fig. S2E), whereas no ITRs were identified in horseradish.
The Origin of the Octoploid N. microphyllum
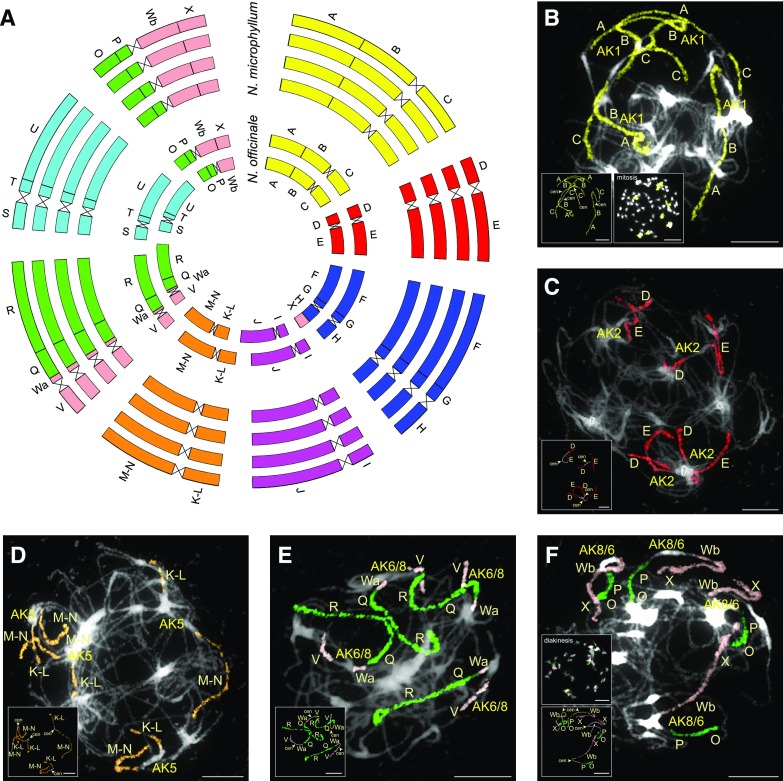
One-rowed watercress, N. microphyllum, was confirmed to be an octoploid species (2n = 8x = 64; Fig. 3A; Supplemental Fig. S2, F to H). CCP with probes designed based on the structure of watercress and horseradish chromosomes revealed that the haploid N. microphyllum genome is composed of eight chromosomes present in four identical copies (Fig. 3, B–F). Structurally, the eight chromosome quartets are identical between watercress and horseradish chromosomes, except for the AK3/8 and AK8/6′ translocation chromosomes, which are characteristic to watercress (Fig. 2A).
Figure 3.
Genome structure of the octoploid N. microphyllum. A, Circos graphic displaying chromosomal collinearity between 16 chromosomes of N. officinale (inner circle) and 32 chromosomes of N. microphyllum (outer circle). Color coding and capital letters correspond to eight chromosomes and 22 genomic blocks of the ACK, respectively. See Supplemental Figure S1 and Supplemental Table S1 for boundaries of all genomic blocks. B–F, Homeologous chromosomes in pachytene complements of N. microphyllum revealed by CCP using Arabidopsis BAC contigs as painting probes. The fluorescence of painting probes was captured as black and white photographs and pseudocolored to match the eight chromosomes in ACK. cen, centromere. Scale bars, 10 μm.
To further elucidate the origin of the octoploid one-rowed watercress, we carried out genomic in situ hybridization (GISH) with the genomic DNA of N. officinale and six other Cardamineae species (Supplemental Fig. S3A) probed to mitotic chromosomes of N. microphyllum. The uniform labeling of all 64 chromosomes of N. microphyllum with the genomic DNA of N. officinale and negative results obtained with probes of the six Cardamineae species (Supplemental Fig. S3, B to H) strongly supported a polyploid origin of N. microphyllum from parental species in the genus Nasturtium.
Two homeologous chromosome pairs bearing 35S rDNA loci and three different 5S rDNA-bearing chromosome pairs were identified in N. microphyllum (Supplemental Fig. S2, F and G). ITRs were identified on approximately 19 chromosomes in N. microphyllum (Supplemental Fig. S2H).
DISCUSSION
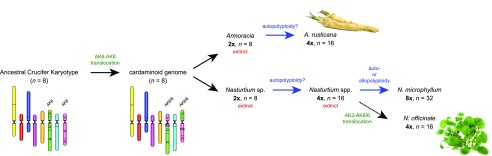
Here, we confirmed that both the Cardamineae crop species horseradish and watercress are tetraploids (x = 8), with two parental subgenomes having identical chromosomal structures (horseradish) or being differentiated by a single approximately 2.4-Mb translocation (watercress). Because the translocation chromosomes AK6/8 and AK8/6 as well as the six remaining chromosomes (AK1 to AK5 and AK7) were identified in various Cardamine species earlier (Mandáková et al., 2013, 2014, 2016; Hay et al., 2014; Gan et al., 2016), we conclude that the origin of the ancestral cardaminoid (n = 8) genome predated the diversification of the three genera and that the clodogenetic/speciation events in these taxa were accompanied by considerable genome stasis (Fig. 4).
Figure 4.
The inferred origin and evolution of horseradish and watercress genomes.
Auto- Versus Allopolyploid Origin of Horseradish and Watercress
As x = 8 is the dominating base number in tribe Cardamineae (Warwick et al., 2006), the tetraploid status (2n = 32) of the horseradish genome is self-evident and was corroborated by the cytogenomic analyses we performed. The structural uniformity of the two parental subgenomes speaks for an autopolyploid origin of horseradish. However, due to the remarkable heterozygosity of 35S rDNA loci (Supplemental Fig. S2, A and B), the possibility of an allotetraploid origin via hybridization between two genomes that are structurally almost identical cannot be ruled out.
Howard and Manton (1946) considered N. officinale to be a diploid species (x = 16; 2n = 2x = 32). Here, we found the watercress genome to be allotetraploid or, perhaps more likely and in agreement with transcriptome data (Voutsina et al., 2016), autotetraploid. The presence of two translocation chromosomes (AK3/8 and AK8/6′) in watercress may provide evidence of an allotetraploid origin of its genome. However, comparison of the two Nasturtium genomes (Fig. 3A), as well as the absence of the two translocation chromosomes in the octoploid N. microphyllum, indicates that in case of an auto- or allopolyploid origin of N. microphyllum from N. officinale, the translocation event in N. officinale occurred only after the formation of N. microphyllum (Fig. 4).
Origin of the Octoploid N. microphyllum
N. microphyllum was first considered to be a tetraploid species (x = 16; 2n = 4x = 64) by Howard and Manton (1946) and then, correctly, an octoploid one (x = 8; e.g., Bleeker et al., 1999). Here, through identification of eight cardaminoid base genomes, we corroborated the octoploid origin of N. microphyllum.
As the chromosome number in N. microphyllum is twice that in N. officinale, the former species was classified as an autopolyploid that originated from the latter species (Manton, 1935). Later, Howard and Manton (1946) concluded that N. microphyllum is an allopolyploid and that N. officinale was one of its parental species. An isozyme electrophoretic study (Bleeker et al., 1999) corroborated the conclusion that N. officinale was one of the parental species, and a Rorippa taxon was purported to be the second parental species (NB Rorippa is a genus closely related to Cardamine and Nasturtium; see Fig. 1). Phylogenetic studies based on molecular markers showed that Nasturtium is more closely related to the nonmonophyletic Cardamine than to Rorippa (Al-Shehbaz and Price, 1998; Olsen et al., 2016). Indeed, our GISH experiments confirmed the phylogenetic distinctiveness of Nasturtium and other cardaminoid genera and clades, and strongly suggested that the octoploid N. microphyllum originated by auto- or allopolyploidization within the genus Nasturtium. Due to the absence of genus- and (mostly) species-specific chromosomal rearrangements in tribe Cardamineae (e.g., Mandáková et al., 2013, 2014 and this study), CCP analyses per se cannot determine whether N. microphyllum has an auto- or allopolyploid origin. Our GISH results can be interpreted as evidence for an auto-octoploid origin of N. microphyllum from N. officinale (Manton, 1935), and this is supported by the geographic co-occurrence of both species (e.g., Al-Shehbaz and Price, 1998; Rasheed et al., 2018); however, the absence of two translocation chromosomes in N. microphyllum and species-specific morphological characters (Al-Shehbaz and Rollins, 1988; Al-Shehbaz and Price, 1998; Rasheed et al., 2018) might support a more ancestral origin of the octoploid species. The one-rowed watercress genome may have originated via autopolyploidization from the immediate tetraploid predecessor of watercress or hybridization between this and another now-extinct tetraploid Nasturtium species (Fig. 4).
Subgenome Stasis in Horseradish and Watercress Polyploids
As concluded above, Armoracia, Cardamine, and Nasturtium species have descended from the common cardaminoid genome and exhibit considerable genome stasis despite a million years of evolution. This is also true for polyploid species in these genera. Postpolyploid diploidization in the allotetraploid C. flexuosa (Mandáková et al., 2014) and the presumably autotetraploid watercress genome is manifested by a single unequal translocation involving two homeologous (C. flexuosa) or nonhomeologous chromosomes (watercress), respectively. Descending dysploidy from n = 16 to n = 15 in the hypotetraploid populations of C. pratensis was mediated by recombination events involving two nonhomeologous chromosomes and accompanied by a centromere loss (Mandáková et al., 2013). Almost complete subgenome stasis in C. flexuosa and C. pratensis stands in stark contrast to the postpolyploid evolution in C. cordifolia (2n = 4x = 24), marked by the origin of four fusion chromosomes mediating descending dysploidy from n = 16 to n = 12 (Mandáková et al., 2016). The diploidized allohexaploid genome of L. alabamica comprises only 11 chromosome pairs (2n = 22), despite the assumption that the ancestral genome had 24 pairs, i.e., 2n = 6x = 48 (Haudry et al., 2013). Hence, polyploid cardaminoid genomes can be broadly divided into three categories: genomes with subgenome stasis (e.g., C. flexuosa, horseradish and watercress species), those with moderate level of diploidization (C. pratensis), and those with progressed postpolyploid diploidization (C. cordifolia, Leavenworthia species). The factors underlying the extent and tempo of postpolyploid diploidization in cardaminoid genomes remain to be identified. For most analyzed polyploids (e.g., horseradish, watercress, C. pratensis) the putative diploid parental genomes as well as the mode of polyploidization (auto- versus allopolyploidy) are unknown (Fig. 4). Besides the probable but unrecognized environmental factors triggering polyploidization, the age of cardaminoid polyploids, and thus, the time span of diploidization processes, can only be hypothesized without dating methods to an accuracy of several thousand years. The available data on postpolyploid evolution in other crucifer allopolyploids suggest that the polyploid chromosome numbers and structure of parental subgenomes can be remarkably stable for up to a few hundred thousand years. The allopolyploid Arabidopsis suecica (2n = 26) show the stability of both parental subgenomes (Comai et al., 2003) since its origin after the last glacial maximum 22,000 years ago (Novikova et al., 2017). A comparable level of subgenome stasis is anticipated for Capsella bursa-pastoris (2n = 32), an allotetraploid genome formed during the past 100,000 to 300,000 years (Douglas et al., 2015), and the three subgenomes of the allohexaploid Camelina sativa (2n = 40; Kagale et al., 2014).
Heterozygosity of rDNA Loci
The peculiar location of 35S rDNA loci on only four different nonhomologous chromosomes in horseradish can be attributed to either a hybrid origin or postpolyploid diploidization of the tetraploid genome. The observed structural heterozygosity offers more support for postpolyploid transposition of NORs to new chromosomal positions, perhaps subsequently fixed by vegetative propagation of horseradish (Sampliner and Miller, 2009). As heterozygosity of rDNA loci in crucifers and other angiosperm plants is not rare, the distribution of rDNA loci can hardly be used as an indicator of auto- versus allopolyploid origin. In the allotetraploid C. flexuosa (Mandáková et al., 2014), both NORs from C. amara were retained on the same chromosomes, whereas all three 35S rDNA loci from C. hirsuta were eliminated. Postpolyploid loss or amplification of 35S rDNA loci was also observed in other Cardamine hybrids and allopolyploids (Zozomová-Lihová et al., 2014) and similar variation in the number of 5S and 35S rDNA loci occurred in synthetic allotetraploid Arabidopsis suecica-like plants (Pontes et al., 2004). In the autotetraploid cytotype of Calepina irregularis (Brassicaceae), we observed heterozygosity of two 35S and one 5S rDNA locus, contrasting with the homozygosity of two 35S rDNA loci and the heterozygosity of one 5S rDNA locus in the autotetraploid cytotype of Golbachia laevigata (Brassicaceae) (Mandáková and Lysak, 2008). Similarly, the mobility of 35S rDNA locus was documented in colchicine-induced autotetraploid plants of Arabidopsis (Weiss and Maluszynska, 2000). The loss of ribosomal DNA sequences after hybridization and/or polyploidization was repeatedly observed in many noncrucifer angiosperm species (e.g., Kotseruba et al., 2010; Liu and Davis, 2011; Huska et al., 2016; Rosato et al., 2017).
MATERIALS AND METHODS
Plant Materials
Plants were either collected in the wild (Armoracia rusticana - Czech Republic, 49.27955N, 16.26686E; Barbarea intermedia - Switzerland, 46.86667N, 8.88333E; Cardamine bellidifolia - Norway, 78.234333N, 15.330639E; Nasturtium officinale - South Africa, 32.99444S, 17.97361E; N. microphyllum - Czech Republic, 49.4540850N, 17.1674319E; Rorippa islandica - Iceland, 64.66361N, 21.40972E, and R. palustris - Slovakia, 47.746594W, 18.163839E) or grown from seeds in a greenhouse (Andrzeiowskia cardamine, seeds obtained from Israel Plant Gene Bank; N. officinale, seeds obtained from Vitacress Salads). Young inflorescences of the sampled plants were collected and fixed in a freshly prepared fixative (ethanol/acetic acid, 3:1) overnight. The fixative was exchanged for 70% (v/v) ethanol and the material stored at −20°C until use. For GISH in N. microphyllum, leaves of A. cardamine, A. rusticana, B. intermedia, C. bellidifolia, N. officinale, R. islandica, and R. palustris were dried in silica gel.
Chromosome Preparations
Chromosome spreads from fixed young flower buds containing immature anthers were prepared according to a published protocol (Mandáková and Lysak, 2016a), with minor modifications described in Mandáková et al. (2016). Ready-to-use chromosome spreads were checked under a phase-contrast microscope for suitable chromosome figures and the amount of cytoplasm. Although extended pachytene chromosomes were required for fine-scale CCP, mitotic chromosomes were used for chromosome counts, fluorescence in situ hybridization with rDNA or telomeric probes, and GISH. When appropriate, preparations were treated with 100 μg/mL RNase (AppliChem) in 2× sodium saline citrate (SSC; 20× SSC: 3 m sodium chloride, and 300 mm trisodium citrate, at pH 7.0) for 60 min and 0.1 mg/mL pepsin (Sigma) in 0.01 m HCl at 37°C for 5 min, then postfixed in 4% (v/v) formaldehyde in 2× SSC for 10 min, washed in 2× SSC twice for 5 min, and dehydrated in an ethanol series (70%, 90%, and 100% [v/v], 2 min each).
DNA Probes
The Arabidopsis thaliana (Arabidopsis) BAC clone T15P10 (AF167571) bearing 35S rRNA gene repeats was used for in situ localization of NORs, and the Arabidopsis clone pCT4.2 (M65137), corresponding to a 500-bp 5S rDNA repeat, was used for localization of 5S rDNA loci. The Arabidopsis-type telomere repeat (TTTAGGG)n, with PCR-amplified fragment sizes up to 25 kb, was prepared according to Ijdo et al. (1991). For CCP, Arabidopsis chromosome-specific BAC clones, grouped into contigs corresponding to 22 genomic blocks of the ACK (Lysak et al., 2016), were used (see Supplemental Fig. S1 and Supplemental Table S1 for the limits of individual blocks). Total genomic DNA of N. officinale and six other Cardamineae species (see Plant materials for the species list) was extracted using a Qiagen DNA isolation kit and used as a GISH probe in N. microphyllum. All DNA probes were labeled with Cy3-, biotin-, or digoxigenin-dUTP by nick translation, as described by Mandáková and Lysak (2016b). Labeled probes were pooled, ethanol-precipitated, desiccated, and dissolved in 20 μL of 50% (v/v) formamide and 10% (v/v) dextran sulfate in 2× SSC per slide overnight.
In Situ Hybridization and Microscopy
For fluorescence in situ hybridization/CCP, 20 μL of the labeled probe was pipetted on a chromosome-containing spread and immediately denatured on a hot plate at 80°C for 2 min. For GISH in N. microphyllum, 20 μL of the probe was denatured in an Eppendorf tube at 90°C for 10 min, placed on ice for 10 min, pipetted on a slide, and denatured on a hot plate at 80°C for 2 min. Hybridization was carried out in a moist chamber at 37°C overnight, followed by posthybridization washing in 20% (v/v) formamide in 2× SSC at 42°C. The immunodetection of hapten-labeled probes was performed as described by Mandáková and Lysak (2016b). Chromosomes were counterstained with DAPI (2 μg/mL) in Vectashield. Fluorescence signals were analyzed and photographed using an Axioimager Z2 epifluorescence microscope (Zeiss) and a CoolCube camera (MetaSystems). Images were acquired separately for all four fluorochromes using appropriate excitation and emission filters (AHF Analysentechnik). The four monochromatic images were pseudocolored and merged using the software Photoshop CS (Adobe Systems), and cropped using the software Image J (National Institutes of Health). Circular visualization of the analyzed genomes was prepared using the software Circos (Krzywinski et al., 2009).
Accession Numbers
The discussed Arabidopsis loci and BAC clones are are listed in Supplemental Table S1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Comparative structure of the horseradish genome.
Supplemental Figure S2. Comparative karyotypes, chromosome counts and chromosomal localization of selected DNA probes in Armoracia and Nasturtium species.
Supplemental Figure S3. Genomic in situ hybridization (GISH) experiments in N. microphyllum (2n = 64).
Supplemental Table S1. Genomic block boundaries defined by Arabidopsis gene loci and BAC clones.
Acknowledgments
We are grateful to Vitacress Salads Ltd. (St Mary Bourne, Andover, UK) for providing seeds of commercially cultivated watercress, P. Mokroš for collecting horseradish inflorescences, B. Trávníček for his advice on the locality of N. microphyllum, and P. Hloušková for her assistance with the Circos software package. We thank I.A. Al-Shehbaz and A. Kovarik for their valuable comments on the manuscript.
Footnotes
This work was supported by a research grant from the Czech Science Foundation (grant no. 17-13029S) and the CEITEC 2020 project (grant no. LQ1601).
References
- Agneta R, Möllers C, Rivelli AR (2013) Horseradish (Armoracia rusticana), a neglected medical and condiment species with a relevant glucosinolate profile: A review. Genet Resour Crop Evol 60: 1923–1943 [Google Scholar]
- Al-Shehbaz IA. (2012) A generic and tribal synopsis of the Brassicaceae (Cruciferae). Taxon 61: 931–954 [Google Scholar]
- Al-Shehbaz IA, Price RA (1998) Delimitation of the genus Nasturtium (Brassicaceae). Novon 8: 124–126 [Google Scholar]
- Al-Shehbaz IA, Rollins RC (1988) A reconsideration of Cardamine curvisiliqua and C. gambellii as species of Rorippa (Cruciferae). J Arnold Arbor 69: 65–71 [Google Scholar]
- Bleeker W, Huthmann M, Hurka H (1999) Evolution of the hybrid taxa in Nasturtium R.Br. (Brassicaceae). Folia Geobot 34: 421–433 [Google Scholar]
- Comai L, Tyagi AP, Lysak MA (2003) FISH analysis of meiosis in Arabidopsis allopolyploids. Chromosome Res 11: 217–226 [DOI] [PubMed] [Google Scholar]
- Douglas GM, Gos G, Steige KA, Salcedo A, Holm K, Josephs EB, Arunkumar R, Ågren JA, Hazzouri KM, Wang W, et al. (2015) Hybrid origins and the earliest stages of diploidization in the highly successful recent polyploid Capsella bursa-pastoris. Proc Natl Acad Sci USA 112: 2806–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X, Hay A, Kwantes M, Haberer G, Hallab A, Ioio RD, Hofhuis H, Pieper B, Cartolano M, Neumann U, et al. (2016) The Cardamine hirsuta genome offers insight into the evolution of morphological diversity. Nat Plants 2: 16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudry A, Platts AE, Vello E, Hoen DR, Leclercq M, Williamson RJ, Forczek E, Joly-Lopez Z, Steffen JG, Hazzouri KM, et al. (2013) An atlas of over 90,000 conserved noncoding sequences provides insight into crucifer regulatory regions. Nat Genet 45: 891–898 [DOI] [PubMed] [Google Scholar]
- Hay AS, Pieper B, Cooke E, Mandáková T, Cartolano M, Tattersall AD, Ioio RD, McGowan SJ, Barkoulas M, Galinha C, et al. (2014) Cardamine hirsuta: A versatile genetic system for comparative studies. Plant J 78: 1–15 [DOI] [PubMed] [Google Scholar]
- Heenan PB. (2017) A taxonomic revision of Cardamine L. (Brassicaceae) in New Zealand. Phytotaxa 330: 1–154 [Google Scholar]
- Howard HW, Manton I (1946) Autopolyploid and allopolyploid watercress with the description of a new species. Ann Bot 10: 1–13 [Google Scholar]
- Huska D, Leitch IJ, De Carvalho JF, Leitch AR, Salmon A, Ainouche M, Kovarik A (2016) Persistence, dispersal and genetic evolution of recently formed Spartina homoploid hybrids and allopolyploids in Southern England. Biol Invasions 18: 2137–2151 [Google Scholar]
- Ijdo JW, Wells RA, Baldini A, Reeders ST (1991) Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res 19: 4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagale S, Koh C, Nixon J, Bollina V, Clarke WE, Tuteja R, Spillane C, Robinson SJ, Links MG, Clarke C, Higgins EE, et al. (2014) The emerging biofuel crop Camelina sativa retains a highly undifferentiated hexaploid genome structure. Nat Commun 5: 3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek-Szczykutowicz M, Szopa A, Ekiert H (2018) Chemical composition, traditional and professional use in medicine, application in environmental protection, position in food and cosmetics industries, and biotechnological studies of Nasturtium officinale (watercress)—a review. Fitoterapia 129: 283–292 [DOI] [PubMed] [Google Scholar]
- Kotseruba V, Pistrick K, Blattner FR, Kumke K, Weiss O, Rutten T, Fuchs J, Endo T, Nasuda S, Ghukasyan A, et al. (2010) The evolution of the hexaploid grass Zingeria kochii (Mez) Tzvel. (2n = 12) was accompanied by complex hybridization and uniparental loss of ribosomal DNA. Mol Phylogenet Evol 56: 146–155 [DOI] [PubMed] [Google Scholar]
- Krainer FW, Glieder A (2015) An updated view on horseradish peroxidases: Recombinant production and biotechnological applications. Appl Microbiol Biotechnol 99: 1611–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener EM, Buettner A (2018) Sensory-analytical comparison of the aroma of different horseradish varieties (Armoracia rusticana). Front Chem 6: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: An information aesthetic for comparative genomics. Genome Res 19: 1639–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Davis TM (2011) Conservation and loss of ribosomal RNA gene sites in diploid and polyploid Fragaria (Rosaceae). BMC Plant Biol 11: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysak MA, Mandáková T, Schranz ME (2016) Comparative paleogenomics of crucifers: Ancestral genomic blocks revisited. Curr Opin Plant Biol 30: 108–115 [DOI] [PubMed] [Google Scholar]
- Mandáková T, Lysak MA (2008) Chromosomal phylogeny and karyotype evolution in x = 7 crucifer species (Brassicaceae). Plant Cell 20: 2559–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandáková T, Lysak MA (2016a) Chromosome preparation for cytogenetic analyses in Arabidopsis. Curr Protoc Plant Biol 1: 43–51 [DOI] [PubMed] [Google Scholar]
- Mandáková T, Lysak MA (2016b) Painting of Arabidopsis chromosomes with chromosome-specific BAC clones. Curr Protoc Plant Biol 1: 359–371 [DOI] [PubMed] [Google Scholar]
- Mandáková T, Kovarík A, Zozomová-Lihová J, Shimizu-Inatsugi R, Shimizu KK, Mummenhoff K, Marhold K, Lysak MA (2013) The more the merrier: Recent hybridization and polyploidy in Cardamine. Plant Cell 25: 3280–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandáková T, Marhold K, Lysak MA (2014) The widespread crucifer species Cardamine flexuosa is an allotetraploid with a conserved subgenomic structure. New Phytol 201: 982–992 [DOI] [PubMed] [Google Scholar]
- Mandáková T, Gloss AD, Whiteman NK, Lysak MA (2016) How diploidization turned a tetraploid into a pseudotriploid. Am J Bot 103: 1187–1196 [DOI] [PubMed] [Google Scholar]
- Manton I. (1935) The cytological history of watercress (Nasturtium officinale R.Br.).Z. indukt. Abstammungs-Vererbungsl 69: 132–157 [Google Scholar]
- Novikova PY, Tsuchimatsu T, Simon S, Nizhynska V, Voronin V, Burns R, Fedorenko OM, Holm S, Säll T, Prat E, et al. (2017) Genome sequencing reveals the origin of the allotetraploid Arabidopsis suecica. Mol Biol Evol 34: 957–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CE, Huang XC, Hansen CIC, Cipollini D, Ørgaard M, Matthes A, Geu-Flores F, Koch MA, Agerbirk N (2016) Glucosinolate diversity within a phylogenetic framework of the tribe Cardamineae (Brassicaceae) unraveled with HPLC-MS/MS and NMR-based analytical distinction of 70 desulfoglucosinolates. Phytochemistry 132: 33–56 [DOI] [PubMed] [Google Scholar]
- Pontes O, Neves N, Silva M, Lewis MS, Madlung A, Comai L, Viegas W, Pikaard CS (2004) Chromosomal locus rearrangements are a rapid response to formation of the allotetraploid Arabidopsis suecica genome. Proc Natl Acad Sci USA 101: 18240–18245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S, Khuroo AA, Ganie AH, Mehraj G, Dar T, Dar GH (2018) Correct taxonomic delimitation of Nasturtium microphyllum Rchb. from Nasturtium officinale R. Br. (Brassicaceae) in Kashmir Himalaya, India. J Asia-Pac Biodivers 11: 154–157 [Google Scholar]
- Rosato M, Álvarez I, Nieto Feliner G, Rosselló JA (2017) High and uneven levels of 45S rDNA site-number variation across wild populations of a diploid plant genus (Anacyclus, Asteraceae). PLoS One 12: e0187131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampliner D, Miller A (2009) Ethnobotany of horseradish (Armoracia rusticana, Brassicaceae) and its wild relatives (Armoracia spp.): Reproductive biology and local uses in their native ranges. Econ Bot 63: 303–313 [Google Scholar]
- Schranz ME, Lysak MA, Mitchell-Olds T (2006) The ABC’s of comparative genomics in the Brassicaceae: Building blocks of crucifer genomes. Trends Plant Sci 11: 535–542 [DOI] [PubMed] [Google Scholar]
- Veitch NC. (2004) Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 65: 249–259 [DOI] [PubMed] [Google Scholar]
- Voutsina N, Payne AC, Hancock RD, Clarkson GJ, Rothwell SD, Chapman MA, Taylor G (2016) Characterization of the watercress (Nasturtium officinale R. Br.; Brassicaceae) transcriptome using RNASeq and identification of candidate genes for important phytonutrient traits linked to human health. BMC Genomics 17: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick SI, Francis A, Shehbaz IA (2006) Brassicaceae: Species checklist and database on CD-ROM. Plant Syst Evol 259: 249–258 [Google Scholar]
- Weiss H, Maluszynska J (2000) Chromosomal rearrangement in autotetraploid plants of Arabidopsis thaliana. Hereditas 133: 255–261 [DOI] [PubMed] [Google Scholar]
- Zozomová-Lihová J, Mandáková T, Kovaříková A, Mühlhausen A, Mummenhoff K, Lysak MA, Kovařík A (2014) When fathers are instant losers: Homogenization of rDNA loci in recently formed Cardamine × schulzii trigenomic allopolyploid. New Phytol 203: 1096–1108 [DOI] [PubMed] [Google Scholar]