Abstract
Background:
Methamphetamine (METH) use poses a barrier to antiretroviral therapy (ART) adherence. We evaluated the efficacy of the individualized texting for adherence building (iTAB) intervention among persons living with HIV (PLWH) who meet criteria for METH use disorder. We examined daily associations between ART adherence and text-reported METH use and depressed mood.
Methods:
We conducted a single site, 2-arm, 6-week, pilot randomized clinical trial comparing a personalized, bidirectional, text messaging system (iTAB; n=50) to an active control condition (n=25). All participants received adherence psychoeducation and daily texts assessing METH use and depressed mood. The iTAB group received personalized daily ART reminder texts. ART adherence was monitored using Medication Event Monitoring System (MEMS) caps.
Results:
Response rates to daily ART reminder texts were high (79%), with good concordance between MEMS-derived and text-reported ART adherence (p<.001). Intervention groups did not differ in MEMS-derived ART adherence (68% iTAB, 70% active control; p=.68); however, participants in the iTAB group had fewer METH use days (median 14.4 iTAB, 22.0 active control; p=.05). Text-reported METH use, but not depressed mood, was associated with poorer MEMS-derived ART adherence.
Conclusions:
High text response rates and good concordance between MEMS-derived and text-reported adherence suggests text messaging is a feasible intervention delivery approach that provides a valid indication of ART adherence. Reductions in METH use among iTAB participants suggest daily health reminders may help attenuate substance use. Further research is needed to substantiate daily text messaging as a harm reduction approach.
Keywords: Medication Adherence, HIV/AIDS, Methamphetamine, Stimulant, mHealth, Behavior Modification
1. Introduction
Antiretroviral therapy (ART) is the standard of care for persons living with HIV infection (PLWH). Suboptimal ART adherence can lead to disease progression and development of drug-resistant strains of HIV (Flandre et al., 2002; Nachega et al., 2011). Persons with substance use disorders demonstrate poorer adherence to ART compared to those without substance use disorders (Hinkin et al., 2004; Meade et al., 2011; Westergaard et al., 2012). Methamphetamine (METH) use is particularly concerning in the context of HIV care, given its high prevalence among PLWH, particularly in San Diego (Wagner, 2014), and its association with poor ART adherence (Hinkin et al., 2007; Marquez et al., 2009; Moore et al., 2012).
Text messaging is a potentially effective and low-cost means of both assessing medication adherence using ecological momentary assessment (EMA) and delivering medication adherence interventions (Horvath et al., 2012). High rates of concordance have been observed in self-reported ART adherence obtained via two-way text messaging and ART adherence assessed by electronic pill bottle caps (Harris et al., 2010). Thus, we developed the Individualized Texting for Adherence Building (iTAB) intervention, an automated, personalized, bidirectional, text messaging system that draws on aspects of the Health Belief Model (HBM), Theory of Planned Behavior (TPB), and Social Cognitive Theory (SCT) (Bandura, 2001; Becker, 1974; Godin & Kok, 1996). In particular, the iTAB message content was structured to fall into domains (e.g., social support, self-esteem) addressing individual and interpersonal factors that may influence ART adherence. Message content was designed to promote self-efficacy and reciprocal determinism (SCT), support adherence behavioral intentions (TPB), and promote the perception of benefits derived from engaging in the target health behavior (HBM). The iTAB intervention demonstrated efficacy for improving ART adherence with respect to dose timing in a study involving PLWH with bipolar disorder (Moore et al., 2015). Other studies have used text messaging to deliver intervention content to support health behaviors among PLWH with comorbid substance use disorders (Glasner-Edwards et al., 2016; Ingersoll et al., 2015).
Based on growing evidence indicating the efficacy of text message interventions to support ART adherence (Pellowski & Kalichman, 2012) and need to better understand daily factors of nonadherence among substance users, the goal of the present study was to evaluate the iTAB intervention among PLWH who use METH. We hypothesized that participants assigned to iTAB (i.e., standard-of-care adherence psychoeducation, daily ART reminder texts, and daily texts assessing METH use and presence of depressed mood) would show better ART adherence rates as compared to participants in the active control condition (i.e., same components as iTAB except the daily ART reminder texts). We also examined whether METH use and depressed mood were related to ART adherence, and we hypothesized that a higher frequency of METH use and presence of depressed mood would correlate with poorer ART adherence among participants in both the active control and iTAB conditions. Secondary analyses examined the frequency of METH use between study arms.
2. Methods
2.1. Trial design
The study was a two-armed, parallel-design pilot randomized clinical trial (RCT). Target enrollment was 75 participants. With a randomization strategy of 2:1 to the iTAB intervention (n = 50) and active control arm (n = 25), we had 80% power to detect an effect size d of .71 for the difference in adherence rates between the two intervention arms (Wilcoxon-Mann-Whitney critical t = 1.99, df = 69.6). The unbalanced design was selected to maximize ability to investigate data within the iTAB condition. The intervention period was six weeks with in-person assessments occurring at pre- and post-intervention. This study length was chosen as an adequate amount of time to assess changes in viral load, based on a published recommendation for measurement of plasma viral load following initiation or modification of ART regimen (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2011). The local Human Research Protection Program approved the current study.
2.2. Participants
Participants were recruited from ongoing studies of HIV infection and substance use conducted at the University of California, San Diego – HIV Neurobehavioral Research Program from November 2011 to May 2014. Inclusion criteria were the capacity to provide informed consent, age 18 years or older at enrollment, documented HIV infection, DSM-IV-TR diagnosis of METH abuse or dependence via the Composite International Diagnostic Interview (World Health Organization, 1997), self-reported METH use within 45 days of baseline, and an active prescription for ART to treat HIV. Plasma HIV viral load detectability was not an inclusionary criterion. Participants with other comorbid substance use conditions were deemed eligible if they met recruitment criteria. Participants had to be willing to respond to text messages and utilize electronic medication tracking devices (i.e., Medication Event System Monitoring TrackCaps, MEMS, AARDEX, Sion, Switzerland). Exclusion criteria were minimal (i.e., unable to show capability of responding to texts at baseline by direction observation) to enhance generalizability.
After meeting study inclusion requirements, interested participants provided written informed consent. Participants were informed that some participants would be randomly selected to receive daily ART reminder texts. Participants received monetary compensation for the initial ($50) and follow-up ($35) assessments and for returning the MEMS device ($25). Participants were reimbursed for additional costs incurred by participating in the study over their regular cell phone use. A mobile phone with a texting plan was loaned to participants who did not own a cell phone or were unable to receive texts on their current phone (n=17 iTAB and n=11 in active control arm received a loaned phone).
2.3. Intervention (iTAB)
The iTAB intervention has been described previously in detail (Moore et al., 2013; Moore et al., 2015); therefore, the following sections briefly describe the components of the RCT.
2.3.1. Personalized ART Reminder and Reinforcement Text Messages.
For this pilot RCT, iTAB was refined through focus groups to derive patient-centered intervention content for use among PLWH who use METH (Montoya et al., 2014). As a result of the focus groups, 40 ART reminder text messages that fall into eight thematic reminder stems (i.e., social support/responsibility to others, self-esteem, dangers of non-adherence, harm reduction, direct reminder, spirituality, celebration of health, and disease control) were developed.
During the first study visit, all participants selected, modified, and/or created ten personalized ART reminder text messages from the list of 40 predetermined ART reminder text messages. For participants in the active control group, these messages were printed out for participants to take with them and use as desired. The active control group did not receive daily ART reminder texts during the intervention. iTAB participants provided their preferred name and a description of their tracked ART medication to be used in the text messages. The ART reminder text was sent during the iTAB participants’ specified dosing time. An example ART reminder text might read, “John, you can have fun and take ur meds. Time 4 ur big blue pill now. Pls reply A) took D) didn’t G) snooze.”
The iTAB system had automated features designed to optimize adherence, including a “snooze” option for the ART reminder text (i.e., the reminder was re-sent one hour later). If iTAB participants indicated they did not take his or her ART, they were sent an additional text to identify the reason for the missed dose. After three consecutive days of non-responses to the ART reminder texts, the automated system sent an “adherence alert” message to the participant and study coordinator. The study coordinator called the participant after five days of failure to respond to the ART reminder texts and each subsequent five days until the participant began responding to the system or the study period closed.
iTAB participants also selected, modified, and/or created ten reinforcement text messages from a list of 20 predetermined messages (e.g., “Great job, every dose helps” and “Keep up the good work.”). The reinforcement texts were sent to reinforce events where the participant reported taking his or her ART medication. A reinforcement text might read, “Great job! Ur current adherence: 75%. Adhr when u take ur next dose: 80% (4/5 doses).”
2.3.2. Text Messages to Assess METH Use.
Both groups received a daily text asking, “Have you done anything in the past 24 hours? Y) yes N) no” to assess for METH use. To protect participants from potential legal or personal ramifications associated with disclosure of METH use, the word “methamphetamine,” or variants thereof, were not included in the texts. At the first study visit, participants were informed this daily text was in reference to METH use. It was emphasized that the response to this inquiry would not impact individuals’ participation in the study. For each participant, we calculated the responsiveness to texts assessing METH use (# of texts responded to / # of texts received) and the proportion of days a participant endorsed METH use (# of days endorsing METH use / # of texts responded to).
2.3.3. Text Messages to Assess Depressed Mood.
Both groups received a daily text assessing depressed mood and had the following response options: 0 – not at all depressed, 1 – a little depressed, 2 – moderately depressed, 3 – very depressed, and 4 – extremely depressed. For each participant, we calculated the responsiveness to texts assessing depressed mood (# of texts responded to / # of texts received) and the average severity of depressed mood endorsed during the study period (range of 0 to 4).
2.3.4. ART Adherence Psychoeducation.
All participants received up to 30-minutes of ART adherence psychoeducation individually delivered by research staff via PowerPoint at the first study visit. The ART adherence psychoeducation component presented the health benefits of adherence, potential adverse ART and METH use effects, potential adherence difficulties, and practical adherence strategies. Participants had an opportunity to ask questions and speak about their own experiences adhering to medications.
2.4. Pre-Intervention Assessments
Substance use and mood disorder diagnoses were determined using the lay-administered Composite International Diagnostic Interview (World Health Organization, 1997). Severity of current depressive symptomatology was assessed via the Beck Depression Inventory-II (Beck et al., 1996), and severity of current anxiety-related symptoms was assessed via the Beck Anxiety Inventory (Beck & Steer, 1993). Participants were administered a detailed substance use interview, recording both frequency and quantity of METH use. Each participant completed a standardized medical history interview, structured neurological and medical examination, and collection of blood and urine samples consistent with previous studies at the HNRP (Heaton et al., 2011). Trained research staff conducted all medical history interviews, and a clinician (i.e., RN, NP or MD) performed the neuromedical examination
2.5. Primary Outcome: ART Adherence
2.5.1. Objective Medication Adherence.
MEMS TrackCaps, which provide an electronic record of the date and time the cap is removed, were used to track ART adherence during the study period. Two primary outcome variables were derived from the MEMS data:
2.5.2. Overall MEMS Adherence.
One of the primary outcome variables for analysis was overall MEMS adherence over the study period. In analyses assessing between-group differences (iTAB vs. active control), overall MEMS Adherence was defined as the percentage of taken doses (i.e., ([# of bottle openings)/ (# of prescribed doses)] *100%). In multilevel models examining daily adherence, overall MEMS adherence was a binary variable that denoted the occurrence (or non-occurrence) of MEMS Trackcap openings.
2.5.3. MEMS Adherence Based on Dose Timing.
The second primary outcome variable for examining between-group differences was MEMS adherence based on dose timing (i.e., [(# of bottle openings within a ± two-hour time window of the intended dosing time)/ (# of prescribed doses)] *100%). In multilevel models examining daily adherence, MEMS adherence based on dose timing was a binary variable that indicated whether or not MEMS Trackcap openings occurred within two hours of intended dosing times.
2.6. Randomization
Prior to study enrollment, a series of participant ID’s were randomly assigned to the iTAB or active control condition by a statistician. Research associates providing the psychoeducational component of the study were blinded to these assignments. A separate research staff member kept the randomization code and informed the participant of their assignment after provision of the adherence psychoeducation (but ahead of the set-up of daily text messages).
2.7. Data Analytic Strategy
Group differences between iTAB and active control participants on MEMS adherence and responsiveness to texts were examined using non-parametric methods (Wilcoxon Rank-Sum test). Two iTAB participants had brief periods when they were unable to respond to texts (e.g., incarceration), and data from these days were treated as missing rather than as non-adherent.
To account for within-subject correlations that are inherent to repeated measurements and to examine daily relationships between text responses and adherence outcomes, multilevel models were used (Bolger & Laurenceau, 2013). These analyses were carried out in Mplus v7.4 (Muthén & Muthén, 1998–2012) using robust maximum likelihood estimation, which provides standard errors for parameters that are robust to non-normality and missing data; non-response to text messages was considered as missing and modeled under standard missing data assumptions (Yuan & Bentler, 2000).
3. Results
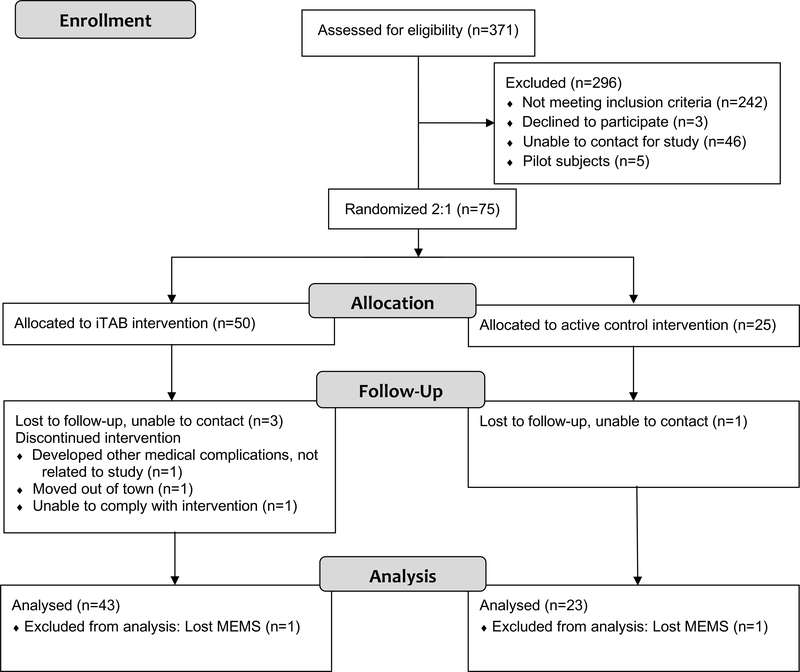
Figure 1 provides a study CONSORT diagram detailing study enrollment and retention (Schulz et al., 2010). The demographic and clinical characteristics of the 66 study participants who completed follow-up and had analyzable MEMS adherence data are provided in Table 1.
Figure 1.
CONSORT 2010 Flow Diagram
Table 1.
Baseline participant characteristics by intervention arm (N = 66)
| iTAB (n = 43) | Active Control (n = 23) | p-value | Effect size | |
|---|---|---|---|---|
| Descriptive | ||||
| Age; mean (SD) | 45.4 (7.7) | 46.0 (8.9) | .78 | g = −0.07 |
| Years of education; mean (SD) | 13.1 (2.8) | 13.7 (2.5) | .45 | g = −0.20 |
| Male; n (%) | 40 (93.0%) | 23 (100%) | .55 | - |
| Sexual orientation | .32 | |||
| Gay/bisexual; n (%) | 37 (86.0%) | 17 (73.9%) | OR = 2.18 | |
| Heterosexual; n (%) | 6 (14.0%) | 6 (26.1%) | OR = 0.46 | |
| Race/Ethnicity | .04 | |||
| Non-Hispanic white; n (%) | 23 (53.5%) | 7 (30.4%) | OR = 2.63 | |
| Black; n (%) | 15 (34.9%) | 7 (30.4%) | OR = 1.22 | |
| Hispanic; n (%) | 5 (11.6%) | 8 (34.8%) | OR = 0.25 | |
| Pacific Islander; n (%) | 0 (0.0%) | 1 (4.3%) | - | |
| Employed; n (%) | 15 (34.9%) | 2 (8.7%) | .04 | OR = 5.63 |
| HCV infected; n (%) | 10 (23.3%) | 4 (17.4%) | .75 | OR = 1.44 |
| HIV Disease Characteristics | ||||
| Estimated duration of HIV (years); median [IQR] a | 10.6 [4.4, 19.6] | 13.5 [4.7, 19.7] | .64 | g = −0.10 |
| AIDS; n (%) b | 25 (62.5%) | 13 (56.5%) | .64 | OR = 1.28 |
| CD4 Count; median [IQR] a | 595 [375, 775] | 479 [178, 605] | .07 | g = 0.49 |
| Nadir CD4 Count; median [IQR] c | 167 [42, 360] | 196 [12, 280] | .48 | g = 0.22 |
| Undetectable plasma viral load (<50 cp/ml); n (%) c | 31 (73.8%) | 14 (63.6%) | .40 | OR = 1.61 |
| Antiretroviral therapy (ART) | ||||
| Prescribed once daily regimen; n (%) | 41 (95.4%) | 21 (91.3%) | .61 | OR = 1.95 |
| Months on current regimen; median [IQR] d | 25.5 [11.9, 50.3] | 17.9 [5.0, 44.5] | .19 | g = 0.36 |
| Months on any ART regimen; median [IQR] b | 75.9 [33.8, 134.1] | 93.6 [49.7, 177.0] | .31 | g = −0.27 |
| Methamphetamine (METH) Use Characteristics | ||||
| Age of first use; median [IQR] e | 23.0 [15.8, 27.8] | 16.5 [9.5, 23.5] | .09 | g = 0.34 |
| Days since last use; median [IQR] f | 4.5 [2.0, 20.8] | 4.0 [1.5, 14.0] | .75 | g = 0.10 |
| Quantity (grams) consumed in past year; median | ||||
| [IQR] d | 8.4 [2.6, 39.3] | 9.8 [1.5, 41.5] | .90 | g = 0.13 |
| Days of use in past year; median [IQR] d | 24.0 [11.0, 52.6] | 19.9 [4.3, 104.2] | .63 | g = 0.34 |
| History of treatment for METH use; # (%) | 33 (76.7%) | 17 (73.9%) | 1.00 | OR = 1.16 |
| Psychiatric | ||||
| BAI Total; median [IQR] | 26 [23, 38] | 27 [22, 32] | .48 | g = 0.32 |
| BDI-II Total; median [IQR] | 13 [3, 19] | 14 [1, 21] | .61 | g = 0.15 |
| Lifetime MDD; # (%) | 29 (67.4%) | 14 (60.9%) | .60 | OR = 1.33 |
| Current MDD; # (%) | 9 (20.9%) | 0 (0%) | .02 | - |
| ADHD; # (%) c | 7 (16.7%) | 0 (0%) | .09 | - |
| ASPD; # (%) c | 14 (33.3%) | 5 (22.7%) | .57 | OR = 1.70 |
Note: Nadir CD4 count is calculated as the lowest of self-reported or laboratory generated value. Effect size compared iTAB to active control. BAI = Beck Anxiety Inventory; BDI-II = Beck Depression Inventory-II; HCV = Hepatitis C Virus; g = hedge’s g, which is based on standardized mean difference; IQR = interquartile range; MDD = Major Depressive Disorder; OR = odds ratio.
n=50
n=63
n=64
n=65
n=58
n=61
3.1. MEMS Adherence by Intervention Arm
We examined MEMS-derived ART adherence by intervention arm and found the iTAB and active control groups did not significantly differ on the MEMS adherence outcome variables (Table 2). Given the intervention arms differed on race/ethnicity, employment status, and proportion meeting criteria for current major depressive disorder (p’s < 0.05), we explored the relationship between these covariates and overall MEMS adherence within the whole sample. Of the three covariates, only employment status was associated with overall MEMS adherence, such that those with employment achieved higher adherence rates (Median: 84.0% [72.6%, 90.5%]) compared to unemployed participants (Median: 69.0% [34.5%, 90.1%]; Z = 2.59, p = 0.01). The interaction of intervention group and employment status was not significantly associated with overall MEMS adherence (p > 0.05).
Table 2:
Between-group differences in MEMS adherence (N = 66)
| iTAB | Active Control | Difference Test | Effect Size | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Wilcoxon-Rank Statistic | ||
| Overall MEMS Adherence (%) | 68.0 (0.3) | 70.4 (0.3) | Z = 0.42, p = 0.68 | r = 0.05 |
| MEMS dose timing (mins) | 181.0 (133.1) | 195.2 (129.9) | Z = 0.51, p = 0.61 | r = 0.06 |
| MEMS adherence based on dose timing (%) | 43.9 (32.2) | 45.5 (30.5) | Z = 0.06, p = 0.95 | r = 0.01 |
Note. The effect size, r, was calculated as Z/sqrt(n) (Rosenthal, 1991)
We examined the association of HIV disease indicators and MEMS-derived ART adherence outcomes. Neither estimated duration of HIV nor current CD4 count were significantly associated with overall MEMS adherence or MEMS adherence based on dose timing (p’s > 0.05). AIDS status was significantly associated with overall MEMS adherence (t (62) = 2.4, p = 0.02, g = −0.61) but not with MEMS adherence based on dose timing (p > 0.05). Having an undetectable plasma viral load was significantly positively associated with overall MEMS adherence (t (63) = 3.3, p = 0.002, g = −0.89) and MEMS adherence based on dose timing [Welch’s t (45.4) = 3.9, p < 0.01, g = −0.94].
3.2. Responsiveness to Text Messages by Intervention Arm
iTAB group participants were sent a median of 42 ART reminder texts. Both iTAB and active control groups received a median of 42 texts assessing METH use and depressed mood across the 42-day study period. iTAB participants responded to a median of 78.6% [64.3%, 90.5%] ART reminder texts. There were no significant differences in the responsiveness rates to METH (iTAB: Median = 79.8% [69.05%, 91.6%] vs. active control: Median = 83.3% [70.0%, 92.7%]; Z = 0.56, p = 0.57) or mood (iTAB: Median = 83.3% [71.4%, 92.9%] vs. active control: Median = 88.1% [69.1%, 90.5%]; Z = 0.10, p = 0.92) texts. Regarding text-reported METH use, participants in the iTAB group reported fewer days of METH use than participants in the active control group (iTAB: Median = 14.4 [0, 33.6] vs. active control: Median: 22.0 [7.3, 65.6]; Z = −1.97, p = 0.05). Severity of depressed mood responses did not differ by intervention arm (iTAB: Median = 0.33 [0.07, 0.93] vs. active control: Median: 0.48 [0.04, 0.86]; Z = −0.09, p = 0.92).
3.3. Daily MEMS adherence and daily ART text responses
Within the iTAB group, the most common response to ART reminder text was ‘Took’, at 68.4%, followed by a non-response at 23.9%, ‘Snooze’ at 5.4%, and ‘Didn’t Take’ at 2.3%. The relationship between MEMS adherence and text-reported ART adherence was examined in correlations and in multilevel regression models that took into account the effect of progression of time in the study. The correlation between overall MEMS adherence and text-reported ART adherence was significant at the within-person (r = 0.19, p < .001) and between-person levels (r = 0.44, p < .001). Similarly, the correlation between MEMS adherence based on dose timing and text-reported ART adherence was significant at the within-person (r = 0.13, p < .001) and between-person levels (r = 0.46, p < .001). When these relationships were examined in multilevel models accounting for time (Table 3), text-reported ART adherence was significantly associated with overall MEMS adherence (B = 2.12, OR = 8.31, p < .001) and MEMS adherence based on dose timing (B = 2.86, OR = 17.48, p = .001). This was such that, on a day on which a participant text-reported ART adherence, he/she had greater odds of a MEMS cap opening and greater odds of the cap opening occurring within 2 hours of his/her dosing time. Associations between MEMS outcomes and text-reported ART adherence were similar at the between-person level such that individuals with a higher rates of text-reported ART adherence had significantly greater overall MEMS adherence (B = 6.27, p = .023) and significantly greater MEMS adherence based on dose timing (B = 8.50, p = .001).
Table 3.
Multilevel Models of Daily ART Text Responses Predicting Daily MEMS Adherence among Participants in the iTAB Arm (n = 43)
| Effect | Odds Ratio | Estimate | Standard Error | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Outcome: MEMS overall adherence | |||||
| Within-person level | |||||
| Text Response | 8.306 | 2.117*** | 0.501 | 1.135 | 3.099 |
| Study day | 0.960 | −0.041*** | 0.009 | −0.059 | −0.024 |
| Between-person level | |||||
| Text Response | - | − 6.269* | 2.753 | 0.874 | 11.665 |
| Outcome: MEMS adherence based on dose timing | |||||
| Within-person level Text Response | |||||
| Text Response | 17.479 | 2.861** | 0.827 | 1.240 | 4.482 |
| Study day | 0.966 | −0.035*** | 0.007 | −0.049 | −0.021 |
| Between-person level | |||||
| Text Response | - | 8.501** | 2.480 | 3.639 | 13.362 |
Note.
p <.10.
p < .05.
p < .01.
p < .001.
3.4. Daily MEMS adherence and daily reports of METH use and depressed mood
We examined associations between MEMS adherence outcomes and text-reported METH use and depressed mood during the study period. Among iTAB participants, text-reported METH use was significantly correlated with lower overall MEMS adherence (r= −0.07, p = 0.03) and lower MEMS adherence based on dose timing (r = −0.05, p = 0.04) at the within-person level, indicating that when individuals reported METH use via text, they were generally non-adherent. At the between-person level, text-reported METH use was marginally associated with MEMS adherence based on dose timing (r = −0.30, p = 0.08), indicating that participants who report METH use also report poorer adherence. Among active control participants, text-reported METH use was significantly correlated with overall MEMS adherence and MEMS adherence based on dose timing at the between-person level only (r = −0.46, p = 0.01 and r = −0.52, p < 0.001). No significant correlations were observed between MEMS adherence outcomes and text-reported severity of depressed mood among either iTAB or active control participants.
When examined in multilevel regression models (Table 4), at the within-person level, text-reported METH use was marginally associated with a decreased same day probability of MEMS adherence based on dose timing (B = −0.26, OR = 0.77, p = .08), above and beyond concurrent depressed mood severity. No significant daily associations were found between text-reported METH use and overall MEMS adherence, controlling for text-reported depressed mood. At the between-person level, individuals with greater reports of METH use tended to have poorer overall MEMS adherence (B = −2.08, p = .01) and poorer MEMS adherence based on dose timing (B = −2.57, p = .002). No significant between-person associations were found for text-reported depressed mood, above the effect of METH use, group assignment, and time.
Table 4.
Multilevel Models of Daily Meth and Depressed Mood Text Responses Predicting Daily MEMS Adherence (N = 66)
| Effect | Odds Ratio | Estimate | Standard Error | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Outcome: MEMS overall adherence | |||||
| Within-person level | |||||
| METH | 0.809 | −0.212 | 0.207 | −0.617 | 0.193 |
| Mood | 0.964 | −0.037 | 0.102 | −0.236 | 0.163 |
| Study day | 0.969 | −0.032*** | 0.008 | −0.047 | −0.016 |
| Between-person level | |||||
| METH | - | −2.081** | 0.791 | −3.631 | −0.532 |
| Mood | - | 0.182 | 0.262 | −0.332 | 0.696 |
| Group | - | −0.606 | 0.470 | −1.527 | 0.315 |
| Outcome: MEMS adherence based on dose timing | |||||
| Within-person level | |||||
| METH | 0.775 | −0.255† | 0.145 | −0.540 | 0.030 |
| Mood | 0.879 | −0.129 | 0.089 | −0.303 | 0.046 |
| Study day | 0.975 | −0.025*** | 0.006 | −0.037 | −0.012 |
| Between-person level | |||||
| METH | - | −2.566** | 0.826 | −4.185 | −0.947 |
| Mood | - | 0.409 | 0.261 | −0.102 | 0.919 |
| Group | - | 0.572 | 0.505 | −1.562 | 0.419 |
Note. N = 66 individuals (23 active control and 43 intervention).
p <.10.
p < .05.
p < .01.
p < .001.
4. Discussion
The present study examined the efficacy of an automated, personalized, bidirectional, text messaging system to support ART adherence among PLWH with recent and ongoing METH use. The iTAB group did not significantly differ from the active control group in overall rates of ART adherence based on monitored pill cap openings; however, participants in the iTAB group reported using METH on fewer days during the intervention period. For the iTAB group, we observed a daily association between text-reported ART adherence and MEMS adherence. Although previous studies have found good concordance between self-reports of adherence and MEMS adherence, these studies have used global retrospective recalls of adherence or daily average text-reported adherence, rather than daily correlations between the two (e.g., Harris et al., 2010; Walsh et al., 2002). Thus, this study is the first to report significant day-day within-person associations between text-reported adherence and MEMS adherence. Last, for the combined pool of iTAB and active control participants, we observed a daily association between text-reported METH use and poorer ART adherence. Overall, this study supports the feasibility of using text messaging to explore the contemporaneous relationship between METH use and ART adherence.
Contrary to the primary hypothesis, the iTAB and active control groups did not significantly differ in their levels of MEMS-derived ART adherence during the study period. Furthermore, intervention arms did not differ in post-study HIV disease characteristics related to ART adherence (i.e., CD4 count and viral load). This lack of a significant effect of daily ART reminder texts and related outcomes in the iTAB group may be the result of our active control condition, which involved psychoeducation and receipt of daily texts assessing METH use and depressed mood. Many of the bidirectional-texting studies for ART adherence that have shown significant effects had passive control conditions without daily texts (e.g., Ingersoll et al., 2015; Finitsis et al., 2014). Results from a recent meta-analysis suggest mobile health interventions with active control conditions generally yield smaller effect sizes than interventions without active control conditions (Firth et al., 2017). Indeed, during debriefing, some participants in the active control arm reported the daily METH use and depressed mood prompts served as daily reminders for ART adherence, which is consistent with participant feedback from a previous study indicating EMA prompts increase self-awareness of behaviors (Freedman et al., 2006).
We hypothesized that METH use and depressed mood would negatively impact ART adherence. We found that METH use was associated with a decrease in probability of on-time ART adherence on the same day, above and beyond depressed mood. Individuals with greater METH use across the study period tended to be those with poorer adherence. These results, showing daily and adverse effects of METH use on adherence, confirm prior research that has shown day specific effects of METH use on ART adherence (Parsons et al., 2013). Interestingly, we observed that participants in the iTAB arm reported fewer days of METH use during the intervention period than those in the active control arm. A possible reason underlying the lower rates of METH use among iTAB participants may be that the daily texts, generally rooted in models of health behavior change, may have promoted greater self-efficacy and awareness of substance use. Thus, although this study did not directly aim to intervene on METH use, these results suggest the possibility that daily reminders to attend to one’s health (e.g., adhering to a medication regimen) may moderate substance use. Additional intervention studies utilizing a harm reduction approach are needed to substantiate this possibility.
Some limitations of this study should be noted. Although MEMS caps are considered to be a gold standard method of assessing medication adherence, their use has some drawbacks. MEMS caps may be potentially inaccurate when individuals use other methods of medication storage (e.g., pillboxes), and use of MEMS caps as a monitoring tool may alter participants’ adherence behaviors. Given the high correlation between text-reported ART adherence and MEMS data among iTAB participants, utilization of the MEMS cap likely approximated actual medication-taking behavior. Although text message content variability and personalization were provided to participants, we hypothesize that greater variability in message content may enhance novelty. Future studies may want to consider greater content variability and text-message systems capable of adaptively responding to the nuances of participant responses. Despite these limitations, results suggest the possibility of modest to good engagement in text messaging interventions among individuals with active substance use.
In summary, this study sought to examine the effect of a bidirectional text messaging system on ART adherence among PLWH with comorbid METH use. Although no between-group differences were found between the intervention and active control group, we found the intervention to be feasible and to have good participant response rates. Additionally, participants in the iTAB group had fewer reported days of METH use than those in the active control group. Furthermore, METH use was observed to have deleterious daily and cumulative effects of on ART adherence, underscoring the necessity of harm reduction as a concomitant goal in interventions to improve medication adherence among those with active substance use.
Footnotes
This trial is registered with the ClinicalTrials.gov # NCT01317277.
References
- Bandura A, 2001. Social cognitive theory: an agentic perspective. Annu Rev Psychol. 52, 1–26. [DOI] [PubMed] [Google Scholar]
- Beck AT, & Steer RA (1993). Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). BDI: Beck Depression Inventory: Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Becker MH (Ed.). (1974). The Health Belief Model and Personal Health Behavior (Vol. 2).
- Bolger N, & Laurenceau JP (2013). Intensive longitudinal methods. New York, NY: Guilford. [Google Scholar]
- Finitsis DJ, Pellowski JA, & Johnson BT, 2014. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One. 9, e88166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J, Torous J, Nicholas J, Carney R, Pratap A, Rosenbaum S, & Sarris J, 2017. The efficacy of smartphone-based mental health interventions for depressive symptoms: a meta-analysis of randomized controlled trials. World Psychiatry. 16, 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandre P, Peytavin G, Meiffredy V, Saidi Y, Descamps D, Delagnes M, … Raffi F, 2002. Adherence to antiretroviral therapy and outcomes in HIV-infected patients enrolled in an induction/maintenance randomized trial. Antivir Ther. 7, 113–121. [PubMed] [Google Scholar]
- Freedman MJ, Lester KM, McNamara C, Milby JB, & Schumacher JE, 2006. Cell phones for ecological momentary assessment with cocaine-addicted homeless patients in treatment. J Subst Abuse Treat. 30, 105–111. [DOI] [PubMed] [Google Scholar]
- Glasner-Edwards S, Patrick K, Ybarra ML, Reback CJ, Rawson RA, Chokron Garneau H, … Venegas A, 2016. A Cognitive Behavioral Therapy-Based Text Messaging Intervention Versus Medical Management for HIV-Infected Substance Users: Study Protocol for a Pilot Randomized Trial. JMIR Res Protoc. 5, e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin G, & Kok G, 1996. The theory of planned behavior: a review of its applications to health-related behaviors. Am J Health Promot. 11, 87–98. [DOI] [PubMed] [Google Scholar]
- Harris LT, Lehavot K, Huh D, Yard S, Andrasik MP, Dunbar PJ, & Simoni JM, 2010. Two-way text messaging for health behavior change among human immunodeficiency virus-positive individuals. Telemed J E Health. 16, 1024–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, … Grant I, 2011. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 17, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, … Longshore D, 2007. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 11, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, & Stefaniak M, 2004. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 18 Suppl 1, S19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath T, Azman H, Kennedy GE, & Rutherford GW, 2012. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database of Systematic Reviews 3, CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll KS, Dillingham RA, Hettema JE, Conaway M, Freeman J, Reynolds G, & Hosseinbor S, 2015. Pilot RCT of bidirectional text messaging for ART adherence among nonurban substance users with HIV. Health Psychol. 34S, 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez C, Mitchell SJ, Hare CB, John M, & Klausner JD, 2009. Methamphetamine use, sexual activity, patient-provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006. AIDS Care. 21, 575–582. [DOI] [PubMed] [Google Scholar]
- Meade CS, Conn NA, Skalski LM, & Safren SA, 2011. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. J Behav Med. 34, 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Georges S, Poquette A, Depp CA, Atkinson JH, Moore DJ, & TMARC Group., 2014. Refining a personalized mHealth intervention to promote medication adherence among HIV+ methamphetamine users. AIDS Care. 26, 1477–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, & Grant I, 2012. Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care. 24, 1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Montoya JL, Blackstone K, Rooney A, Gouaux B, Georges S, … TMARC Group, 2013. Preliminary Evidence for Feasibility, Use, and Acceptability of Individualized Texting for Adherence Building for Antiretroviral Adherence and Substance Use Assessment among HIV-Infected Methamphetamine Users. AIDS Res Treat. 2013, 585143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Poquette A, Casaletto KB, Gouaux B, Montoya JL, Posada C, … HNRP Group, 2015. Individualized texting for adherence building (iTAB): improving antiretroviral dose timing among HIV-infected persons with co-occurring bipolar disorder. AIDS Behav. 19, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2012). Mplus User’s Guide. Seventh Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nachega JB, Marconi VC, van Zyl GU, Gardner EM, Preiser W, Hong SY, … Gross R, 2011. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets. 11, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KD, 2014. Drug abuse patterns and trends in San Diego County -- Update: January 2014. National Institute on Drug Abuse. Retrieved 03/07/2018, from https://www.drugabuse.gov/about-nida/organization/workgroups-interest-groups-consortia/community-epidemiology-work-group-cewg/highlights-summaries-january-2014-reports/san-diego-county
- Panel on Antiretroviral Guidelines for Adults and Adolescents, 2011. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Retrieved 03/07/2018, from https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL002111.pdf
- Parsons JT, Kowalczyk WJ, Botsko M, Tomassilli J, & Golub SA, 2013. Aggregate versus day level association between methamphetamine use and HIV medication non-adherence among gay and bisexual men. AIDS Behav. 17, 1478–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellowski JA, & Kalichman SC, 2012. Recent advances (2011–2012) in technology-delivered interventions for people living with HIV. Curr HIV/AIDS Rep. 9, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, 1991. Meta-analytic Procedures for Social Research. Newbury Park, CA: Sage. [Google Scholar]
- Schulz KF, Altman DG, Moher D, & Group C, 2010. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 8, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JC, Mandalia S, & Gazzard BG, 2002. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 16, 269–277. [DOI] [PubMed] [Google Scholar]
- Westergaard RP, Ambrose BK, Mehta SH, & Kirk GD, 2012. Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: a survey of North American HIV providers. J Int AIDS Soc. 15, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, (1997). Composite International Diagnostic Interview (CIDI, version 2.1). Geneva, Switzerland: World Health Organization. [Google Scholar]
- Yuan KH, & Bentler PM, 2000. Three likelihoodbased methods for mean and covariance structure analysis with nonnormal missing data. Sociological methodology. 30, 165–200. [Google Scholar]