Key Points
Question
Which approach is best to reduce surgical site infections and anastomotic leak in colorectal surgery: mechanical bowel preparation with oral antibiotics, oral antibiotics only, mechanical bowel preparation only, or no preparation?
Findings
Among 38 randomized clinical trials (8458 patients) in this network meta-analysis, mechanical bowel preparation with oral antibiotics was associated with the lowest rate of surgical site infections, reducing both incisional and organ/space infections. There was no significant difference in anastomotic leak rate between the 4 approaches.
Meaning
Mechanical bowel preparation with oral antibiotics is the best approach to reduce surgical site infections in patients undergoing colorectal surgery.
This network meta-analysis of randomized clinical trials assesses which approach in colorectal surgery (mechanical bowel preparation with and without oral antibiotics) is associated with the lowest rate of surgical site infections.
Abstract
Importance
There has been a resurgence of interest in the use of mechanical bowel preparation (MBP) and oral antibiotics (OAB) before elective colorectal surgery. Until now, clinical trials and meta-analyses have not compared all 4 approaches (MBP with OAB, OAB only, MBP only, or no preparation) simultaneously.
Objective
To perform a network meta-analysis to clarify which approach in colorectal surgery is associated with the lowest rate of surgical site infection (SSI).
Data Sources
Five electronic databases were searched, including PubMed, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, ACP Journal Club. and Database of Abstracts of Review of Effectiveness from database inception to November 27, 2017.
Study Selection
Only data from randomized clinical trials were included. Inclusion criteria were RCTs that reported on SSI rates or other complications based on MBP or OAB status. Quality of studies was appraised by the Cochrane Collaboration risk of bias tool.
Data Extraction and Synthesis
The study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Main Outcomes and Measures
Total, incisional, and organ/space SSI rates. Secondary outcomes included rates of anastomotic leak, mortality, readmissions/reoperations, urinary tract infection, and pulmonary complications.
Results
Thirty-eight randomized clinical trials among 8458 patients (52.1% male) were included, providing 4 direct comparisons and 2 indirect comparisons for 8 outcome measures. On Bayesian analysis, MBP with OAB vs MBP only was associated with reduced SSI (odds ratio [OR], 0.71; 95% equal-tail credible interval [CrI], 0.57-0.88). There was no significant difference between MBP with OAB vs OAB only (OR, 0.95; 95% CrI, 0.56-1.62). Oral antibiotics without MBP was not associated with a statistically significant reduction in SSI compared with any other group (except for a risk reduction in organ/space SSI when indirectly compared with no preparation) (OR, 0.13; 95% CrI, 0.02-0.55). There was no difference in SSI between MBP only vs no preparation (OR, 0.84; 95% CrI, 0.69-1.02).
Conclusions and Relevance
In this network meta-analysis of randomized clinical trials, MBP with OAB was associated with the lowest risk of SSI. Oral antibiotics only was ranked as second best, but the data available on this approach were limited. There was no difference between MBP only vs no preparation. In addition, there was no difference in rates of anastomotic leak, readmissions, or reoperations between any groups.
Introduction
There has been a resurgence of interest in the use of mechanical bowel preparation (MBP) and oral antibiotics (OAB) before elective colorectal surgery (CRS). This is a current and controversial topic, not owing to a lack of high-quality evidence but because studies on this topic have reported a diverse range of outcomes.
Despite the American Society for Enhanced Recovery and the Perioperative Quality Initiative joint consensus statement recommending the “routine use of a combined isosmotic mechanical bowel preparation with oral antibiotics before elective CRS”1(p6) to reduce surgical site infection (SSI), there has been a lack of consensus between international guidelines in United States, Europe, and Asia-Pacific. The recent Australian guidelines recommended that “mechanical bowel preparation should not be used routinely in colonic surgery.”2
Furthermore, a 2017 survey of European colorectal surgeons found that few European surgeons used OAB despite recent evidence suggesting that preoperative OAB reduced SSI.3 Less than 10% of the European colorectal surgeons who participated in the survey prescribed preoperative OAB with perioperative intravenous antibiotics (IVAB), with 96% choosing to prescribe perioperative IVAB only. Most also indicated that they used MBP before rectal surgery, and 30% used MBP before colonic surgery3 despite a large body of evidence showing no benefit with MBP.4
Several meta-analyses4,5,6,7,8 have already been published on MBP and OAB before CRS. However, traditional meta-analysis techniques have only provided comparisons between 2 approaches (MBP vs no preparation, OAB vs no preparation, MBP with OAB vs no preparation, and MBP with OAB vs OAB). Therefore, it has been difficult to compare the results of studies that have reported on different permutations of MBP and OAB regimens. To compare all 4 approaches simultaneously (MBP with OAB, OAB only, MBP only, or no preparation), we performed the first network meta-analysis (NMA) to date, to our knowledge, of randomized clinical trials (RCTs) only on this topic.
The advantage of performing an NMA over traditional meta-analysis on this topic was the ability to compare all 4 treatment arms of trials that assessed MBP and OAB. As a result, we were able to rank all 4 MBP and OAB regimens simultaneously, facilitating side-by-side comparisons of the 4 approaches. By comparing multiple treatment arms using only evidence from RCTs, this NMA synthesizes and clarifies the existing evidence on MBP and OAB before elective CRS.
Methods
Literature Search Strategy
The present study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline9,10 (eFigure 1 in the Supplement). Five electronic databases were searched, including PubMed, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, ACP Journal Club, and Database of Abstracts of Review of Effectiveness from database inception to November 27, 2017. To minimize the risk of overlooking relevant studies and given the wide variety of procedural nomenclature, it was necessary to combine a large number of keywords and Medical Subject Headings, resulting in the following search terms: mechanical bowel preparation, oral antibiotics, colon, rectal, colorectal, and surgery. Moreover, reference lists of relevant literature were examined for any further studies. Literature search strategy, selection process, data extraction, and assessment of quality of studies are reported in the eAppendix in the Supplement.
Inclusion and Exclusion Criteria
Inclusion criteria were RCTs that reported on SSI rates or other complications based on MBP or OAB status. When institutions published subsequent studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment at each interval. Exceptions to this protocol were studies by Zmora et al11 in 2003 and by Zmora et al12 in 2006, both of which reported on MBP with OAB vs OAB only. The 2006 study12 reported on left-sided CRS, whereas the 2003 study11 reported on both sides. The 2003 study11 recruited more patients, but the 2006 study12 was included in this NMA because it provided good qualitative data on the role of MBP and OAB in left-sided colonic surgery and because the direct comparison between MBP with OAB vs OAB only had limited data.
Studies13,14 involving the use of the older oral antiprotozoan antibiotic tinidazole were excluded from analysis. Tinidazole is commonly used to treat protozoan, amebic, and parasitic infections.
All publications were limited to those involving human participants and in the English language. Abstracts, case reports, conference presentations, editorials, and expert opinions were excluded. Review articles were omitted because of potential publication bias and duplication of the results.
Data Extraction and Quality Assessment of Studies
Two reviewers (J.W.T.T. and K.P.) independently reviewed and appraised studies using a standard form and extracted data on methods and outcome measures. Discrepancies between the 2 reviewers were resolved by discussion and consensus. In addition, quality of studies was appraised by the Cochrane Collaboration risk of bias tool (version 5.0.1) (eTable 1 in the Supplement). This tool included the following components: selection bias (defined as random sequence generation and allocation concealment), performance bias (masking of both participants and investigators), detection bias (masking of evaluators), attrition bias (incomplete outcome data), and reporting bias (selective outcome reporting). Each component was judged to be of low, unclear, or high risk of bias. Discrepancies between reviewers were resolved by discussion and consensus.
Outcome Assessment
The primary outcome measures were total, incisional, and organ/space SSI rates as defined by the US Centers for Disease Control and Prevention.15 Secondary outcomes included rates of anastomotic leak, mortality, readmissions/reoperations, urinary tract infection (UTI), and pulmonary complications.
Statistical Analysis
We conducted an NMA using a Bayesian Markov chain Monte Carlo method in WinBUGS 1.4.3 (MRC Biostatistics Unit, Cambridge, United Kingdom) through the conduit of the Microsoft Excel–based macro NetMetaXL 1.6.1 (Canadian Agency for Drugs and Technologies in Health).16 A convergence test for each analysis was conducted by checking whether the Monte Carlo error was less than 5% of the SD of the effect estimates or the variance between the studies. Convergence was achieved for all analyses at 20 000 “burn in” runs and 30 000 model runs. A random-effects model with informative priors was used to best minimize the consequences of the diversity of the assorted patient populations and designs for each study.
Clinical postoperative outcomes were examined by calculating the pooled estimates of odds ratios (ORs) and 95% CIs of direct comparisons between any 2 MBP and OAB approaches. Direct evidence and indirect evidence for all MBP and OAB approaches were combined to estimate the examined outcomes, with a 95% equal-tail credible interval (CrI). We used NetMetaXL for rank probabilities to be plotted against the possible ranks for a treatment to result in the production of a graphical rankogram. This method of visually representing probabilities was combined with a surface under the cumulative ranking line for each surgical intervention.
We explored the comparison network by representing each of the 4 bowel preparation approaches as a node, with lines between nodes representing a comparison between 2 linked treatments. We considered the distribution of effect modifiers to be the same in all of the pairwise comparisons according to the transitivity assumption. Where there were inconsistencies between direct and indirect evidence, we evaluated clinical and methodological variables to identify possible causes of inconsistency (Table). Publication bias was assessed with funnel plots (eFigure 2 in the Supplement). The network plots (Figure 1) and funnel plots were generated by the gemtc package in STATA (Stata MP, version 15; StataCorp LP).
Table. Characteristics of Included Studies and Randomized Patientsa.
| Source | Study Period | Male, No.:Female, No.b | Treatment 1, Patients, No. | Treatment 2, Patients, No. | Oral Solution | Left, Right, or Mixed Location | Laparoscopic, Open, or Mixed Approach | Intravenous Antibiotic Type | Oral Antibiotic Type |
|---|---|---|---|---|---|---|---|---|---|
| Randomized to MBP vs No Preparation | |||||||||
| Ali,17 2007 | NA | NA | 109 | 101 | PEG | Mixed | Open | Ceftriaxone 2 g, metronidazole 1 g | NA |
| Bertani et al,18 2011 | 2007-2010 | 65:49 60:55 |
114 | 115 | PEG | Mixed | Mixed | Cefoxitin 2 g and then 1 g administered at 4, 12, and 24 h | NA |
| Bhat and Chakraborty,19 2016 | 2012-2014 | 56:42 57:44 |
98 | 104 | PEG | Mixed | Open | Ceftriaxone 1 g, metronidazole 500 mg, continued for 48 h | NA |
| Bhattacharjee et al,20 2015 | 2010-2013 | 21:17 20:13 |
38 | 33 | PEG | Mixed | Open | Cefuroxime 1.5 g, metronidazole 500 mg, 1 h before surgery | NA |
| Bretagnol et al,21 2010 | 2007-2009 | 56:33 46:43 |
89 | 89 | Senna, povidone-iodine enema | Left | Mixed | Ceftriaxone1 g, metronidazole 500 mg, continued every 2 h during the surgical procedure | NA |
| Bucher et al,22 2005 | 2001-2003 | 47:31 34:41 |
78 | 75 | PEG | Left | Open | Ceftriaxone 1 g, metronidazole 500 mg, continued for at least 24 h | NA |
| Burke et al,23 1994 | 1988-1992 | 52:30 43:44 |
82 | 87 | Sodium picosulfate | Left | Open | Ceftriaxone 1 g, metronidazole 500 mg, then metronidazole 500 mg administered at 8 h and 16 h | NA |
| Contant et al,24 2007 | 1998-2004 | 337:333 345:339 |
670 | 684 | PEG, bisacodyl or sodium phosphate |
Mixed | Open | As per institution guidelines | NA |
| Fa-Si-Oen et al,25 2005 | 1998-2002 | 58:67 56:69 |
125 | 125 | PEG | Mixed | Open | Cephazolin 2 g, metronidazole 1.5 g or gentamicin 240 mg, metronidazole 1.5 g |
NA |
| Khan et al,26 2011 | NA | NA | 51 | 51 | PEG | Mixed | Open | Ceftriaxone 2 g, metronidazole 1 g | NA |
| Miettinen et al,27 2000 | 1994-1996 | 68:70 62:67 |
138 | 129 | PEG | Mixed | Open | Ceftriaxone 2 g, metronidazole 1 g | NA |
| Pena-Soria et al,28 2008 | 2001-2007 | 35:29 33:22 |
65 | 64 | PEG | Mixed | Open | Gentamicin 80 mg, metronidazole 500 mg, repeat dose at 8, 16, and 24 h | NA |
| Platell et al,29 2006 | 2000-2005 | NA | 147 | 147 | PEG | Mixed | Open | Ticarcillin-clavulanate 3.1 g or gentamicin 2 mg/kg, metronidazole 500 mg |
NA |
| Ram et al,30 2005 | 1999-2002 | 99:65 102:63 |
164 | 165 | Sodium phosphate | Mixed | Open | Ceftriaxone 1 g, metronidazole 500 mg, continued for 48 h | NA |
| Saha et al,31 2014 | 2008-2010 | NA | 32 | 31 | PEG | Left | Open | Ceftriaxone 1 g, metronidazole 500 mg, continued for 36 h | NA |
| Santos et al,32 1994 | 1991-1992 | NA | 72 | 77 | Laxative, mannitol | Mixed | Open | Cephalothin 1 g, metronidazole 500 mg, then cephalothin 1 g given at 6 h and 12 h and metronidazole 500 mg at 8 h and 16 h | NA |
| Sasaki et al,33 2012 | 2009 | 17:21 24:17 |
38 | 41 | PEG | Mixed | Mixed (laparoscopic data given) | Flomoxef 1 g, continued every 3 h during surgery | NA |
| Randomized to MBP With OAB vs OAB | |||||||||
| Reddy et al,34 2007 | NA | 22:20 11:11 |
42 | 22 | Sodium picosulfate, magnesium citrate | Mixed | Open | NA | Neomycin 1 g, 3 doses 1 d before surgery, with or without synbiotic |
| Zmora et al,11 2003 | 1997-2000 | 103:84 94:99 |
187 | 193 | PEG | Mixed | Open | Intravenous antibiotics, type not specified, at induction of anesthesia and continued at least 24 h | Neomycin, erythromycin, 3 doses before surgery |
| Zmora et al,12 2006 | 1997-2001 | 67:53 65:64 |
120 | 129 | PEG | Left | Open | Metronidazole 500 mg, gentamicin 240 mg, ampicillin 1 g | Neomycin 1 g, erythromycin 1 g, 3 doses 1 d before surgery |
| Randomized to MBP With OAB vs MBP | |||||||||
| Beggs et al,35 1982 | NA | 25:21 26:25 |
46 | 51 | As per surgeon | Mixed | Open | Metronidazole 500 mg, then continued every 8 h for a further 5 doses | Metronidazole 200 mg for 4 d before surgery (all patients received neomycin 1 g orally 4 times daily for 5 d before surgery) |
| Dion et al,36 1980 | NA | NA | 39 | 39 | Magnesium citrate | Mixed | Open | Metronidazole 1 g, then 500 mg at 8 h and 16 h | Metronidazole 750 mg 3 times daily for 2 d before surgery (all patients received 1 g of neomycin 3 times daily 1 d before surgery) |
| Espin-Basany et al,37 2005 | NA | 130:70 62:38 |
200 | 100 | Sodium phosphate | Mixed | Open | Cefoxitin 1 g, then continued 1 g at 8 h and 16 h | Neomycin 1 g, metronidazole 1 g, either 3 doses or 1 dose 1 d before surgery |
| Hata et al,38 2016 | 2007-2012 | 153:136 175:115 |
289 | 290 | Picosulfate, magnesium citrate | Mixed | Laparoscopic | Metronidazole 750 mg, cefmetazole 1 g, then every 3 h during surgery | Kanamycin 1 g, metronidazole 750 mg, 2 doses 1 d before surgery |
| Ikeda et al,39 2016 | 2013-2014 | 142:113 141:115 |
255 | 256 | Picosulfate, magnesium citrate | Mixed | Laparoscopic | Cefmetazole 1 g, then every 3 h during surgery, continued for 24 h | Kanamycin 1 g, metronidazole 750 mg, 2 doses 1 d before surgery |
| Ishida et al,40 2001 | 1998-2000 | 47:25 42:29 |
72 | 71 | PEG | Mixed | Open | Cefotiam 1 g, then 1 g at completion of surgery, then 1 g twice daily for 2 d (6 doses in total) | Kanamycin 2 g/d, erythromycin 1.6 g/d, 4 doses for 2 d before surgery |
| Kling and Dahlgren,41 1989 | 1985-1986 | 14:13 11:16 |
27 | 27 | Bisacodyl, magnesium sulfate | Mixed | Open | Metronidazole 1.5 g, ceftriaxone 2 g | Neomycin 1 g, erythromycin 1 g, 3 doses 1 d before surgery |
| Kobayashi et al,42 2007 | 2001-2004 | 154:88 137:105 |
242 | 242 | PEG | Mixed | Open | Cefmetazole 1 g, then every 3 h during surgery, continued daily for 72 h | Kanamycin 1 g, erythromycin 400 mg, 3 doses 1 d before surgery |
| Lau et al,43 1988 | 1981-1987 | 1.3:1 1.3:1 |
65 | 67 | Bisacodyl, magnesium sulfate | Mixed | Open | Metronidazole 500 mg, gentamicin 2 mg/kg, then repeated at 8 h and 16 h | Neomycin 1 g, erythromycin 1 g, 3 doses 1 d before surgery |
| Lazorthes et al,44 1982 | 1979-1980 | 20:10 14:16 |
30 | 30 | Magnesium sulfate | Mixed | Open | Cephradine 2 g, metronidazole 500 mg, with or without gentamicin 2 mg/kg | Kanamycin 1 g, metronidazole 250 mg, 4 doses daily for 3 d before surgery |
| Lewis,45 2002 | 1992-1995 | NA | 109 | 106 | Sodium phosphate | Mixed | Open | Amikacin 1 g, metronidazole 1 g | Neomycin 2 g, metronidazole 2 g, 2 doses 1 d before surgery |
| Oshima et al,46 2013 | 2006-2009 | 55:42 57:41 |
97 | 98 | Magnesium citrate | Mixed | Open | Flomoxef, then every 3 h during surgery | Kanamycin 500 mg, metronidazole 500 mg, 3 doses 1 d before surgery |
| Playforth et al,47 1988 | NA | 31:30 32:26 |
61 | 58 | Mannitol | Mixed | Open | Metronidazole 500 mg | Neomycin 1 g every 6 h, metronidazole 200 mg every 8 h, 1 d before surgery |
| Raahave et al,48 1988 | NA | 21:29 24:26 |
50 | 50 | Bisacodyl, magnesium sulfate | Mixed | Open | Cefotaxime 2 g, then 2 g at 6 h and 12 h | Neomycin 1 g, erythromycin 1 g, 3 doses 1 d before surgery (ampicillin 2 g powdered in wound at closure) |
| Reddy et al,34 2007 | NA | 22:20 11:13 |
42 | 24 | Picosulfate, magnesium citrate | Mixed | Open | NA | Neomycin 1 g, 3 doses 1 d before surgery, with or without synbiotic |
| Sadahiro et al,49 2014 | 2008-2011 | 49:51 51:44 |
100 | 95 | Picosulfate, PEG | Mixed | Mixed | Flomoxef 1 g | Kanamycin 500 mg, metronidazole 500 mg, 3 doses 1 d before surgery |
| Stellato et al,50 1990 | 1987 | NA | 38 | 45 | Picosulfate, PEG | Mixed | Open | Cefoxitin | Neomycin, erythromycin |
| Weaver et al,51 1986 | NA | NA | 29 | 31 | As per surgeon | Mixed | Open | Ceftriaxone 2 g, metronidazole 1.5 g | Neomycin 1 g, erythromycin 1 g, 3 doses 1 d before surgery |
| Yabata et al,52 1997 | NA | 23:17 29:22 |
40 | 51 | PEG | Mixed | Open | Cefmetazole 1 g, then every 3 h during surgery | Tobramycin 30 mg, metronidazole 250 mg, 3 doses daily for 3 d before surgery (tobramycin 30 mg with saline instilled into lumen during surgery) |
| Randomized to MBP vs OAB | |||||||||
| Reddy et al,34 2007 | NA | 11:13 11:11 |
24 | 22 | Picosulfate, magnesium citrate | Mixed | Open | NA | Neomycin 1 g, 3 doses 1 d before surgery, with or without synbiotic |
Abbreviations: MBP, mechanical bowel preparation; NA, not applicable; OAB, oral antibiotics; PEG, polyethylene glycol.
The study by Reddy et al34 reports on MBP with OAB, MBP only, and OAB only.
Sex breakdown for the fourth and fifth columns.
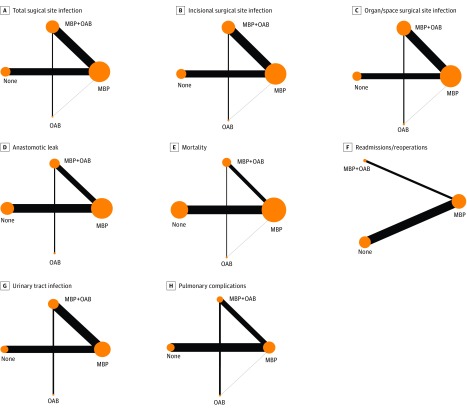
Figure 1. Network Plots of the 8 Outcomes Showing Direct Comparisons and Indirect Comparisons Between Treatment Groups Based on Mechanical Bowel Preparation (MBP) and Oral Antibiotic (OAB) Status.
Comparison networks were explored by representing each of the 4 bowel preparation approaches as a node, with lines between nodes representing a comparison between 2 linked treatments. Size of the node is proportional to the number of patients randomized to that bowel preparation approach, and the thickness of the lines is proportional to the number of studies comparing the 2 approaches.
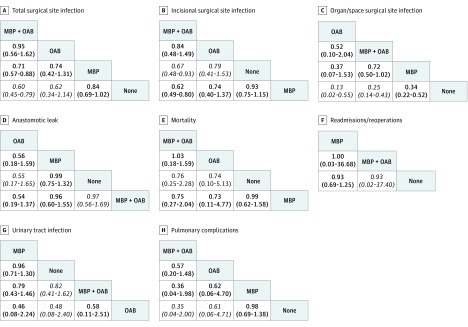
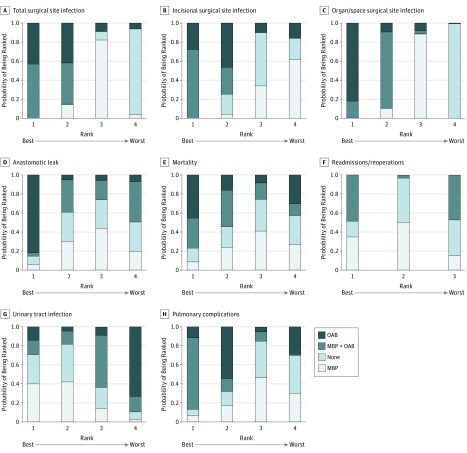
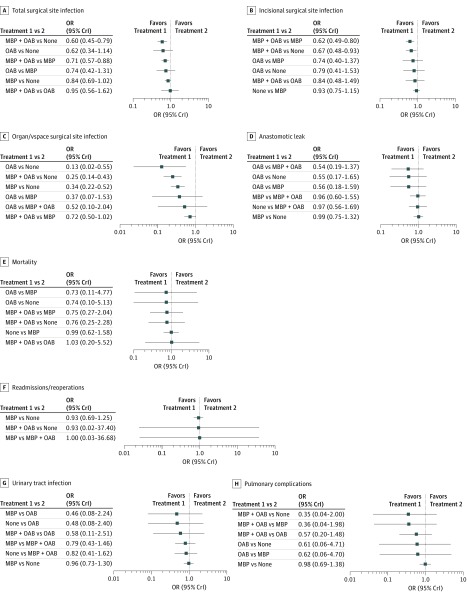
All results are presented as relative effects and Bayesian estimates of the probability of each technique being the best to the worst relating to every studied outcome using rankograms (Figure 2), league tables (Figure 3), and forest plots (Figure 4).
Figure 2. League Tables of the 8 Outcomes Showing Direct Comparisons and Indirect Comparisons Between Treatment Groups Based on Mechanical Bowel Preparation (MBP) and Oral Antibiotic (OAB) Status.
Outcomes are shown as odds ratios (95% equal-tail credible intervals); direct comparisons are represented in bold, and indirect comparisons are represented in italics.
Figure 3. Rankograms of the 8 Outcomes Showing the Probability of Being Ranked the Best vs the Worst Based on Mechanical Bowel Preparation (MBP) and Oral Antibiotic (OAB) Status.
Outcomes on the far left of the x-axis are ranked best; far right, worst.
Figure 4. Forest Plots of the 8 Outcomes Showing Direct Comparisons and Indirect Comparisons Based on Mechanical Bowel Preparation (MBP) and Oral Antibiotic (OAB) Status.
Outcomes are shown as odds ratios (ORs) (95% equal-tail credible intervals [CrIs]).
Results
A total of 1198 studies were identified through 5 electronic database searches and from other sources, such as reference lists. After applying inclusion and exclusion criteria and removal of duplicate studies, there were 17 studies17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,53,54 identified comparing MBP vs no preparation (2117 vs 2128 patients), 3 studies11,12,34 identified comparing MBP with OAB vs OAB (349 vs 344 patients), and 19 studies34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52 identified comparing MBP with OAB vs MBP (1831 vs 1731 patients). In total, 38 RCTs among 8458 patients (52.1% male) were included. Features of included studies are summarized in the Table. Raw results reported in studies for SSI, anastomotic leak, and mortality are listed in eTables 2, 3, and 4 in the Supplement.
Most studies in this NMA used IVAB as part of the routine protocol, regardless of type of MBP and OAB approach. However, several studies compared OAB with IVAB (ie, patients receiving OAB with or without MBP did not receive IVAB). These trials included studies by Beggs et al,35 Dion et al,36 Kling and Dahlgren,41 Raahave et al,48 Reddy et al,34 and Weaver et al.51 Furthermore, several studies had 2 separate groups of patients who received OAB with and without IVAB. These trials included studies by Lau et al,43 Lazorthes et al,44 and Stellato et al.50
Most studies used a combination of either ceftriaxone, cefoxitin, cefuroxime, flomoxef, cephazolin, amikacin with or without metronidazole with or without gentamicin, or ticarcillin-clavulanate as the IVAB of choice. Two studies11,12 using IV ticarcillin-clavulanate were excluded because their comparison OAB group was tinidazole. The OAB regimens used were most commonly neomycin with or without erythromycin (9 studies), followed by kanamycin or neomycin with metronidazole (6 studies) (the Table lists the specific OAB used in each study). Among most studies in which OAB was used, at least 3 doses of OAB were prescribed for a duration of 1 to 3 days before surgery.
Most studies reported outcomes for combined right and left procedures except for 5 studies12,21,22,23,31 that reported on left-sided outcomes. Most studies used an open approach to CRS except for 2 studies38,39 using a laparoscopic approach and 4 studies18,21,33,49 reporting on data from a mix of open and laparoscopic procedures. Quality appraisal of included RCTs is summarized in eTable 1 in the Supplement.
There were 4 direct comparisons (MBP with OAB vs MBP, MBP with OAB vs OAB, MBP vs no preparation, and MBP vs OAB) and 2 indirect comparisons (MBP with OAB vs no preparation and OAB vs no preparation) based on the transitivity assumption (if A > B and B > C, then A > C). Figure 1 shows the network plots for direct comparisons and indirect comparisons. Direct comparisons have been boldfaced on the rankograms and league tables, and indirect comparisons have been italicized.
Total SSI
Our NMA demonstrated a statistically significant reduction in total SSI for MBP with OAB compared with MBP only (OR, 0.71; 95% CrI, 0.57-0.88). Indirectly, there was also a risk reduction in SSI for MBP with OAB compared with no preparation (OR, 0.60; 95% CrI, 0.45-0.79). There was no difference in SSI between MBP only vs no preparation (OR, 0.84; 95% CrI, 0.69-1.02).
There was no significant difference in SSI between MBP with OAB vs OAB only (OR, 0.95; 95% CrI, 0.56-1.62). However, on Bayesian rankogram analysis, MBP with OAB was associated with a higher probability of the lowest total SSI rate after surgery than OAB only.
The reduction in SSI rate for OAB compared with MBP was not statistically significant (OR, 0.74; 95% CrI, 0.42-1.31). Similarly, the reduction in SSI rate for OAB compared with no preparation was not statistically significant (OR, 0.62; 95% CrI, 0.34-1.14).
The rankogram analysis, league tables, and forest plots all indicated that MBP with OAB had the highest probability of having the lowest total postoperative SSI rate, followed by OAB only, MBP only, and no preparation. These results are shown in Figures 2, 3, and 4.
Incisional SSI
From the NMA model, MBP with OAB had a statistically significant lower rate of incisional SSI compared with MBP only (OR, 0.62; 95% CrI, 0.49-0.80) and no preparation (OR, 0.67; 95% CrI, 0.48-0.93). The difference in incisional SSI rate with MBP with OAB vs OAB only was not statistically significant (OR, 0.84; 95% CrI, 0.48-1.49). The difference in SSI rate between OAB only vs MBP only was not statistically significant (OR, 0.74; 95% CrI, 0.40-1.37). Similarly, the difference in incisional SSI rate between OAB vs no preparation was not statistically significant (OR, 0.79; 95% CrI, 0.41-1.53).
Both the rankogram analysis and the league tables demonstrated that MBP with OAB had the highest probability of having the lowest incisional SSI rate, followed by OAB only, no preparation, and MBP only. These results are shown in Figures 2 and 3.
Organ/Space SSI
The NMA model revealed a statistically significant reduction in organ/space SSI for OAB only (OR, 0.13; 95% CrI, 0.02-0.55), MBP with OAB (OR, 0.25; 95% CrI, 0.25-0.43), and MBP only (OR, 0.34; 95% CrI, 0.22-0.52) over no preparation, respectively. It is important to note that OAB vs no preparation and MBP with OAB vs no preparation were indirect comparisons.
The difference in organ/space SSI rate between MBP with OAB vs MBP only did not reach statistical significance (OR, 0.72; 95% CrI, 0.50-1.02), but this analysis included studies in which patients were randomized to OAB without IVAB. When we excluded studies reporting on OAB without IVAB, the result reached statistical significance. The difference between OAB only and MBP with OAB did not reach statistical significance, with a wide 95% CrI (OR, 0.52; 95% CrI, 0.10-2.04).
Based on rankogram results, OAB only had the highest probability of having the lowest postoperative organ/space SSI rate, followed by MBP with OAB, MBP only, and no preparation. This NMA clearly showed that no preparation was associated with a statistically significant increase in organ/space SSI compared with the other 3 groups.
Anastomotic Leak
Our NMA was unable to demonstrate a statistically significant difference in anastomotic leak rates between any of the 4 approaches. For MBP with OAB, this NMA did not show a risk reduction in anastomotic leaks compared with MBP only (OR, 0.96; 95% CrI, 0.60-1.55) and compared with no preparation (OR, 0.97; 95% CrI, 0.56-1.69). There was also no risk reduction in anastomotic leaks for MBP only vs no preparation (OR, 0.99; 95% CrI, 0.75-1.32). None of the following comparisons between OAB only and other approaches reached statistical significance: OAB only vs MBP with OAB (OR, 0.54; 95% CrI, 0.19-1.37), OAB only vs MBP only (OR, 0.56; 95% CrI, 0.18-1.59), and OAB only vs no preparation (OR, 0.55; 95% CrI, 0.17-1.65). The rankogram analysis and league tables also demonstrated that OAB only had the highest probability of having the lowest rate of postoperative anastomotic leak. However, the small numbers in the OAB only group are a caveat to this result. There was virtually no difference in anastomotic leak rates between the other 3 approaches on NMA and Bayesian Monte Carlo rankogram.
Mortality
There was no difference in perioperative mortality between the 4 approaches from the NMA. Comparing MBP with OAB vs OAB only, no difference was observed (OR, 1.03; 95% CrI, 0.20-5.52). The difference between MBP with OAB and OAB only compared with both MBP only and no preparation was not statistically significant. The wide 95% CrI when comparing all groups was likely associated with the low perioperative mortality rate in all groups, and it is difficult to draw valid conclusions in terms of perioperative mortality. There was no difference in the perioperative mortality rate, with a narrow 95% CrI between MBP only and no preparation (OR, 0.99; 95% CrI, 0.62-1.58).
Readmissions/Reoperations
There was no difference in readmissions/reoperations between the groups. Insufficient data were obtained to compare OAB only with other approaches in terms of readmissions/reoperations. For MBP with OAB vs MBP only, the OR was 1.00 (95% CrI, 0.03-36.68). For MBP with OAB vs no preparation, the OR was 0.93 (95% CrI, 0.02-37.40). Although there was no difference between these approaches, the wide 95% CrI suggests that the data are inconclusive. No difference was found between MBP only and no preparation, with a narrow 95% CrI (OR, 0.93; 95% CrI, 0.69-1.25).
Urinary Tract Infection
The NMA demonstrated no significant difference in UTI rates between the 4 approaches. The strategies of MBP with OAB and OAB only did not reduce UTI rates after surgery. When MBP with OAB was compared with OAB only, MBP with OAB was associated with lower rates of UTI, although the 95% CrI was wide and did not reach statistical significance (OR, 0.58; 95% CrI, 0.11-2.51). Because of the wide 95% CrI, it is difficult to draw accurate conclusions from the NMA between the groups in terms of UTI rates.
Pulmonary Complications
The NMA demonstrated no significant differences in terms of pulmonary complications, including aspiration pneumonia, between the 4 approaches. Although the 95% CrIs were wide when comparing OAB only and no preparation (OR, 0.61; 95% CrI, 0.06-4.71) and when comparing OAB only and MBP only (OR, 0.62; 95% CrI, 0.06-4.70), the rankogram analysis and league tables strongly ranked MBP with OAB as the best approach to reduce pulmonary complications. The rate of pulmonary complications when comparing MBP with OAB vs OAB only was not statistically significant (OR, 0.57; 95% CrI, 0.20-1.48). The difference in pulmonary complications between MBP with OAB vs MBP only was also not statistically significant (OR, 0.36; 95% CrI, 0.04-1.98). In addition, the indirect comparison between MBP with OAB vs no preparation was not statistically significant (OR, 0.35; 95% CrI, 0.04-2.00).
Discussion
This NMA provided 4 direct comparisons (MBP with OAB vs MBP, MBP with OAB vs OAB, MBP vs no preparation, and MBP vs OAB) and 2 indirect comparisons (MBP with OAB vs no preparation and OAB vs no preparation) of MBP and OAB approaches before elective CRS, with the following 8 short-term outcomes evaluated: total SSI, incisional SSI, organ/space SSI, anastomotic leak, mortality, readmissions/reoperations, UTI, and pulmonary complications. Like other meta-analyses4,5,6,55,56,57 on this topic, most of the RCTs included in this study reported on outcomes based on open CRS.
The 2 studies included in this NMA that reported data based on laparoscopic cohorts had different findings. Hata et al38 found a reduced risk of SSI (5.5%) associated with MBP with OAB vs MBP. However, Ikeda et al39 found no difference in infectious complications between MBP with OAB vs MBP (5.9% for both).
This NMA identified only 5 RCTs12,21,22,23,31 reporting on left-sided CRS. The study by Bretagnol et al21 showed that there was a higher risk of infectious complications without MBP before elective rectal cancer sphincter-saving surgery. In contrast, Bucher et al22 reported increased morbidity with MBP, and both Saha et al31 and Burke et al23 reported no difference in anastomotic leak rate with and without MBP.
The results of our Bayesian analysis support the findings of a recent meta-analysis of RCTs by Chen et al5 that compared MBP with OAB vs MBP and showed that MBP with OAB was associated with reduced infectious complications. This finding is also similar to conclusions drawn from recent, large population-based American College of Surgeons–National Surgical Quality Improvement Program (ACS-NSQIP) studies58,59,60,61 and the Cochrane reviews by Nelson et al.7,8 Furthermore, our study reported findings similar to other meta-analyses,4,6 including the Cochrane review by Güenaga et al4 comparing MBP vs no preparation, which showed no difference between the 2 approaches in terms of mortality, anastomotic leak, SSI, and reoperation. However, our NMA was not able to replicate the increased risk of harm with MBP reported in the meta-analyses by Slim et al55 and by Bucher et al.57
To clarify the most important network findings of our NMA, the rest of this section focuses mainly on 3 direct comparisons reported in this NMA (MBP vs no preparation, MBP with OAB vs OAB, and MBP with OAB vs MBP) and characterizes the limitations and strengths of the studies included, as well as the results from the Bayesian analysis. Because there was limited direct comparison between MBP vs OAB and because comparisons between MBP with OAB vs no preparation and OAB vs no preparation were indirect comparisons made on the transitivity assumption, the results reported for these comparisons were not based on strong evidence and represent gaps in the literature.
MBP vs No Preparation (17 Studies) Among 4245 Patients
Most studies17,18,21,22,23,24,25,26,27,28,29,30,31,32,33,53,54 included in this NMA reported no difference in infectious complications when comparing MBP vs no preparation.17,18,23,25,27,28,30,31,33,53,54 In contrast, Bretagnol et al,21 Contant et al,24 and Platell et al29 reported a lower risk of infectious complications with MBP. Bucher et al22 and Santos et al32 reported increased morbidity and infectious complications, respectively, with MBP. Most RCTs were single-center trials. Studies ranged from a single-surgeon trial28 to multicenter trials.21,24,25 Intravenous antibiotics were administered at induction of anesthesia in all trials comparing MBP vs no preparation. A broad-spectrum cephalosporin with and without metronidazole was the most common regimen, followed by gentamicin and metronidazole. In the multicenter trial reported by Contant et al,24 the choice of IVAB prophylaxis regimen was according to the guideline for prevention of SSI issued by the infectious diseases department at each participating hospital.
Of the studies that reported better outcomes with MBP vs no preparation, Bretagnol et al21 reported specifically on elective rectal cancer sphincter-preserving surgery from the French Research Group of Rectal Cancer Surgery (GRECCAR) III RCT. The main advantages were decreased infectious complications after anastomotic leakage reported by Contant et al24 and decreased risk of anastomotic leakage requiring reoperation reported by Platell et al.29 In contrast, the study by Bucher et al22 that reported on elective left-sided CRS found increased risk of morbidity with MBP, and the study by Santos et al32 reported increased risk of wound infection with MBP vs no preparation (24% vs 12%) but no difference in risk of anastomotic leak.
On Bayesian analysis, our NMA was unable to demonstrate a statistically significant benefit of MBP vs no preparation for most of the outcome measures except for a reduction in the rate of organ/space SSI. There was no significant reduction in total SSI, incisional SSI, anastomotic leak, perioperative mortality, readmission rates, UTI, or pulmonary complications. These outcomes are consistent with most of the RCTs analyzed in this NMA and with existing meta-analyses12,21,24,28,62,63,64 that have shown no benefit associated with MBP in the context of CRS. Our NMA is consistent with the findings from the 2011 Cochrane review4 (18 RCTs among 5805 patients) that demonstrated no significant reduction in anastomotic leak rates or wound infections with MBP over no preparation. The conclusion of the meta-analysis by Dahabreh et al6 (18 RCTs, 7 nonrandomized comparative studies, and 6 single-group cohorts) was that MBP was similar to no preparation with respect to mortality, anastomotic leakage, and wound infection. Our NMA confirmed no benefit of MBP vs no preparation on Bayesian analysis.
MBP With OAB vs OAB (3 Studies) Among 693 Patients
Only 3 RCTs11,12,34 comparing MBP with OAB vs OAB were included in this NMA. These comprise the study by Reddy et al34 and 2 studies by Zmora et al.11,12 Zmora et al12 in 2006 reported on left-sided CRS. In 2003, Zmora et al11 reported on both right-sided and left-sided colon and rectal surgery. While the 2003 study11 included more patients, the 2006 study12 provided important data on left-sided CRS. While other overlapping studies were excluded, the 2006 study12 was included for qualitative analysis, and including and excluding that study for quantitative synthesis did not change the outcome of the Bayesian analysis. In the study by Reddy et al,34 patients were randomized to the following 4 groups: MBP only, neomycin plus MBP, synbiotics plus neomycin plus MBP, and synbiotics plus neomycin but no MBP. None of the 3 studies in this subsection showed a statistically significant difference between MBP with OAB vs OAB. The results of the study by Reddy et al34 suggested that synbiotics plus neomycin plus MBP reduced fecal Enterobacteriaceae and bacterial translocation, but there was no clinically relevant difference when comparing the 4 groups.
On Bayesian analysis, none of the differences in outcome measures reached statistical significance. The rankogram analysis and league tables ranked MBP with OAB better than OAB in terms of total SSI, incisional SSI, UTI, and pulmonary complications. In terms of organ/space SSI and anastomotic leak, OAB was ranked better than MBP with OAB. Compared with no preparation, MBP with OAB, MBP, and OAB were associated with a statistically significant reduction in organ/space SSI. There was no difference on the rankogram between MBP with OAB and OAB in terms of mortality and readmissions/reoperations. The difficulty in deciding between MBP with OAB and OAB has been demonstrated by the lack of consensus between ACS-NSQIP studies. While 2 recent studies59,65 have shown that MBP with OAB is associated with the lowest risk of infectious complications, other ACS-NSQIP studies66,67 have recommended OAB on the basis that addition of MBP with OAB provided no additional benefit.
This NMA was not able to show a statistically significant difference between MBP with OAB and OAB. That group was small, and the direct comparison between MBP with OAB and OAB was limited. However, MBP with OAB consistently ranked as the best regimen in 4 of 8 outcome measures and as second best in 2 of the 8 outcome measures. In 2 of the 8 outcome measures, OAB ranked best, and OAB was associated with a statistically significant reduction in organ/space SSI. Neither MBP with OAB nor OAB reduced the rate of UTI.
MBP With OAB vs MBP (19 Studies) Among 3562 Patients
Nineteen studies34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52 comparing MBP with OAB vs MBP were included in this NMA. In this group, there was methodological diversity. A range of IVAB and OAB for varying durations was used in these RCTs. Intravenous antibiotics administered in RCTs included metronidazole, cephalosporins, combination cephalosporin with metronidazole, and amikacin with metronidazole. Oral antibiotics included metronidazole, neomycin, kanamycin, kanamycin with erythromycin, neomycin with erythromycin, kanamycin with metronidazole, neomycin with metronidazole, and tobramycin with metronidazole. Studies used different doses of antibiotics for various durations before surgery.
While most studies used IVAB at induction of anesthesia (regardless of OAB status), the studies by Beggs et al,35 Dion et al,36 Kling and Dahlgren,41 Raahave et al,48 Reddy et al,34 and Weaver et al51 compared OAB with IVAB. In these studies, the OAB group did not receive IVAB. In the studies by Beggs et al35 and by Dion et al,36 all patients received oral neomycin, and patients were randomized to IV metronidazole vs oral metronidazole. In the studies by Weaver et al51 and Kling and Dahlgren,41 patients were randomized to either IV ceftriaxone and metronidazole or oral neomycin and erythromycin.51 The study by Raahave et al48 compared IV cefotaxime with oral neomycin-erythromycin. As stated earlier, Reddy et al34 randomized patients into the following 4 groups: MBP only, oral neomycin plus MBP, synbiotics plus neomycin plus MBP, and synbiotics plus neomycin without MBP. In these studies,35,36,41,48,51 there was no benefit reported for OAB over IVAB, and the study by Weaver et al51 was discontinued after 60 patients were enrolled because of the high rate of infection (41%) in the oral neomycin and erythromycin group. As already mentioned, the study by Reddy et al34 showed no reduction in SSI with oral neomycin but reported reduced fecal Enterobacteriaceae and bacterial translocation in the MBP, oral neomycin, and synbiotic groups. Furthermore, a subgroup of patients receiving OAB without IVAB in the studies by Lau et al,43 Lazorthes et al,44 and Stellato et al50 had increased risk of septic complications. In the study by Lazorthes et al,44 the rates of septic complications for patients receiving either oral kanamycin and metronidazole vs IV cephradine and metronidazole vs both oral and IVAB were 30%, 23%, and 3.3%, respectively. In the study by Lau et al,43 the rates of postoperative septic complications in patients receiving oral neomycin and erythromycin vs IV gentamicin and metronidazole vs both were 27.4%, 11.9%, and 12.3%, respectively. Comparing oral neomycin and erythromycin only vs IV cefoxitin only vs both OAB and IVAB, Stellato et al50 reported rates of postoperative septic complications of 11.4%, 11.7%, and 7.8%, respectively.
In contrast, a large number of studies38,40,44,45,46,47,49 comparing MBP with OAB vs MBP reported benefit of MBP with OAB over MBP in reducing SSI. In studies reporting a benefit of MBP with OAB, it is important to note that OAB was given in addition to IVAB at induction of anesthesia rather than as a replacement for IVAB. In summary, studies in which MBP with OAB was administered without IVAB showed no benefit in terms of infectious complications over MBP.
On Bayesian analysis, this NMA showed a statistically significant improvement in total SSI and incisional SSI when MBP with OAB was used compared with MBP. For organ/space SSI, the OR was 0.72 (95% CrI, 0.50-1.02). There was no statistically significant difference in rates of mortality, UTI, and pulmonary complications.
Limitations and Strengths
Our study had several limitations. First, there were only 2 studies38,39 reporting specifically on a laparoscopic approach and 4 studies18,21,33,49 reporting on a combination of open and laparoscopic techniques, and surgery in most studies was performed with an open approach. This does not reflect the current status in which most colorectal surgical procedures are performed laparoscopically. Second, we were not able to report outcomes based on colon vs rectum, side of resection, and formation of stoma because of a lack of available data. Third, there were only 3 studies4,44,45 comparing MBP with OAB vs OAB (among a total of 693 patients), and this may have led to failure to detect statistically significant differences between MBP with OAB and OAB. Fourth, there were variations in IVAB and OAB, as well as type of MBP used (most studies used ethylene glycol or sodium phosphate as the preferred agent). There were also variations in the dosage, duration of antibiotics used before surgery, and duration continued after surgery. Fifth, several of the comparisons in this NMA were indirect based on the transitivity assumption rather than actual data from RCTs. For this reason, as stated earlier, we boldfaced the direct comparisons in the rankogram analysis and league tables and italicized the indirect comparisons to easily distinguish direct and indirect comparisons.
Despite these limitations, our study has several key strengths. First, by using the network NMA, we were able to combine large quantitative evidence from RCTs to simultaneously compare MBP with OAB, MBP, OAB, and no preparation, which would be unlikely to be addressed by an RCT with 4 arms. Second, we used Bayesian Monte Carlo modeling to rank the 4 preparation approaches using both a rankogram analysis and league tables, providing a simple visual representation of which technique is better for each outcome measure. Third, we included only RCTs to reduce the risk of bias and ensure standardization and the validity of the results, and we appraised quality of studies by the Cochrane Collaboration risk of bias tool. Fourth, our results are consistent with the results of the Cochrane review4 comparing MBP vs no preparation and with the ACS-NSQIP studies59,60 on MBP with OAB and OAB. Furthermore, our results draw together the findings of previous meta-analyses4,5,6 comparing different approaches of MBP and OAB regimens into a comprehensive network study with clear graphical visualization of how each approach compares and ranks with one another. The next step may be a full factorial design with all 4 approaches included in the same trial, which would be the ideal way to address the issue at hand.
Conclusions
This NMA demonstrated that MBP with OAB is associated with the lowest risk of SSI in patients undergoing elective CRS. Oral antibiotics only may be beneficial, but there was insufficient evidence in the existing literature to draw conclusive results. In this NMA, MBP with OAB was ranked as the best approach for most of the outcome measures evaluated herein. In terms of SSI, there was no difference between MBP and no preparation before elective CRS. Both MBP and no preparation were associated with increased SSI compared with MBP with OAB and OAB. Evidence from RCTs comparing MBP with OAB vs OAB is limited, and further research should concentrate on increasing this evidence base. The long-standing practice of MBP and no preparation is still a common approach and should be revisited after careful reconsideration of the growing body of evidence from RCTs and population-based studies.
eFigure 1. PRISMA Flow Diagram
eFigure 2. Funnel Plots
eAppendix. Search Strategy, Selection Process and Data Extraction and Method of Network Analysis
eTable 1. Cochrane Collaboration Risk of Bias Tool (Version 5.0.1): Risk of Bias in the Individual Randomized Controlled Trials Assessed by Cochrane Risk of Bias Checklist
eTable 2. Presentation of Outcome Data for Total Surgical Site Infection, by Included Study
eTable 3. Presentation of Outcome Data for Anastomotic Leak, by Included Study
eTable 4. Presentation of Outcome Data for Mortality, by Included Study
References
- 1.Holubar SD, Hedrick T, Gupta R, et al. ; Perioperative Quality Initiative (POQI) I Workgroup . American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med (Lond). 2017;6:. doi: 10.1186/s13741-017-0059-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Systematic Review Report for question PRP2-5,7. Clinical question: can peri operative management be optimised? https://wiki.cancer.org.au/australiawiki/images/9/9e/CRC_PRP2-5%2C7_systematic_review_report.pdf. Accessed November 7, 2017.
- 3.Devane LA, Proud D, O’Connell PR, Panis Y. A European survey of bowel preparation in colorectal surgery. Colorectal Dis. 2017;19(11):O402-. doi: 10.1111/codi.13905 [DOI] [PubMed] [Google Scholar]
- 4.Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011;(9):CD001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Song X, Chen LZ, Lin ZD, Zhang XL. Comparing mechanical bowel preparation with both oral and systemic antibiotics versus mechanical bowel preparation and systemic antibiotics alone for the prevention of surgical site infection after elective colorectal surgery: a meta-analysis of randomized controlled clinical trials. Dis Colon Rectum. 2016;59(1):70-78. doi: 10.1097/DCR.0000000000000524 [DOI] [PubMed] [Google Scholar]
- 6.Dahabreh IJ, Steele DW, Shah N, Trikalinos TA. Oral mechanical bowel preparation for colorectal surgery: systematic review and meta-analysis. Dis Colon Rectum. 2015;58(7):698-707. doi: 10.1097/DCR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 7.Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev. 2014;(5):CD001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson RL, Glenny AM, Song F. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev. 2009;(1):CD001181. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan K, Tian DH, Cao C, Black D, Yan TD. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg. 2015;4(2):112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zmora O, Mahajna A, Bar-Zakai B, et al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Ann Surg. 2003;237(3):363-367. doi: 10.1097/01.SLA.0000055222.90581.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zmora O, Mahajna A, Bar-Zakai B, et al. Is mechanical bowel preparation mandatory for left-sided colonic anastomosis? results of a prospective randomized trial. Tech Coloproctol. 2006;10(2):131-135. doi: 10.1007/s10151-006-0266-1 [DOI] [PubMed] [Google Scholar]
- 13.University of Melbourne Colorectal Group Clinical trial of prophylaxis of wound sepsis in elective colorectal surgery comparing ticarcillin with tinidazole. Aust N Z J Surg. 1986;56(3):209-213. doi: 10.1111/j.1445-2197.1986.tb06137.x [DOI] [PubMed] [Google Scholar]
- 14.University of Melbourne Colorectal Group Systemic Timentin is superior to oral tinidazole for antibiotic prophylaxis in elective colorectal surgery. Dis Colon Rectum. 1987;30(10):786-789. doi: 10.1007/BF02554628 [DOI] [PubMed] [Google Scholar]
- 15.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606-608. doi: 10.1017/S0195941700015241 [DOI] [PubMed] [Google Scholar]
- 16.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012;41(3):818-827. doi: 10.1093/ije/dys041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali M. Randomized prospective clinical trial of no preparation versus mechanical bowel preparation before elective colorectal surgery. Med Channel. 2007;13:32-35. [Google Scholar]
- 18.Bertani E, Chiappa A, Biffi R, et al. Comparison of oral polyethylene glycol plus a large volume glycerine enema with a large volume glycerine enema alone in patients undergoing colorectal surgery for malignancy: a randomized clinical trial. Colorectal Dis. 2011;13(10):e327-e334. doi: 10.1111/j.1463-1318.2011.02689.x [DOI] [PubMed] [Google Scholar]
- 19.Bhat AP, Parray FQ, Chowdri NA, et al. Mechanical bowel preparation versus no preparation in elective colorectal surgery: a prospective randomized study. Int J Surg Open. 2016;2:26-30. doi: 10.1016/j.ijso.2016.02.010 [DOI] [Google Scholar]
- 20.Bhattacharjee PK, Chakraborty S. An open-label prospective randomized controlled trial of mechanical bowel preparation vs nonmechanical bowel preparation in elective colorectal surgery: personal experience. Indian J Surg. 2015;77(suppl 3):1233-1236. doi: 10.1007/s12262-015-1262-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bretagnol F, Panis Y, Rullier E, et al. ; French Research Group of Rectal Cancer Surgery (GRECCAR) . Rectal cancer surgery with or without bowel preparation: the French GRECCAR III multicenter single-blinded randomized trial. Ann Surg. 2010;252(5):863-868. doi: 10.1097/SLA.0b013e3181fd8ea9 [DOI] [PubMed] [Google Scholar]
- 22.Bucher P, Gervaz P, Soravia C, Mermillod B, Erne M, Morel P. Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery [published correction appears in Br J Surg. 2005;92(8):1051]. Br J Surg. 2005;92(4):409-414. doi: 10.1002/bjs.4900 [DOI] [PubMed] [Google Scholar]
- 23.Burke P, Mealy K, Gillen P, Joyce W, Traynor O, Hyland J. Requirement for bowel preparation in colorectal surgery. Br J Surg. 1994;81(6):907-910. doi: 10.1002/bjs.1800810639 [DOI] [PubMed] [Google Scholar]
- 24.Contant CM, Hop WC, van’t Sant HP, et al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet. 2007;370(9605):2112-2117. doi: 10.1016/S0140-6736(07)61905-9 [DOI] [PubMed] [Google Scholar]
- 25.Fa-Si-Oen P, Roumen R, Buitenweg J, et al. Mechanical bowel preparation or not? outcome of a multicenter, randomized trial in elective open colon surgery. Dis Colon Rectum. 2005;48(8):1509-1516. doi: 10.1007/s10350-005-0068-y [DOI] [PubMed] [Google Scholar]
- 26.Khan SA, Hadi A, Ahmad S, et al. Mechanical bowel preparation in elective colorectal surgery. J Med Sci. 2011;19(1):31-34. [Google Scholar]
- 27.Miettinen RP, Laitinen ST, Mäkelä JT, Pääkkönen ME. Bowel preparation with oral polyethylene glycol electrolyte solution vs. no preparation in elective open colorectal surgery: prospective, randomized study. Dis Colon Rectum. 2000;43(5):669-675. doi: 10.1007/BF02235585 [DOI] [PubMed] [Google Scholar]
- 28.Pena-Soria MJ, Mayol JM, Anula R, Arbeo-Escolar A, Fernandez-Represa JA. Single-blinded randomized trial of mechanical bowel preparation for colon surgery with primary intraperitoneal anastomosis. J Gastrointest Surg. 2008;12(12):2103-2108. doi: 10.1007/s11605-008-0706-5 [DOI] [PubMed] [Google Scholar]
- 29.Platell C, Barwood N, Makin G. Randomized clinical trial of bowel preparation with a single phosphate enema or polyethylene glycol before elective colorectal surgery. Br J Surg. 2006;93(4):427-433. doi: 10.1002/bjs.5274 [DOI] [PubMed] [Google Scholar]
- 30.Ram E, Sherman Y, Weil R, Vishne T, Kravarusic D, Dreznik Z. Is mechanical bowel preparation mandatory for elective colon surgery? a prospective randomized study. Arch Surg. 2005;140(3):285-288. doi: 10.1001/archsurg.140.3.285 [DOI] [PubMed] [Google Scholar]
- 31.Saha AK, Chowdhury F, Jha AK, Chatterjee S, Das A, Banu P. Mechanical bowel preparation versus no preparation before colorectal surgery: a randomized prospective trial in a tertiary care institute. J Nat Sci Biol Med. 2014;5(2):421-424. doi: 10.4103/0976-9668.136214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos JC Jr, Batista J, Sirimarco MT, Guimarães AS, Levy CE. Prospective randomized trial of mechanical bowel preparation in patients undergoing elective colorectal surgery. Br J Surg. 1994;81(11):1673-1676. doi: 10.1002/bjs.1800811139 [DOI] [PubMed] [Google Scholar]
- 33.Sasaki J, Matsumoto S, Kan H, et al. Objective assessment of postoperative gastrointestinal motility in elective colonic resection using a radiopaque marker provides an evidence for the abandonment of preoperative mechanical bowel preparation. J Nippon Med Sch. 2012;79(4):259-266. doi: 10.1272/jnms.79.259 [DOI] [PubMed] [Google Scholar]
- 34.Reddy BS, Macfie J, Gatt M, Larsen CN, Jensen SS, Leser TD. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br J Surg. 2007;94(5):546-554. doi: 10.1002/bjs.5705 [DOI] [PubMed] [Google Scholar]
- 35.Beggs FD, Jobanputra RS, Holmes JT. A comparison of intravenous and oral metronidazole as prophylactic in colorectal surgery. Br J Surg. 1982;69(4):226-227. doi: 10.1002/bjs.1800690419 [DOI] [PubMed] [Google Scholar]
- 36.Dion YM, Richards GK, Prentis JJ, Hinchey EJ. The influence of oral versus parenteral preoperative metronidazole on sepsis following colon surgery. Ann Surg. 1980;192(2):221-226. doi: 10.1097/00000658-198008000-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espin-Basany E, Sanchez-Garcia JL, Lopez-Cano M, et al. Prospective, randomised study on antibiotic prophylaxis in colorectal surgery: is it really necessary to use oral antibiotics? Int J Colorectal Dis. 2005;20(6):542-546. doi: 10.1007/s00384-004-0736-8 [DOI] [PubMed] [Google Scholar]
- 38.Hata H, Yamaguchi T, Hasegawa S, et al. Oral and Parenteral Versus Parenteral Antibiotic Prophylaxis in Elective Laparoscopic Colorectal Surgery (JMTO PREV 07-01): a phase 3, multicenter, open-label, randomized trial. Ann Surg. 2016;263(6):1085-1091. doi: 10.1097/SLA.0000000000001581 [DOI] [PubMed] [Google Scholar]
- 39.Ikeda A, Konishi T, Ueno M, et al. Randomized clinical trial of oral and intravenous versus intravenous antibiotic prophylaxis for laparoscopic colorectal resection. Br J Surg. 2016;103(12):1608-1615. doi: 10.1002/bjs.10281 [DOI] [PubMed] [Google Scholar]
- 40.Ishida H, Yokoyama M, Nakada H, Inokuma S, Hashimoto D. Impact of oral antimicrobial prophylaxis on surgical site infection and methicillin-resistant Staphylococcus aureus infection after elective colorectal surgery: results of a prospective randomized trial. Surg Today. 2001;31(11):979-983. doi: 10.1007/s005950170006 [DOI] [PubMed] [Google Scholar]
- 41.Kling PA, Dahlgren S. Oral prophylaxis with neomycin and erythromycin in colorectal surgery: more proof for efficacy than failure. Arch Surg. 1989;124(6):705-707. doi: 10.1001/archsurg.1989.01410060075015 [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi M, Mohri Y, Tonouchi H, Miki C, Nakai K, Kusunoki M; Mie Surgical Infection Research Group . Randomized clinical trial comparing intravenous antimicrobial prophylaxis alone with oral and intravenous antimicrobial prophylaxis for the prevention of a surgical site infection in colorectal cancer surgery. Surg Today. 2007;37(5):383-388. doi: 10.1007/s00595-006-3410-7 [DOI] [PubMed] [Google Scholar]
- 43.Lau WY, Chu KW, Poon GP, Ho KK. Prophylactic antibiotics in elective colorectal surgery. Br J Surg. 1988;75(8):782-785. doi: 10.1002/bjs.1800750819 [DOI] [PubMed] [Google Scholar]
- 44.Lazorthes F, Legrand G, Monrozies X, et al. Comparison between oral and systemic antibiotics and their combined use for the prevention of complications in colorectal surgery. Dis Colon Rectum. 1982;25(4):309-311. doi: 10.1007/BF02553603 [DOI] [PubMed] [Google Scholar]
- 45.Lewis RT. Oral versus systemic antibiotic prophylaxis in elective colon surgery: a randomized study and meta-analysis send a message from the 1990s. Can J Surg. 2002;45(3):173-180. [PMC free article] [PubMed] [Google Scholar]
- 46.Oshima T, Takesue Y, Ikeuchi H, et al. Preoperative oral antibiotics and intravenous antimicrobial prophylaxis reduce the incidence of surgical site infections in patients with ulcerative colitis undergoing IPAA. Dis Colon Rectum. 2013;56(10):1149-1155. doi: 10.1097/DCR.0b013e31829f71a0 [DOI] [PubMed] [Google Scholar]
- 47.Playforth MJ, Smith GM, Evans M, Pollock AV. Antimicrobial bowel preparation: oral, parenteral, or both? Dis Colon Rectum. 1988;31(2):90-93. doi: 10.1007/BF02562635 [DOI] [PubMed] [Google Scholar]
- 48.Raahave D, Hesselfeldt P, Pedersen TB. Cefotaxime i.v. versus oral neomycin-erythromycin for prophylaxis of infections after colorectal operations. World J Surg. 1988;12(3):369-373. doi: 10.1007/BF01655676 [DOI] [PubMed] [Google Scholar]
- 49.Sadahiro S, Suzuki T, Tanaka A, et al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery. 2014;155(3):493-503. doi: 10.1016/j.surg.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 50.Stellato TA, Danziger LH, Gordon N, et al. Antibiotics in elective colon surgery: a randomized trial of oral, systemic, and oral/systemic antibiotics for prophylaxis. Am Surg. 1990;56(4):251-254. [PubMed] [Google Scholar]
- 51.Weaver M, Burdon DW, Youngs DJ, Keighley MR. Oral neomycin and erythromycin compared with single-dose systemic metronidazole and ceftriaxone prophylaxis in elective colorectal surgery. Am J Surg. 1986;151(4):437-442. doi: 10.1016/0002-9610(86)90097-8 [DOI] [PubMed] [Google Scholar]
- 52.Yabata E, Okabe S, Endo M. A prospective, randomized clinical trial of preoperative bowel preparation for elective colorectal surgery: comparison among oral, systemic, and intraoperative luminal antibacterial preparations. J Med Dent Sci. 1997;44(4):75-80. [PubMed] [Google Scholar]
- 53.Bhattacharjee PK, Chakraborty S. An open-label prospective randomized controlled trial of mechanical bowel preparation vs nonmechanical bowel preparation in elective colorectal surgery: personal experience. Indian J Surg. 2015;77(suppl 3):1233-1236. doi: 10.1007/s12262-015-1262-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhat AH, Parray FQ, Chowdri NA, et al. Mechanical bowel preparation versus no preparation in elective colorectal surgery: a prospective randomized study. Int J Surg Open. 2016;2(suppl C):26-30. doi: 10.1016/j.ijso.2016.02.010 [DOI] [Google Scholar]
- 55.Slim K, Vicaut E, Launay-Savary MV, Contant C, Chipponi J. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg. 2009;249(2):203-209. doi: 10.1097/SLA.0b013e318193425a [DOI] [PubMed] [Google Scholar]
- 56.Slim K, Vicaut E, Panis Y, Chipponi J. Meta-analysis of randomized clinical trials of colorectal surgery with or without mechanical bowel preparation. Br J Surg. 2004;91(9):1125-1130. doi: 10.1002/bjs.4651 [DOI] [PubMed] [Google Scholar]
- 57.Bucher P, Mermillod B, Gervaz P, Morel P. Mechanical bowel preparation for elective colorectal surgery: a meta-analysis. Arch Surg. 2004;139(12):1359-1364. doi: 10.1001/archsurg.139.12.1359 [DOI] [PubMed] [Google Scholar]
- 58.Moghadamyeghaneh Z, Hanna MH, Carmichael JC, et al. Nationwide analysis of outcomes of bowel preparation in colon surgery. J Am Coll Surg. 2015;220(5):912-920. doi: 10.1016/j.jamcollsurg.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 59.Kiran RP, Murray AC, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg. 2015;262(3):416-425. doi: 10.1097/SLA.0000000000001416 [DOI] [PubMed] [Google Scholar]
- 60.Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg. 2015;262(2):331-337. doi: 10.1097/SLA.0000000000001041 [DOI] [PubMed] [Google Scholar]
- 61.Morris MS, Graham LA, Chu DI, Cannon JA, Hawn MT. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann Surg. 2015;261(6):1034-1040. doi: 10.1097/SLA.0000000000001125 [DOI] [PubMed] [Google Scholar]
- 62.Young Tabusso F, Celis Zapata J, Berrospi Espinoza F, Payet Meza E, Ruiz Figueroa E. Mechanical preparation in elective colorectal surgery, a usual practice or a necessity? [in Spanish]. Rev Gastroenterol Peru. 2002;22(2):152-158. [PubMed] [Google Scholar]
- 63.Kale TI, Kuzu MA, Tekeli A, Tanik A, Aksoy M, Cete M. Aggressive bowel preparation does not enhance bacterial translocation, provided the mucosal barrier is not disrupted: a prospective, randomized study. Dis Colon Rectum. 1998;41(5):636-641. doi: 10.1007/BF02235274 [DOI] [PubMed] [Google Scholar]
- 64.Cao F, Li J, Li F. Mechanical bowel preparation for elective colorectal surgery: updated systematic review and meta-analysis. Int J Colorectal Dis. 2012;27(6):803-810. doi: 10.1007/s00384-011-1361-y [DOI] [PubMed] [Google Scholar]
- 65.Klinger AL, Green H, Monlezun DJ, et al. The role of bowel preparation in colorectal surgery: results of the 2012-2015 ACS-NSQIP data [published online October 23, 2017]. Ann Surg. doi: 10.1097/SLA.0000000000002568 [DOI] [PubMed] [Google Scholar]
- 66.Atkinson SJ, Swenson BR, Hanseman DJ, et al. In the absence of a mechanical bowel prep, does the addition of pre-operative oral antibiotics to parenteral antibiotics decrease the incidence of surgical site infection after elective segmental colectomy? Surg Infect (Larchmt). 2015;16(6):728-732. doi: 10.1089/sur.2014.215 [DOI] [PubMed] [Google Scholar]
- 67.Garfinkle R, Abou-Khalil J, Morin N, et al. Is there a role for oral antibiotic preparation alone before colorectal surgery? ACS-NSQIP analysis by coarsened exact matching. Dis Colon Rectum. 2017;60(7):729-737. doi: 10.1097/DCR.0000000000000851 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Diagram
eFigure 2. Funnel Plots
eAppendix. Search Strategy, Selection Process and Data Extraction and Method of Network Analysis
eTable 1. Cochrane Collaboration Risk of Bias Tool (Version 5.0.1): Risk of Bias in the Individual Randomized Controlled Trials Assessed by Cochrane Risk of Bias Checklist
eTable 2. Presentation of Outcome Data for Total Surgical Site Infection, by Included Study
eTable 3. Presentation of Outcome Data for Anastomotic Leak, by Included Study
eTable 4. Presentation of Outcome Data for Mortality, by Included Study