Abstract
The KardiaBand, which records a rhythm strip from an AppleWatch, was paired with an app for automated detection of atrial fibrillation. A new study by Bumgarner and colleagues is one of the first studies to examine the feasibility of using a smartwatch to discriminate between sinus rhythm and atrial fibrillation.
In a paper published in the Journal of the American College of Cardiology, Bumgarner and colleagues describe the performance of an electrocardiogram (ECG) watch band (KardiaBand, AliveCor, USA) that is connected to an AppleWatch (Apple, USA) for the detection of atrial fibrillation (AF)1. The commercially available KardiaBand was introduced in November 2017 as the first FDA- approved AppleWatch accessory for the diagnosis of AF. The device records a 30-s segment of single- lead ECG data when the user places his or her finger on the electrode embedded in the smartwatch band; these data are then transmitted via Bluetooth to a smartphone application. Bumgarner and colleagues explored the performance of the KardiaBand in a small, prospective, observational study including 100 patients with AF who were scheduled to undergo electrical cardioversion. The objective of the study was to investigate whether the smartwatch system could accurately differentiate between sinus rhythm (after cardioversion) and AF (before cardioversion) when compared with contemporaneous, physician- interpreted 12-lead ECG data.
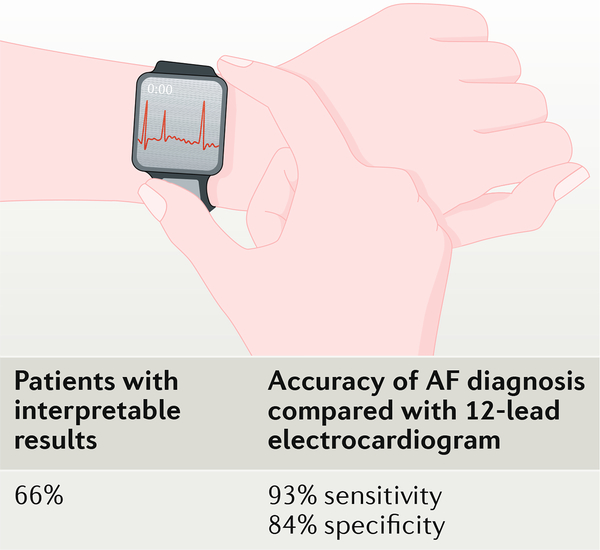
The AF detection approach of the KardiaBand is based on a proprietary algorithm (AliveCor, USA) that detects AF on the basis of rhythm irregularity and absence of P waves. Several approaches for automated detection of AF from ECG signals, including an approach using the AliveCor KardiaMobile device, have already been validated and are quite accurate, with sensitivity and specificity values >95% in large and diverse cohorts2–4. In the Bumgarner study that used the standard AliveCor criteria for AF detection discussed above, the algorithm for AF detection correctly identified AF with 93% sensitivity and 84% specificity when compared with the electrophysiologist- interpreted 12-lead ECGs1 (FIG. 1). Of the total 169 paired 12-lead ECG and ECG- band recordings, 57 of the band recordings were deemed unclassified by the algorithm owing to baseline artefact or low-amplitude recording, recording <30 s in duration, heart rate <50 bpm, heart rate >100 bpm, or unknown reasons. The short nature of the ECG recording (30 s) might, in part, explain the lower accuracy of this study for AF discrimination compared with previous studies with ECG data (typically using 1-min or 2-min segments).
Fig. 1|. Performance of an electrocardiogram watch band that is connected to an AppleWatch for the detection of AF in patients undergoing cardioversion.
The algorithm for detection of atrial fibrillation (AF) correctly identified AF with 93% sensitivity and 84% specificity when compared with the electrophysiologist- interpreted 12-lead electrocardiograms.
The data from many studies on automated AF detection, including the study by Bumgarner and colleagues, were collected in controlled clinical settings, in which contamination from motion or noise artefacts is often minimal; therefore, the discrepancy in sensitivity and specificity observed in the results is not likely to originate solely from poor signal- to-noise ratios. Premature atrial and ventricular contractions, which are common among patients with AF and often confound the accuracy of AF detection, might not have been fully factored into the algorithm used by Bumgarner and colleagues, resulting in lower sensitivity and specificity values than when algorithms designed to address frequent premature beats are used. Algorithms for the detection of premature atrial and ventricular contractions in ECG data are widely available and can be implemented in real time; therefore, if this issue is indeed the reason for the shortcoming, an improvement in accuracy when using a smartwatch for AF detection can be expected in the future.
Wearable devices such as smartphones and smartwatches show great promise for AF screening and monitoring. AF detection via these smart devices offers the potential for early diagnosis, but adoption of the technology by both clinicians and patients requires that these devices are easy to use and accurate, and provide clinically meaningful results in a manner that is compatible with the workflow of clinicians. The study by Bumgarner and colleagues has already partially fulfilled this promise by demonstrating that an ECG-based smartwatch band can detect AF in patients with known AF. However, to be used to screen for paroxysmal AF, wearable devices need to be accurate in ambulatory settings and provide more than just intermittent monitoring. Consequently, algorithms for AF detection need to be motion-artefact tolerant and, ideally, passive. The use of an ECG band for AF detection in its present form, which requires the user to touch a metal sensor on the wrist band with their non-watch hand, is also not the optimal approach for detection of paroxysmal AF. This user- involved measurement could too easily miss minimally symptomatic and brief paroxysms of AF. Although frequent and active screening using a watch is potentially feasible, few studies have examined long- term adherence to this type of system. More studies are needed to clearly define the ideal population for the use of this system as well as to help to inform on the optimal frequency of smartwatch- based screening for AF, in terms of both adherence and yield for AF detection.
“Wearable devices such as smartphones and smartwatches show great promise for AF screening and monitoring”
Photoplethysmographic (PPG) signals derived from the video camera of a smartphone have also been used for AF detection using an application that has been shown to provide high sensitivity and specificity (both >98%) for patients undergoing cardioversion for AF, as reported in a study by McManus and colleagues5. The lower cost of using a smartphone PPG signal that comes from the built- in camera is a trade-off with a better acceptance of the smartwatch ECG band by clinicians because they are more familiar with ECG data than with PPG signals. Given that some smartwatches already contain PPG sensors, including the AppleWatch used in the study by Bumgarner and colleagues, PPG signals could be used to passively and near- continuously monitor for pulse irregularity suggestive of AF. When AF is detected using PPG, the user could be prompted to take a confirmatory and diagnostic- grade ECG measurement. A similar approach was used by the AliveCor application and holds greater promise for real- world detection of paroxysmal AF3,6. However, novel signal processing approaches to motion artefact resilience are needed. Machine- learning algorithms to discriminate between clean signals and motion artefacts, and between various normal and AF rhythms, have the potential to support more accurate, ubiquitous, and near- continuous monitoring of paroxysmal AF with excellent coverage in the not- too-distant future.
As reported by Bumgarner and colleagues, the use of a smartwatch to screen for AF might reduce health- care costs by guiding appropriate use of medical procedures, including cardioversion. Innovators such as Bumgarner and colleagues are to be congratulated for demonstrating a convincing use- case for wearable smart devices in contemporary clinical practice. These investigators have also generated much excitement about the promise of smart devices for better AF diagnosis and connected patient care.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Bumgarner JM et al. Smartwatch algorithm for automated detection of atrial fibrillation. J. Am. Coll. Cardiol 71, 2381–2388 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Dash S, Chon KH, Lu S & Raeder EA Automatic real time detection of atrial fibrillation. Ann. Biomed. Eng 37, 1701–1709 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Halcox JPJ et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE- AF study. Circulation 136, 1784–1794 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Soni A et al. Study protocol for smartphone monitoring for atrial fibrillation in real- time in India (SMART- India): a community- based screening and referral programme. BMJ Open 7, e017668 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McManus DD et al. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm 10, 315–319 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tison GH et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 3, 409–416 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]