Key Points
Question
Among patients with a hip fracture, what is the frequency and effectiveness of initiating osteoporosis medications for prevention of subsequent fractures?
Findings
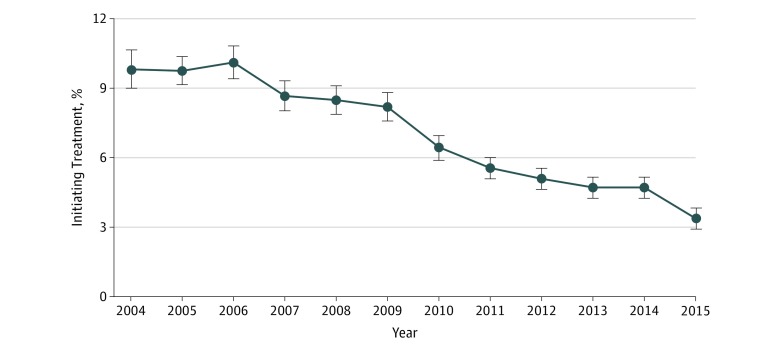
In this cohort study of 97 169 patients with hip fracture, a continuous decline was observed in osteoporosis medication initiation rates, from 9.8% in 2004 to 3.3% in 2015. After adjusting for measured and unmeasured confounding with an instrumental variable approach, a difference of 4.2 events per 100 person-years was observed in the rate of subsequent fractures associated with treatment initiation.
Meaning
The findings of low initiation rates of osteoporosis medications after hip fracture despite evidence of effectiveness highlight a need for interventions to increase patient and physician awareness and to promote innovative collaborative care models that can increase adherence to evidence-based prescribing practices.
Abstract
Importance
Osteoporosis medication treatment is recommended after hip fracture, yet contemporary estimates of rates of initiation and clinical benefit in the patient population receiving routine care are not well documented.
Objectives
To report osteoporosis treatment initiation rates between January 1, 2004, and September 30, 2015, and to estimate the risk reduction in subsequent nonvertebral fractures associated with treatment initiation in patients with hip fracture.
Design, Setting, and Participants
In this cohort study, data from a commercial insurance claims database from the United States were analyzed. Patients 50 years and older who had a hip fracture and were not receiving treatment with osteoporosis medications before their fracture were included.
Exposure
Prescription dispensing of an osteoporosis medication within 180 days of a hip fracture hospitalization.
Main Outcomes and Measures
Each initiation episode was matched with 10 nonuse episodes on person-time after the index hip fracture event to preclude immortal time bias and followed up for the outcome of nonvertebral fracture until change in exposure or a censoring event. An instrumental variable analysis using 2-stage residual inclusion method was conducted using calendar year, specialist access, geographical variation in prescribing patterns, and hospital preference.
Results
Among 97 169 patients with a hip fracture identified, the mean (SD) age was 80.2 (10.8) years, and 64 164 (66.0%) were women. A continuous decline over the study years was observed in osteoporosis medication initiation rates from 9.8% (95% CI, 9.0%-10.6%) in 2004 to 3.3% (95% CI, 2.9%-3.8%) in 2015. In the effectiveness analyses, the hospital preference instrumental variable had a stronger association with treatment (pseudo R2 = 0.20) than the other 3 instrumental variables (specialist access: pseudo R2 = 0.04; calendar year: pseudo R2 = 0.05; and geographic variation: pseudo R2 = 0.07). Instrumental variable analysis with hospital preference suggested a rate difference of 4.2 events (95% CI, 1.1-7.3) per 100 person-years in subsequent fractures associated with osteoporosis treatment initiation compared with nonuse in an additive hazard model.
Conclusions and Relevance
Low rates of osteoporosis treatment initiation after a hip fracture in recent years were observed. Clinically meaningful reduction in subsequent nonvertebral fracture rates associated with treatment suggests that improving prescriber adherence to guidelines and patient adherence to prescribed regimens may result in notable public health benefit.
This cohort study reports osteoporosis medication initiation rates among adults with hip fracture and estimates the association with risk reduction in subsequent nonvertebral fractures.
Introduction
Recurrent fractures after an initial osteoporotic fracture is a major public health burden, as 15% to 25% of patients experience a second fracture within 10 years.1 Therefore, treatment with osteoporosis medication is recommended in patients with hip fracture to prevent subsequent fractures.2 However, treatment rates in this population are reported to be low and decreasing over time, with recent studies suggesting rates of any treatment use after hip fracture in the range of 13% to 21% in the United States in 2012.3,4 Poor adherence to prescribing recommendations in a high-risk patient population has raised substantial concerns in the medical community owing to a potential increase in preventable subsequent fracture cases resulting from undertreatment.5 A 2018 study6 found that among Medicare enrollees, after a decade of decline, rates of hip fractures have remained stable at approximately 7.4 cases per 1000 enrollees after 2012, and this plateau may have resulted in more than 11 000 additional estimated hip fractures between 2012 and 2015.
To our knowledge, only 2 randomized clinical trials (RCTs) have quantified estimates of risk reduction in subsequent nonvertebral fracture after a primary hip fracture with osteoporosis treatments.7,8 Since patients who qualify for participation in RCTs may not adequately represent complex patients seen in routine care,9 it is critical to evaluate potential benefits of treatment with osteoporosis medications vs no treatment in observational studies conducted in general patient populations. However, the threat of unmeasured confounding is particularly severe in observational studies comparing the effect of a drug treatment with no treatment. If the decision to treat is based on unmeasured patient characteristics, including patient frailty or underlying subclinical conditions, observational studies relying on standard confounding control approaches that only account for measured confounding, such as regression modeling, propensity scores, and disease risk scores, are likely to result in biased estimates.10
This study provides contemporary estimates for trends in initiation of osteoporosis treatments after hip fracture and evaluates the association of treatment initiation with the risk of subsequent osteoporotic fractures compared with no use using an instrumental variable approach. Since the selected instrument serves as an unconfounded substitute for actual study treatment, this approach offers the opportunity to account for both measured and unmeasured confounding and provide unbiased estimates of treatment effects in observational studies.11 As instrumental variable analyses are known to result in high variance in treatment effect estimation,12 we evaluated multiple instrumental variables based on factors related to health care access, trends, and preferences, with the ultimate goal of selecting the most appropriate instrumental variables alone or in combination to optimize the bias-variance trade-off.
Methods
Study Design and Data Source
We designed an observational cohort study using data from Truven MarketScan commercial claims, which include data from employer-sponsored health insurance plans for commercially insured employees and their dependents as well as Medicare-eligible retirees with employer-sponsored Medicare supplemental plans, between January 1, 2004, and September 30, 2015. Data from inpatient services file, outpatient services file, outpatient drug claims files, and enrollment files were merged using unique patient identifiers. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The institutional review board of Brigham and Women’s Hospital approved this study protocol. Patient consent was waived because the data used in this study were fully deidentified.
Study Cohort
We identified patients 50 years and older who were hospitalized with a hip fracture and defined the date of hospitalization as the cohort entry date. We required patients to have continuous enrollment in their health plan for 6 months before the cohort entry date to ensure sufficient availability of baseline data for covariate assessment. Patients with prevalent use of osteoporosis medications in the baseline period were excluded to focus on treatment initiation. A period of 180 days after hip fracture hospitalization was used to identify a newly filled prescription of an osteoporosis treatment (ie, bisphosphonates [alendronate, ibandronate, risedronate, and zolendronic acid], teriparatide, and denosumab) in this cohort, and rates of initiation were described over calendar time in this cohort.
We sampled a subset of patients from the overall study cohort for the effectiveness analysis. First, to preclude bias associated with immortal person-time, which is common in studies evaluating effectiveness of treatments against a nonuser comparator group,13 we deemed it necessary to identify a specific time point among nonuse episodes to mark their study index date. To achieve that, we matched each initiation episode on its treatment initiation date with 10 episodes in which an osteoporosis medication was not initiated up to that point and assigned the study index date to the entire matched set on the day of matching. Of note, the nonusers were sampled randomly from the pool of all eligible patients without conditioning on future initiation of osteoporosis medications. In other words, patients initiating an osteoporosis medication at later time points (eg, day 100 after cohort entry) were eligible to be sampled as nonusers in earlier periods (eg, days 1-99 after cohort entry) and contributed person-time to the nonuser group (if they were randomly chosen) until initiation of their osteoporosis medication. eFigure 1 in the Supplement summarizes our study design. We further restricted the study sample to the patients for whom we could reliably identify all the instrumental variables.
Variable Measurement
Nonvertebral Fractures
The primary outcome of interest was time to event of a composite nonvertebral osteoporotic, fracture end point (eTable in the Supplement) including humerus, radius, ulna, hip, or pelvis fracture.14 Follow-up for the outcome began on the day following the study index date and continued until change in exposure status (initiation of osteoporosis medication for nonuser patients and discontinuation of medication for using patients, defined as no new prescription filled for 90 days), recorded in-hospital mortality, end of enrollment, or end of data availability.
Instrumental Variables
We identified 4 potential instrumental variables.
Calendar year: Calendar year of cohort entry was evaluated as an instrumental variable, as this variable is expected to be a strong determinant of treatment3,4 but otherwise unlikely to be associated with fractures, since the patient population at risk for fractures may not vary much across calendar time.
Specialist access: Access to specialists was also evaluated as an instrumental variable, as it has been previously demonstrated to be associated with osteoporosis treatment receipt.3,15 This variable was defined as a binary indicator of access vs no access to specialists based on patients’ medical visit with specialists who are most likely to prescribe osteoporosis treatments (rheumatologists or endocrinologists) in a period of 6 months prior to the study index date.
Geographic variation in prescribing patterns: Regional variation in prescription medication use for osteoporosis treatments is well documented.3,16 We identified patients’ geographical location using metropolitan statistical area codes from enrollment files and excluded patients for whom metropolitan statistical area codes were not recorded. To derive regional preference-based instrumental variables, we computed rates of osteoporosis treatment prescribing from the entire population of patients with hip fracture hospitalization meeting our age and insurance enrollment criteria at the metropolitan statistical area level using mixed-effects logistic regression models, with age and sex as fixed effects (to adjust for case mix) and geographical region as random effects (eAppendix in the Supplement). Deciles were created based on these adjusted rates and were used as instrumental variables in the study population.
Hospital preference: Preference-based instrumental variables can be a powerful tool to control confounding in observational studies because preferences are clearly associated with the treatment of interest, without any apparent association with the outcome. While past studies have used the choice of a specific treatment option in the most recent patient seen by a particular prescriber to define preference-based instrumental variables,17,18 we modified this approach to create a more robust preference-based instrumental variable that can take into account the patient case mix. Further, we posited that focusing on just 1 clinician may not adequately explain variation in treatment initiation because appropriately treating a patient with hip fracture for osteoporosis involves care coordination from multiple physicians. Therefore, we defined preferences at the hospital level. In this approach, we first identified a primary hospital for each patient based on the unique identifiers recorded on his or her hip fracture hospitalization claims at cohort entry. Next, we excluded patients if their primary hospitals had fewer than 3 patients from the entire population of patients with hip fracture hospitalization meeting our age and insurance enrollment criteria. We computed primary hospital–specific osteoporosis treatment prescribing rates controlling for patient age and sex using a mixed-effects logistic regression model. The rates were used to create deciles of hospital preference, which were used as instrumental variables in the study population.
Confounding Variables
We measured several factors, including demographic characteristics (age and sex); factors related with fracture risk, including osteoporosis diagnosis, osteoporotic fractures, and orders of bone mineral density test; comorbid conditions, including Parkinson disease, Alzheimer disease or other dementia, obesity, diabetes, rheumatoid arthritis, or history of falls, syncope, or gait abnormality; and use of medications potentially associated with bone metabolism or fall risk, including anticonvulsants, benzodiazepines, selective serotonin reuptake inhibitors, β-blockers, proton pump inhibitors, opioids, or glucocorticoids. Additionally, health care use factors, including number of physician visits, acute care hospitalizations, number of different medications, and number of emergency department visits during the baseline period, were also measured as a marker for general health and contact with the health care system. All confounding variables were measured in a 6-month period immediately preceding the cohort entry date. Additionally, we adjusted for time between the index hip fracture and follow-up initiation as a linear term in our models.
Statistical Analysis
We provided crude rates of treatment initiation along with 95% confidence intervals calculated for binomial proportions over study years in the overall study cohort. Among patients sampled for the effectiveness analysis, we reported patient characteristics by treatment initiation status and compared characteristics between users and nonusers using standardized differences, which are equal to the difference in means or proportions of a variable between 2 groups divided by the pooled standard deviation of the variable.19 Among the patients sampled for effectiveness analysis, we used logistic regression models with osteoporosis medication initiation as the dependent variable and each of the proposed instruments along with other measured confounders as independent variables in the first-stage instrumental variable models. An additional model was also considered where all instrumental variables were used simultaneously as independent variables along with other confounding factors. To evaluate the strength of each instrument, we reported crude proportion of patients initiating treatment in each stratum of the instrumental variable, pseudo R2 values for overall model fit,20 discrimination indices, and partial F statistics.10 We further reported bias component plots to evaluate the potential relative bias resulting from omitting unmeasured confounding across various instrumental variable analyses.21
We fit the second-stage instrumental variable model using a control function approach based on residual errors to account for any variation in the hazard function owing to unobserved covariates.22 Accordingly, we calculated the difference between actually received treatment and model-computed probabilities of treatment from stage 1 to derive residuals. The outcome model was constructed with time to fracture as the event of interest and included treatment residuals in addition to actually received treatment as an independent variable. We estimated the treatment effect from an additive hazard model.23 Under the additive hazard model, constancy in the treatment effect over time was evaluated by plotting the cumulative regression function for the treatment variable over follow-up time and inspecting the slope.24 To safeguard against the possibility of instrumental variables being associated with the outcome indirectly through measured confounders (in other words, violation of the exclusion restriction assumption of instrumental variables), we added all the measured confounding factors in this regression model.25 See the eAppendix in the Supplement for instrumental variable model equations.
To contrast our instrumental variable–based estimates with standard methods, we conducted an analysis with multivariable regression using additive hazard models with actual study treatment as the main exposure of interest adjusting for confounders. Further, we also conducted 1-to-1 propensity score matching of initiators with nonusers with a caliper of .025 on the probability scale26,27 as an alternative method to control for measured confounders and provided treatment effect estimates. Analyses were conducted in SAS statistical software, version 9.4 (SAS Institute Inc), and the additive hazards model was implemented in the timereg package of R software version 3.0.2 (R Foundation for Statistical Computing).
Results
Treatment Initiation Rates
We included 97 169 patients 50 years and older who were not taking osteoporosis medications at the time of hip fracture hospitalization. The mean (SD) age in this cohort was 80.2 (10.8) years, and 64 164 (66.0%) were women. A total of 6743 patients (6.9%; 95% CI, 6.8%-7.1%) initiated treatment with an osteoporosis medication within 180 days of their hip fracture. We observed a continuous decline over the study years in osteoporosis medication initiation rates among patients with hip fracture, from 9.8% (95% CI, 9.0%-10.6%) in 2004 to 3.3% (95% CI, 2.9%-3.8%) in 2015 (Figure 1). In the effectiveness analyses, the hospital preference instrumental variable had a stronger association with treatment (pseudo R2 = 0.20) than the other 3 instrumental variables (specialist access: pseudo R2 = 0.04; calendar year: pseudo R2 = 0.05; and geographic variation: pseudo R2 = 0.07).
Figure 1. Osteoporosis Treatment Initiation Over Time in Patients With Hip Fracture Hospitalizations.
Data in this figure are from a total of 97 169 patients 50 years and older with hip fracture who were not taking any osteoporosis treatment prior to the hip fracture, of whom 6743 (6.9%) initiated treatment. Error bars indicate 95% confidence intervals.
Effectiveness Analysis
Study Sample
Of the 97 169 total patients, we matched 6743 initiations to 67 430 nonuse episodes to mark their study index date. We further restricted our sample to 18 685 episodes for which we were able to reliably define instrumental variables, of which 2116 (11.3%) had osteoporosis medication use initiated. Alendronate (3918 [58.1%]) was taken by most patients who initiated osteoporosis medication, followed by risedronate (1746 [25.9%]) and ibandronate (634 [9.4%]). The mean (SD) follow-up time in this cohort was 1.6 (1.8) years (median [interquartile range], 341 [114-833] days). Table 1 summarizes the baseline characteristics of our study cohort. The mean (SD) age was 78 (10.4) years among the initiator group and 80 (10.7) years among the nonuser group. Only 416 patients (19.7%) in the osteoporosis treatment initiators were men, while 5756 nonusers (34.7%) were men. Proportion of patients with osteoporosis diagnoses was higher among initiators (261 [12.3%]) compared with nonusers (1119 [6.8%]).
Table 1. Baseline Characteristics of the Study Cohort Stratified by Osteoporosis Medication Exposure.
| Variable | No. (%) | Standardized Difference | |
|---|---|---|---|
| Initiators (n = 2116) | Nonusers (n = 16 569) | ||
| Demographic characteristics | |||
| Age, mean (SD), y | 78 (10.4) | 80 (10.7) | −13.7 |
| Male | 416 (19.7) | 5756 (34.7) | −34.4 |
| Fracture risk factors | |||
| Osteoporosis | 261 (12.3) | 1119 (6.8) | 19.1 |
| Prior vertebral fracture | 61 (2.9) | 503 (3.0) | −0.9 |
| Prior humerus fracture | 69 (3.3) | 515 (3.1) | 0.9 |
| Prior radius or ulna fracture | 53 (2.5) | 472 (2.8) | −2.1 |
| Prior pelvic fracture | 99 (4.7) | 941 (5.7) | −4.5 |
| Any other prior fracture | 656 (31.0) | 5478 (33.1) | −4.4 |
| Bone mineral density procedure ordered | 66 (3.1) | 259 (1.6) | 10.3 |
| Medication use potentially affecting fracture risk | |||
| Anticonvulsants | 223 (10.5) | 1843 (11.1) | −1.9 |
| Benzodiazepines | 429 (20.3) | 3162 (19.1) | 3 |
| Selective serotonin reuptake inhibitors | 482 (22.8) | 3654 (22.1) | 1.7 |
| β-Blockers | 675 (31.9) | 5045 (30.4) | 3.1 |
| Proton pump inhibitors | 491 (23.2) | 3796 (22.9) | 0.7 |
| Opioids | 872 (41.2) | 5780 (34.9) | 13.1 |
| Oral glucocorticoids | 72 (3.4) | 394 (2.4) | 6.1 |
| Comorbid conditions | |||
| Parkinson disease | 64 (3.0) | 586 (3.5) | −2.9 |
| Alzheimer disease | 242 (11.4) | 2985 (18.0) | −18.6 |
| Obesity | 40 (1.9) | 316 (1.9) | −0.1 |
| Diabetes | 408 (19.3) | 3621 (21.9) | −6.4 |
| Rheumatoid arthritis | 66 (3.1) | 330 (2.0) | 7.1 |
| Falls, syncope, or gait disturbances | 615 (29.1) | 5592 (33.7) | −10.1 |
| Health care use factors, mean (SD), No. | |||
| Distinct medications | 4 (3.7) | 4 (3.8) | 6.3 |
| Emergency department visits | 1 (0.8) | 1 (1.0) | −11.3 |
| Hospitalizations | 1 (0.4) | 1 (0.5) | −8.2 |
| Office visits | 5 (4.3) | 4 (4.3) | 5.6 |
Instrumental Variables
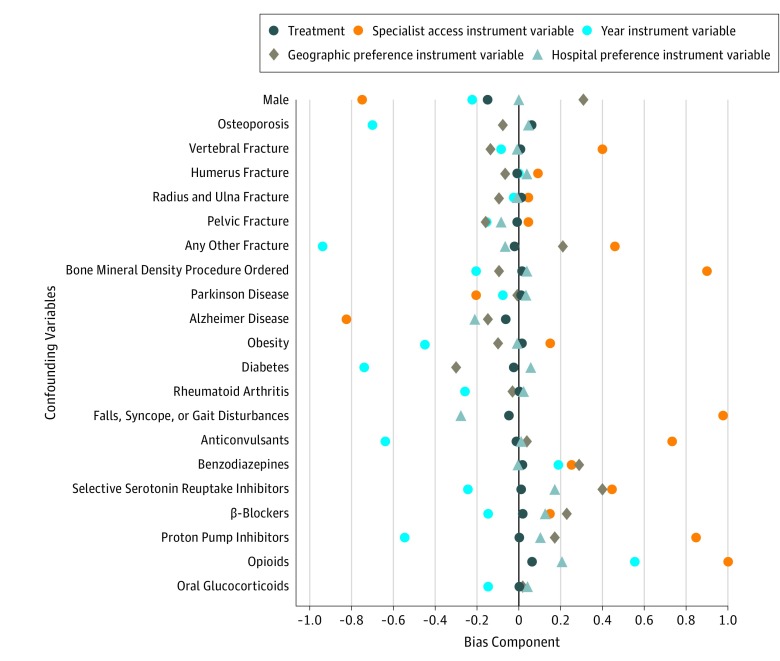
The variation in the proportion initiating treatment across instrumental variable strata was more pronounced for the hospital preference instrumental variable (2% in the first decile vs 25% in the last decile) compared with the other 3 instrumental variables (eFigure 2 in the Supplement). Measures of instrument strengths are reported in Table 2. Compared with the base model without instrumental variables, addition of calendar year, specialist access, or geographical variation instrumental variables resulted in small improvement in C statistics and pseudo R2 values; however, the hospital preference instrumental variable substantially increased explanatory power and discriminatory ability of the model. The partial F statistic was lowest for the specialist access instrumental variable (F = 19.9) and highest for the hospital preference instrumental variable (F = 1289.9). Adding all 4 instrumental variables together decreased the partial F statistic to 358.1, suggesting potential for finite sample bias because of combining weak instruments with a strong instrument. Finally, visual inspection of the bias component plots revealed that after scaling for instrument strength, the hospital preference–based instrumental variable analyses would often be less biased than other instrumental variable analyses when a covariate is omitted, suggesting that this instrumental variable is more likely to account for unmeasured confounding compared with other instrumental variables (Figure 2). Based on these observations from stage 1 instrumental variable models, we selected the hospital preference as the most appropriate instrumental variable for our analysis.
Table 2. Measures of Instrument Strength Based on Stage 1 Models.
| Model | C Statistic | Pseudo R2 | F Statistic |
|---|---|---|---|
| Baseline covariates onlya | 0.64 | 0.04 | NA |
| Baseline plus calendar year | 0.65 | 0.06 | 96.2 |
| Baseline plus specialist access | 0.64 | 0.05 | 19.9 |
| Baseline plus geographic variation | 0.67 | 0.07 | 197.7 |
| Baseline plus hospital preferences | 0.78 | 0.19 | 1289.9 |
| Baseline plus calendar year, specialist access, geographic variation, and hospital preferences | 0.79 | 0.20 | 358.1 |
Abbreviation: NA, not applicable.
Baseline covariates include all factors in Table 1.
Figure 2. Scaled Covariate Balance (Bias Component) by Levels of Treatment and Proposed Instrumental Variables.
To demonstrate balance, we dichotomized the categorical instrumental variables (calendar year, regional variation, and hospital preference) based on extreme strata. However, this dichotomization is for illustrative purpose only, and in stage 1 of instrumental variable modeling, these variables were included as categorical variables to preserve maximum information. These plots aim to visually compare the absolute covariate prevalence difference by treatment and by the proposed instruments after scaling for instrument strength. An instrument with bias components closer to 0 is expected to account for unmeasured confounding more efficiently than instruments with large bias components.21
Unadjusted and Adjusted Estimates of Fracture Risk
A total of 203 nonvertebral fracture events were observed among patients who initiated osteoporosis medication over 3798 person-years of follow-up, whereas 1737 events were observed among nonusers over 26 688 person-years follow-up. Of the 1940 total events, hip and pelvis fractures were most frequent, accounting for 758 events (39.1%) and 770 events (39.7%), respectively. Radius or ulna and humerus fractures occurred in 222 patients (11.4%) and 190 patients (9.8%). The incidence rates of fracture among osteoporosis medication initiators and nonusers were 5.34 (95% CI, 4.63-6.13) per 100 person-years and 6.50 (95% CI, 6.21-6.82) per 100 person-years, respectively.
Under the additive hazard model, slope of the cumulative regression function for the treatment effect did not change appreciably over the follow-up period (eFigure 3 in the Supplement). Therefore, the treatment effect was modeled assuming constancy. In the unadjusted model, initiation of osteoporosis medication was associated with a lower rate of fractures by a magnitude of 1.2 events (95% CI, 0.3-1.9) per 100 person-years (Table 3). The hospital preference instrumental variable model resulted in treatment effect estimates further downward and below the null, suggesting a rate difference of 4.2 events (95% CI, 1.1-7.3) per 100 person-years between initiators and nonusers, whereas multivariable adjustment and 1-to-1 propensity score–matched analysis suggested differences of 1.3 (95% CI, 0.5-2.1) and 0.8 (95% CI, −2.2 to 0.6), respectively (Table 3).
Table 3. Association of Osteoporosis Medication Initiation After Hip Fracture With Subsequent Nonvertebral Fracture Risk.
| Model | Estimate | |
|---|---|---|
| Coefficient (SE)a | 95% CI | |
| Crude | −1.1 (0.4) | −1.9 to −0.3 |
| Multivariable adjustedb | −1.3 (0.4) | −2.1 to −0.5 |
| 1:1 Propensity score matched | −0.8 (0.7) | −2.2 to 0.6 |
| Instrumental variable hospital preferenceb | −4.2 (1.6) | −7.3 to −1.1 |
Interpreted as difference between rates of nonvertebral fractures in the osteoporosis medication initiation group compared with the nonuser group per 100 person-years of follow-up.
Adjusted for all factors in Table 1.
Discussion
In this large observational cohort study, we noted low rates of treatment initiation with osteoporosis medications in patients with hip fracture not receiving treatment before their fracture. An instrumental variable analysis, which accounted for measured and unmeasured confounding, suggested that osteoporosis treatment initiation in patients with a hip fracture may result in a rate of subsequent fractures that is lower by a magnitude of 4.2 events per 100 person-years compared with no treatment.
We noted that by 2015, only 3.3% of patients initiated any osteoporosis treatment within 6 months of a hip fracture hospitalization. These results extend observations from previous studies, which reported rates of any treatment in the range of 13% to 21% after a hip fracture in the United States in 2012.3,4 Treatment rates reported in this study are lower than previous estimates because, unlike previous studies, we focused on a select population of patients who were not taking any osteoporosis medication in a 6-month period before their initial hip fracture event. We further noted that although nonusers had a higher prevalence of certain comorbid conditions, including Alzheimer disease and higher age, compared with initiators, this difference was not substantial. This observation underscores that decision to not treat individuals is unlikely to be completely explained by high frailty and low benefit-to-risk ratio in this group.
Estimates of effectiveness reported in this study are in line with evidence from RCTs evaluating similar outcomes. In a large RCT, Lyles et al7 reported a hazard ratio of 0.73 (95% CI, 0.55-0.98) for nonvertebral fractures among 1065 patients treated with zoledronic acid after hip fracture compared with 1062 patients treated with placebo over 1.9 years. While some other RCTs were underpowered to detect differences in fracture risk with osteoporosis treatments,28 a 2016 meta-analysis29 synthesizing evidence from 4 studies (2 RCTs and 2 prospective cohort studies) reported a 40% lower risk of subsequent hip fractures in hospitalized patients with hip fracture receiving osteoporosis treatment compared with placebo (hazard ratio, 0.60; 95% CI, 0.39-0.93). Our findings of low use of these treatments, despite clear evidence of effectiveness, highlight a need for interventions to increase patient and physician awareness and to promote innovative collaborative care models that can increase adherence to evidence-based prescribing practices.
Use of an instrumental variable analysis is a major strength of this investigation. Accounting for measured and unmeasured confounding through approaches such as instrumental variable analysis30,31,32 is critical while comparing treatment initiators with nonusers because standard approaches (ie, multivariable regression and 1-to-1 propensity score matching in our study) may result in substantial underestimation of the treatment effect owing to unmeasured confounding by indication. Use of appropriate instrumental variable diagnostics, including regularly reported measures such as R2 and partial F statistics, as well as innovative approaches, such as bias component plots that take into account the association between instrumental variables and actual treatment assignment,21 readily enables identification of the most appropriate instrumental variable out of a larger set of eligible instrumental variables.
Limitations
Our study had limitations. Our database only contains data on medication dispensing where claims were paid through health insurance programs, so this study relies on the assumption that the comparison group (nonusers) did not receive medications through alternate sources, such as out-of-pocket payments or free samples. Further, although we used instrumental variable analysis to account for unmeasured confounding, it must be noted that these methods rely on several assumptions, including the empirically untestable assumption of exclusion restriction, which requires that the instrumental variable must not be associated with the outcome indirectly through common causes. If some of these common causes are unmeasured, the instrumental variable methods may result in bias. Further, the patients with hip fracture represented in this sample are somewhat younger and have relatively fewer comorbid conditions compared with fee-for-service Medicare cohorts described in some previous studies.4,33 Therefore, the magnitude of the effect reported in our article may not extend to traditional Medicare patients, if the treatment effect is heterogeneous by patient characteristics. Our study did not evaluate the association of duration of treatment with the risk of fractures or compared effectiveness of various classes of osteoporosis medications. Future research is recommended to provide insights into these clinically relevant questions.
Conclusions
In conclusion, we observed continually decreasing rates, which reached a nadir of 3% by 2015, in osteoporosis medication initiation after a hip fracture in the United States. We also documented clinically meaningful reduction in subsequent nonvertebral fracture rate of approximately 4.2 events per 100 person-years among patients who initiated osteoporosis treatment compared with nonusers in an instrumental variable analysis, suggesting that improving prescriber adherence to prescribing guidelines and patient adherence to prescribed regimen may result in notable public health benefit.
eFigure 1. Study Design
eFigure 2. Proportion of Patients Receiving Treatment With Osteoporosis Medications by Instrumental Variable Strata
eFigure 3. Cumulative Regression Function With Pointwise 95% Confidence Interval for the Treatment Effect Under the Additive Hazard Model
eTable. Components of the Composite Outcome
eAppendix. Model Equations
References
- 1.Hodsman AB, Leslie WD, Tsang JF, Gamble GD. 10-Year probability of recurrent fractures following wrist and other osteoporotic fractures in a large clinical cohort: an analysis from the Manitoba Bone Density Program. Arch Intern Med. 2008;168(20):-. doi: 10.1001/archinte.168.20.2261 [DOI] [PubMed] [Google Scholar]
- 2.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res. 2014;29(9):1929-1937. doi: 10.1002/jbmr.2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SC, Kim MS, Sanfelix-Gimeno G, et al. Use of osteoporosis medications after hospitalization for hip fracture: a cross-national study. Am J Med. 2015;128(5):519-526.e1. doi: 10.1016/j.amjmed.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khosla S, Cauley JA, Compston J, et al. Addressing the crisis in the treatment of osteoporosis: a path forward. J Bone Miner Res. 2016;32(3):424-430. doi: 10.1002/jbmr.3074 [DOI] [PubMed] [Google Scholar]
- 6.Michael Lewiecki E, Wright NC, Curtis JR, et al. Hip fracture trends in the United States, 2002 to 2015. Osteoporos Int. 2018;29(3):717-722. doi: 10.1007/s00198-017-4345-0 [DOI] [PubMed] [Google Scholar]
- 7.Lyles KW, Colón-Emeric CS, Magaziner JS, et al. ; HORIZON Recurrent Fracture Trial . Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809. doi: 10.1056/NEJMoa074941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22(3):983-991. doi: 10.1007/s00198-010-1411-2 [DOI] [PubMed] [Google Scholar]
- 9.Reyes C, Pottegård A, Schwarz P, et al. Real-life and RCT participants: alendronate users versus FITs’ trial eligibility criterion. Calcif Tissue Int. 2016;99(3):243-249. doi: 10.1007/s00223-016-0141-7 [DOI] [PubMed] [Google Scholar]
- 10.Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19(6):537-554. doi: 10.1002/pds.1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angrist J, Imbens G, Rubin D. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91(434):444-455. doi: 10.1080/01621459.1996.10476902 [DOI] [Google Scholar]
- 12.Ionescu-Ittu R, Delaney JA, Abrahamowicz M. Bias-variance trade-off in pharmacoepidemiological studies using physician-preference-based instrumental variables: a simulation study. Pharmacoepidemiol Drug Saf. 2009;18(7):562-571. doi: 10.1002/pds.1757 [DOI] [PubMed] [Google Scholar]
- 13.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492-499. doi: 10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]
- 14.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45(7):703-714. doi: 10.1016/0895-4356(92)90047-Q [DOI] [PubMed] [Google Scholar]
- 15.Majumdar SR, Lix LM, Yogendran M, Morin SN, Metge CJ, Leslie WD. Population-based trends in osteoporosis management after new initiations of long-term systemic glucocorticoids (1998-2008). J Clin Endocrinol Metab. 2012;97(4):1236-1242. doi: 10.1210/jc.2011-2645 [DOI] [PubMed] [Google Scholar]
- 16.Liu SK, Munson JC, Bell JE, et al. Quality of osteoporosis care of older Medicare recipients with fragility fractures: 2006 to 2010. J Am Geriatr Soc. 2013;61(11):1855-1862. doi: 10.1111/jgs.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rassen JA, Brookhart MA, Glynn RJ, Mittleman MA, Schneeweiss S. Instrumental variables II: instrumental variable application-in 25 variations, the physician prescribing preference generally was strong and reduced covariate imbalance. J Clin Epidemiol. 2009;62(12):1233-1241. doi: 10.1016/j.jclinepi.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneeweiss S, Solomon DH, Wang PS, Rassen J, Brookhart MA. Simultaneous assessment of short-term gastrointestinal benefits and cardiovascular risks of selective cyclooxygenase 2 inhibitors and nonselective nonsteroidal antiinflammatory drugs: an instrumental variable analysis. Arthritis Rheum. 2006;54(11):3390-3398. doi: 10.1002/art.22219 [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 20.Nagelkerke NJ. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691-692. doi: 10.1093/biomet/78.3.691 [DOI] [Google Scholar]
- 21.Jackson JW, Swanson SA. Toward a clearer portrayal of confounding bias in instrumental variable applications. Epidemiology. 2015;26(4):498-504. doi: 10.1097/EDE.0000000000000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tchetgen Tchetgen EJ, Walter S, Vansteelandt S, Martinussen T, Glymour M. Instrumental variable estimation in a survival context. Epidemiology. 2015;26(3):402-410. doi: 10.1097/EDE.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Fine J, Brookhart A. Instrumental variable additive hazards models. Biometrics. 2015;71(1):122-130. doi: 10.1111/biom.12244 [DOI] [PubMed] [Google Scholar]
- 24.Aalen O, Borgan O, Gjessing H. Survival and Event History Analysis: A Process Point of View. New York, NY: Springer Science & Business Media; 2008. doi: 10.1007/978-0-387-68560-1 [DOI] [Google Scholar]
- 25.Rassen JA, Brookhart MA, Glynn RJ, Mittleman MA, Schneeweiss S. Instrumental variables I: instrumental variables exploit natural variation in nonexperimental data to estimate causal relationships. J Clin Epidemiol. 2009;62(12):1226-1232. doi: 10.1016/j.jclinepi.2008.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):69-80. doi: 10.1002/pds.3263 [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 28.Cecilia D, Jodar E, Fernandez C, Resines C, Hawkins F. Effect of alendronate in elderly patients after low trauma hip fracture repair. Osteoporos Int. 2009;20(6):903-910. doi: 10.1007/s00198-008-0767-z [DOI] [PubMed] [Google Scholar]
- 29.Peng J, Liu Y, Chen L, et al. Bisphosphonates can prevent recurrent hip fracture and reduce the mortality in osteoporotic patient with hip fracture: a meta-analysis. Pak J Med Sci. 2016;32(2):499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29(4):722-729. doi: 10.1093/ije/29.4.722 [DOI] [PubMed] [Google Scholar]
- 31.Brookhart MA, Wang PS, Solomon DH, Schneeweiss S. Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology. 2006;17(3):268-275. doi: 10.1097/01.ede.0000193606.58671.c5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernán MA, Robins JM. Instruments for causal inference: an epidemiologist’s dream? Epidemiology. 2006;17(4):360-372. doi: 10.1097/01.ede.0000222409.00878.37 [DOI] [PubMed] [Google Scholar]
- 33.Cenzer IS, Tang V, Boscardin WJ, et al. One-year mortality after hip fracture: development and validation of a prognostic index. J Am Geriatr Soc. 2016;64(9):1863-1868. doi: 10.1111/jgs.14237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study Design
eFigure 2. Proportion of Patients Receiving Treatment With Osteoporosis Medications by Instrumental Variable Strata
eFigure 3. Cumulative Regression Function With Pointwise 95% Confidence Interval for the Treatment Effect Under the Additive Hazard Model
eTable. Components of the Composite Outcome
eAppendix. Model Equations