Key Points
Question
Does the presence of polyvascular disease affect the risk of ischemic events, both cardiac and limb, and bleeding in patients with peripheral artery disease?
Findings
In this secondary analysis of a randomized clinical trial of 13 885 patients with peripheral artery disease randomized to receive ticagrelor or clopidogrel compared with patients with isolated peripheral artery disease, those with concomitant peripheral artery disease and polyvascular disease had an increased risk of major adverse cardiac events and lower-extremity revascularization. Polyvascular disease was not associated with an increased risk of Thrombolysis in Myocardial Infarction major bleeding.
Meaning
These data highlight the increased risk of coronary artery disease and cerebrovascular disease among patients with peripheral artery disease and the need to identify potent preventive antithrombotic therapies.
This secondary analysis of the Examining Use of Ticagrelor in Peripheral Artery Disease (EUCLID) trial examines the risk of ischemic events in patients with peripheral artery disease (PAD) and polyvascular disease (PAD with coronary artery disease [CAD] and/or cerebrovascular disease [CVD]).
Abstract
Importance
The effect of polyvascular disease on cardiovascular outcomes in the background of peripheral artery disease (PAD) is unclear.
Objective
To determine the risk of ischemic events (both cardiac and limb) among patients with PAD and polyvascular disease.
Design, Setting, and Participants
In this post hoc secondary analysis of the international Examining Use of Ticagrelor in Peripheral Artery Disease (EUCLID) trial, outcomes were compared among 13 885 enrolled patients with PAD alone, PAD + coronary artery disease (CAD), PAD + cerebrovascular disease (CVD), and PAD + CAD + CVD. Adjusted Cox proportional hazards regression models were implemented to determine the risk associated with polyvascular disease and outcomes, and intention-to-treat analysis was performed. The EUCLID trial was conducted from December 31, 2012, to March 7, 2014; the present post hoc analysis was performed from June 1, 2017, to February 5, 2018.
Interventions
EUCLID evaluated ticagrelor vs clopidogrel in preventing major adverse cardiac events (cardiovascular death, myocardial infarction [MI], or ischemic stroke) and major bleeding in patients with PAD.
Main Outcomes and Measures
The primary end point was a composite of cardiovascular death, MI, or ischemic stroke. Key secondary end points included the individual components of the primary end point and acute limb ischemia leading to hospitalization, major amputation, and lower-extremity revascularization. The primary end point of Thrombolysis in Myocardial Infarction (TIMI) major bleeding was also evaluated.
Results
The EUCLID trial randomized 13 885 patients with a median age of 66 years (interquartile range, 60-73 years), of whom 3888 (28.0%) were women. At baseline, 7804 patients (56.2%) had PAD alone; 2639 (19.0%) had PAD + CAD; 2049 (14.8%) had PAD + CVD; and 1393 (10.0%) had PAD + CAD + CVD. Compared with patients with isolated PAD, the adjusted hazard ratios (aHRs) for major adverse cardiac events were 1.34 (95% CI, 1.15-1.57; P < .001) for PAD + CVD, 1.65 (95% CI, 1.43-1.91; P < .001) for PAD + CAD, and 1.99 (95% CI, 1.69-2.34; P < .001) for PAD + CAD + CVD. The aHRs for lower-extremity revascularization were 1.17 (95% CI, 1.03-1.34; P = .01) for PAD + CAD, 1.17 (95% CI, 1.02-1.35; P = .02) for PAD + CVD, and 1.34 (95% CI, 1.15-1.57; P < .001) for PAD + CAD + CVD. Polyvascular disease was not associated with an increased risk of acute limb ischemia (aHR for PAD + CVD, 0.91; 95% CI, 0.62-1.34, P = .63; PAD + CAD, 0.93; 95% CI, 0.64-1.34, P = .69; and PAD + CAD + CVD, 0.98; 95% CI, 0.63-1.53, P = .93), major amputation (aHR for PAD + CVD, 0.83; 95% CI, 0.54-1.27, P = .40; PAD + CAD, 0.74; 95% CI, 0.47-1.16, P = .19; and PAD + CAD + CVD, 1.12; 95% CI, 0.69-1.80, P = .65), or TIMI major bleeding (PAD + CVD, 0.98; 0.66-1.44, P = .91; PAD + CAD, 1.04; 0.74-1.48, P = .81; and PAD + CAD + CVD, 0.96; 95% CI, 0.62-1.51, P = .88).
Conclusions and Relevance
Compared with patients with PAD alone, the risk of major adverse cardiac events and lower-extremity revascularization increased with multiple vascular bed involvement. There was no clear increased risk of bleeding associated with polyvascular disease.
Introduction
Patients with peripheral artery disease (PAD) are a high-risk population.1 Peripheral artery disease enhances the risk of major adverse cardiac events (MACEs), defined as cardiovascular death, myocardial infarction (MI), and ischemic stroke.2 Observational studies report that among patients with PAD, the risk of cardiac events is greatest in those with concomitant coronary artery disease (CAD) and/or cerebrovascular disease (CVD), a condition known as polyvascular disease.3 In addition to MACEs, patients with PAD are also at risk for major adverse limb events, which include but are not limited to acute limb ischemia (ALI), major amputation, and lower-extremity revascularization.4
Antithrombotic strategies are considered the cornerstone therapy for preventing cardiovascular events in patients with PAD.5 To date, studies evaluating the use of intense antithrombotic therapies in patients with PAD have all demonstrated a heightened risk of bleeding with varying degrees of efficacy in reducing both cardiac and limb events.6,7,8,9,10 Antithrombotic therapy in patients with PAD can be particularly problematic as the same comorbidities conferring an increased risk for atherothrombosis, such as older age, diabetes, and renal dysfunction, also predispose this population to bleeding.11,12
In the Examining Use of Ticagrelor in Peripheral Artery Disease (EUCLID) trial, monotherapy with ticagrelor compared with clopidogrel did not reduce the rates of MACEs in patients with symptomatic PAD.13,14 Using the PAD study population from the EUCLID trial, the present study aims to evaluate the association between polyvascular disease and MACEs as well as major adverse limb events and determine the risk of bleeding associated with polyvascular disease.
Methods
Study Population
This is a post hoc secondary analysis of the EUCLID trial (ClinicalTrials.gov identifier: NCT01732822). The study design and results of the EUCLID trial have been previously published.13,14 Briefly, EUCLID was a double-blind, active-comparator trial of ticagrelor vs clopidogrel in patients with symptomatic PAD. Between December 31, 2012, and March 7, 2014, patients with symptomatic PAD were randomized in a 1:1 double-blind manner via an interactive voice- or web-response system to receive ticagrelor, 90 mg twice daily, or clopidogrel, 75 mg once daily. Patients were followed up until May 9, 2016. On screening, patients were eligible if they were aged 50 years or older and had symptomatic PAD, defined as an ankle-brachial index of 0.80 or less or lower-extremity revascularization more than 30 days before randomization. With regard to ankle-brachial index eligibility criteria, a confirmation measurement of 0.85 or less was also required at the time of randomization. In settings in which the ankle-brachial index was 1.40 or greater at screening, a toe-brachial index cutoff level of 0.60 or lower was implemented, with a required second reading of 0.65 or lower at randomization.
Exclusion criteria were as follows: (1) current or planned use of dual antiplatelet therapy; (2) history of poor clopidogrel metabolism, defined as 2 loss-of-function cytochrome P450 enzyme 2C19 allele variants; (3) need for long-term anticoagulation; and (4) elevated bleeding risk. In the present study, patients were grouped according to baseline history of CAD or CVD in the following manner: PAD alone, PAD + CAD, PAD + CVD, and PAD + CAD + CVD. History of CAD or CVD was based on clinical data obtained at the start of the trial. Prior CAD was defined on the case report form as a history of percutaneous coronary intervention, coronary artery bypass grafting, or MI. Prior CVD was defined based on the case report form as a history of stroke or transient ischemic attack, carotid stenosis, or carotid revascularization.
All patients provided written informed consent, and the trial protocol was reviewed and approved by appropriate ethics committees at participating sites. The EUCLID publication committee approved the post hoc analysis with waiver of informed consent.
End Points
The primary end point in the EUCLID trial was a composite of cardiovascular death, MI, or ischemic stroke. For the present study, key secondary end points included the individual components of the primary end point and ALI leading to hospitalization, major amputation, and lower-extremity revascularization. The primary end point of Thrombolysis in Myocardial Infarction (TIMI) major bleeding was also evaluated.
Statistical Analysis
The present analysis was conducted from June 1, 2017, to February 5, 2018. Continuous variables are presented as medians with interquartile ranges and were assessed using the Kruskal-Wallis test. Categorical variables are reported as counts and percentages and were assessed using χ2 tests. End points were assessed according to the following groups: PAD alone (reference group), PAD + CAD, PAD + CVD, and PAD + CAD + CVD. Analyses were performed according to the intention-to-treat principle and included all randomized patients. The interaction between the number of diseased vascular beds and treatment cohorts—ticagrelor or clopidogrel—for the primary end points was assessed. A Cox proportional hazards regression model was applied to determine the association between polyvascular disease and outcomes. Results of the models include adjusted hazard ratios (aHRs), 95% CIs, and P values.
Adjustment variables for each outcome were chosen using a stepwise selection process with α level = .05. Results for the primary composite efficacy end point were adjusted for age, weight, sex, estimated glomerular filtration rate, baseline ankle-brachial index, randomized treatment, inclusion criteria, critical limb ischemia, diabetes (type 1 or 2), statin use before randomization, smoking status, prior major amputation, and prior minor amputation. Cardiovascular death was adjusted for age, weight, estimated glomerular filtration rate, baseline ankle-brachial index, randomized treatment, sex, geographic region, critical limb ischemia, diabetes, use of statins before randomization, and prior minor amputation. Myocardial infarction was adjusted for age, estimated glomerular filtration rate, baseline ankle-brachial index, randomized treatment, inclusion criteria, geographic region, diabetes, smoking status, and prior minor amputation. Ischemic stroke was adjusted for age, baseline ankle-brachial index, randomized treatment, geographic region, diabetes, smoking status, and prior minor amputation.
For safety events, the results for the primary safety end point were adjusted for age, randomized treatment, and geographic region. Acute limb ischemia was adjusted for baseline ankle-brachial index, randomized treatment, inclusion criteria, use of statins before randomization, and use of angiotensin receptor blockers before randomization. Lower-extremity revascularization was adjusted for baseline ankle-brachial index, estimated glomerular filtration rate, inclusion criteria, diabetes, use of aspirin before randomization, use of clopidogrel before randomization, and smoking status. Amputation was adjusted for weight, baseline ankle-brachial index, inclusion criteria, critical limb ischemia, diabetes, use of statins before randomization, prior major amputation, and prior minor amputation. Major bleeding was adjusted for age, randomized treatment, and geographic region.
The proportional hazards assumption was evaluated using weighted Schoenfeld residuals, and there were no major violations of the assumption for any outcome. Kaplan-Meier curves are presented for the composite end point of cardiovascular death, MI, or ischemic stroke according to polyvascular disease phenotype. The P value for the log-rank test is presented on each plot. Two-sided P values ≤.05 were considered statistically significant.
All statistical analyses were performed by the Duke Clinical Research Institute (Duke University School of Medicine, Durham, NC), using SAS, version 9.4 (SAS Institute Inc).
Results
Patients
The EUCLID trial screened 16 237 patients with symptomatic PAD. After implementing inclusion and exclusion criteria, 13 885 patients, of whom 3888 (28.0%) were women, with a median age of 66 years (interquartile range, 60-73 years) underwent randomization. Polyvascular disease was present in 43.8% of the patients: 7804 patients (56.2%) had PAD alone, 2639 (19.0%) had PAD + CAD, 2049 (14.8%) had PAD + CVD, and 1393 (10.0%) had PAD + CAD + CVD. Most randomized patients (56.7%) met inclusion criteria owing to a history of lower-extremity revascularization.
Compared with patients with PAD alone, those with polyvascular disease were older; more likely to have multiple comorbidities, such as diabetes, hypertension, or hyperlipidemia; and more likely to have undergone prior lower-extremity revascularization (Table 1).
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | P Value | |||
|---|---|---|---|---|---|
| PAD Only | PAD + CAD | PAD + CVD | PAD + CAD + CVD | ||
| Patients | 7804 (56.2) | 2639 (19.0) | 2049 (14.8) | 1393 (10.0) | |
| Age, median (IQR), y | 65 (59-72) | 66 (61-73) | 67 (61-73) | 68 (63-74) | <.001 |
| Women | 2364 (30.3) | 588 (22.3) | 621 (30.3) | 315 (22.6) | <.001 |
| Region | |||||
| Asia | 1002 (12.8) | 201 (7.6) | 292 (14.3) | 107 (7.7) | <.001 |
| Central/South America | 1131 (14.5) | 358 (13.6) | 160 (7.8) | 91 (6.5) | |
| Europe | 4525 (58.0) | 1251 (47.4) | 1135 (55.4) | 587 (42.1) | |
| North America | 1146 (14.7) | 829 (31.4) | 462 (22.5) | 608 (43.6) | |
| Weight, median (IQR), kg | 75 (65-86) | 80 (69-91) | 75 (65-86) | 80 (70-90) | <.001 |
| Inclusion criteria for randomization | <.001 | ||||
| Previous revascularization | 4238 (54.3) | 1541 (58.4) | 1193 (58.2) | 903 (64.8) | |
| ABI or TBI criteria, mean (SD) | 3566 (45.7) | 1098 (41.6) | 856 (41.8) | 490 (35.2) | |
| ABI value | 0.63 (0.16) | 0.64 (0.14) | 0.62 (0.14) | 0.63 (0.13) | .004 |
| TBI value | 0.52 (0.14) | 0.54 (0.34) | 0.48 (0.19) | 0.53 (0.26) | .49 |
| Limb symptoms | |||||
| Asymptomatic | 1557 (20.0) | 479 (18.2) | 350 (17.1) | 215 (15.4) | <.001 |
| Mild or moderate claudication | 4168 (53.4) | 1415 (53.6) | 1093 (53.3) | 734 (52.7) | |
| Severe claudication | 1678 (21.5) | 638 (24.2) | 525 (25.6) | 387 (27.8) | |
| Pain while at rest | 217 (2.8) | 70 (2.7) | 47 (2.3) | 44 (3.2) | |
| Minor tissue loss | 135 (1.7) | 29 (1.1) | 31 (1.5) | 12 (0.9) | |
| Major tissue loss | 47 (0.6) | 7 (0.3) | 3 (0.1) | 1 (0.1) | |
| Major amputation above the ankle | 212 (2.7) | 47 (1.8) | 41 (2.0) | 39 (2.8) | .02 |
| Minor amputation | 414 (5.3) | 83 (3.1) | 74 (3.6) | 34 (2.4) | <.001 |
| Medical history | |||||
| Stroke | 0 | 0 | 736 (35.9) | 407 (29.2) | NA |
| Transient ischemic attack | 0 | 0 | 300 (14.6) | 207 (14.9) | NA |
| Myocardial infarction | 0 | 1692 (64.1) | 0 | 830 (59.6) | NA |
| Carotid stenosis or carotid revascularization | 0 | 0 | 1423 (69.4) | 1104 (79.3) | NA |
| Diabetes, type 1 or 2 | 2724 (34.9) | 1199 (45.4) | 756 (36.9) | 666 (47.8) | <.001 |
| Hypertension | 5529 (70.8) | 2304 (87.3) | 1743 (85.1) | 1281 (92.0) | <.001 |
| Hyperlipidemia | 5241 (67.2) | 2345 (88.9) | 1607 (78.4) | 1287 (92.4) | <.001 |
| Chronic kidney disease, No./total No. (%)a | 1558/7531 (20.7) | 719/2564 (28.0) | 545/1996 (27.3) | 483/1358 (35.6) | <.001 |
| Tobacco use | n = 7744 | n = 2627 | n = 2042 | n = 1390 | <.001 |
| Current | 2555 (33.0) | 723 (27.5) | 649 (31.8) | 362 (26.0) | <.001 |
| Former | 3365 (43.5) | 1401 (53.3) | 965 (47.3) | 799 (57.5) | <.001 |
| Never | 1824 (23.6) | 503 (19.1) | 428 (21.0) | 229 (16.5) | <.001 |
| Medications | |||||
| Aspirin | 4802 (61.5) | 2045 (77.5) | 1344 (65.6) | 1080 (77.5) | <.001 |
| Clopidogrel | 2045 (26.2) | 978 (37.1) | 782 (38.2) | 668 (48.0) | <.001 |
| DAPT | 841 (10.8) | 617 (23.4) | 356 (17.4) | 453 (32.5) | <.001 |
| Statin | 5123 (65.6) | 2299 (87.1) | 1531 (74.7) | 1228 (88.2) | <.001 |
| ACE inhibitor | 2741 (35.1) | 1318 (49.9) | 872 (42.6) | 704 (50.5) | <.001 |
| Angiotensin receptor blocker | 1850 (23.7) | 705 (26.7) | 571 (27.9) | 362 (26.0) | <.001 |
| Cilostazol | 1254 (16.1) | 363 (13.8) | 297 (14.5) | 181 (13.0) | .002 |
Abbreviations: ABI, ankle-brachial index; ACE, angiotensin-converting enzyme; CAD, coronary artery disease; CVD, cerebrovascular disease; DAPT, dual antiplatelet therapy; IQR, interquartile range; NA, not applicable; PAD, peripheral artery disease; TBI, toe-brachial index.
Chronic kidney disease was defined as estimated glomerular filtration rate less than 60 mL/min/1.73 m2.
Before randomization, patients with polyvascular disease were more frequently treated with aspirin, clopidogrel, dual antiplatelet therapy, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers. In contrast, patients with PAD alone were more frequently treated with cilostazol (Table 1).
Outcomes
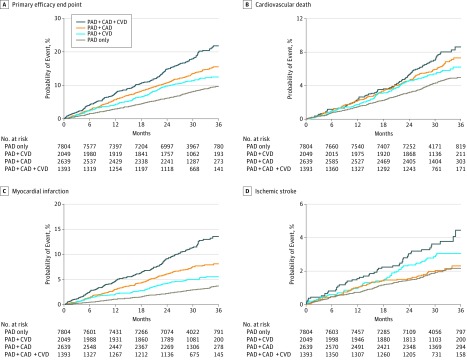
The rate of the primary end point of the composite of cardiovascular death, MI, or ischemic stroke, defined as events per 100 patient-years, was greatest in patients with polyvascular disease, with the lowest event rates occurring in patients with PAD alone (3.4), followed by PAD + CVD (4.8), PAD + CAD (5.7), and PAD + CAD + CVD (8.1) (log-rank P < .001) (Table 2 and Figure 1A). Rates for the individual components of cardiovascular death and MI increased in a similar incremental fashion with each additional diseased vascular bed. Rates for ischemic stroke were heightened only among patients with CVD—those with PAD + CVD or PAD + CAD + CVD. Figure 1B, C, and D depict the 36-month Kaplan-Meier rates for the individual components of the primary composite end point (cardiovascular death, MI, and ischemic stroke, respectively) according to vascular disease phenotype. There was no significant difference in rates of ALI requiring hospitalization and major amputation among patients with polyvascular disease compared with patients with PAD alone.
Table 2. Unadjusted Polyvascular Disease Outcomes: Efficacy and Safety .
| Outcome | PAD Only (n = 7804) | PAD + CAD (n = 2639) | PAD + CVD (n = 2049) | PAD + CAD + CVD (n = 1393) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate (Count)a | Rate (Count)a | HR (95% CI) | P Valueb | Rate (Count)a | HR (95% CI) | P Valueb | Rate (Count)a | HR (95% CI) | P Valueb | ||
| Composite end points | |||||||||||
| CV death, MI, stroke | 3.37 (639) | 5.74 (357) | 1.70 (1.50-1.94) | <.001 | 4.78 (234) | 1.42 (1.22-1.65) | <.001 | 8.12 (261) | 2.41 (2.09-2.78) | <.001 | |
| Efficacy end points | |||||||||||
| CV death | 1.70 (331) | 2.48 (162) | 1.46 (1.21-1.76) | <.001 | 2.18 (111) | 1.28 (1.03-1.59) | .02 | 2.96 (102) | 1.74 (1.39-2.17) | <.001 | |
| MI | 1.22 (232) | 3.03 (190) | 2.49 (2.06-3.02) | <.001 | 2.06 (102) | 1.70 (1.34-2.14) | <.001 | 4.90 (159) | 4.03 (3.30-4.94) | <.001 | |
| Ischemic stroke | 0.75 (144) | 0.79 (51) | 1.06 (0.77-1.45) | .74 | 1.12 (56) | 1.49 (1.09-2.03) | .01 | 1.45 (49) | 1.93 (1.40-2.67) | <.001 | |
| ALI requiring hospitalization | 0.71 (136) | 0.62 (40) | 0.87 (0.61-1.24) | .45 | 0.66 (33) | 0.92 (0.63-1.35) | .69 | 0.68 (23) | 0.96 (0.61-1.49) | .84 | |
| Major amputation | 0.72 (138) | 0.42 (27) | 0.58 (0.38-0.88) | .01 | 0.56 (28) | 0.77 (0.51-1.16) | .22 | 0.68 (23) | 0.94 (0.61-1.46) | .79 | |
| LER | 4.82 (870) | 6.03 (361) | 1.25 (1.10-1.41) | <.001 | 5.90 (274) | 1.22 (1.07-1.40) | .004 | 7.58 (233) | 1.57 (1.35-1.81) | <.001 | |
| Safety end points | |||||||||||
| TIMI major bleeding | 0.70 (118) | 0.84 (46) | 1.20 (0.86-1.69) | .28 | 0.76 (33) | 1.09 (0.74-1.60) | .67 | 0.89 (25) | 1.27 (0.83-1.96) | .27 | |
| TIMI minor bleeding | 0.43 (73) | 0.48 (26) | 1.10 (0.70-1.72) | .67 | 0.60 (26) | 1.39 (0.89-2.17) | .15 | 0.93 (26) | 2.14 (1.36-3.34) | .001 | |
Abbreviations: ALI, acute limb ischemia; CAD, coronary artery disease; CV, cardiovascular; CVD, cerebrovascular disease; HR, hazard ratio; LER, lower-extremity revascularization; MI, myocardial infarction; PAD, peripheral artery disease; TIMI, Thrombolysis in Myocardial Infarction.
Event rates defined as number of events per 100 patient-years of follow-up.
P values are from the log-rank test comparing the Kaplan-Meier rates across groups.
Figure 1. Outcomes for the Efficacy End Points.
Outcomes for the primary efficacy end point (composite of cardiovascular death, myocardial infarction, and ischemic stroke) (A), cardiovascular death (B), myocardial infarction (C), and ischemic stroke (D). CAD indicates coronary artery disease; CVD, cerebrovascular disease; and PAD, peripheral artery disease. For all panels P < .001.
The rate of lower-extremity revascularization increased per 100 patient-years with each additional diseased vascular bed, with the lowest rates in patients with PAD alone (4.8), followed by PAD + CVD (5.9), PAD + CAD (6.0), and PAD + CAD + CVD (7.6) (log-rank P < .001). The rate of TIMI major bleeding, defined as events per 100 patient-years, was similar among all 4 cohorts: PAD alone (0.70); PAD + CVD (0.76); PAD + CAD (0.84); and PAD + CAD + CVD (0.89) (log-rank P = .58) (Table 2). The ticagrelor vs clopidogrel comparison among all of the polyvascular disease subgroups (PAD + CAD, PAD + CVD, and PAD + CAD + CVD) did not demonstrate an interaction P value of significance for the primary end points of cardiovascular death, MI, or ischemic stroke (P = .71), and major bleeding (P = .31).
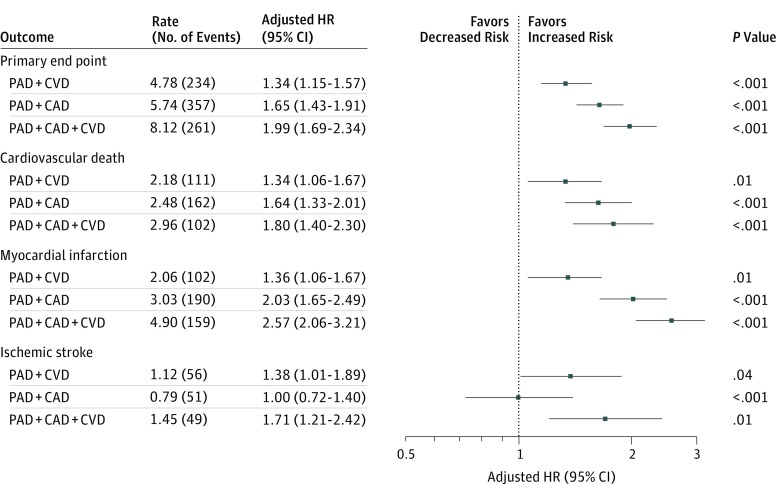
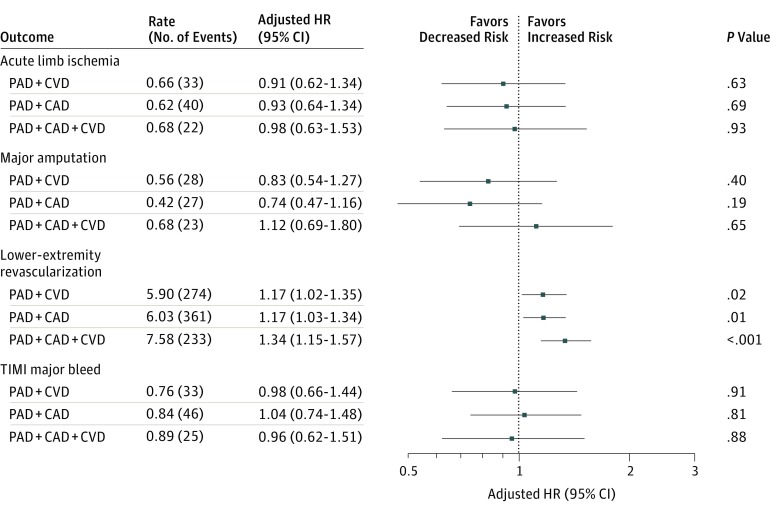
Compared with patients with PAD alone and following adjustment for baseline variables, a stepwise increase in risk of the primary end point was observed with each additional diseased vascular bed: PAD + CVD (aHR, 1.34; 95% CI, 1.15-1.57; P < .001), PAD + CAD (aHR, 1.65; 95% CI, 1.43-1.91; P < .001), and PAD + CAD + CVD (aHR, 1.99; 95% CI, 1.69-2.34; P < .001). A comparable increase in risk was observed for cardiovascular death and MI with each additional diseased vascular bed (Figure 2). With regard to key limb end points, the risk of acute limb ischemia requiring hospitalization and major amputation was similar among all cohorts regardless of the presence of polyvascular disease. The risk of lower-extremity revascularization increased in a stepwise fashion with each additional diseased vascular bed: PAD + CAD (aHR, 1.17; 95% CI, 1.03-1.34; P = .01), PAD + CVD (aHR, 1.17; 95% CI, 1.02-1.35; P = .02), and PAD + CAD + CVD (aHR, 1.34; 95% CI, 1.15-1.57; P < .001). The risk of TIMI major bleeding was consistent among all cohorts (Figure 3).
Figure 2. Major Adverse Cardiac Events According to Polyvascular Disease Phenotype .
Adjusted rates presented as events per 100 patient-years. Reference group was patients with peripheral artery disease (PAD) alone. The primary end point (composite of cardiovascular death, myocardial infarction, and ischemic stroke) was adjusted for age, weight, sex, estimated glomerular filtration rate (eGFR), baseline ankle-brachial index (ABI), randomized treatment, sex, inclusion criteria, geographic region, critical limb ischemia, diabetes (type 1 or 2), statin use before randomization, smoking status, prior major amputation, and prior minor amputation. Cardiovascular death was adjusted for age, weight, eGFR, baseline ABI, randomized treatment, sex, geographic region, critical limb ischemia, diabetes, use of statins before randomization, and prior minor amputation. Myocardial infarction was adjusted for age, eGFR, baseline ABI, randomized treatment, inclusion criteria, geographic region, diabetes, smoking status, and prior minor amputation. Ischemic stroke was adjusted for age, baseline ABI, randomized treatment, geographic region, diabetes, smoking status, and prior minor amputation. CAD indicates coronary artery disease; CVD, cerebrovascular disease; and HR, hazard ratio.
Figure 3. Major Adverse Limb Events and Safety Events According to Polyvascular Disease Phenotype .
Adjusted rates presented as events per 100 patient-years. Reference group was patients with peripheral artery disease (PAD) alone. Acute limb ischemia was adjusted for baseline ankle-brachial index (ABI), randomized treatment, inclusion criteria, use of statins before randomization, and use of angiotensin receptor blockers before randomization. Lower-extremity revascularization was adjusted for baseline ABI, estimated glomerular filtration rate, inclusion criteria, diabetes (type 1 or 2), use of aspirin before randomization, use of clopidogrel before randomization, and smoking status. Amputation was adjusted for weight, baseline ABI, inclusion criteria, critical limb ischemia, diabetes, use of statins before randomization, prior major amputation, and prior minor amputation. Major bleeding was adjusted for age, randomized treatment, and geographic region. Minor bleeding was adjusted for age, baseline ABI, inclusion criteria, sex, randomized treatment, and geographic region. CAD indicates coronary artery disease; CVD, cerebrovascular disease; HR, hazard ratio; and TIMI, Thrombolysis in Myocardial Infarction.
Polyvascular disease was not associated with an increased risk of ALI (aHR for PAD + CVD, 0.91; 95% CI, 0.62-1.34, P = .63; PAD + CAD, 0.93; 95% CI, 0.64-1.34, P = .69; and PAD + CAD + CVD, 0.98; 95% CI, 0.63-1.53, P = .93), major amputation (aHR for PAD + CVD, 0.83; 95% CI, 0.54-1.27, P = .40; PAD + CAD, 0.74; 95% CI, 0.47-1.16, P = .19; and PAD + CAD + CVD, 1.12; 95% CI, 0.69-1.80, P = .65), or TIMI major bleeding (aHR for PAD + CVD, 0.98; 0.66-1.44, P = .91; PAD + CAD, 1.04; 0.74-1.48, P = .81; and PAD + CAD + CVD, 0.96; 95% CI, 0.62-1.51, P = .88).
Discussion
In this study of 13 885 patients with PAD after adjustment for significant baseline variables, polyvascular disease (>1 diseased vascular bed at baseline) was associated with an increased risk of MACEs, defined as cardiovascular death, MI, or stroke, in patients in whom the reference is established isolated PAD. The risk of ALI requiring hospitalization and major amputation was similar in patients with PAD with polyvascular disease compared with patients with PAD alone. The risk of lower-extremity revascularization, however, was increased among patients with polyvascular disease. The risk of the primary safety end point of TIMI major bleeding after adjustment was similar in patients with polyvascular disease compared with those with PAD alone. These findings have both mechanistically and clinically important implications.
To our knowledge, the effect of polyvascular disease on both major adverse cardiac and limb events has not been previously evaluated in a contemporary clinical trial of patients with symptomatic PAD. Previous studies involving a large international registry of patients with stable atherosclerotic disease (CAD, CVD, or PAD) have found the existence of polyvascular disease to confer a heightened risk of ischemic events.3,15 Consistent with these findings, patients with PAD and polyvascular disease from the EUCLID trial demonstrated an incremental rise in MACE risk with each additional diseased vascular territory. The risk of the individual components of cardiovascular death and MI also rose in a stepwise fashion with each additional diseased vascular territory. The novelty of the present analysis is the finding that polyvascular disease is independently associated with an increase in lower-extremity revascularization. In addition, among the EUCLID trial population, geographic location has been demonstrated to also affect the rate of lower-extremity revascularization.16
In light of an observed increased risk of adverse events associated with polyvascular disease, one would expect to detect a greater clinical benefit among these subpopulations owing to a greater opportunity for treatment benefit; however, this outcome was not the case in the present study. The interpretation for this difference likely stems from the overall findings of the EUCLID trial, which were neutral. Close examination of these previously published results demonstrates event rates and times-to-event analyses for efficacy and safety end points to be similar among both ticagrelor and clopidogrel cohorts.14
Our findings expand our knowledge of polyvascular disease in several ways. First, in the setting of PAD, not all polyvascular disease is the same. Studies have previously evaluated outcomes stratified according to the number of diseased vascular beds—0, 1, 2, or 3—with the coronary vascular bed being the reference in all patients. In the present analysis, evaluation of all 4 phenotypes of cardiovascular disease among patients with PAD (defined as PAD alone, PAD + CAD, PAD + CVD, or PAD + CAD + CVD) showed that the highest rates of ischemic events occurred in patients with PAD + CAD.
Second, among patients with polyvascular disease, the increase in ischemic events is related to the polyvascular disease phenotype. For example, patients with PAD + CAD experienced the highest rates of cardiovascular death and MI, while patients with PAD + CVD experienced the highest rates of ischemic stroke. The addition of CAD or CVD was not associated with a higher risk of the adverse limb events of ALI or major amputation; however, CAD and CVD were linked to a heightened risk of lower-extremity revascularization. These data highlight the increased risk of CAD and CVD among patients with PAD and the need to identify potent preventive antithrombotic therapies.
In addition, we evaluated the risk of major bleeding in patients with polyvascular disease on a background of PAD. Evaluation of a large registry of patients presenting with acute coronary syndromes found polyvascular disease to be associated with increased rates of blood transfusion among patients who did not undergo coronary artery bypass grafting.2 In our analysis, there was no clear evidence that polyvascular disease conferred an increased risk of major bleeding among patients with PAD.
Limitations
The present study has limitations. First, it is a post hoc secondary analysis of subgroups of the overall EUCLID trial; as such, results are considered hypothesis generating, and caution should be used when interpreting all findings. Second, the median follow-up period was 36 months; therefore, any potential benefit or risk of ticagrelor therapy in a stable outpatient PAD population beyond this time frame is uncertain.
Conclusions
In EUCLID, a randomized trial of ticagrelor vs clopidogrel in patients with symptomatic PAD, polyvascular disease was present in 43.8% of the study population. Compared with patients with PAD alone, the risk of MACEs and lower-extremity revascularization increased with atherosclerosis across multiple vascular territories. Polyvascular disease, however, was not associated with an increased risk of TIMI major bleeding compared with patients with PAD alone. These data highlight the increased risk of CAD and CVD among patients with PAD and the need to identify potent preventive antithrombotic therapies.
References
- 1.Subherwal S, Patel MR, Kober L, et al. . Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol. 2015;22(3):-. doi: 10.1177/2047487313519344 [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DL, Peterson ED, Harrington RA, et al. ; CRUSADE Investigators . Prior polyvascular disease: risk factor for adverse ischaemic outcomes in acute coronary syndromes. Eur Heart J. 2009;30(10):1195-1202. doi: 10.1093/eurheartj/ehp099 [DOI] [PubMed] [Google Scholar]
- 3.Bhatt DL, Eagle KA, Ohman EM, et al. ; REACH Registry Investigators . Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304(12):1350-1357. doi: 10.1001/jama.2010.1322 [DOI] [PubMed] [Google Scholar]
- 4.Bonaca MP, Gutierrez JA, Creager MA, et al. . Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: results from the Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients With Atherosclerosis-Thrombolysis in Myocardial Infarction 50 (TRA2°P-TIMI 50). Circulation. 2016;133(10):997-1005. doi: 10.1161/CIRCULATIONAHA.115.019355 [DOI] [PubMed] [Google Scholar]
- 5.Gerhard-Herman MD, Gornik HL, Barrett C, et al. . 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e726-e779. doi: 10.1161/CIR.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt DL, Flather MD, Hacke W, et al. ; CHARISMA Investigators . Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49(19):1982-1988. doi: 10.1016/j.jacc.2007.03.025 [DOI] [PubMed] [Google Scholar]
- 7.Belch JJ, Dormandy J, Biasi GM, et al. ; CASPAR Writing Committee . Results of the randomized, placebo-controlled Clopidogrel and Acetylsalicylic Acid in Bypass Surgery for Peripheral Arterial Disease (CASPAR) trial. J Vasc Surg. 2010;52(4):825-833, 833.e1-833.e2. doi: 10.1016/j.jvs.2010.04.027 [DOI] [PubMed] [Google Scholar]
- 8.Bonaca MP, Scirica BM, Creager MA, et al. . Vorapaxar in patients with peripheral artery disease: results from TRA2°P-TIMI 50. Circulation. 2013;127(14):1522-1529, 1529e1-6. doi: 10.1161/CIRCULATIONAHA.112.000679 [DOI] [PubMed] [Google Scholar]
- 9.Bonaca MP, Bhatt DL, Storey RF, et al. . Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67(23):2719-2728. doi: 10.1016/j.jacc.2016.03.524 [DOI] [PubMed] [Google Scholar]
- 10.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 11.Yeh RW, Secemsky EA, Kereiakes DJ, et al. ; DAPT Study Investigators . Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315(16):1735-1749. doi: 10.1001/jama.2016.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez A, Bhatt DL. Medical management of peripheral artery disease in diabetes mellitus. Eur Heart J. 2015;36(24):1494-1495. [PubMed] [Google Scholar]
- 13.Berger JS, Katona BG, Jones WS, et al. . Design and rationale for the Effects of Ticagrelor and Clopidogrel in Patients With Peripheral Artery Disease (EUCLID) trial. Am Heart J. 2016;175:86-93. doi: 10.1016/j.ahj.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 14.Hiatt WR, Fowkes FG, Heizer G, et al. ; EUCLID Trial Steering Committee and Investigators . Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376(1):32-40. doi: 10.1056/NEJMoa1611688 [DOI] [PubMed] [Google Scholar]
- 15.Suárez C, Zeymer U, Limbourg T, et al. ; REACH Registry Investigators . Influence of polyvascular disease on cardiovascular event rates: insights from the REACH Registry. Vasc Med. 2010;15(4):259-265. doi: 10.1177/1358863X10373299 [DOI] [PubMed] [Google Scholar]
- 16.Jones WS, Baumgartner I, Hiatt WR, et al. ; International Steering Committee and Investigators of the EUCLID Trial . Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation. 2017;135(3):241-250. doi: 10.1161/CIRCULATIONAHA.116.025880 [DOI] [PubMed] [Google Scholar]