Sir: Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) comprises 5–10% of all Hodgkin lymphomas (HLs), with usually excellent survival despite the propensity for multiple relapses.1 Histologically, the nodules in typical NLPHL are rich in small B cells (CD20+ ⁄ IgD+) with uniform CD20 and CD79a (80%) expression on the lymphocyte-predominant (LP) cells, reflective of a well-preserved B-cell ⁄ germinal centre programme.2 In a large series of 219 NLPHLs examined as part of the European Task Force on Lymphoma (ETFL) project,3 both CD30 and CD15 were reportedly negative in all cases of NLPHL. Since this study, three other groups have reported CD15 expression on LP cells of otherwise typical NLPHL.4–6
Herein, we report eight cases of NLPHL with CD15+ LP cells identified among 105 NLPHLs in which both CD30 and CD15 were examined. This study was approved by the Institutional Review Board of the National Cancer Institute (NCI). All eight CD15+ cases were diagnostic lymph node biopsies submitted for consultation ⁄ review to the Hematopathology Section of the Laboratory of Pathology, NCI, between 2002 and 2008. Six patients had stage I ⁄ II disease, and two had stage III disease; all had only nodal disease.
Scattered LP cells were identified within and outside the nodules, amidst a background of lymphocytes and histiocytes. We further characterized the cases morphologically according to the various patterns of NLPHL described previously by Fan et al.4 (Table 1).
Table 1.
Clinicopathological summary of the eight CD15+ nodular lymphocyte-predominant Hodgkin lymphomas (NLPHLs)
| Case no. |
Age ⁄ Sex | Pattern(s)* | Treatment(s) | Response | Outcome | Follow-up (months) |
|---|---|---|---|---|---|---|
| 1 | 36 ⁄ M | C ⁄ D | – | – | – | – |
| 2 | 49 ⁄ M | A | – | – | – | – |
| 3 | 77 ⁄ F | C ⁄ E | R-CEOP | – | Dead | 5 |
| 4 | 55 ⁄ M | D | ABVD ⁄ RT | – | Dead | 1 |
| 5 | 62 ⁄ M | A ⁄ D | RICE | – | Dead | 18 |
| 6 | 46 ⁄ M | E ⁄ A | R-CHOP | – | Alive | 3 |
| 7 | 68 ⁄ M | D ⁄ E | ABVD | Partial response | Dead | 0.7 |
| 8 | 26 ⁄ M | C ⁄ D | ABVD ⁄ EPOCH-R ⁄ RT ⁄ BMT | No response | Alive | 60 |
Cases were classified on the basis of the patterns of NLHPL described by Fan et al., denoting predominant/less predominant pattern.
ABVD, Doxorubicin, bleomycin, vinblastine and dacarbazine; EPOCH-R, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin plus rituximab; R-CEOP, rituximab with cisplastin, etoposide, cyclophosphamide and vincristine; R-CHOP, rituximab with cyclophosphamide, adriamycin, vincristine, and prednisone with rituximab; RICE, rituximab, ifosfamide, carboplatin and etoposide; RT, radiotherapy; BMT, bone marrow transplant.
By immunostaining, LP cells in all cases had a strong B-cell phenotype (CD20+ ⁄ PAX-5+ ⁄ Oct-2+ ⁄ CD79a+ ⁄ BCL-6+) and were negative for CD30 and Epstein–Barr virus by RNA in-situ hybridization (Figure 1). All eight cases displayed strong CD15 expression on the LP cells. The CD15+ LP cells were distributed in the nodular and diffuse areas, and were not spatially restricted to any specific pattern by the Fan schema.4 Case 7 (Table 1), which was clinically stage III, focally displayed increased numbers of large cells with cytological atypia. Strong CD15 expression was restricted to these areas (Figure 2). Treatment and outcome information was available in six patients (Table 1).
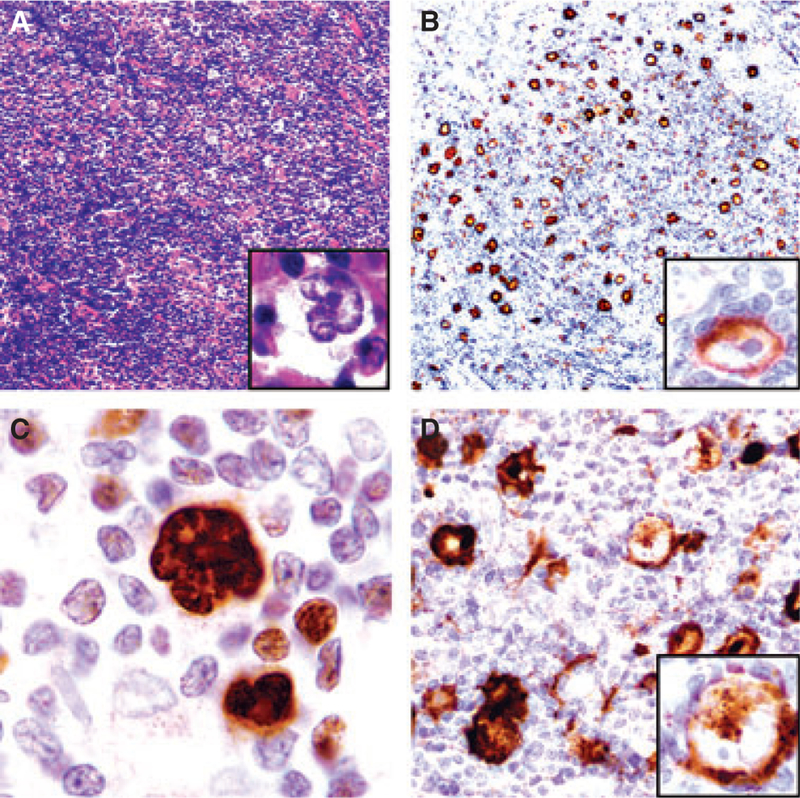
Figure 1.
IgD+ nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) expressing CD15 in a 36-year-old male (case 1). A, Low-power view showing mottled areas with scattered lymphocyte-predominant (LP) cells (inset). B, CD20 highlights LP cells at the periphery of small B-cell-poor nodules (Pattern C / D); the inset shows IgD expression in LP cells. C, LP cells strongly express Oct-2. D, Cluster of CD15+ LP cells (inset: LP cell with CD15 expression in the cytoplasm).
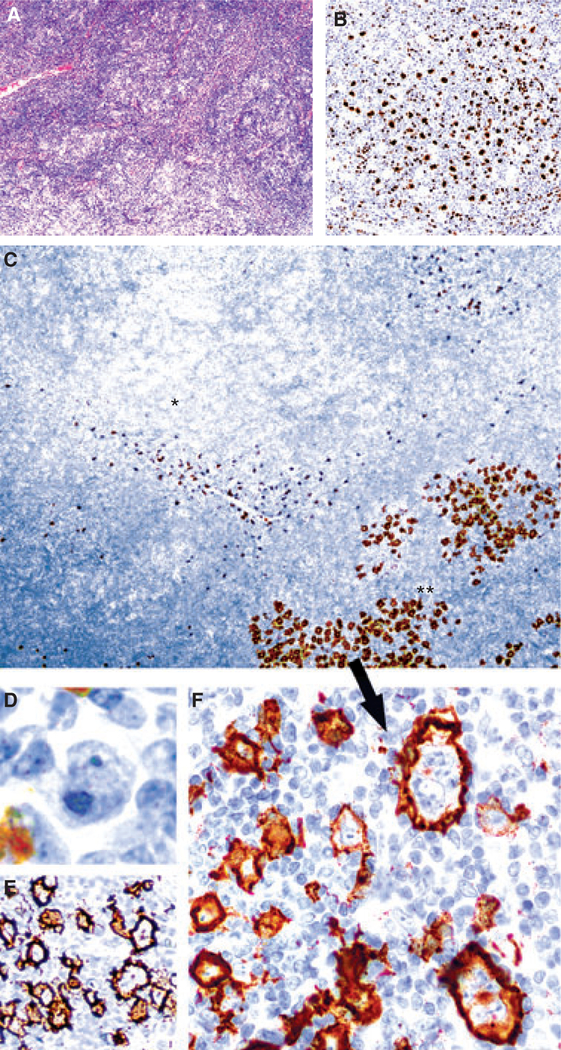
Figure 2.
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) with increased numbers of large cells (case 7). A, Areas of typical NLPHL with many singly scattered lymphocyte-predominant (LP) cells (inset) amidst a vaguely nodular background of lymphocytes and few histiocytes. B, Oct-2 stain highlights LP cells admixed with small B cells within nodules. C, Low-power view of CD15 stain showing numerous CD15+ LP cells in areas with increased numbers of large cells (**, bottom right) in contrast to adjacent areas of typical NLPHL clearly lacking CD15 expression (*, upper left). Only scattered granulocytes stain intensely in these latter areas. D, These cells are CD30) and strongly CD20+ (E). F, High-power view showing cytological atypia of the CD15+ large cells.
Estimation of the incidence of CD15 expression in NLPHL from studies prior to the past decade is difficult, as older series probably represented an impure mix that included cases of the lymphocyte-rich classical variant of HL (LRCHL) – which would be expected to be CD15+. In a landmark study by the ETFL that led to the acceptance of LRCHL as a distinct entity, only 219 ⁄ 388 assessable cases submitted as NLPHL for review were found by the panel to represent NLPHL. Most of the other excluded cases were classified as classical HL, including 115 that were reclassified as nodular LRCHL.3 Notably, none of the NLPHLs in this reclassification study were CD15+, as all cases that expressed CD15 were categorized as classical HL. Cases representing CD15+ NLPHL in this study may have been grouped either under the nine unclassified cases or with the two cases of LRCHL in which CD30 was reported to be negative. In an additional seven cases, CD30 expression was uninterpretable. This study did not incorporate additional markers such as Oct-2 and BCL-6, which are valuable in further resolving the classification as LRCHL or NLPHL.
The clear-cut lack of CD30 and EBER (Epstein-Barr Virus)-Encoded RNA (Ribonucleic Acid)) coupled with the presence of a strong B-cell phenotype excludes the diagnosis of classical HL in our cases. The 6% (8 ⁄ 105) rate of positivity for CD15 that we identified is in agreement with the 4 ⁄ 66 (6%) rate found by Nam-Cha et al.5 in their series of NLPHLs (personal communication with Syong Hyun Nam-Cha), although this incidence may still be an overestimation, since we see cases as part of a consultation service, enriched for more atypical cases. On the other hand, one of our cases with IgD-expressing LP cells (Figure 1) is notable, and bears a striking similarity to two CD15-expressing NLPHLs submitted to a workshop on grey-zone lymphomas. Both cases also were reportedly positive for IgD on the LP cells and negative for CD30 (personal communication with Leticia Quintanilla-Martinez) as well as EBER.6
When compared with historical cohorts of NLPHL, our preliminary data indicate a more aggressive clinical course for CD15+ NLPHL, although these findings are limited by the variety of the treatment regimens, disease stage and lack of accurate information pertaining to the primary end-point (disease-specific mortality). We recommend that CD15 be routinely assessed in the diagnosis of both classical HL and NLPHL, to better determine the clinical impact of CD15 expression in an unselected clinical series. From a diagnostic standpoint, it is important to appreciate that CD15 expression does not exclude the diagnosis of NLPHL.
ACKNOWLEDGEMENT
This work was supported by the intramural research program of the Center for Cancer Research.
References
- 1.Diehl V, Sextro M, Franklin J et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J. Clin. Oncol 1999; 17; 776–783. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL. WHO classification of tumours of haematopoietic and lymphoid tissues, 4th edn. Lyons: IARC Press, 2008. [Google Scholar]
- 3.Anagnostopoulos I, Hansmann ML, Franssila K et al. European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood 2000; 96; 1889–1899. [PubMed] [Google Scholar]
- 4.Fan Z, Natkunam Y, Bair E, Tibshirani R, Warnke RA. Characterization of variant patterns of nodular lymphocyte predominant Hodgkin lymphoma with immunohistologic and clinical correlation. Am. J. Surg. Pathol 2003; 27; 1346–1356. [DOI] [PubMed] [Google Scholar]
- 5.Nam-Cha SH, Montes-Moreno S, Salcedo MT, Sanjuan J, Garcia JF, Piris MA. Lymphocyte-rich classical Hodgkin’s lymphoma: distinctive tumor and microenvironment markers. Mod. Pathol 2009; 22; 1006–1015. [DOI] [PubMed] [Google Scholar]
- 6.Quintanilla-Martinez L, de Jong D, de Mascarel A et al. Gray zones around diffuse large B cell lymphoma. Conclusions based on the workshop of the XIV meeting of the European Association for Hematopathology and the Society of Hematopathology in Bordeaux, France. J. Hematop 2009; 2; 211–236. [DOI] [PMC free article] [PubMed] [Google Scholar]