Abstract
Primary cutaneous T-cell lymphomas (CTCL) comprise a heterogeneous group of neoplasms with diverse clinical behavior. Mycosis fungoides (MF) is the most common type of CTCL. Immunophenotypical shift during progression of the disease is a rare event and its significance is unknown. We present three primary CTCL cases that showed an immunophenotypical shift and poor prognosis. Conventional hematoxylin/eosin and immunohistochemical-stained sections were examined in all the cases. Molecular analysis for rearrangement of the T-cell receptor (TCR) gene was performed in two cases. One case was classified as MF, while the other two lacked epidermotropism, and were considered primary cutaneous peripheral T-cell lymphoma (PTCL), NOS. Two cases were CD3+/CD4+ and one case was CD3+/CD8+ at diagnosis. The first two patients suffered many relapses and eventually, new CTCL lesions with a CD3+/CD8+ phenotype were observed. Both cases revealed identical clonal TCR rearrangements on the initial and late lesions, supporting the interpretation of a single clonal proliferation with different phenotypes. The third case progressed with skin recurrences and pulmonary lesions with a predominant CD3+/CD4+/CD8− phenotype. All cases manifested poor prognosis and two patients died of lymphoma. Immunophenotypical shift between CD4 and CD8 in CTCL seems to be a rare phenomenon that may be associated with disease progression.
Keywords: cutaneous T-cell lymphoma (CTCL), immunohistochemistry, immunophenotype, mycosis fungoides (MF), T-cell receptor gene rearrangement (PCR technique)
Immunophenotypic shift from CD4+ to CD8+ and vice versa in the progression of cutaneous T-cell lymphoma (CTCL) is a rarely reported phenomenon. To the authors’ knowledge, only one such case has been previously published.1 Herein, we describe three cases of CTCL that demonstrated a switch of CD4 or CD8 phenotype during the course of the disease. All cases had a relatively poor prognosis. Lesions with different immunophenotypes demonstrated clonal T-cell receptor (TCR) gene rearrangement with PCR products of identical size, in keeping with the same clonal origin.
CTCL comprises a number of entities primarily involving skin. From these, mycosis fungoides (MF) is the most common, accounting for almost 50% of all primary cutaneous lymphomas.2,3 Extracutaneous dissemination can occur in advanced stages, mainly to lymph nodes, liver, spleen, bone marrow, and lungs.1,2,4 The typical immunophenotype of MF by immunohistochemistry is that of a T helper/inducer lymphoproliferative process, that is CD3+, CD4+ and CD8−. 5 T-suppressor/cytotoxic (CD3+, CD4− and CD8+) and aberrant T-cell phenotypes are also described.5 The significance of these rare phenotypes is uncertain; while some reports suggest that CD8+ and aberrant T-cell phenotypes are associated, at least in some cases, with a more rapid growth and chronicity,6,7 other studies suggest that a CD8+ immunophenotype in early MF has the same prognosis as a CD4+ T helper/inducer phenotype.8 At any rate, the meaning of immunophenotypic switch during CTCL progression is still unknown.
Material and methods
Three patients, ranging in age from 41 to 67 years, were included in the study. Sequential skin biopsies were obtained and examined by routine hematoxylin/eosin staining and immunohistochemistry. Immunohistochemical studies for CD3, CD4, CD8, CD5, CD2, CD7, CD20, CD45RO, TIA1, Granzyme A/B, βF1, PAX-5 and CD30 were performed at the different collaborating institutions using standard immunoperoxidase methods. Molecular studies for rearrangement of the TCR genes using the polymerase chain reaction (PCR) technique were performed on selected specimens.
Results
Patient #1
A 67-year-old Caucasian male with a reported clinical history of peripheral T-cell lymphoma (PTCL), NOS presented at the University of Texas MD Anderson Cancer Center with scattered pink plaques on his forearms, upper arms and right flanks. He also had a history of bladder urothelial carcinoma, treated with multiple sessions of radiation and chemotherapy. A biopsy taken from his left upper arm showed a CD3+, CD4+ T cell dense infiltrate occupying dermis and subcutis, interpreted as PTCL, NOS. A month later, two punch biopsy specimens were reviewed from new skin tumors of the patient’s right forearm and right flank. Both biopsies showed a dense lymphocytic infiltrate in dermis extending to subcutaneous adipose tissue. Minimal epidermotropism was identified. The infiltrate was mainly composed of mediumlarge atypical cells with few small lymphocytes (Fig. 1A). Immunohistochemical studies revealed a predominance of CD3+ T lymphocytes (Fig. 1B), positive for CD4 (Fig. 1C). CD8 stained mostly small reactive lymphocytes (Fig. 1D). The approximate CD4 to CD8 ratio was 6 : 1. Interestingly, a few cells showed aberrant expression of PAX5 and CD20.
Fig. 1.
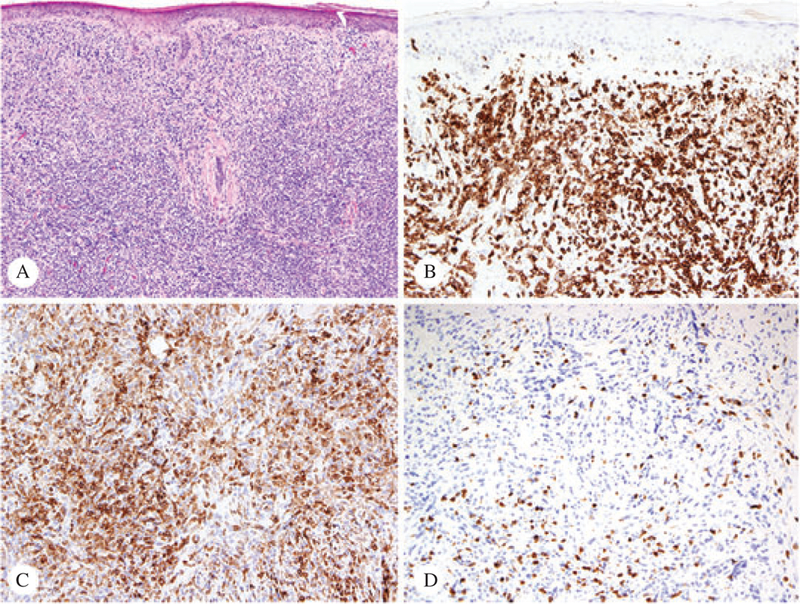
Patient #1 – Sections of the initial skin biopsy from the right forearm, stained with hematoxylin/eosin (A, ×4), CD3 (B, ×10), CD4 (C, ×10) and CD8 (D, ×10). The CD4/CD8 ratio was approximately 6 : 1 (immunoperoxidase technique, hematoxylin counterstain).
Twenty days later, a biopsy from a new lesion on the right forearm was taken. This specimen displayed similar histomorphology but a different immunophenotype. Most CD3+ atypical cells were negative for CD4 and CD8. Only rare cells were positive for either CD4 or CD8. The approximate CD4 to CD8 ratio was 1 : 1. Five months later, the patient presented with a right orbital mass, which was histopathologically and immunohistochemically similar to the specimen from the right forearm.
Five months later, a new biopsy from a right forearm lesion was obtained. The atypical lymphocytes in this biopsy were less pleomorphic and smaller than those seen in previous specimens (Fig. 2A). While only rare cells displayed CD4 positivity (Fig. 2C), the cells were predominantly CD8+ (Fig. 2D), with an approximate CD4 : CD8 ratio of 1 : 6. The lymphoma cells expressed TIA-1 and Granzyme B; focal CD30 expression was noted in a few larger cells.
Fig. 2.
Patient #1 – Sections of a late skin biopsy from the right forearm, stained with hematoxylin/eosin (A, ×4), CD3 (B, ×10), CD4 (C, ×10) and CD8 (D, ×10). The CD4/CD8 ratio is in this case reversed compared to that seen in Fig. 1, and it is approximately 1 : 6 (immunoperoxidase technique, hematoxylin counterstain)
The patient was diagnosed as advanced stage PTCL, NOS. Staging workup discovered a presacral mass (that was composed of CD8+ atypical lymphoid cells) and CNS involvement. TCR gene rearrangement analysis using the PCR technique revealed same monoclonal peak sizes for both beta and gamma chains of TCR on the initial CD4+ and the late CD8+ lesions (Fig. 3A–D). The patient died of disease 17 months after his initial biopsy.
Fig. 3.
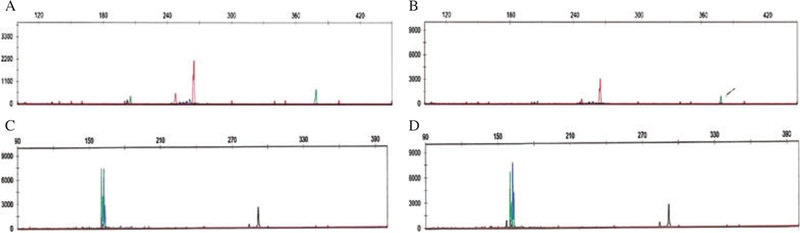
Patient #1 – Molecular analysis for T-cell receptor gene rearrangement using PCR. The top panel (A, B) shows TCR beta chain PCR products revealing peaks of identical sizes (260 bp) in both CD4+ (left) and CD8+ (right) lesions. The bottom (C, D) panel shows the TCR gamma chain analysis. Again, two clonal peaks of identical size (160 bp) were identified in both CD4+ (left) and CD8+ (right) lesions.
Patient #2
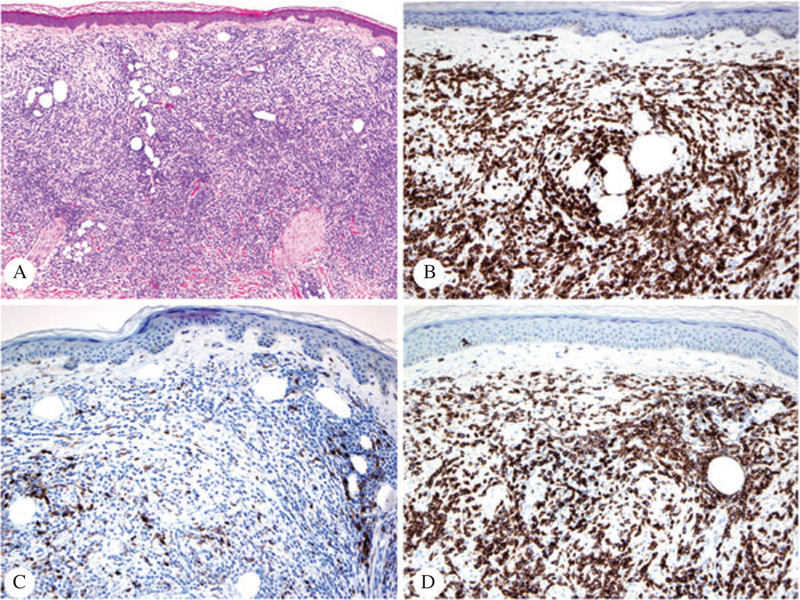
A 54-year-old Spanish female presented to the Hospital Universitari de Bellvitge, Barcelona, Spain with a history of CTCL diagnosed in 2004. On physical examination, the patient had multiple irregular slight scaling and erythematous patches on breasts and lower back. Histopathologic examination of a lesion on her breast showed an atypical perivascular lymphocytic infiltrate with epidermotropism and folliculotropism, consistent with MF (Fig. 4A). The phenotype of this lesion was CD3+/CD4+ (Fig. 4B,C, respectively), CD8− (Fig. 4D), with loss of expression of CD5 and CD2. The CD4 and CD8 ratio was approximately 5 : 1 in both dermis and epidermis.
Fig. 4.
Patient #2 – Sections of the conventional plaque of MF from the breast initially received and stained with hematoxylin/eosin (A, ×4), CD3 (B, ×20), CD4 (C, ×20) and CD8 (D, ×20). The CD4/CD8 ratio is, in this biopsy, of approximately 5:1 (immunoperoxidase technique, hematoxylin counterstain).
After receiving local radiation therapy, the patient had several recurrences in 2006 and 2009, consistent with conventional MF in plaques and controlled with local radiation/phototherapy. In 2010, the patient presented with a massive tumor on her right leg. The lesion showed a lymphocytic infiltrate extending into the subcutaneous adipose tissue. The immunophenotype in this case revealed an almost equal expression of CD4 and CD8, with a CD4/CD8 ratio of approximately 1 : 1. A new course of radiotherapyplus oral bexarotene initially showed a good partial response. However, in 2011 the patient presented with new painful scaly erythematous plaques with superficial ulceration affecting both lower extremities, especially the soles (Fig. 5). Biopsies taken from these lesions revealed different histopathologic findings. Prominent epidermotropism with pagetoid pattern of growth was seen (Fig. 6A). The atypical lymphocytes expressed CD3 (Fig. 6B), but lacked positivity for CD4 (Fig. 6C) while gained expression of CD8 (Fig. 6D). The complete immunoprofile wasCD7+/βF1+/Granzyme B+, CD2 partially lost and CD5(−)/CD45RO(−)/CD30(−)/EBER(−).
Fig. 5.
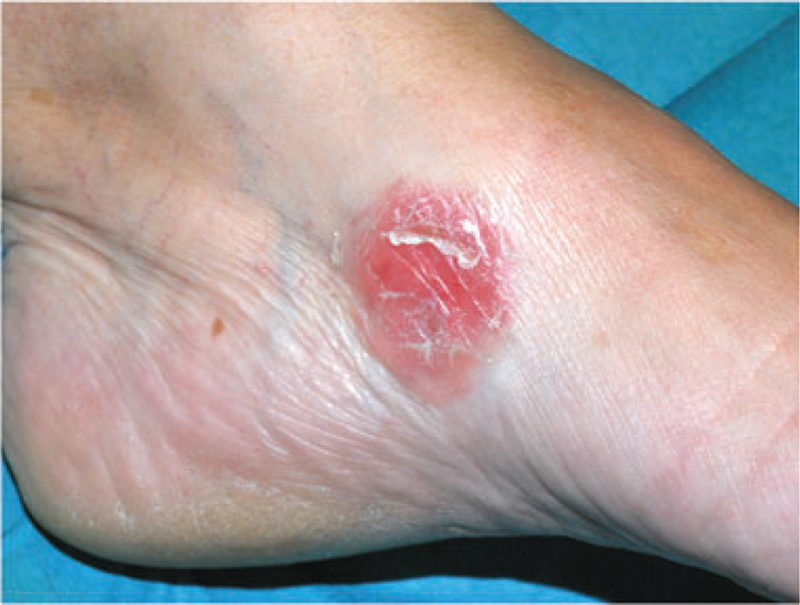
Patient #2 – Clinical appearance of a scaly erythematous plaque corresponding to a epidermotropic CD8+lesion.
Fig. 6.
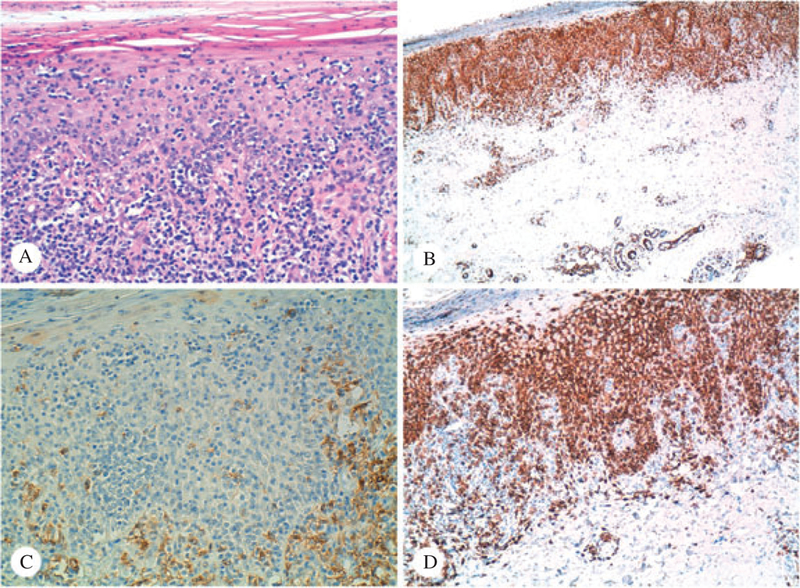
Patient #2 – Sections of the skin biopsy from the sole received two years after that seen in Fig. 4, stained with hematoxylin/eosin (A, ×10), CD3 (B, ×10), CD4 (C, ×20) and CD8 (D, ×10). There is an obvious predominance of CD8 cells in this biopsy, with a CD4/CD8 ratio of approximately 1:10 (immunoperoxidase technique, hematoxylin counterstain)
TCR beta chain gene rearrangement was performed on biopsies from different anatomical sites and corresponding to lesions expressing CD4 and CD8. Identical peaks were identified on both initial CD4+ and late CD8+ lesions. New restaging disclosed systemic involvement. In October 2011, the patient received an allogenic stem cell transplantation from an related donor. Currently the patient is in complete remission.
Patient #3
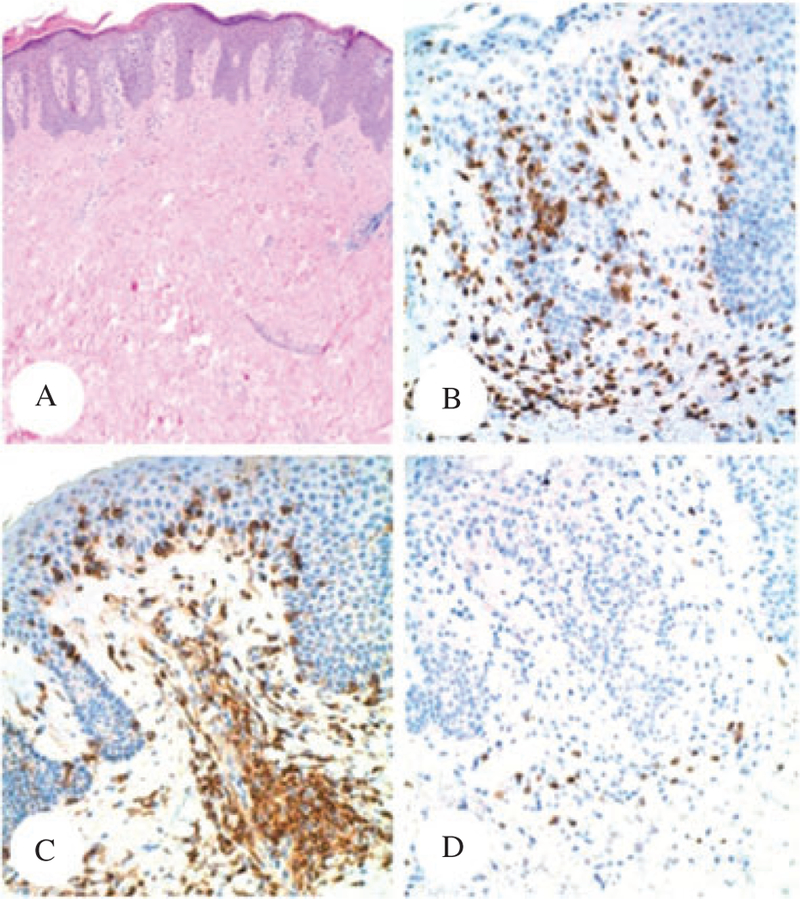
In 1974, a 41-year-old gentleman was diagnosed with PTCL, NOS based on a skin biopsy. The patient was referred to the National Cancer Institute in 1979, at which time a biopsy of erythematous skin plaques on the posterior neck was performed. The patient also had scattered pink plaques and patches on his lower back. The biopsy showed a dense atypical lymphocytic infiltrate involving the dermis. There was only focal exocytosis of small lymphocytes, and the lesion was classified as PTCL, NOS (Fig. 7A). The infiltrate was predominantly composed of CD3+ T cells (Fig. 7B), and showed a CD4−, CD8+ phenotype (Fig. 7C,D, respectively). The patient was treated with the Promace MOPP chemotherapy regimen. Multiple relapses were recorded in 1980 and 1981. In 1983, he had developed diffuse pulmonary infiltrates on chest X-ray. An open lung biopsy revealed PTCL (Fig. 8A,B). At this time the tumor cells displayed diffuse labeling for CD4 (Fig. 8C) and only scattered small lymphocytes were positive for CD8 (Fig. 8D). The patient eventually died of lymphoma.
Fig. 7.
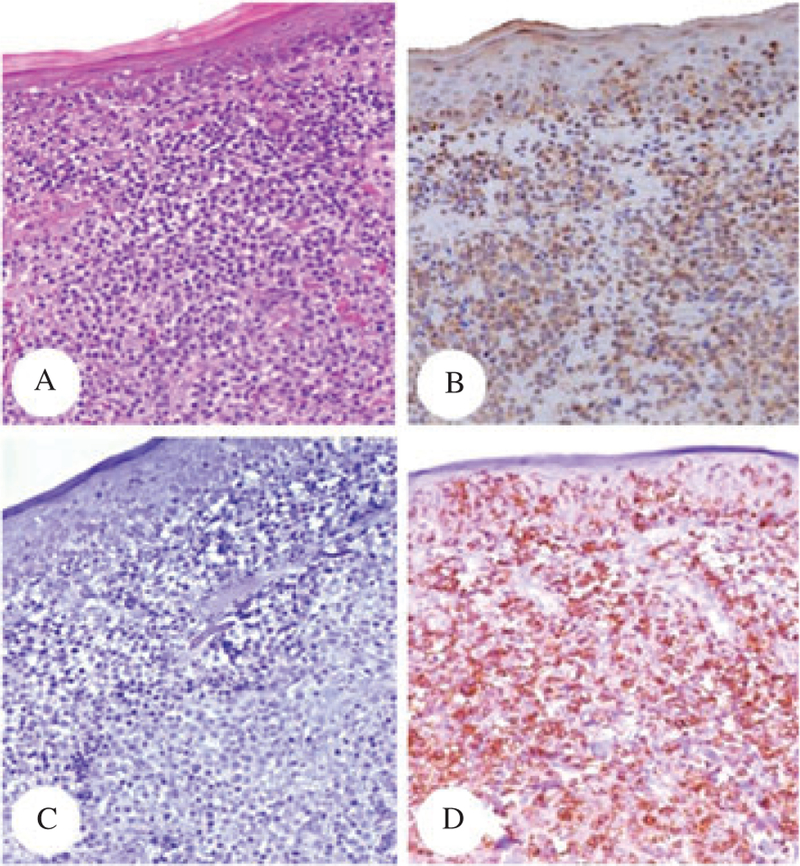
Patient #3 – Sections of the initial skin biopsy from the posterior neck, stained with hematoxylin/eosin (A, ×4), CD3 (B, ×10), CD4 (C, ×10) and CD8 (D, ×10). The CTCL displayed aCD3+/CD4−/CD8+ phenotype (immunoperoxidase technique, hematoxylin counterstain).
Fig. 8.
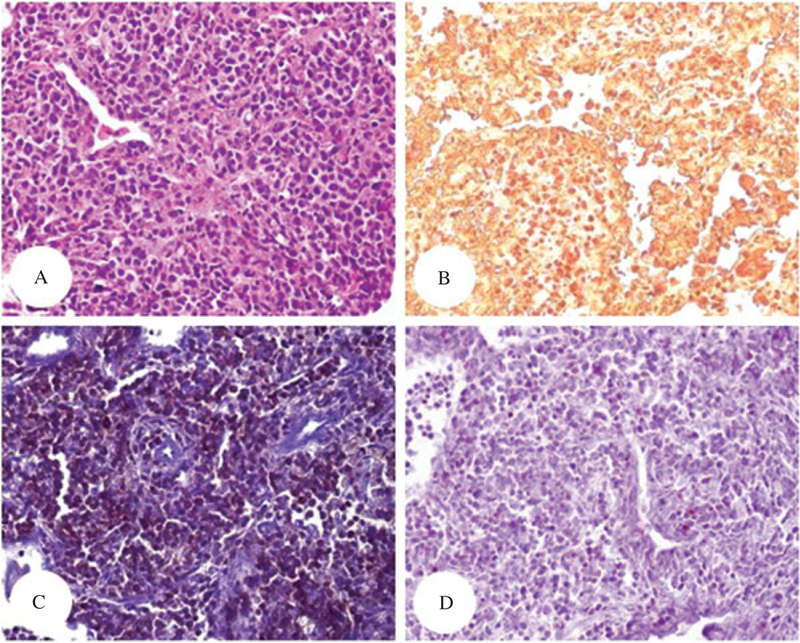
Patient #3 – Sections of lung showing involvement by lymphoma, 4 years after the initial biopsy seen in Fig. 7. The sections were stained with hematoxylin/eosin (A, ×4), CD3 (B, ×10), CD4 (C, ×10) and CD8 (D, ×10). There is diffuse and weak expression of CD4 by the lymphoma cells. Only scattered small CD8+T cells are seen (immunoperoxidase technique, hematoxylin counterstain).
Discussion
CTCL comprises a clinically, histomorphologically and immunophenotypically heterogenous group of lymphoid proliferations. Although these lymphomas exhibit different immunophenotypes, switch of expression of CD4 and CD8 during disease progression in these entities is rarely recorded.
Immunophenotypic shifts with or without lineage change have been recognized as frequent events in certain leukemias of childhood, such as precursor B-acute lymphoblastic leukemia (B-ALL), precursor T-acute lymphoblastic leukemia (T-ALL) and acute myeloid leukemia (AML), especially at relapse9–12 TdT and CD99 loss in precursor T-cell ALL has been reported to occur during therapy.13 In these precursor hematopoietic malignancies differentiation from an immature stem cell has beenpostulated as a possible explanation for the observed immunophenotypical shift.
A single case of T-cell prolymphocytic leukemia with spontaneous immunophenotypical switch from CD4 to CD8 has been reported.14 Diminution in CD4 and increase in CD8 expression was noted when the patient presented with cutaneous involvement after refusing therapy. After failing chemotherapy, the patient showed a predominantly CD8+ leukemia. Poor response associated with acquisition of CD8+ phenotype was suggested.
Mature B or T cell lymphomas displaying immunophenotypical switch are rarely mentioned in the literature. Among B cell lymphomas, one case of diffuse large B cell lymphoma that switched from germinal center to non-germinal center phenotype and showed an aggressive clinical course has been published.15 A case of mantle cell lymphoma with loss of CD5 and aberrant expression of CD10 has also been reported.16 Loss of CD20 following rituximab therapy is a well-known phenomenon and should be considered as an induced immunophenotypic shift.17 A case of T-cell chronic lymphocytic leukemia (CLL) presenting as an intracerebellar tumor has been described to have switched from an initial CD4+ phenotype to a more variegated process showing a subpopulation of CD8+ large atypical cells, negative for CD4, before becoming more aggressive and nonresponsive to chemotherapy.18
Agnarsson et al., described an immunophenotypic switch between CD7 and CD2 during CTCL progression.19 In their study, the authors found that the rapidly progressive CD8+ lesions show a CD2−, CD3+, CD5+/− and CD7+ phenotype. CD2 loss is unusual because it is seldom lost in CTCL, even in tumor stage lesions, whereas the persistence of expression of CD7 is also notable because this antigen is frequently lost in CD4+ CTCL, even in early stages of disease. In contrast, the immunophenotype of the chronic CD8+ lesions were CD2+, CD3+, CD5−/+ and CD7(−), which is more similar to that usually found in CD4+ CTCL. This aberrant expression of CD2 and CD7 may explain the discordant data of prognosis in CD8+ CTCL cases and it could be considered as another type of immunophenotypic shift during progression of CTCL.
Only one case of MF displaying immunophenotypical shift of CD4 and CD8 has been reported in the literature.1 The patient presented with classical lesions of CD4+ MF, successfully controlled with PUVA and radiation therapy. After 5 years of initial diagnosis, subcutaneous nodules, consistent with tumor stage MF were noted along with decreasedvision in his left eye. A vitreous biopsy revealed infiltration by atypical lymphocytes expressing CD8 with a CD4 : CD8 ratio of 1 : 4. About 40% of the atypical T cells co-expressed CD79a. The patient died soon after ocular involvement by lymphoma. Clonality studies, such as analysis of TCR gene rearrangement, are not mentioned in the case report. However, clinical progression of the disease to tumor stage and concomitant intra-ocular compromise is suggestive of the same lymphoid neoplasm involving both the skin and the eye. In this case, switch in phenotype seemed to be associated with disease progression and poor outcome.
In two of our three CTCL cases T cell clones of the same peak size were identified on TCR gene rearrangement analyses by PCR performed on samples obtained from different anatomical sites and at different times of evolution. These results suggested that in both cases lesions with different morphologies and immunophenotypes were clonally related. Along with the immunophenotypical shift, a morphological change was also noted in all our cases. With progression of the disease, the lesional lymphoid cells became either more pleomorphic andcytologically atypical, as seen in our cases #2 and #3, or adopted a more homogeneous appearance, with less variation in size and shape, as noted in case #1. In case #1, a gradual loss of expression of CD4 with concomitant gain of CD8 positivity was recorded, similar to that seen in the CLL case mentioned above.18
Although our series of cases is small and clinically meaningful conclusions are difficult to establish, it seems that, based on the outcome of two of our patients and in the previous reported case, the phenotype switch phenomenon in CTCL may herald or be associated with disease progression. Clonal selection due to therapeutic intervention or development of secondary lymphoid proliferations may also account for such clinical outcome. Immunophenotypical shift in CTCL seems to be extremely rare. However, its recognition by pathologists is crucial for the correct interpretation of lesions occurring at different times in the progression of the disease. This phenomenon may constitute a potential pitfall in the diagnosis of phenotypedivergent lymphoid proliferations occurring in patients with a previous history of CTCL.
References
- 1.Lois N, Hiscott PS, Nash J, Wong D. Immunophenotypic shift in a case of mycosis fungoides with vitreous invasion. Arch Ophthalmol 2000; 118: 1692. [DOI] [PubMed] [Google Scholar]
- 2.Ralfkiaer E, Cerroni L, Sander CA, Smoller BR, Willemze R. Mycosis fungoides. WHO classification of tumors of hematopoietic and lymphoid tissues, 4th ed. Lyon, France: IARC Press, 2008; 296. [Google Scholar]
- 3.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005; 105: 3768. [DOI] [PubMed] [Google Scholar]
- 4.Lynch PJ. Lymphoma, leukemia, and related diseases. In Sams WM, Lynch PJ, eds. Principles and Practice of Dermatology New York: Churchill Livingstone, 1996; 308. [Google Scholar]
- 5.Massone C, Kodama K, Kerl H, Cerroni L. Histopathologic features of early (patch) lesions of mycosis fungoides: a morphologic study on 745 biopsy specimens from 427 patients. Am J Surg Pathol 2005; 29: 550. [DOI] [PubMed] [Google Scholar]
- 6.Izban KF, Hsi ED, Alkan S. Immunohistochemical analysis of mycosis fungoides on paraffin-embedded tissue sections. Mod Pathol 1998; 11: 978. [PubMed] [Google Scholar]
- 7.van der Putte SC, Toonstra J, van Wichen DF, van Unnik JA, van Vloten WA. Aberrant immunophenotypes in mycosis fungoides. Arch Dermatol 1988; 124: 373. [DOI] [PubMed] [Google Scholar]
- 8.Massone C, Crisman G, Kerl H, Cerroni L. The prognosis of early mycosis fungoides is not influenced by phenotype and T-cell clonality. Br J Dermatol 2008; 159: 881. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Du W, Liu W, Li X, Li H, Huang S-A. Comprehensive flow cytometry phenotype in acute leukemia at diagnosis and at relapse. APMIS 2010; 118: 353. [DOI] [PubMed] [Google Scholar]
- 10.van der Velden VH, van Lom K, Hoogeveen PG, Beverloo HB, Breems DA. Unusual immunophenotypic shift in a patient with Tcell acute lymphoblastic leukemia. Leukemia 2006; 20: 1626. [DOI] [PubMed] [Google Scholar]
- 11.Guglielmi C, Cordone I, Boecklin F, et al. Immunophenotype of adult and childhood acute lymphoblastic leukemia: changes at first relapse and clinico-prognostic implications. Leukemia 1997; 11: 1501. [DOI] [PubMed] [Google Scholar]
- 12.van Wering ER, Beishuizen A, Roeffen ET, et al. Immunophenotypic changes between diagnosis and relapse in childhood acute lymphoblastic leukemia. Leukemia 1995; 9: 1523. [PubMed] [Google Scholar]
- 13.Roshal M, Fromm JR, Winter S, Dunsmore K, Wood BL. Immaturity associated antigens are lost during induction for T cell lymphoblastic leukemia: implications for minimal residual disease detection. Cytometry 2010; 78: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tse E, So CC, Cheung WW, Kwong YL. Tcell prolymphocytic leukaemia: spontaneous immunophenotypical switch from CD4 to CD8 expression. Ann Hematol 2011; 90: 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo JJ, Rizack T, Treaba D. Immunophenotypical switch versus tumor heterogeneity in a patient with HIV-associated diffuse large B-cell lymphoma. Patholog Res Int 2011: 563216. [DOI] [PMC free article] [PubMed]
- 16.Morice WG, Hodnefield JM, Kurtin PJ, Hanson CA. An unusual case of leukemic mantle cell lymphoma with a blastoid component showing loss of CD5 and aberrant expression of CD10. Am J Clinic Pathol 2004; 122: 122. [DOI] [PubMed] [Google Scholar]
- 17.Maeshima AM, Taniguchi H, Nomoto J, et al. Histological and immunophenotypic changes in 59 cases of B-cell non-Hodgkin’s lymphoma after rituximab therapy. Cancer Sci 2009; 100: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubota K, Tanaka H, Suda K, et al. Morphological and immunological change in the predominant type of leukaemic cells in a patient with T-cell chronic lymphocytic leukaemia. Scand J Haematol 1986; 36: 439. [DOI] [PubMed] [Google Scholar]
- 19.Agnarsson BA, Vonderheid EC, Kadin ME. Cutaneous T cell lymphoma with suppressor/cytotoxic (CD8) phenotype: identification of rapidly progressive and chronic subtypes. J Am Acad Dermatol 1990; 22: 569. [DOI] [PubMed] [Google Scholar]


