Key Points
Question
Is omega-3 polyunsaturated fatty acid treatment associated with an improvement in anxiety symptoms?
Findings
In this systematic review and meta-analysis of 19 clinical trials including 2240 participants from 11 countries, improvement in anxiety symptoms was associated with omega-3 polyunsaturated fatty acid treatment compared with controls in both placebo-controlled and non–placebo-controlled trials. The anxiolytic effects of omega-3 polyunsaturated fatty acids were also stronger in participants with clinical conditions than in subclinical populations.
Meaning
Omega-3 polyunsaturated fatty acid treatment for anxiety might be effective in clinical settings.
Abstract
Importance
No systematic review or meta-analysis has assessed the efficacy of omega-3 polyunsaturated fatty acids (PUFAs) for anxiety.
Objective
To evaluate the association of anxiety symptoms with omega-3 PUFA treatment compared with controls in varied populations.
Data Sources
PubMed, Embase, ProQuest, ScienceDirect, Cochrane Library, ClinicalKey, Web of Science, and ClinicalTrials.gov databases were searched up to March 4, 2018.
Study Selection
A search was performed of clinical trials assessing the anxiolytic effect of omega-3 PUFAs in humans, in either placebo-controlled or non–placebo-controlled designs. Of 104 selected articles, 19 entered the final data extraction stage.
Data Extraction and Measures
Two authors independently extracted the data according to a predetermined list of interests. A random-effects model meta-analysis was performed and this study was conducted based on Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Main Outcomes and Measures
Changes in the severity of anxiety symptoms after omega-3 PUFA treatment.
Results
In total, 1203 participants with omega-3 PUFA treatment (mean age, 43.7 years; mean female proportion, 55.0%; mean omega-3 PUFA dosage, 1605.7 mg/d) and 1037 participants without omega-3 PUFA treatment (mean age, 40.6 years; mean female proportion, 55.0%) showed an association between clinical anxiety symptoms among participants with omega-3 PUFA treatment compared with control arms (Hedges g, 0.374; 95% CI, 0.081-0.666; P = .01). Subgroup analysis showed that the association of treatment with reduced anxiety symptoms was significantly greater in subgroups with specific clinical diagnoses than in subgroups without clinical conditions. The anxiolytic effect of omega-3 PUFAs was significantly better than that of controls only in subgroups with a higher dosage (at least 2000 mg/d) and not in subgroups with a lower dosage (<2000 mg/d).
Conclusions and Relevance
This review indicates that omega-3 PUFAs might help to reduce the symptoms of clinical anxiety. Further well-designed studies are needed in populations in whom anxiety is the main symptom.
This systematic review and meta-analysis of 19 clinical trials evaluates whether improvement in anxiety symptoms is associated with anxiolytic effects of omega-3 polyunsaturated fatty acid (PUFA) treatment compared with control treatments in both placebo-controlled and non–placebo-controlled trials.
Introduction
Anxiety, the most commonly experienced psychiatric symptom, is a psychological state derived from inappropriate or exaggerated fear leading to distress or impairment. The lifetime prevalence of any anxiety disorder is reported to be approximately 1 in 3.1 Anxiety is often comorbid with depressive disorders2 and is associated with lower health-related quality of life3 and increased risk of all-cause mortality.4 Treatment options include psychological treatments, such as cognitive-behavioral therapy and pharmacological treatments, mainly with selective serotonin reuptake inhibitors.5 Individuals with anxiety and related disorders tend to be more concerned about the potential adverse effects of pharmacological treatments (eg, sedation or drug dependence) and may be reluctant to engage in psychological treatments that can be time-consuming and costly, as well as sometimes limited in availability.6 Thus, evidence-based and safer treatments are required, especially for anxious patients with comorbid medical conditions.
Omega-3 polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are essential nutrients that have potential preventive and therapeutic effects on psychiatric disorders, such as anxiety and depression,7,8,9,10,11,12,13,14,15 as well as comorbid depression and anxiety in physically ill patients,16,17,18,19 patients with coronary heart disease,20,21 and pregnant women.22,23 Preclinical data support the effectiveness of omega-3 PUFAs as treatment for anxiety disorders. Song et al24,25 found that an EPA-rich diet could reduce the development of anxiety-like behaviors in rats as well as normalize dopamine levels in the ventral striatum. In addition, Yamada et al26 showed that a high dietary omega-3 to omega-6 PUFA ratio reduced contextual fear behaviors in mice and that these effects were abolished by a cannabinoid CB1 receptor antagonist.
A number of trials have found that omega-3 PUFAs might reduce anxiety under serious stressful situations. Case-controlled studies have shown low peripheral omega-3 PUFA levels in patients with anxiety disorders.27,28,29,30,31 A cohort study found that high serum EPA levels were associated with protection against posttraumatic stress disorder.32 In studies of therapeutic interventions, while a randomized clinical trial of adjunctive EPA treatment in patients with obsessive-compulsive disorder revealed that EPA augmentation had no beneficial effect on symptoms of anxiety, depression, or obsessive-compulsiveness,33 a randomized clinical trial involving participants with substance abuse showed that EPA and DHA administration was accompanied by significant decreases in anger and anxiety scores compared with placebo.34 In addition, a randomized clinical trial found that omega-3 PUFAs had additional effects on decreasing depressive and anxiety symptoms in patients with acute myocardial infarction,35 and a randomized clinical trial demonstrated that omega-3 PUFAs could reduce inflammation and anxiety among healthy young adults facing a stressful major examination.36 Despite the largely positive findings of these trials, the clinical application of the findings is unfortunately limited by their small sample sizes.
We hypothesized that omega-3 PUFAs might have anxiolytic effects in patients with significant anxiety- and fear-related symptoms. However, there have been no systematic reviews of this topic to date. Thus, we examined the anxiolytic effects of omega-3 PUFAs in participants with elevated anxiety symptoms in the results of clinical trials to determine the overall efficacy of omega-3 PUFAs for anxiety symptoms irrespective of diagnosis.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.37 The study protocol adhered to the requirements of the institutional review board of Tri-Service General Hospital.
Literature Search and Screening
Two psychiatrists (P.-T.T. and T.-Y.C.) separately performed a systematic literature search of the PubMed, Embase, ProQuest, ScienceDirect, Cochrane Library, ClinicalKey, Web of Science, and ClinicalTrials.gov databases to March 4, 2018. Because we presumed some clinical trials would use investigating scales for some other mood symptoms but also contain symptoms of anxiety, we tried to use some nonspecific medical subject heading terms to include those clinical trials. Therefore, we used the following keywords: omega-3, eicosapentaenoic acid, EPA, DHA, or docosahexaenoic acid; and anxiety, anxiety disorder, generalized anxiety disorder, agoraphobia, panic disorder, or posttraumatic stress disorder. After removing duplicate studies, the same 2 authors screened the search results according to the title and abstract to evaluate eligibility. List of potentially relevant studies were generated for a full-text review. Any inconsistencies were discussed with a third author to achieve final consensus. To expand the list of potentially eligible articles, we performed a manual search of the reference lists of review articles in this area.12,38,39
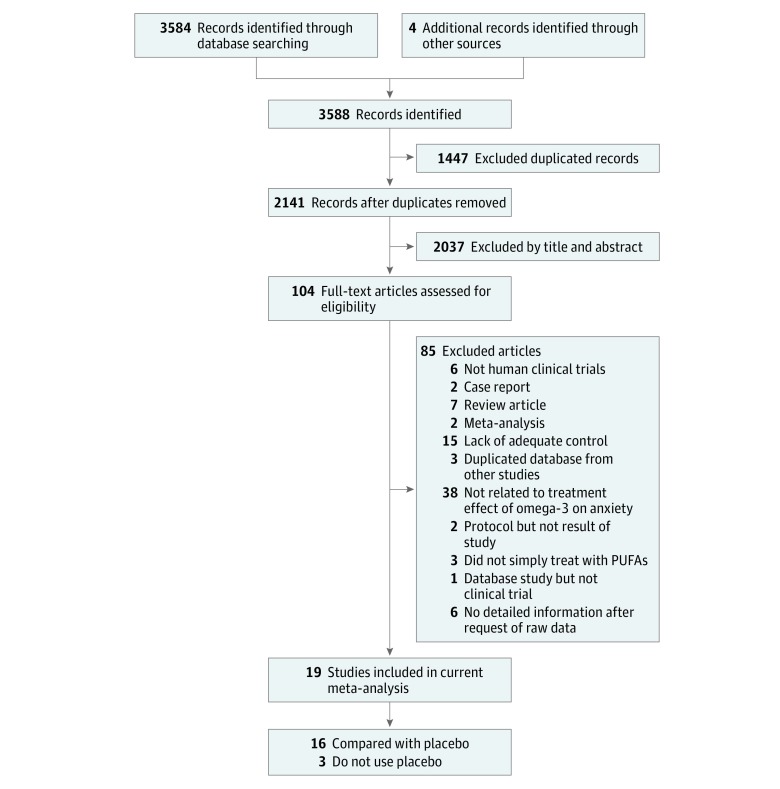
Because of the preliminary state of knowledge on the effects of omega-3 PUFA treatment on anxiety, we decided to include as many studies as possible and not to set further limitations on specific characteristics, such as length of study, diagnosis, omega-3 PUFA dosage, omega-3 PUFA preparation (EPA to DHA ratio), rated anxiety coding scale, or type of control. Therefore, we chose to make the inclusion criteria as broad as possible to avoid missing any potentially eligible studies. The inclusion criteria included clinical trials in humans (randomized or nonrandomized), studies investigating the effects of omega-3 PUFA treatment on anxiety symptoms, and formal published articles in peer-reviewed journals. The clinical trials could be placebo controlled or non–placebo controlled. The target participants could include healthy volunteers, patients with psychiatric illness, and patients with physical illnesses other than psychiatric illnesses. The exclusion criteria included case reports or series, animal studies or review articles, and studies not investigating the effects of omega-3 PUFA treatment on anxiety symptoms. We did not set any language limitation to increase the number of eligible articles. Figure 1 shows the literature search and screening protocol.
Figure 1. Flowchart of the Selection Strategy and Inclusion and Exclusion Criteria for This Meta-analysis.
PUFAs indicates polyunsaturated fatty acids.
Meta-analysis and Data Extraction and Input
Due to the anticipated heterogeneity, a random-effects meta-analysis was chosen rather than a fixed-effects meta-analysis because random-effects modeling is more stringent and incorporates an among-study variance in the calculations. The entire meta-analysis procedure was performed on the platform of Comprehensive Meta-analysis statistical software, version 3 (Biostat). Under the preliminary assumption that the scales for anxiety symptoms are heterogeneous among the recruited studies, we chose Hedges g and 95% confidence intervals to combine the effect sizes, in accordance with the manual of the Comprehensive Meta-analysis statistical software, version 3. Regarding the interpretation of effect sizes, we defined Hedges g values 0 or higher as a better association of treatment with reduced anxiety symptoms of omega-3 PUFAs than in controls. For each analysis, a 2-tailed P value less than .05 was considered to indicate statistical significance. When more than 1 anxiety scale was used in a study, we chose the one with the most informative data (ie, mean and standard deviation [SD] before and after treatment). We entered the primary outcome provided in the included articles or obtained from the original authors. As for the variance imputation, we mainly chose the mean and SD before and after treatment. Later, we entered the mean and SD and calculated the effect sizes based on the software option, standardized by post score SD. In the case of studies with 2 active treatment arms, we merged the 2 active treatment arms into 1 group. If these 2 active treatment arms belonged to different subgroups (ie, different PUFA dosage subgroups), we kept them separate. Regarding the numbers of participants counted, we chose intention-to-treat as our priority. If there were insufficient data in the intention to treat group (ie, some studies only provided the changes in anxiety severity in those participants completing trials), we chose instead the per-protocol numbers of participants.
The quality of the included clinical trials were assessed using the Jadad score,40 which was designed to evaluate the risk of bias in interventional trials in 3 specific domains: randomization, blindness, and cohort follow-up.
The primary outcome was analyzed by changes in anxiety symptoms in patients receiving omega-3 PUFA treatment compared with those not receiving omega-3 PUFA treatment.
Heterogeneity, Publication Bias, and Sensitivity Testing
Heterogeneity was examined using the Q statistic and the corresponding P values,41 and the I2 statistic was used to evaluate the proportion of variation resulting from among-study differences. Any possible publication bias was detected with both funnel plots and Egger regression in the main part of the meta-analysis.42 By using Duval and Tweedie’s trim-and-fill test, we adjusted the effect sizes for potential publication bias if there was evidence of publication bias detected by this test in the Comprehensive Meta-analysis statistical software, version 3.43 To investigate the potential confounding effects of any outliers within the recruited studies, sensitivity testing was conducted with the 1-study removal method to detect the potential outliers.44
Metaregression and Subgroup Meta-analysis
To exclude the possible confounding effects of clinical variables on the Hedges g, metaregression analysis was conducted with an unrestricted maximum likelihood random-effects model of single variables when there were more than 10 data sets available. Specifically, the clinical variables of interest included mean age, female proportion, sample size, mean body mass index, daily omega-3 PUFA dosage, EPA to DHA ratio, treatment duration, dropout rate, and others. In addition, a subgroup meta-analysis was conducted to investigate potential sources of heterogeneity, specifically, a further subgroup meta-analysis focused on those trials that were placebo controlled or non–placebo controlled. To more clearly uncover the differences in the meta-analysis results among the recruited studies, a further subgroup meta-analysis was performed according to the presence of a specific clinical diagnosis or no specific clinical condition, mean omega-3 PUFA daily dosage, and mean age. In addition, in a previous study, the EPA percentage (ie, ≥60%) in the PUFA regimens had different effects on depression treatment.9 Therefore, we also arranged the subgroup meta-analysis based on the EPA percentage. Furthermore, we arranged subgroup meta-analysis procedures only when there were at least 3 data sets included.45 To investigate the potentially different estimated effect sizes between subgroups, we performed an interaction test and calculated the corresponding P values.46
Results
Characteristics of the Included Studies
After the initial screening process, a total of 104 articles were considered for full-text review (Figure 1; eFigure 1 in the Supplement); 85 were excluded according to the exclusion criteria (eAppendix in the Supplement), leaving 19 articles for analysis in this study (Table).33,34,35,36,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61
Table. Characteristics of Recruited Studies.
| Source | Diagnosis | Comparison | Participants, No. | Anxiety Scale | Age, Mean (SD), y | Female, No. (%) | Omega-3 Dosage, mg/d | Dropout Rate, No./Total No. | Treatment Duration, wk | Country |
|---|---|---|---|---|---|---|---|---|---|---|
| Watanabe et al,47 2018 | Junior nurses work in hospital | Omega-3 PUFA Placebo |
40 40 |
HADS-A | 29.6 (9.1) 30.5 (7.8) |
40 (100.0) 40 (100.0) |
1800.0 | 0/40 3/40 |
13 | Japan |
| Cornu et al,48 2018 | Children with ADHD | Omega-3 PUFA Placebo |
80 82 |
Conners | 10.2 (2.8) 9.7 (2.5) |
19 (23.7) 16 (19.5) |
600.0 | 3/80 1/82 |
12 | France |
| Matsuoka et al,49 2015 | Severe accidental injury | Omega-3 PUFA Placebo |
53 57 |
CAPS | 38.1 (13.5) 40.9 (17.3) |
9 (17.0) 11 (19.3) |
2100.0 | 8/53 6/57 |
12 | Japan |
| Bellino et al,50 2014 | Borderline personality disorders | Omega-3 PUFA + valproate Control + valproate |
18 16 |
HAM-A | 25.2 (6.4) | 26 (76.5) | 2000.0 | 5/23 4/20 |
12 | Italy |
| Cohen et al,51 2014 | Generally healthy participants | Omega-3 PUFA Placebo |
177 178 |
GAD-7 | 54.7 (3.7) | 177 (100.0) 178 (100.0) |
1800.0 | 4/177 5/178 |
12 | United States |
| Pomponi et al,52 2014 | Parkinson disease | Omega-3 PUFA Placebo |
12 12 |
HAM-A | 64.0 (4.9) 64.0 (9.8) |
5 (41.7) 6 (50.0) |
2000.0 | 0/12 0/12 |
24 | Italy |
| Widenhorn-Müller et al,53 2014 | Children with ADHD | Omega-3 PUFA Placebo |
46 49 |
CBCL-A | 8.9 (1.5) 8.9 (1.2) |
11 (23.9) 10 (20.4) |
720.0 | 7/55 6/55 |
16 | Germany |
| Haberka et al,35 2013 | AMI | Omega-3 PUFA + AMI treatment Control + AMI treatment |
26 26 |
STAI | 56.4 59.6 (6.0) |
3 (11.5) 4 (15.4) |
1000.0 | 0/26 0/26 |
4 | Poland |
| Nishi et al,54 2013 | Disaster-related trauma | Omega-3 PUFA + education Education |
86 86 |
IES-R | 37.9 (7.4) 37.4 (7.4) |
24 (27.9) 23 (26.7) |
2240.0 | 0/86 1/86 |
12.6 | Japan |
| Sauder et al,55 2013 | Healthy, nonsmoking men and postmenopausal women with moderate hypertriglyceridemia | Omega-3 PUFA (3.4 g/d) Omega-3 PUFA (0.85 g/d) Placebo |
26 26 26 |
STAI-state | 44.0 | 3 (11.5) | 3400.0 850.0 |
0/26 0/26 0/26 |
8 | United States |
| Sohrabi et al,56 2013 | Women with premenstrual syndrome | Omega-3 PUFA Placebo |
63 61 |
VASA | 31.2 (6.5) 31.6 (8.4) |
63 (100.0) 61 (100.0) |
1000.0 | 7/70 8/69 |
12 | Iran |
| Gabbay et al,57 2012 | Tourette syndrome | Omega-3 PUFA Placebo |
17 16 |
C-YBOCS | 11.9 (3.6) 10.6 (2.3) |
3 (17.6) 3 (18.8) |
4074.0 | 3/17 5/16 |
20 | United States |
| Kiecolt-Glaser et al,36 2011 | Generally healthy participants | Omega-3 PUFA Placebo |
34 34 |
BAI | 23.9 (2.0) 23.4 (1.7) |
16 (47.1) 14 (41.2) |
2496.0 | 0/34 0/34 |
12 | United States |
| Buydens-Branchey et al,34 2008 | Substance abuse | Omega-3 PUFA Placebo |
11 11 |
POMS | NA | 0 0 |
3000.0 | 0/11 0/11 |
12 | United States |
| Freund-Levi et al,58 2008 | Alzheimer disease | Omega-3 PUFA Placebo |
89 85 |
NPI | 72.6 (9.0) 72.9 (8.6) |
51 (57.3) 39 (45.9) |
2320.0 | 12/103 14/101 |
24 | Sweden |
| Rogers et al,59 2008 | Mild to severe depression | Omega-3 PUFA Placebo |
109 109 |
DASS | 38.0 (13.5) 38.2 (13.7) |
85 (78.0) 83 (76.1) |
2369.5 | 13/109 15/109 |
12 | United Kingdom |
| van de Rest et al,60 2008 | Elderly volunteers | Omega-3 PUFA (1.8 g/d) Omega-3 PUFA (0.4 g/d) Placebo |
96 100 106 |
HADS-A | 69.9 (3.4) 69.5 (3.2) 70.1 (3.7) |
43 (44.8) 45 (45.0) 47 (44.3) |
1800.0 400.0 |
0/96 0/100 3/106 |
26 | Netherlands |
| Yehuda et al,61 2005 | Undergraduate college students with test anxiety | Omega-3 PUFA Placebo |
88 38 |
TAS | NA | NA | 225.0 | 0/88 0/38 |
3 | Israel |
| Fux et al,33 2004 | Obsessive-compulsive disorder | Omega-3 PUFA Placebo |
6 5 |
YBOCS | 33.5 (5) | 8 (72.7) | 2000.0 | 1/11 | 6 | Israel |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; AMI, acute myocardial infarction; BAI, Beck anxiety index; CAPS, clinician-administered posttraumatic stress disorder scale; CBCL-A, Child Behavior Checklist anxiety subscale; C-YBOCS, children’s Yale-Brown obsessive-compulsive scale; DASS, depression, anxiety, and stress scales; GAD-7, generalized anxiety disorder questionnaire; HADS-A, Hospital Anxiety and Depression Scale anxiety subscale; HAM-A, Hamilton anxiety rating scale; IES-R, impact of event scale-revised; NA, not available; NPI, Neuropsychiatric Inventory; POMS, profiles of mood states; PUFA, polyunsaturated fatty acid; STAI, state-trait anxiety inventory; TAS, test anxiety severity; VASA, visual analog scale of anxiety; YBOCS, Yale-Brown obsessive-compulsive scale.
In the 19 recruited studies,33,34,35,36,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61 there were a total of 1203 participants with omega-3 PUFA treatment (mean age, 43.7 years; mean female proportion, 55.0%; mean omega-3 PUFA dosage, 1605.7 mg/d) and 1037 participants without omega-3 PUFA treatment (mean age, 40.6 years; mean female proportion, 55.0%).
Various scales were used in these studies to evaluate the target outcome of anxiety symptoms: the Yale-Brown Obsessive-Compulsive Scale, Profile of Mood States, State-Trait Anxiety Inventory, Hamilton Anxiety Rating Scale, Generalized Anxiety Disorder questionnaire, Depression, Anxiety, and Stress Scales, Clinician-Administered Posttraumatic Stress Disorder Scale, Beck Anxiety Inventory, visual analog scale of anxiety, Impact of Event Scale–Revised, Conners score anxiety subscale, Neuropsychiatric Inventory, test anxiety severity, Hospital Anxiety and Depression Scale anxiety subscale, and Child Behavior Checklist anxiety subscale. The psychiatric and physical health conditions of the recruited participants also varied widely: general population without specific clinical conditions,36,47,51,55,60 participants with acute myocardial infarction,35 borderline personality disorder,2 mild to severe depression,59 obsessive-compulsive disorder,33 severe accidental injury,49 participants who were traumatized by disaster,54 participants with substance abuse disorder,34 women with premenstrual syndrome,56 children with attention-deficit/hyperactivity disorder,48,53 Alzheimer disease,58 generally healthy undergraduate college students but with test anxiety,61 Parkinson disease,52 and participants with Tourette syndrome.57 Sixteen studies compared the effect of omega-3 PUFA treatment with that of the placebo33,34,36,47,48,49,51,52,53,55,56,57,58,59,60,61; the other 3 studies were non–placebo controlled trials.35,50,54 The mean (SD) Jadad score of the recruited studies was 3.8 (1.0) (eTable in the Supplement).
Meta-analysis of Changes in Anxiety Symptoms in Patients Receiving and Not Receiving Omega-3 PUFA Treatment
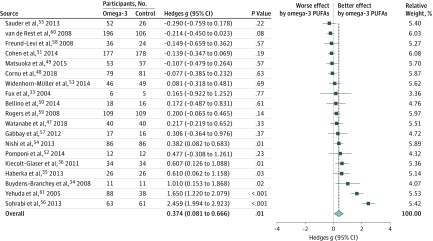
In total, 19 articles with 19 data sets revealed the main results of the meta-analysis, namely that there was a significantly better association of treatment with reduced anxiety symptoms in patients receiving omega-3 PUFA treatment than in those not receiving it (k, 19; Hedges g, 0.374; 95% CI, 0.081-0.666; P = .01; Figure 2), with significant heterogeneity (Cochran Q, 178.820; df, 18; I2, 89.934%; P < .001) but no significant publication bias via Egger regression (t, 1.736; df, 17; P = .10) or inspection of the funnel plot (eFigure 2 in the Supplement). According to the trim-and-fill test, there was no need for adjustment for publication bias. The meta-analysis results remained significant after removal of any one of the included studies, which indicated that the significant results are not owing to any single study.
Figure 2. Meta-Analysis Forest Plot of the Association of Treatment With Reduced Anxiety Symptoms in Patients Receiving and Not Receiving omega-3 PUFAs.
There was a significant improvement in anxiety symptoms in patients receiving omega-3 PUFAs than in those not receiving omega-3 PUFAs (k, 19; Hedges g, 0.374; 95% CI, 0.081-0.666; P = .01).
There was no significant association between the Hedges g and mean age (k, 17; P = .51), female proportion (k, 18; P = .32), mean omega-3 PUFA dosage (k, 19; P = .307), EPA to DHA ratio (k, 17; P = .86), dropout rate in the omega-3 PUFA group (k, 18; P = .71), duration of omega-3 PUFA treatment (k, 19; P = .14), Jadad score of randomization (k, 19; P = .10), Jadad score of blindness (k, 19; P = .57), or total Jadad score (k, 19; P = .18).
Subgroup Meta-analysis When Focusing on Placebo-Controlled Trials or Non–Placebo-Controlled Trials
Among the 16 studies comparing the effect of omega-3 PUFA treatment with that of the placebo,33,34,36,47,48,49,51,52,53,55,56,57,58,59,60,61 the main results revealed a significantly greater association of treatment with reduced anxiety symptoms in patients receiving omega-3 PUFA treatment than in those not receiving it (k, 16; Hedges g, 0.372; 95% CI, 0.032-0.712; P = .03; eFigure 3 in the Supplement). The meta-analysis of the subgroup focusing on non–placebo-controlled trials also showed a significantly greater association of treatment with reduced anxiety symptoms in patients receiving omega-3 PUFA treatment than in those not receiving it (k, 3; Hedges g, 0.399; 95% CI, 0.154-0.643; P = .001).35,50,54
Subgroup Meta-analysis When Focusing on Trials Recruiting Participants Without Specific Clinical Conditions or Trials Recruiting Participants With Specific Clinical Diagnoses
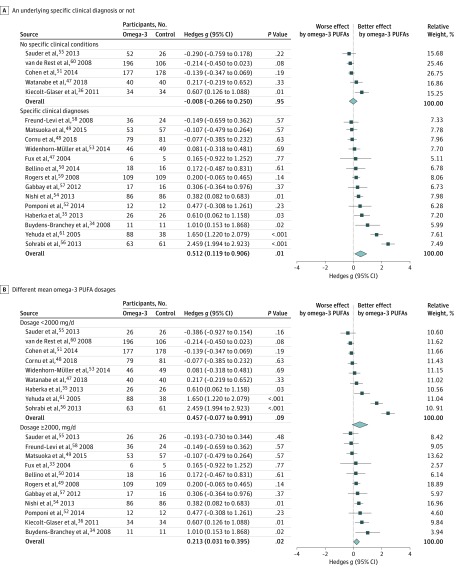
Five studies with 7 data sets recruited participants without specific clinical conditions.36,47,51,55,60 The main results revealed that there was no significant difference in the association of treatment with reduced anxiety symptoms between patients receiving omega-3 PUFA treatment and those not receiving it (k, 5; Hedges g, –0.008; 95% CI, –0.266 to 0.250; P = .95) (Figure 3A). Fourteen studies with 14 data sets recruited participants with specific clinical diagnoses.33,34,35,48,49,50,52,53,54,56,57,58,59,61 The main results revealed a significantly greater association of treatment with reduced anxiety symptoms in patients receiving omega-3 PUFA treatment than in those not receiving it (k, 14; Hedges g, 0.512; 95% CI, 0.119-0.906; P = .01) (Figure 3A). Furthermore, according to the interaction test, the association of omega-3 PUFA treatment with reduced anxiety symptoms was significantly stronger in subgroups with specific clinical diagnoses than in subgroups without specific clinical conditions (P = .03).
Figure 3. Forest Plot of Subgroup Meta-analysis.
A, Subgroup meta-analysis of the anxiolytic effect of omega-3 polyunsaturated fatty acids (PUFAs) based on an underlying specific clinical diagnosis or not. The anxiolytic effect of omega-3 PUFAs was not significant in the subgroup of participants without specific clinical conditions (k, 5; Hedges g, –0.008; 95% CI, –0.266 to 0.250; P = .95) but was significant in the subgroup of participants with specific clinical diagnoses (k, 14; Hedges g, 0.512; 95% CI, 0.119-0.906; P = .01). Furthermore, the association of treatment with reduced anxiety symptoms of omega-3 PUFAs were significantly stronger in subgroups with specific clinical diagnoses than in subgroups without specific clinical conditions (P = .03). B, Subgroup meta-analysis of the anxiolytic effect of omega-3 PUFAs based on different mean omega-3 PUFA dosages. The anxiolytic effect of omega-3 PUFAs was not significant in subgroups of mean omega-3 PUFA dosages less than 2000 mg/d (k, 9; Hedges g, 0.457; 95% CI, –0.077 to 0.991; P = .09) but was significant in the subgroup of mean omega-3 PUFA dosage of at least 2000 mg/d (k, 11; Hedges g, 0.213; 95% CI, 0.031-0.395; P = .02).
Subgroup Meta-analysis When Focusing on Trials With Omega-3 PUFA Dosages of Less Than 2000 mg/d or at Least 2000 mg/d
Nine studies with 10 data sets used omega-3 PUFA dosages of less than 2000 mg/d.35,47,48,51,53,55,56,60,61 The main results revealed that there was no significant difference in the association of treatment with reduced anxiety symptoms between patients receiving omega-3 PUFA treatment and those not receiving it (k, 9; Hedges g, 0.457; 95% CI, –0.077 to 0.991; P = .09) (Figure 3B). Ten studies with 10 data sets used omega-3 PUFA dosages of at least 2000 mg/d.33,34,36,49,50,52,54,55,57,58,59 The main results revealed a significantly greater association of treatment with reduced anxiety symptoms in patients receiving omega-3 PUFA treatment than in those not receiving it (k, 11; Hedges g, 0.213; 95% CI, 0.031-0.395; P = .02) (Figure 3B). Furthermore, there was no significantly different estimated effect sizes between these 2 subgroups by the interaction test (P = .40).
Subgroup Meta-analysis of Trials With an EPA Percentage Less Than 60% or an EPA Percentage of at Least 60%
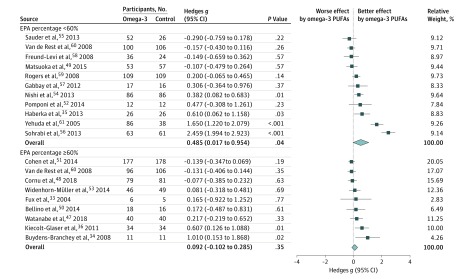
There was a significantly greater association of treatment with reduced anxiety symptoms in participants receiving omega-3 PUFAs than in those not receiving omega-3 PUFAs in the subgroup with an EPA percentage less than 60% (k, 11; Hedges g, 0.485; 95% CI, 0.017-0.954; P = .04; Figure 4)35,49,52,54,55,56,57,58,59,60,61 but no significant difference in the association of treatment with reduced anxiety symptoms between participants receiving omega-3 PUFAs and those not receiving omega-3 PUFAs in the subgroup with an EPA percentage of at least 60% (k, 9; Hedges g, 0.092; 95% CI, –0.102 to 0.285; P = .35) (Figure 4).33,34,36,47,48,50,51,53,60 There were no significantly different estimated effect sizes between these 2 subgroups by the interaction test (P = .13).
Figure 4. Subgroup Meta-analysis With Different Eicosapentaenoic Acid (EPA) Percentages.
Subgroup meta-analysis of the anxiolytic effects of omega-3 polyunsaturated fatty acids (PUFAs) based on different EPA percentages. The anxiolytic effects of omega-3 PUFAs were significant in the subgroup with an EPA percentage less than 60% (k, 11; Hedges g = 0.485; 95% CI, 0.017 to 0.954; P = .04) but not significant in the subgroups with an EPA percentage of at least 60% (k, 9; Hedges g, 0.092; 95% CI, –0.102 to 0.285; P = .35).
Other Subgroup Meta-analyses of Changes in Anxiety Symptoms in Patients Receiving and Not Receiving Omega-3 PUFA Treatment
In addition, there was no significant difference in the association of treatment with reduced anxiety symptoms between participants receiving omega-3 PUFAs and those not receiving omega-3 PUFAs in the adolescent subgroup (aged <18 years) (k, 3; Hedges g, 0.020; 95% CI, –0.209 to 0.250; P = .86),48,53,57 in the adult subgroup (aged ≥18 years but <60 years) (k, 11; Hedges g, 0.388; 95% CI, –0.012 to 0.788; P = .06),33,35,36,47,49,50,51,54,55,56,59 or in the elderly subgroup (aged ≥60 years) (k, 3; Hedges g, –0.112; 95% CI, –0.406 to 0.181; P = .45).52,58,60 These insignificant results might be due to the smaller sample sizes in each subgroup.
Discussion
To our knowledge, this is the first systematic review and meta-analysis to examine the anxiolytic effects of omega-3 PUFAs in individuals with anxiety symptoms. The overall findings revealed modest anxiolytic effects of omega-3 PUFAs in individuals with various neuropsychiatric or major physical illnesses. Although participants and diagnoses were heterogeneous, the main finding of this meta-analysis was that omega-3 PUFAs were associated with significant reduction in anxiety symptoms compared with controls; this effect persisted vs placebo controls. Furthermore, the association of treatment with reduced anxiety symptoms of omega-3 PUFA were significantly higher in subgroups with specific clinical diagnoses than in subgroups without clinical conditions.
Interestingly, the results are also consistent with our recent findings that somatic anxiety is associated with omega-3 PUFA deficits and the genetic risks of PUFA metabolic enzyme cytosolic phospholipase A2 in major depressive disorder62,63 and interferon α–induced neuropsychiatric syndrome.63,64 Brain membranes contain a high proportion of omega-3 PUFAs and their derivatives and most animal and human studies suggest that a lack of omega-3 PUFAs in the brain might induce various behavioral and neuropsychiatric disorders,16,65,66,67,68,69,70 including anxiety-related behaviors.12,18,19,32,49,71 Emerging evidence suggests that omega-3 PUFAs interfere with and possibly control several neurobiological processes, such as neurotransmitter systems, neuroplasticity, and inflammation,12,72 which is postulated to be the mechanism underlying anxiety and depression.
In our analysis, most of the included studies showed a positive Hedges g toward a beneficial effect of omega-3 PUFAs in anxiety reduction, although not all findings were statistically significant. However, after merging of these effect sizes from all of the included studies, the main result showed significant findings in our meta-analysis. Despite the significant heterogeneity, no significant publication bias was found among these 19 studies.
To evaluate the potential placebo effect, we made further subgrouping analyses. In the subgroups of studies using placebo controls, the omega-3 PUFAs still revealed a consistent positive anxiolytic association with anxiety symptoms. These phenomena meant that the anxiolytic effect of omega-3 PUFAs is probably not entirely owing to the placebo effect.
Further, according to subgroup results based on the presence of specific clinical diagnoses or not, the association of omega-3 PUFA treatment with reduced anxiety symptoms was significantly higher in subgroups with specific clinical diagnoses than in subgroups without clinical conditions. Among 6 studies included in a meta-analysis of the effect of omega-3 PUFAs on depressive symptoms, the analysis showed a nearly null effect of omega-3 PUFAs on depressive symptoms in healthy participants.73 Although the reason for the null effect of omega-3 PUFAs on anxiety and depressive symptoms remains unclear, certain pathophysiological conditions might be required for omega-3 PUFAs to exert an association of treatment with reduced anxiety symptoms.
Participants treated with a daily dose of 2000 mg or more of omega-3 PUFAs showed a significantly greater association of treatment with reduced anxiety symptoms. In addition, participants receiving supplements containing less than 60% EPA showed a significant association, but not those receiving supplements containing 60% or more EPA. The depression literature supports the clinical benefits of EPA-enriched formulations (≥60% or ≥50%) compared with placebo for the treatment of clinical depression.9,13,73,74,75 This opposite effect of EPA-enriched formations on anxiety and depression is intriguing and possibly linked to a distinct underlying mechanism of omega-3 PUFAs. Exploration of the effects of omega-3 PUFAs on anxiety symptoms is just beginning and studies assessing the dose response anxiolytic effects of omega-3 PUFAs have not yet been performed. Further phase 2 trials of anxiety symptoms among participants with neuropsychiatric illness or physical illness should aim to determine the optimal dose.
Although there was significant heterogeneity among the included studies (Cochran Q, 178.820; df, 18; I2, 89.934%; P < .001), the sensitivity test suggested that the main significant results of the meta-analysis would not change after removal of any of the included studies. However, through direct inspection of the forest plot, we detected the potential influence of some outliers, such as the studies by Sohrabi et al56 and Yehuda et al.61 These 2 studies evaluated anxiety symptoms with a visual analog scale of anxiety and test anxiety severity, which are seldom used in psychiatric research and lack a definite report to prove their equivalent sensitivity and specificity to some other frequently used anxiety rating scales, such as depression, anxiety, and stress scales or the Hamilton anxiety rating scale. Therefore, these studies might have affected the interpretation of the current meta-analysis.
Finally, to investigate the potential confounding effects of some clinical variables, we tried to conduct further exploratory subgroup analyses based on age. However, there were no significant findings from these subgroups. These results might be due to the smaller sample sizes after subgrouping.
Limitations
This article had several limitations and the findings need to be considered with caution. First, our participant population is too heterogeneous because of our broad inclusion criteria, which might be true if considering current Diagnostic and Statistical Manual of Mental Disorders or International Classification of Diseases diagnostic systems. However, the novel Research Domain Criteria consider anxiety to be one of the major domains in Negative Valence Systems. Trials should be conducted in populations in which anxiety is the main symptom irrespective of the presence or absence of diagnosis of anxiety disorder. Second, because of the limited number of recruited studies and their modest sample sizes, the results should not be extrapolated without careful consideration. Third, the significant heterogeneity among the included studies (Cochran Q, 178.820; df, 18; I2, 89.934%; P < .001) with potential influence by some outlier studies, such as the studies by Sohrabi et al56 and Yehuda et al,61 would be another major concern. Therefore, clinicians should pay attention to this aspect when applying the results of the current meta-analysis to clinical practice, particularly when considering the subgroups of these 2 studies (ie, subgroups with specific clinical diagnoses, with <2000 mg/d, with EPA <60%, and with placebo-controlled trials).
Conclusions
This systematic review and meta-analysis of clinical trials conducted on participants with clinical anxiety symptoms provides the first meta-analytic evidence, to our knowledge, that omega-3 PUFA treatment may be associated with anxiety reduction, which might not only be due to a potential placebo effect, but also from some associations of treatment with reduced anxiety symptoms. The beneficial anxiolytic effects of omega-3 PUFAs might be stronger in participants with specific clinical diagnoses than in those without specific clinical conditions. Larger and well-designed clinical trials should be performed with high-dose omega-3 PUFAs, provided as monotherapy and as adjunctive treatment to standard therapy.
eAppendix. Excluded Studies and Reasons
eTable. Study Design and Jadad Scores of Recruited Studies
eFigure 1. Whole Flowchart of Current Meta-Analysis
eFigure 2. Funnel Plot of Changes in Anxiety Symptoms in Patients With and Without n-3 PUFA Treatment
eFigure 3. Subgroup MA of Anxiolytic Effect Based Upon Placebo Controlled or Non–Placebo Controlled Design
References
- 1.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):-. doi: 10.1002/mpr.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strine TW, Mokdad AH, Balluz LS, et al. . Depression and anxiety in the United States: findings from the 2006 Behavioral Risk Factor Surveillance System. Psychiatr Serv. 2008;59(12):1383-1390. doi: 10.1176/ps.2008.59.12.1383 [DOI] [PubMed] [Google Scholar]
- 3.Stein MB, Roy-Byrne PP, Craske MG, et al. . Functional impact and health utility of anxiety disorders in primary care outpatients. Med Care. 2005;43(12):1164-1170. doi: 10.1097/01.mlr.0000185750.18119.fd [DOI] [PubMed] [Google Scholar]
- 4.Tolmunen T, Lehto SM, Julkunen J, Hintikka J, Kauhanen J. Trait anxiety and somatic concerns associate with increased mortality risk: a 23-year follow-up in aging men. Ann Epidemiol. 2014;24(6):463-468. doi: 10.1016/j.annepidem.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 5.Katzman MA, Bleau P, Blier P, et al. ; Canadian Anxiety Guidelines Initiative Group on behalf of the Anxiety Disorders Association of Canada/Association Canadienne des troubles anxieux and McGill University . Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(suppl 1):S1. doi: 10.1186/1471-244X-14-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon RP, Brandish EK, Baldwin DS. Anxiety disorders, post-traumatic stress disorder, and obsessive–compulsive disorder. Medicine (Baltimore). 2016;44(11):664-671. doi: 10.1016/j.mpmed.2016.08.010 [DOI] [Google Scholar]
- 7.Su KP, Shen WW, Huang SY. Effects of polyunsaturated fatty acids on psychiatric disorders. Am J Clin Nutr. 2000;72(5):1241. doi: 10.1093/ajcn/72.5.1241 [DOI] [PubMed] [Google Scholar]
- 8.Lin P-Y, Huang S-Y, Su K-P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68(2):140-147. doi: 10.1016/j.biopsych.2010.03.018 [DOI] [PubMed] [Google Scholar]
- 9.Lin PY, Mischoulon D, Freeman MP, et al. . Are omega-3 fatty acids antidepressants or just mood-improving agents? the effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry. 2012;17(12):1161-1163. doi: 10.1038/mp.2012.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarris J, Logan AC, Akbaraly TN, et al. ; International Society for Nutritional Psychiatry Research . Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274. doi: 10.1016/S2215-0366(14)00051-0 [DOI] [PubMed] [Google Scholar]
- 11.Sarris J, Logan AC, Akbaraly TN, et al. . International Society for Nutritional Psychiatry Research consensus position statement: nutritional medicine in modern psychiatry. World Psychiatry. 2015;14(3):370-371. doi: 10.1002/wps.20223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su KP, Matsuoka Y, Pae CU. Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci. 2015;13(2):129-137. doi: 10.9758/cpn.2015.13.2.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72(12):1577-1584. doi: 10.4088/JCP.10m06634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mischoulon D, Nierenberg AA, Schettler PJ, et al. . A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. J Clin Psychiatry. 2015;76(1):54-61. doi: 10.4088/JCP.14m08986 [DOI] [PubMed] [Google Scholar]
- 15.Freeman MP, Hibbeln JR, Wisner KL, et al. . Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67(12):1954-1967. doi: 10.4088/JCP.v67n1217 [DOI] [PubMed] [Google Scholar]
- 16.Frasure-Smith N, Lespérance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55(9):891-896. doi: 10.1016/j.biopsych.2004.01.021 [DOI] [PubMed] [Google Scholar]
- 17.Su KP, Lai HC, Yang HT, et al. . Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry. 2014;76(7):559-566. doi: 10.1016/j.biopsych.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 18.Matsumura K, Noguchi H, Nishi D, Hamazaki K, Hamazaki T, Matsuoka YJ. Effects of omega-3 polyunsaturated fatty acids on psychophysiological symptoms of post-traumatic stress disorder in accident survivors: a randomized, double-blind, placebo-controlled trial. J Affect Disord. 2017;224:27-31. doi: 10.1016/j.jad.2016.05.054 [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka YJ, Hamazaki K, Nishi D, Hamazaki T. Change in blood levels of eicosapentaenoic acid and posttraumatic stress symptom: a secondary analysis of data from a placebo-controlled trial of omega3 supplements. J Affect Disord. 2016;205:289-291. doi: 10.1016/j.jad.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 20.Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA. 2009;302(15):1651-1657. doi: 10.1001/jama.2009.1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carney RM, Steinmeyer BC, Freedland KE, Rubin EH, Rich MW, Harris WS. Baseline blood levels of omega-3 and depression remission: a secondary analysis of data from a placebo-controlled trial of omega-3 supplements. J Clin Psychiatry. 2016;77(2):e138-e143. doi: 10.4088/JCP.14m09660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman MP, Hibbeln JR, Wisner KL, Brumbach BH, Watchman M, Gelenberg AJ. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr Scand. 2006;113(1):31-35. doi: 10.1111/j.1600-0447.2005.00660.x [DOI] [PubMed] [Google Scholar]
- 23.Lin PY, Chang CH, Chong MF, Chen H, Su KP. Polyunsaturated fatty acids in perinatal depression: a systematic review and meta-analysis. Biol Psychiatry. 2017;82(8):560-569. doi: 10.1016/j.biopsych.2017.02.1182 [DOI] [PubMed] [Google Scholar]
- 24.Song C, Li X, Leonard BE, Horrobin DF. Effects of dietary omega-3 or n-6 fatty acids on interleukin-1beta-induced anxiety, stress, and inflammatory responses in rats. J Lipid Res. 2003;44(10):1984-1991. doi: 10.1194/jlr.M300217-JLR200 [DOI] [PubMed] [Google Scholar]
- 25.Song C, Li X, Kang Z, Kadotomi Y. Omega-3 fatty acid ethyl-eicosapentaenoate attenuates IL-1beta-induced changes in dopamine and metabolites in the shell of the nucleus accumbens: involved with PLA2 activity and corticosterone secretion. Neuropsychopharmacology. 2007;32(3):736-744. doi: 10.1038/sj.npp.1301117 [DOI] [PubMed] [Google Scholar]
- 26.Yamada D, Takeo J, Koppensteiner P, Wada K, Sekiguchi M. Modulation of fear memory by dietary polyunsaturated fatty acids via cannabinoid receptors. Neuropsychopharmacology. 2014;39(8):1852-1860. doi: 10.1038/npp.2014.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross BM. Omega-3 polyunsaturated fatty acids and anxiety disorders. Prostaglandins Leukot Essent Fatty Acids. 2009;81(5-6):309-312. doi: 10.1016/j.plefa.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 28.Green P, Hermesh H, Monselise A, Marom S, Presburger G, Weizman A. Red cell membrane omega-3 fatty acids are decreased in nondepressed patients with social anxiety disorder. Eur Neuropsychopharmacol. 2006;16(2):107-113. doi: 10.1016/j.euroneuro.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 29.Liu JJ, Galfalvy HC, Cooper TB, et al. . Omega-3 polyunsaturated fatty acid (PUFA) status in major depressive disorder with comorbid anxiety disorders. J Clin Psychiatry. 2013;74(7):732-738. doi: 10.4088/JCP.12m07970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalinić D, Borovac Štefanović L, Jerončić A, Mimica N, Dodig G, Delaš I. Eicosapentaenoic acid in serum lipids could be inversely correlated with severity of clinical symptomatology in Croatian war veterans with posttraumatic stress disorder. Croat Med J. 2014;55(1):27-37. [PubMed] [Google Scholar]
- 31.de Vries G-J, Mocking R, Lok A, Assies J, Schene A, Olff M. Fatty acid concentrations in patients with posttraumatic stress disorder compared to healthy controls. J Affect Disord. 2016;205:351-359. doi: 10.1016/j.jad.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 32.Matsuoka Y, Nishi D, Hamazaki K. Serum levels of polyunsaturated fatty acids and the risk of posttraumatic stress disorder. Psychother Psychosom. 2013;82(6):408-410. doi: 10.1159/000351993 [DOI] [PubMed] [Google Scholar]
- 33.Fux M, Benjamin J, Nemets B. A placebo-controlled cross-over trial of adjunctive EPA in OCD. J Psychiatr Res. 2004;38(3):323-325. doi: 10.1016/S0022-3956(03)00077-3 [DOI] [PubMed] [Google Scholar]
- 34.Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma omega-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):568-575. doi: 10.1016/j.pnpbp.2007.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haberka M, Mizia-Stec K, Mizia M, et al. . Effects of omega-3 polyunsaturated fatty acids on depressive symptoms, anxiety and emotional state in patients with acute myocardial infarction. Pharmacol Rep. 2013;65(1):59-68. [DOI] [PubMed] [Google Scholar]
- 36.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25(8):1725-1734. doi: 10.1016/j.bbi.2011.07.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. 2015;(11):CD004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bozzatello P, Brignolo E, De Grandi E, Bellino S. Supplementation with omega-3 fatty acids in psychiatric disorders: a review of literature data. J Clin Med. 2016;5(8):E67. doi: 10.3390/jcm5080067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jadad AR, Moore RA, Carroll D, et al. . Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1-12. doi: 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 41.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 42.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 44.Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. 1999;8(47):15-17. [Google Scholar]
- 45.Davey J, Turner RM, Clarke MJ, Higgins JP. Characteristics of meta-analyses and their component studies in the Cochrane Database of Systematic Reviews: a cross-sectional, descriptive analysis. BMC Med Res Methodol. 2011;11:160. doi: 10.1186/1471-2288-11-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe N, Matsuoka Y, Kumachi M, Hamazaki K, Horikoshi M, Furukawa TA. Omega-3 fatty acids for a better mental state in working populations—Happy Nurse Project: a 52-week randomized controlled trial. J Psychiatr Res. 2018;102:72-80. doi: 10.1016/j.jpsychires.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 48.Cornu C, Mercier C, Ginhoux T, et al. . A double-blind placebo-controlled randomised trial of omega-3 supplementation in children with moderate ADHD symptoms. Eur Child Adolesc Psychiatry. 2018;27(3):377-384. doi: 10.1007/s00787-017-1058-z [DOI] [PubMed] [Google Scholar]
- 49.Matsuoka Y, Nishi D, Hamazaki K, et al. . Docosahexaenoic acid for selective prevention of posttraumatic stress disorder among severely injured patients: a randomized, placebo-controlled trial. J Clin Psychiatry. 2015;76(8):e1015-e1022. doi: 10.4088/JCP.14m09260 [DOI] [PubMed] [Google Scholar]
- 50.Bellino S, Bozzatello P, Rocca G, Bogetto F. Efficacy of omega-3 fatty acids in the treatment of borderline personality disorder: a study of the association with valproic acid. J Psychopharmacol. 2014;28(2):125-132. doi: 10.1177/0269881113510072 [DOI] [PubMed] [Google Scholar]
- 51.Cohen LS, Joffe H, Guthrie KA, et al. . Efficacy of omega-3 for vasomotor symptoms treatment: a randomized controlled trial. Menopause. 2014;21(4):347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pomponi M, Loria G, Salvati S, et al. . DHA effects in Parkinson disease depression. Basal Ganglia. 2014;4(2):61-66. doi: 10.1016/j.baga.2014.03.004 [DOI] [Google Scholar]
- 53.Widenhorn-Müller K, Schwanda S, Scholz E, Spitzer M, Bode H. Effect of supplementation with long-chain ω-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): a randomized placebo-controlled intervention trial. Prostaglandins Leukot Essent Fatty Acids. 2014;91(1-2):49-60. doi: 10.1016/j.plefa.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 54.Nishi D, Koido Y, Nakaya N, et al. . PTSD and the attenuating effects of fish oils: results of supplementation after the 2011 great east Japan earthquake In: Durbano F, ed. New Insights Into Anxiety Disorders. London, United Kingdom: InTechOpen; 2013:407-425. doi: 10.5772/52134 [DOI] [Google Scholar]
- 55.Sauder KA, Skulas-Ray AC, Campbell TS, Johnson JA, Kris-Etherton PM, West SG. Effects of omega-3 fatty acid supplementation on heart rate variability at rest and during acute stress in adults with moderate hypertriglyceridemia. Psychosom Med. 2013;75(4):382-389. doi: 10.1097/PSY.0b013e318290a107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sohrabi N, Kashanian M, Ghafoori SS, Malakouti SK. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial.” Complement Ther Med. 2013;21(3):141-146. doi: 10.1016/j.ctim.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 57.Gabbay V, Babb JS, Klein RG, et al. . A double-blind, placebo-controlled trial of ω-3 fatty acids in Tourette’s disorder. Pediatrics. 2012;129(6):e1493-e1500. doi: 10.1542/peds.2011-3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freund-Levi Y, Basun H, Cederholm T, et al. . Omega-3 supplementation in mild to moderate Alzheimer’s disease: effects on neuropsychiatric symptoms. Int J Geriatr Psychiatry. 2008;23(2):161-169. doi: 10.1002/gps.1857 [DOI] [PubMed] [Google Scholar]
- 59.Rogers PJ, Appleton KM, Kessler D, et al. . No effect of omega-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421-431. doi: 10.1017/S0007114507801097 [DOI] [PubMed] [Google Scholar]
- 60.van de Rest O, Geleijnse JM, Kok FJ, et al. . Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2008;88(3):706-713. doi: 10.1093/ajcn/88.3.706 [DOI] [PubMed] [Google Scholar]
- 61.Yehuda S, Rabinovitz S, Mostofsky DI. Mixture of essential fatty acids lowers test anxiety. Nutr Neurosci. 2005;8(4):265-267. doi: 10.1080/10284150500445795 [DOI] [PubMed] [Google Scholar]
- 62.Chang JP, Guu TW, Chen YC, Gałecki P, Walczewska A, Su KP. BanI polymorphism of cytosolic phospholipase A2 gene and somatic symptoms in medication-free acute depressed patients. Prostaglandins Leukot Essent Fatty Acids. 2017;S0952-3278(16):30155-30157. [DOI] [PubMed] [Google Scholar]
- 63.Su K-P, Huang S-Y, Peng C-Y, et al. . Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry. 2010;67(6):550-557. doi: 10.1016/j.biopsych.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang JP, Lai HC, Yang HT, et al. . Polyunsaturated fatty acids levels and initial presentation of somatic symptoms induced by interferon-alpha therapy in patients with chronic hepatitis C viral infection. Nutr Neurosci. 2017;20(5):291-296. doi: 10.1080/1028415X.2015.1123378 [DOI] [PubMed] [Google Scholar]
- 65.Carlezon WA Jr, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry. 2005;57(4):343-350. doi: 10.1016/j.biopsych.2004.11.038 [DOI] [PubMed] [Google Scholar]
- 66.Levant B, Radel JD, Carlson SE. Reduced brain DHA content after a single reproductive cycle in female rats fed a diet deficient in OMEGA-3 polyunsaturated fatty acids. Biol Psychiatry. 2006;60(9):987-990. doi: 10.1016/j.biopsych.2005.12.013 [DOI] [PubMed] [Google Scholar]
- 67.Freeman MP. Omega-3 fatty acids and perinatal depression: a review of the literature and recommendations for future research. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4-5):291-297. doi: 10.1016/j.plefa.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 68.Brookes KJ, Chen W, Xu X, Taylor E, Asherson P. Association of fatty acid desaturase genes with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;60(10):1053-1061. doi: 10.1016/j.biopsych.2006.04.025 [DOI] [PubMed] [Google Scholar]
- 69.McNamara RK, Hahn CG, Jandacek R, et al. . Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62(1):17-24. doi: 10.1016/j.biopsych.2006.08.026 [DOI] [PubMed] [Google Scholar]
- 70.Bentsen H, Solberg DK, Refsum H, et al. . Bimodal distribution of polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. Biol Psychiatry. 2011;70(1):97-105. doi: 10.1016/j.biopsych.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 71.Müller CP, Reichel M, Mühle C, Rhein C, Gulbins E, Kornhuber J. Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta. 2015;1851(8):1052-1065. doi: 10.1016/j.bbalip.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 72.Hashimoto M, Maekawa M, Katakura M, Hamazaki K, Matsuoka Y. Possibility of polyunsaturated fatty acids for the prevention and treatment of neuropsychiatric illnesses. J Pharmacol Sci. 2014;124(3):294-300. doi: 10.1254/jphs.13R14CP [DOI] [PubMed] [Google Scholar]
- 73.Grosso G, Pajak A, Marventano S, et al. . Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9(5):e96905. doi: 10.1371/journal.pone.0096905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hallahan B, Ryan T, Hibbeln JR, et al. . Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry. 2016;209(3):192-201. doi: 10.1192/bjp.bp.114.160242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin CR, Blanco PG, Keach JC, et al. . The safety and efficacy of oral docosahexaenoic acid supplementation for the treatment of primary sclerosing cholangitis—a pilot study. Aliment Pharmacol Ther. 2012;35(2):255-265. doi: 10.1111/j.1365-2036.2011.04926.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Excluded Studies and Reasons
eTable. Study Design and Jadad Scores of Recruited Studies
eFigure 1. Whole Flowchart of Current Meta-Analysis
eFigure 2. Funnel Plot of Changes in Anxiety Symptoms in Patients With and Without n-3 PUFA Treatment
eFigure 3. Subgroup MA of Anxiolytic Effect Based Upon Placebo Controlled or Non–Placebo Controlled Design