Abstract
Introduction
The objective of this study was to determine whether sex or education level affects the longitudinal rate of cognitive decline in Japanese patients in the Alzheimer's disease Neuroimaging Initiative study with defined mild cognitive impairment (MCI).
Methods
We accessed the entire Japanese Alzheimer's Disease Neuroimaging Initiative data set of 537 individuals, among whom 234 had MCI and 149 had Alzheimer's disease. We classified participants into three categories of educational history: (1) low, 0 to 9 years; (2) moderate, 10 to 15 years; and (3) high ≥16 years. We examined the main effects and interactions of visit, sex, and educational achievement on scores for the Clinical Dementia Rating Sum of Boxes, Alzheimer's Disease Assessment Scale–cognitive subscale 13, Mini-Mental State Examination, and Functional Activities Questionnaire in a longitudinal manner.
Results
Women with MCI had a significantly faster rate of decline than men over a 3-year period. Highly educated men showed a significantly slower rate of decline than the other groups. Sex differences in the rates of decline remained after stratification by amyloid or apolipoprotein E (APOE) ε4 status but were absent in Alzheimer's disease over a 2-year period. Subtle differences in chronic kidney disease grade affected the rate of decline. A higher Fazekas periventricular hyperintensity score was associated with a lower estimated glomerular filtration rate in women only.
Discussion
In patients with MCI, sex and educational history significantly affected the rate of change in cognitive and clinical assessments. Furthermore, a subtle decline in chronic kidney disease grade was associated with a faster rate of decline regardless of amyloid pathology in women.
Keywords: MCI, J-ADNI, Cognition, Sex, Education, CKD
1. Background
Successful completion of the Japanese Alzheimer's Disease Neuroimaging Initiative (J-ADNI) project yielded a data set comprising 537 individuals, including 154 cognitively normal individuals, 234 individuals with late mild cognitive impairment (LMCI), and 149 individuals with mild Alzheimer's disease (AD). Previous publication has reported that the Japanese data set is generally comparable to the North American (NA)-ADNI data set, supporting the feasibility of a clinical trial bridging the prodromal stage of AD between Eastern and Western countries [1]. Moreover, the J-ADNI is the most comprehensive data set of this size, reflecting cognitive changes in older individuals from a Japanese (or Asian) population.
It is known that the incidence of AD does not seem to be affected by sex [2], whereas women have a higher life-time risk of AD owing to their longer life span than men [3]. In general, there is a lower incidence of AD among highly educated individuals compared with less educated individuals, suggesting that educational achievement confers some resistance to the development of the disease [4], [5]. Nonetheless, highly educated subjects are thought to decline more quickly after they are diagnosed [6]. However, there has been almost no research into these factors among the elderly in the Japanese population. Therefore, we believe it is important to conduct an analysis of cognitive and functional changes in particular subgroups of Japanese people to explore the factors influencing them.
After publishing the initial analysis of the J-ADNI data set [1], we performed subanalyses with the aim of determining the effects of sex and education level on longitudinal decline in individuals with LMCI and AD. We analyzed various cognitive scales after stratifying them by sex and education level. Subsequently, we further analyzed the data according to amyloid positivity and other clinical and laboratory factors obtained in the study. Finally, we compared the data from the J-ADNI and NA-ADNI cohorts to investigate whether our findings are unique to the J-ADNI data set.
2. Methods
2.1. Sample
We obtained the J-ADNI data set from the National Bioscience Database Center with approval from its data access committee (https://humandbs.biosciencedbc.jp/en/hum0043-v1). The inclusion criteria for J-ADNI were identical to those for NA-ADNI [7]. The follow-up period was 3 years for NC and LMCI and 2 years for AD. The antidementia drug prescription history of participants was obtained from the J-ADNI database (in preparation for publicization). We obtained the NA-ADNI data sets from ADNI1, ADNIGO (Grand Opportunities), and ADNI2 from the Laboratory of Neuro Imaging with their approval. The data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu) (downloaded on June 15, 2017). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging, positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD.
2.2. Assessments and clinical variables
From the several neuropsychological tests used in the J-ADNI cohort, we chose the Clinical Dementia Rating Sum of Boxes (CDR-SOB), Alzheimer's Disease Assessment Scale–cognitive subscale 13 (ADAS-cog13), Mini-Mental State Examination (MMSE), and Functional Activities Questionnaire (FAQ) to assess the longitudinal cognitive and functional status of participants. The clinical and laboratory data used for analysis included participants' sex, age, family history of dementia, marital status, smoking habits, Geriatric Depression Scale scores, fasting blood sugar level, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, glomerular filtration rate (GFR) calculated from serum creatinine, Fazekas periventricular hyperintensity (PVH) score, and deep and subcortical white matter hyperintensity scores from the screening magnetic resonance imaging scan (scored by a trained radiologist blind to clinical information), apolipoprotein E (APOE) ε4 allele status, and amyloid positivity. Amyloid positivity was clinically defined by visual inspection of amyloid PET images with Pittsburgh compound B as a ligand by trained radiologists who were blinded to any clinical information [8] and/or a cerebrospinal fluid amyloid-beta 42 (Aβ42) value ≤ 333 pg/mL at baseline, using the cutoff value determined in a previous report [1]. When both data were available, we defined “positive” as at least one test having met “positive” criteria. When calculating the GFR, we used estimated glomerular filtration rate (eGFR) for J-ADNI participants [9] and GFR-EPI (epidemiology collaboration) for the NA-ADNI participants [10]. These formulas are well established and validated in both the regions [9], [10].
2.3. Statistical analyses
Data were analyzed using the JMP program (SAS Institute Inc, Cary, NC) or Prism 6.0c (GraphPad, La Jolla, CA). We assessed differences with chi-squared tests when analyzing demographic data, whereas longitudinal changes were analyzed using linear mixed-effects models. The interactive effect of group (high education vs. moderate education vs. low education, men vs. women, G1 vs. ≥G2) and time (months) on cognition and function was examined. Age at baseline and APOE ε4 carriage were included as covariates because they are known to be associated with cognition. Individuals were included as a random effect in the analyses. All P values shown in the figures were generated for the interactive effect between group and time. Differences with a P value of <.05 were considered significant.
2.4. Ethics
The study protocol was approved by the University of Tokyo Ethics Committee (11628).
3. Results
3.1. Sample characteristics
Table 1 presents detailed demographic data for the participants included in this study. In the included cohort, 49.6% of those with LMCI and 43.0% of those with AD were men. The mean ± standard deviation for the length of education was 13.0 ± 2.8 years in those with LMCI and 12.4 ± 3.1 years in those with AD, and there was no significant difference between the groups. We divided participants into three groups: (1) low education level (0–9 years), (2) moderate education level (10–15 years), and (3) high education level (≥16 years). Regarding the subtypes of MCI, 2 out of 116 of the men and 4 out of 118 of the women had nonamnestic features. During the 3-year follow-up period, 50% of male and 54.7% of female LMCI subjects completed the study. For AD, 64.1% of male and 63.4% of female subjects completed the 2-year follow-up. Amyloid data were obtained from amyloid PET in 74 subjects with MCI and 57 with AD, from cerebrospinal fluid data in 88 with MCI and 55 with AD, and for both in 45 with MCI and 24 with AD. The positivity rate was 65.8% for MCI and 89.8% for AD. There were no sex differences in terms of average age, family history of dementia, amyloid positivity, or APOE ε4 frequency. However, we observed that significantly more men than women were highly educated in both the LMCI and AD groups.
Table 1.
Detailed demographic data from participants with LMCI and AD from the J-ADNI cohort
| Characteristics | LMCI |
AD |
P value LMCI versus AD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | P value male versus female | Male | Female | Total | P value male versus female | ||
| Total, n (%) | 116 (49.6) | 118 (50.4) | 234 (100) | 64 (43.0) | 85 (57.0) | 149 (100) | .2099 | ||
| Average age years (SD) | 72.8 (5.9) | 72.9 (6.0) | 72.8 (5.9) | .8403 | 73.3 (5.8) | 73.8 (7.1) | 73.6 (6.6) | .6522 | .2253 |
| Family history of dementia, n (%) | 32 (27.6) | 28 (23.7) | 60 (25.6) | .5505 | 19 (29.7) | 23 (27.1) | 42 (28.2) | .8542 | .6356 |
| Educational years (SD) | 14.1 (2.9) | 11.9 (2.3) | 13.0 (2.8) | <.0001 | 13.7 (3.7) | 11.5 (2.3) | 12.4 (3.1) | <.0001 | .0663 |
| Educational level, n (%) | |||||||||
| 0–9 years | 12 (10.3) | 17 (14.4) | 29 (12.4) | <.0001 | 10 (15.6) | 21 (24.7) | 31 (20.8) | <.0001 | NA |
| 10–15 years | 43 (37.1) | 88 (74.6) | 131 (56.0) | 27 (42.2) | 58 (68.2) | 85 (57.0) | |||
| ≥16 years | 61 (52.6) | 13 (11.0) | 74 (31.6) | 27 (42.2) | 6 (7.1) | 33 (22.1) | |||
| Amyloid positive, n (%) | 39 (62.9) | 38 (69.1) | 77 (65.8) | .5596 | 37 (90.2) | 42 (89.4) | 79 (89.8) | 1 | <.0001 |
| Total available, n | 62 | 55 | 117 | 41 | 47 | 88 | |||
| APOE ε4 positive, n (%) | 57 (50.0) | 64 (54.2) | 121 (52.2) | .5992 | 41 (65.1) | 47 (55.3) | 88 (59.5) | .2417 | .2051 |
| Total available, n | 114 | 118 | 232 | 63 | 85 | 148 | |||
Abbreviations: AD, Alzheimer's disease; LMCI, late mild cognitive impairment; SD, standard deviation; NA, not available.
3.2. Effects of sex and educational achievement on cognitive and functional decline
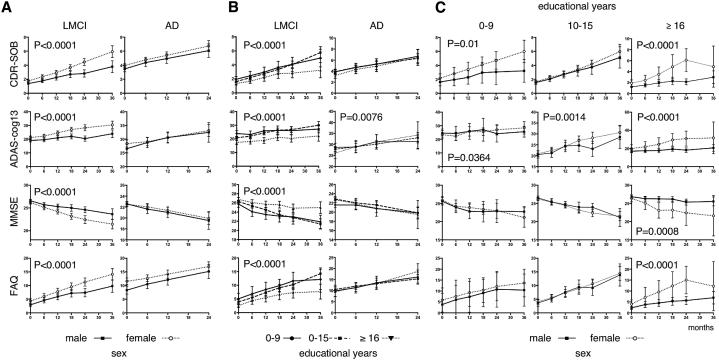
To evaluate the effect of sex on rates of cognitive and functional decline, we analyzed longitudinal changes in the CDR-SOB, ADAS-cog13, MMSE, and FAQ. As shown in Fig. 1A, ratings on all of these scales declined significantly more quickly in women with LMCI. There were no significant differences in longitudinal changes between the sexes for any of the scales in people with AD. These results imply that in the J-ADNI data set, women with LMCI experienced a faster rate of decline than men in terms of both cognition and function.
Fig. 1.
(A) Longitudinal changes in CDR-SOB, ADAS-cog13, MMSE, and FAQ in participants with LMCI and AD, stratified by sex. The dotted lines represent women, and the solid lines represent men. The error bars represent 95% confidence intervals. The P values represent the results from an analysis of variance (ANOVA) investigating the main effects of visits and sex, as well as their interaction. (B) Longitudinal changes in participants with LMCI and AD, stratified by educational level. The solid lines represent participants with an education level of 0–9 years, the broken lines represent those with 10–15 years of education, and the dotted lines represent those with ≥16 years of education. The P values represent the results from an ANOVA investigating the main effects of visits and educational level, as well as their interaction. (C) Longitudinal changes in LMCI stratified by educational level and sex. The dotted lines represent women, and the solid lines represent men. Abbreviations: CDR-SOB, Clinical Dementia Rating Sum of Boxes; ADAS-cog13, Alzheimer's Disease Assessment Scale–cognitive subscale 13; MMSE, Mini-Mental State Examination; FAQ, Functional Activities Questionnaire; LMCI, late mild cognitive impairment; AD, Alzheimer's disease.
We next examined the effect of educational achievement on cognitive and functional changes. We observed significant differences in the rates of decline between the groups with different educational levels for all of the scales evaluated in those with LMCI (Fig. 1B). Specifically, the low and moderately educated groups showed similar trends, whereas the highly educated group showed a slower rate of decline in cognition and function. Again, there were no significant differences in longitudinal changes between the groups with different educational levels for any of the scales in those with AD, with the exception of slight differences in the ADAS-cog13 scores.
Given that there were significantly more highly educated men than women with LMCI (Table 1), sex differences in cognition and function may simply reflect educational differences between the sexes. To assess this issue, we further stratified the MCI group by educational level and sex. As shown in Fig. 1C, the trend for women to have a faster rate of decline remained for all scales, regardless of educational level. Highly educated women showed a significantly faster rate of decline in all scales than less educated women. Similarly, women with a low level of education showed a faster rate of decline in the CDR-SOB and ADAS-cog13 scales. Further analyses stratifying men and women by educational level revealed that in men, the CDR, MMSE, and FAQ showed a significant effect of educational level (P < .0001). However, none of the scales showed a significant effect of educational level in women (data not shown). This indicates that differences in the effects of educational achievement are not necessarily the reason for women showing a faster rate of decline than men.
3.3. Influence of amyloid positivity and APOE ε4 status
Subsequently, we aimed to understand the mechanisms underlying the differences between the sexes by examining whether amyloid positivity or the presence of the APOE ε4 allele affected the rates of cognitive and functional decline.
Among the participants with LMCI, 117 had amyloid data obtained using either amyloid PET or via cerebrospinal fluid Aβ42. The rates of amyloid positivity were 62.9% and 69.1% in men and women, respectively, and these values were not significantly different between the groups (Table 1). Data regarding the presence of the APOE genotype were available for 232 participants with LMCI. We observed that 50.0% of men and 54.2% of women were APOE ε4 carriers, and these proportions did not differ significantly between the groups (Table 1). We further analyzed amyloid pathology and APOE ε4 positivity by educational level and observed no significant differences between the groups (Table 2).
Table 2.
Amyloid and APOE ε4 status stratified by educational level
| Characteristics | Educational level years | Male |
Female |
Total, n (%) | P value male versus female | ||||
|---|---|---|---|---|---|---|---|---|---|
| Positive, n (%) | Negative, n (%) | Total, n | Positive, n (%) | Negative, n (%) | Total, n | ||||
| Amyloid pathology | 0–9 | 0 | 0 | 0 | 8 (66.7) | 4 (33.3) | 12 | 8 (66.7) | NA |
| 10–15 | 14 (66.7) | 7 (33.3) | 21 | 27 (75.0) | 9 (25.0) | 36 | 41 (71.9) | .5506 | |
| ≥16 | 25 (61.0) | 16 (39.0) | 41 | 3 (42.9) | 4 (57.1) | 7 | 28 (58.3) | .4294 | |
| APOE ε4 | 0–9 | 6 (54.5) | 5 (45.5) | 11 | 10 (58.8) | 7 (41.2) | 17 | 16 (57.1) | 1 |
| 10–15 | 22 (51.2) | 21 (48.8) | 43 | 47 (53.4) | 41 (46.6) | 88 | 69 (52.7) | .8535 | |
| ≥16 | 29 (48.3) | 31 (51.7) | 60 | 7 (53.8) | 6 (46.2) | 13 | 36 (49.3) | .7676 | |
Abbreviations: NA, not available; APOE, apolipoprotein E.
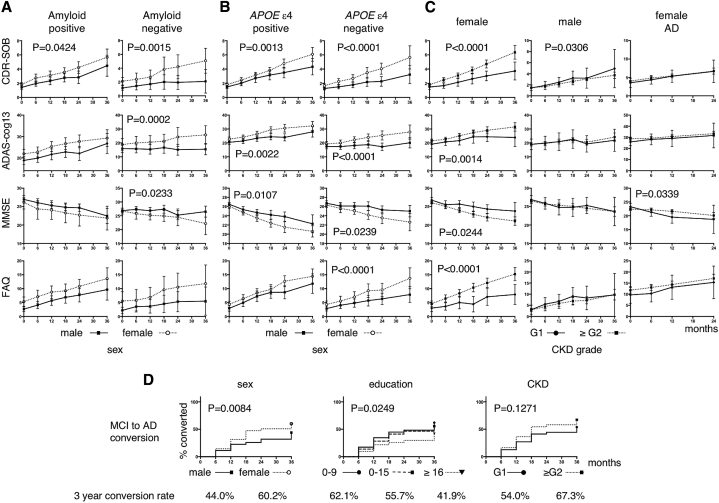
To directly explore the effect of amyloid pathology and the presence of the APOE ε4 allele on the rate of cognitive decline, we stratified participants with LMCI according to these two components and compared longitudinal changes in cognition between the sexes. As shown in Fig. 2A, women still showed a significantly faster rate of decline in the CDR-SOB scale than men, regardless of amyloid positivity. Amyloid-negative women also showed a faster rate of decline than men in the ADAS-cog13 and MMSE scales. Moreover, women with LMCI showed a significantly faster rate of decline than men in all the scales, except the FAQ, regardless of their APOE ε4 status (Fig. 2B).
Fig. 2.
(A) Longitudinal changes in LMCI stratified by sex and amyloid positivity. The dotted lines represent women, and the solid lines represent men. (B) Longitudinal changes in LMCI stratified by APOE ε4 allele status. (C) Longitudinal changes in LMCI and AD stratified by CKD grade. The solid lines represent CKD grade G1 and the dotted lines represent CKD grade ≥ G2. (D) Kaplan-Meyer plot of MCI to AD conversion stratified by sex, educational level, and CKD grades. Statistically significant differences were assessed using the log-rank test for sex and education; the log-rank test showed a trend toward significance for education level. The overall conversion rate is shown below the graphs. Abbreviations: LMCI, late mild cognitive impairment; AD, Alzheimer's disease; CKD, chronic kidney disease; APOE, apolipoprotein E.
We observed that statistical significance was not maintained for several scales after further stratification. However, we speculate that this is due to the relatively small number of participants in each group resulting from stratification because the trend toward significance for women to show a faster rate of decline remained for all scales, regardless of amyloid positivity or APOE ε4 status. Thus, we concluded that there was a rationale to seek further reasons for this trend.
3.4. Further factors leading to faster functional and cognitive decline
To elucidate the factors leading to faster functional and cognitive decline in women with LMCI, we divided female participants into quartiles according to the rate of decline in the CDR-SOB, ADAS-cog13, MMSE, or FAQ scores. Subsequently, we compared the age, years of education, family history, smoking history, body weight, body height, body mass index, blood test results (fasting blood sugar, creatinine, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, C-reactive protein, homocysteine, vitamin B12, and folic acid), amyloid positivity, APOE ε4 status, and chronic kidney disease (CKD) grade between the top and bottom quartiles.
The rate of decline was determined for each score from the baseline visit and last visit (this period was not necessarily 36 months for early dropouts, but all participants completed at least two visits). The results of this analysis suggested that individuals showing a fast rate of decline tended to have a lower CKD grade than participants showing a slower rate of decline. There were no other significant findings (data not shown).
Because there were few participants with a CKD grade above G3a (Table 3), we combined the CKD grades into a single group of ≥G2 (eGFR < 90 mL/min/1.73 m2) and analyzed patterns of declining cognition and function. As shown in Fig. 2C, female participants with a CKD ≥ G2 tended to show a faster rate of decline than those with a CKD of G1 for all the scales.
Table 3.
Participants' CKD grades
| CKD grade | LMCI |
AD |
||
|---|---|---|---|---|
| Male, n (%) | Female, n (%) | Male, n (%) | Female, n (%) | |
| G1 | 16 (13.8) | 20 (16.9) | 10 (15.6) | 10 (11.8) |
| ≥G2 | 100 (86.2) | 98 (83.1) | 54 (84.4) | 75 (88.2) |
| G2 | 82 (70.7) | 79 (66.9) | 43 (67.2) | 62 (72.9) |
| G3a | 13 (11.2) | 18 (15.3) | 10 (15.6) | 10 (11.8) |
| G3b | 5 (4.3) | 0 (0) | 1 (1.6) | 3 (3.5) |
| G4 | 0 (0) | 1 (0.8) | 0 (0) | 0 (0) |
| G5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 116 (100) | 118 (100) | 64 (100) | 85 (100) |
Abbreviations: AD, Alzheimer's disease; LMCI, late mild cognitive impairment; CKD, chronic kidney disease.
We then tested if sex, educational level, and CKD grade affected the MCI to AD conversion. As shown in Fig. 2D, sex and educational level had significant effects on conversion, whereas the effect of CKD grade was not significant.
3.5. Comparisons of J-ADNI and NA-ADNI
Finally, we investigated if similar findings could be observed in the NA-ADNI data set of 562 participants with LMCI (women, n = 344; men, n = 218).
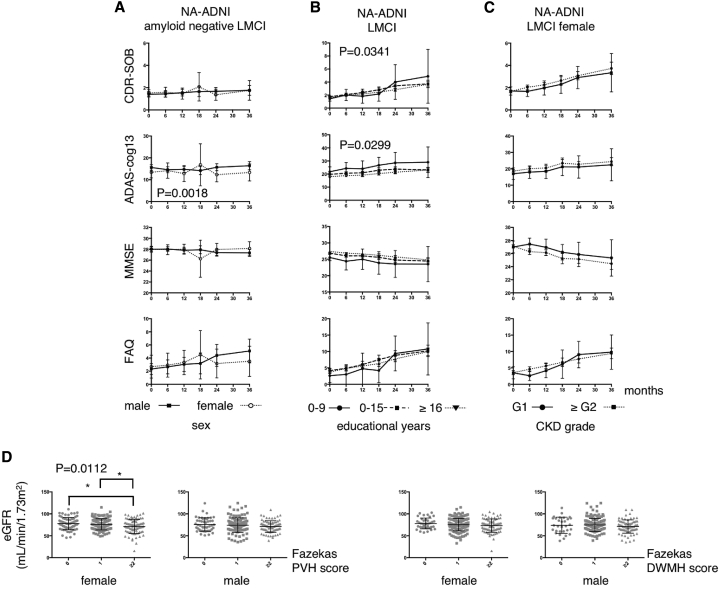
As shown in Fig. 3A, we did not observe a trend for women who were amyloid negative to perform worse within the 3-year follow-up period than male participants, although it has been reported that “overall,” women with MCI declined faster in NA-ADNI population in the 8-year follow-up [11].
Fig. 3.
(A) Longitudinal changes in CDR-SOB, ADAS-cog13, MMSE, and FAQ from participants who were amyloid negative from the NA-ADNI cohort. The dotted lines represent women, and the solid lines represent men. (B) Scale scores from participants with LMCI in the NA-ADNI cohort stratified by educational level. The solid lines represent participants with 0–9 years of education, the broken lines represent those with 10–15 years of education, and the dotted lines represent those with ≥16 years of education. The P values represent the results from an ANOVA investigating the main effects of visit and sex, as well as their interaction. (C) Scale scores of women with LMCI in the NA-ADNI cohort. The solid lines represent those with CKD grade G1 and the dotted lines represent those with CKD grade ≥ G2. The P values represent the results from an ANOVA investigating the main effect of sex throughout the visits. (D) Fazekas PVH and DWMH scores at baseline in patients from the J-ADNI cohort, with participants' CKD grade plotted. Differences were assessed using an ANOVA and the Tukey-Kramer method for multiple comparisons. ∗P = .0112. Abbreviations: CDR-SOB, Clinical Dementia Rating Sum of Boxes; ADAS-cog13, Alzheimer's Disease Assessment Scale–cognitive subscale 13; MMSE, Mini-Mental State Examination; FAQ, Functional Activities Questionnaire; LMCI, late mild cognitive impairment; PVH, periventricular hyperintensity; DWMH, deep white matter hyperintensity; CKD, chronic kidney disease; ANOVA, analysis of variance.
We observed a significant difference between the education level groups with regard to the scores for the CDR-SOB and ADAS-cog13 (Fig. 3B). Female participants with CKD ≥ G2 (according to the GFR-EPI formula) scored consistently worse on the ADAS-cog13 and MMSE scales than those with a CKD of G1, but there was no significant difference in the rate of decline (Fig. 3C).
These results indicate that educational achievement influenced cognition and function in individuals with LMCI from both the J-ADNI and NA-ADNI data sets, but the effects occurred in a slightly different manner. In addition, higher CKD grade was related to a faster rate of cognitive decline in female participants from both the cohorts. However, there was a more prominent difference in the rate of decline in the J-ADNI population.
Therefore, it remains to be determined what underlies these patterns. We speculate that a low eGFR could reflect chronic high blood pressure. Because the subjects' comprehensive past medical history was not available from the J-ADNI database, we attempted to determine whether there was any relationship between the Fazekas score obtained at the baseline magnetic resonance imaging scan and CKD grade. As shown in Fig. 3D, participants with a higher Fazekas PVH score had a lower eGFR, but this was only observed in women.
4. Discussion
Our findings from subanalyses of the J-ADNI data set suggest that women with LMCI showed a faster rate of decline than men with LMCI. Educational achievement also affected the speed of cognitive decline, and women with an eGFR of 90 mL/min/1.73 m2 or less also showed a faster rate of decline. We did not observe the same differences in participants from the J-ADNI with AD, and these findings were only partially present in the NA-ADNI data set.
According to a previous systematic review, there are no sex differences in rates of cognitive decline in the normal aging process [12]. In contrast, the incidence of MCI is reported to be higher in men, although this is still controversial [13], [14]. The precise pathological mechanisms underlying these patterns remain unknown. However, pathophysiological changes mediated by endocrine transition states, such as the menopause, which is both female-specific and age-related, may play a role [15]. Several previous reports have suggested that women with MCI tend to decline more quickly than do men across both a 1-year follow-up period [16] and a longer follow-up period of 8 years [11]. Our current results corroborate these previous findings in a Japanese population.
Several previous studies have investigated the relationship between educational level and risk of dementia. In general, lower educational achievement is related to a higher incidence of dementia, and risk decreases with each year increase in education [17], [18]. In terms of cognitive decline among individuals with MCI, there are also several reports from cross-sectional studies indicating that higher education levels are associated with higher scores on cognitive scales [19], [20]. In contrast, there have been very few reports regarding the longitudinal rate of cognitive decline and educational level in MCI populations [5]. Our current findings show that education level appears to affect the rate of cognitive decline in LMCI in both the J-ADNI and NA-ADNI cohorts. One thing we should discuss here is that the protective effect of education was only positive in male LMCI subjects. The exact reason for this remains to be elucidated, but one explanation could be that highly educated women lack the protection that comes from occupational complexity. Indeed, among the 13 female subjects with an educational history of 16 years or more, seven subjects spent most of their lives as housewives.
It should be noted that in the J-ADNI data set, the rate of amyloid positivity was 66.7%, 70.7%, and 58.3%, in low, moderate, and highly educated individuals, respectively. There were fewer participants who were amyloid positive in the highly educated group, although this was not statistically significant. This could affect the progression rate of LMCI among those in the highly educated group, regardless of their educational achievement. We attempted to account for this in our evaluation of cognitive decline; however, the number of participants was insufficient to perform statistical analyses. In addition, the number of participants with low education levels was very small in the NA-ADNI cohort. The percentages of low, moderate, and highly educated participants were 2.3%, 31.9%, and 65.8%, respectively, among those with LMCI, and 3.2%, 41.2%, and 55.6%, respectively, in those with AD. As such, the NA-ADNI cohort includes significantly more highly educated participants and fewer participants with a low level of education compared with the J-ADNI cohort. This limits comparisons between the two cohorts. Even in the J-ADNI cohort, which included more participants with a low level of education than the NA-ADNI cohort, the percentages of participants in each educational group were not equal (Table 1).
According to the Japanese government census, the male-to-female ratio of people over 65 years of age is 1:1.35, and the average years of education of people aged 65 to 85 years is approximately 10.9 years in men and 10.2 years in women [21]. Therefore, in J-ADNI cohort, the average age of participants was still higher than in the general population; compared to NA-ADNI cohort, it was much closer to that of the general population. Thus, further study is needed to evaluate the progression of LMCI in the general population in both the regions.
Our findings also suggest that women with LMCI show a faster rate of decline regardless of their amyloid or APOE ε4 status, indicating that there could be an independent factor affecting female cognitive decline other than amyloid pathology. Further analysis revealed that in women with LMCI, a CKD stage of G2 or higher was related to a faster rate of decline. This is of interest because a CKD stage of G2 reflects an eGFR lower than 90 mL/min/1.73 m2, which does not necessarily indicate that these participants had CKD or even impaired renal function [22]. Indeed, only 7 women and 16 men fulfilled the criteria for CKD in the entire cohort of participants with LMCI. This indicates that even a subtle decline in eGFR is related to faster cognitive decline in Japanese women with LMCI. Because the serum creatinine levels used for the eGFR calculation could reflect low muscular volume, we tested the effects of differences in body height, body weight, and body mass index among women with CKD grade G1 and ≥G2 in the J-ADNI cohort but found no statistical difference between the two groups (body weight [mean ± standard deviation]: G1, 49.5 ± 7.9 kg and ≥G2, 48.1 ± 6.5 kg; body height: G1, 150.8 ± 5.1 cm and ≥G2, 150.7 ± 5.8 cm; body mass index: G1, 21.9 ± 3.7 and ≥G2, 21.2 ± 3.1). Another explanation was that there were fewer APOE ε4 carriers in the female G1 group than in other groups, but our analysis did not support this hypothesis (G1, 50.0% and ≥G2, 55.1%). Thus, eGFR seems to be related to cognitive decline in women with LMCI, independent of amyloid pathology. Because antidementia drugs, such as donepezil, galantamine, rivastigmine, or memantine, could be prescribed to MCI subjects even before conversion to AD, we tested if prescription of those drugs affected the rate of cognitive decline. As shown in Table S1, the prescription rates, stratified by sex, educational level, or CKD grade, were not significantly different.
These findings were not replicated in the NA-ADNI data set. When analyzing the J-ADNI data set, we used eGFR to evaluate renal function [9] and GFR-EPI for the NA-ADNI data set [10]. These two GFR evaluation methods are widely used in each region. However, they gave completely different values when the data used for calculations were transposed. Nonetheless, even when we evaluated J-ADNI subjects with GFR-EPI and NA-ADNI subjects with eGFR, the results did not change.
In cross-sectional studies, CKD has been shown to be associated with cognitive decline [23], [24], [25] and cerebral cortical thinning [26]. Longitudinal studies of mild to moderate CKD found that an elevated serum creatinine level increased the risk of dementia [27]. We tried to explain this phenomenon by relating low eGFR to brain small vessel disease and found that in women with MCI only, participants with a high Fazekas PVH score had a significantly lower eGFR. The effect of high CKD grade on Fazekas PVH score was small, so other factors may underlie this finding, such as increased levels of inflammatory cytokines, oxidative stress, and alterations in lipid and homocysteine metabolism [27]. Alternatively, the simple Fazekas grading may not be precise enough to evaluate white matter disease, and precise volumetric measurements may be needed. It can be questioned why only women are affected by a low eGFR. One explanation could be that women with hypertension tend to have a lower GFR than men [28], or women may be more vulnerable to inflammatory cytokines or oxidative stress. There are sex differences in cardiovascular pathology. Plaque morphology of coronary arteries differs between men and women [29]. It has been reported that smoking and diabetes have a greater impact on atherosclerosis in women than in men [30]. Thus, this sex difference in atherosclerosis could also underlie this finding.
Moreover, it can be questioned why these relationships were only observed in Japanese women. At this point, it is not possible to answer this question satisfactorily. Further study is needed to determine whether the current results can be replicated in other cohorts.
Our study has several limitations that should be borne in mind. First, the number of participants with amyloid data was insufficient to further analyze the effects of this factor on cognitive decline in LMCI and AD cases. Second, the education level of participants was higher than that in the general population, which may make it difficult to generalize the findings. Third, we evaluated eGFR with serum creatinine levels only. Future studies could improve the evaluation by also incorporating serum cystatin C [31]. In participants with LMCI in the J-ADNI cohort, there was no information about the disease duration in the database, and this could also be a limitation of our findings regarding differences in progression rate.
In this study, we successfully performed subanalyses of the J-ADNI data set. Our findings provide basic knowledge about Japanese individuals with MCI, which should provide a basis for future drug development in Alzheimer's disease and other dementias.
Research in context.
-
1.
Systematic review: We downloaded Japanese Alzheimer's Disease Neuroimaging Initiative (J-ADNI) data and analyzed the cognitive decline rate of mild cognitive impairment (MCI) subjects to see if there is any effect of sex and educational level in the Japanese population similar to previously published studies from Western countries. The findings in J-ADNI data set were evaluated in North American (NA)-ADNI data set for comparison.
-
2.
Interpretation: Female subjects declined faster than males. High educational status protected the subjects from worsening in men. Amyloid-negative subjects tend to decline faster when their kidney function was impaired more than G2 in chronic kidney disease (CKD) grade. Higher Fazekas scores in magnetic resonance imaging (MRI) were associated with higher CKD grade only in female MCI subjects, suggesting vascular factor in accelerated decline. This finding was not replicated in NA-ADNI data set.
-
3.
Future directions: Findings partially corroborated with previously published results regarding sex and educational level. The effect of CKD grading and cognitive decline could be evaluated in a different cohort.
Acknowledgments
Funding sources: This study was funded by the Translational Research Promotion Project from the New Energy and Industrial Technology Development Organization of Japan; Research on Dementia, Health Labor Sciences Research Grant; Life Science Database Integration Project of Japan Science and Technology Agency; Research Association of Biotechnology, Japan; and a grant from an anonymous foundation. This research was supported by AMED under the grant number 16dk0207028h0001.
Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health grant: U01 AG024904) and DOD ADNI (Department of Defense award: W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Authors' contributions: A.I., T.I., K.S., and R.I. conceptualized the study. A.I., T.I., K.S., R.I., H.A., N.T., Y.M., and H.M. analyzed and interpreted the data. T.I., H.A., Kengo.I., M.S., Kenji.I., T.I., R.K., and H.M. designed and organized J-ADNI. A.I. and T.I. wrote the article.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2018.06.008.
Supplementary data
References
- 1.Iwatsubo T., Iwata A., Suzuki K., Ihara R., Arai H., Ishii K. Japanese and North American Alzheimer's Disease Neuroimaging Initiative studies: harmonization for international trials. Alzheimers Dement. 2018 doi: 10.1016/j.jalz.2018.03.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Mielke M.M., Vemuri P., Rocca W.A. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seshadri S., Wolf P.A., Beiser A., Au R., McNulty K., White R. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 4.Letenneur L., Launer L.J., Andersen K., Dewey M.E., Ott A., Copeland J.R. Education and the risk for Alzheimer's disease: sex makes a difference. EURODEM pooled analyses. EURODEM Incidence Research Group. Am J Epidemiol. 2000;151:1064–1071. doi: 10.1093/oxfordjournals.aje.a010149. [DOI] [PubMed] [Google Scholar]
- 5.Vadikolias K., Tsiakiri-Vatamidis A., Tripsianis G., Tsivgoulis G., Ioannidis P., Serdari A. Mild cognitive impairment: effect of education on the verbal and nonverbal tasks performance decline. Brain Behav. 2012;2:620–627. doi: 10.1002/brb3.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarmeas N., Albert S.M., Manly J.J., Stern Y. Education and rates of cognitive decline in incident Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77:308–316. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen R.C., Aisen P.S., Beckett L.A., Donohue M.C., Gamst A.C., Harvey D.J. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamane T., Ishii K., Sakata M., Ikari Y., Nishio T., Ishii K. Inter-rater variability of visual interpretation and comparison with quantitative evaluation of (11)C-PiB PET amyloid images of the Japanese Alzheimer's Disease Neuroimaging Initiative (J-ADNI) multicenter study. Eur J Nucl Med Mol Imaging. 2017;44:850–857. doi: 10.1007/s00259-016-3591-2. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin K.A., Choudhury K.R., Rathakrishnan B.G., Marks D.M., Petrella J.R., Doraiswamy P.M. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y) 2015;1:103–110. doi: 10.1016/j.trci.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira L., Ferreira Santos-Galduroz R., Ferri C.P., Fernandes Galduroz J.C. Rate of cognitive decline in relation to sex after 60 years-of-age: a systematic review. Geriatr Gerontol Int. 2014;14:23–31. doi: 10.1111/ggi.12093. [DOI] [PubMed] [Google Scholar]
- 13.Caracciolo B., Palmer K., Monastero R., Winblad B., Backman L., Fratiglioni L. Occurrence of cognitive impairment and dementia in the community: a 9-year-long prospective study. Neurology. 2008;70:1778–1785. doi: 10.1212/01.wnl.0000288180.21984.cb. [DOI] [PubMed] [Google Scholar]
- 14.Roberts R.O., Geda Y.E., Knopman D.S., Cha R.H., Pankratz V.S., Boeve B.F. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78:342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinton R.D., Yao J., Yin F., Mack W.J., Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11:393–405. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland D., Desikan R.S., Dale A.M., McEvoy L.K., Alzheimer's Disease Neuroimaging I Higher rates of decline for women and apolipoprotein E epsilon4 carriers. AJNR. 2013;34:2287–2293. doi: 10.3174/ajnr.A3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu W., Tan L., Wang H.F., Tan M.S., Tan L., Li J.Q. Education and risk of dementia: dose-response meta-analysis of prospective cohort studies. Mol Neurobiol. 2016;53:3113–3123. doi: 10.1007/s12035-015-9211-5. [DOI] [PubMed] [Google Scholar]
- 18.Sharp E.S., Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25:289–304. doi: 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones R.N., Gallo J.J. Education and sex differences in the mini-mental state examination: effects of differential item functioning. J Gerontol B Psychol Sci Soc Sci. 2002;57:P548–P558. doi: 10.1093/geronb/57.6.p548. [DOI] [PubMed] [Google Scholar]
- 20.Doraiswamy P.M., Krishen A., Stallone F., Martin W.L., Potts N.L., Metz A. Cognitive performance on the Alzheimer's Disease Assessment Scale: effect of education. Neurology. 1995;45:1980–1984. doi: 10.1212/wnl.45.11.1980. [DOI] [PubMed] [Google Scholar]
- 21.Japan PCPCBCToI. Population 15 years of age and over, by school attendance and type of last school completed (6 groups), age (five-year groups), marital status (4 groups) and sex - Japan*. Statistics of Japan.
- 22.Chapter 1: definition and classification of CKD. Kidney Int Suppl. 2011;2013:19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurella M., Yaffe K., Shlipak M.G., Wenger N.K., Chertow G.M. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45:66–76. doi: 10.1053/j.ajkd.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 24.Khatri M., Nickolas T., Moon Y.P., Paik M.C., Rundek T., Elkind M.S. CKD associates with cognitive decline. JASN. 2009;20:2427–2432. doi: 10.1681/ASN.2008101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martens R.J., Kooman J.P., Stehouwer C.D., Dagnelie P.C., van der Kallen C.J., Koster A. Estimated GFR, albuminuria, and cognitive performance: the Maastricht Study. Am J Kidney Dis. 2017;69:179–191. doi: 10.1053/j.ajkd.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Chen C.H., Chen Y.F., Chiu M.J., Chen T.F., Tsai P.H., Chen J.H. Effect of kidney dysfunction on cerebral cortical thinning in elderly population. Sci Rep. 2017;7:2337. doi: 10.1038/s41598-017-02537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurella M., Chertow G.M., Fried L.F., Cummings S.R., Harris T., Simonsick E. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. JASN. 2005;16:2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 28.Muiesan M.L., Ambrosioni E., Costa F.V., Leonetti G., Pessina A.C., Salvetti M. Sex differences in hypertension-related renal and cardiovascular diseases in Italy: the I-DEMAND study. J Hypertens. 2012;30:2378–2386. doi: 10.1097/HJH.0b013e328359b6a9. [DOI] [PubMed] [Google Scholar]
- 29.Yahagi K., Davis H.R., Arbustini E., Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239:260–267. doi: 10.1016/j.atherosclerosis.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Mathur P., Ostadal B., Romeo F., Mehta J.L. Gender-related differences in atherosclerosis. Cardiovasc Drugs Ther. 2015;29:319–327. doi: 10.1007/s10557-015-6596-3. [DOI] [PubMed] [Google Scholar]
- 31.Inker L.A., Schmid C.H., Tighiouart H., Eckfeldt J.H., Feldman H.I., Greene T. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.