Abstract
Peripheral T-cell and NK-cell lymphomas are functionally, pathologically, and clinically complex. Most nodal T-cell lymphomas belong to the adaptive immune system, whereas many extranodal T-cell and NK-cell lymphomas are derived from innate immune cells. The pathological manifestations often reflect the functional attributes of the neoplastic cells. Several forms of peripheral T-cell lymphoma are derived from T-follicular helper cells (TFH), and include angioimmunoblastic T-cell lymphoma, the follicular variant of peripheral T-cell lymphoma, not otherwise specified, and primary cutaneous small/medium CD4-positive T-cell lymphoma. TFH-derived neoplasms are often associated with atypical and clonal B-cell proliferations, which take a number of forms, sometimes mimicking classical Hodgkin’s lymphoma, and sometimes showing marked plasmacytic differentiation. Most extranodal T-cell lymphomas are cytotoxic and often arise in mucosal-associated sites. They can be derived from either αβ or γδ cytotoxic T cells, and include subcutaneous panniculitis-like T-cell lymphoma, and enteropathy-associated T-cell lymphomas, both Type I and Type II. Type I enteropathy-associated lymphomas occur in association with celiac disease, whereas Type II lymphomas are more often sporadic. For some T-cell lymphomas, such as hepatosplenic T-cell lymphoma, immunophenotypic heterogeneity is seen within a single disease entity. New data are emerging on the molecular pathogenesis of T-cell and NK-cell lymphoma, but most tumor types remain poorly characterized.
Keywords: anaplastic large cell lymphoma, Epstein Barr virus, NK-cell lymphoma, peripheral T-cell lymphoma, T-follicular helper cells, T regulatory cells
A biologically relevant and clinically useful classification for mature T-cell and NK-cell lymphomas has been difficult to accomplish. In part, this is due to the rarity of these neoplasms, the relative lack of understanding of the molecular pathogenesis for most tumors, and their morphological and immunophenotypic complexity. These neoplasms are uncommon, representing fewer than 15% of all non-Hodgkin’s lymphomas, although the relative frequency varies in different geographic regions and racial populations.1 The most common subtypes of mature T-cell lymphomas are peripheral T-cell lymphoma, not otherwise specified, and anaplastic large cell lymphoma (ALCL).
The classification of T- and NK-cell neoplasms proposed by the WHO emphasizes a multiparameter approach, integrating morphologic, immunopheno-typic, genetic, and clinical features (Table 1).2 Clinical features have particular importance in the subclassification of these tumors, in part due to the lack of specificity of other parameters. In recent years, immunophenotypic markers capable of delineating certain functional subsets have been recognized, such as lymphomas derived from T regulatory cells (Treg), or T-follicular helper cells (TFH). Presently, specific genetic features have not been identified for many of the T- and NK-cell neoplasms, although there are a limited number of exceptions.
Table 1.
WHO classification of tumors of hematopoietic and lymphoid tissues (2008)
| Mature T-cell and NK-cell neoplasms |
|---|
| T-cell prolymphocytic leukemia |
| T-cell large granular lymphocytic leukemia |
| Chronic lymphoproliferative disorder of NK cells |
| Aggressive NK leukemia |
| Systemic EBV-positive T-cell lymphoproliferative disease of childhood childhood |
| Hydroa vacciniforme-like lymphoma |
| Adult T-cell leukemia/lymphoma |
| Extranodal NK/T-cell lymphoma, nasal type |
| Enteropathy-associated T-cell lymphoma |
| Hepatosplenic T-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Mycosis fungoides |
| Sézary syndrome |
| Primary cutaneous CD30-positive T-cell lymphoproliferative disorders |
| Lymphomatoid papulosis |
| Primary cutaneous anaplastic large cell lymphoma |
| Primary cutaneous γδ T-cell lymphoma |
| Primary cutaneous CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma |
| Primary cutaneous CD4-positive small/medium T-cell lymphoma |
| Peripheral T-cell lymphoma, NOS |
| Angioimmunoblastic T-cell lymphoma |
| Anaplastic large cell lymphoma, ALK positive |
| Anaplastic large cell lymphoma, ALK negative |
Most common entities are underlined. Provisional entities are in italics.
Pathophysiology of T-cell subsets
Mature T-cell lymphomas manifest the immuno-phenotypic features of post-thymic T lymphocytes, being derived from both αβ T cells and γδ T cells.3 This distinction is based on the structure of the T-cell receptor.4 γδ T cells, along with NK cells are components of the innate immune system, and do not require antigen sensitization to be active.5,6 The innate immune system is functional based only on genes encoded in the host genome. It is distinguished from the adaptive or antigen-specific immune system, which is characterized by both memory and specificity of antigen recognition. Most T cells in peripheral blood and peripheral lymphoid organs belong to the adaptive immune system. γδ T cells comprise fewer than 5% of all normal T cells, and show a restricted distribution, being found mainly in the splenic red pulp, intestinal epithelium, and other epithelial sites. It is notable that these sites are more commonly affected by γδ T-cell lymphomas, which are rare in nodal sites.4,7–9 γδ T cells are not MHC restricted in their function, and represent a first line of defense against bacterial peptides, such as heat-shock proteins.3
Cells of the innate immune system represent a first line of defense, a more primitive type of immune response, and have a role in both mucosal and cutaneous immunity. It is interesting that many T-cell and NK-cell lymphomas observed commonly in the pediatric and young adult age group are derived from cells of the innate immune system (Table 2).10 These include aggressive NK-cell leukemia, systemic EBV-positive T-cell lymphoproliferative disease of childhood, hepatosplenic T-cell lymphoma, and γδ T-cell lymphomas affecting muco-cutaneous sites.5 ALCL is the most common pediatric T-cell lymphoma, and is also of cytotoxic origin, although not shown to be derived from innate immune cells.
Table 2.
NK and T-cell subsets and the classification of peripheral T-cell and NK-cell neoplasms
| Innate immune system | Adaptive immune system |
|---|---|
| Does not require antigen sensitization | Characterized by specificity and memory |
| NK cells, NK/T cells, γδ T cells | Effector and memory T cells |
| Cell-mediated cytotoxicity | Act principally through cytokines and chemokines |
| Mainly cutaneous and other extranodal sites | Mainly nodal lymphomas |
| Children and adults | More often in adults |
The T cells of the adaptive immune system are heterogeneous and functionally complex, and include naїve, effector (regulatory and cytotoxic), and memory T cells. CD4-positive T cells are primarily regulatory, acting via cytokine production, while CD8-positive (and double negative) T cells are primarily cytotoxic. Recently, much has been learned about a unique T-cell subset found in the normal germinal center. These cells, termed as follicular T-helper cells (TFH), provide help to B cells in the context of the germinal center reaction.11,12 They have a unique phenotype, expressing the germinal center-associated markers BCL6 and CD10, normally found on B cells. TFH express CD4, PD-1 (CD279), SAP (SH2D1A), IL-21, and ICOS, and produce the chemokine receptor CXCR5 and chemokine CXCL13. CXCL13 causes induction and proliferation of follicular dendritic cells, and is involved in B-cell recruitment to the lymph node, by facilitating the adhesion of B cells to high endothelial venules and allowing them to transit the vessel wall. Angioimmunoblastic T-cell lymphoma, the prototypic TFH neoplasm, is associated with B-cell expansion. In contrast, adult T-cell leukemia/lymphoma has been linked to Treg cells based on expression of both CD25 and FoxP3.13 Treg cells are a specialized type of regulatory T cell that suppresses the immune response, possibly explaining the marked immuno-suppression associated with this disease.
There have been relatively few studies correlating the subclassification of T-cell lymphomas with specific profiles of cytokine or chemokine expression.14 Nevertheless, data are now emerging that can relate the pathological or clinical manifestations of T-cell lymphomas to cytokine or chemokine expression by the neoplastic cells, or accompanying accessory cells. The hemophagocytic syndrome seen in some T-cell and NK-cell malignancies has been associated with secretion of both cytokines and chemokines, in the setting of defective cytolytic function.15 Most recently, the chemokine CXCL13 has been identified in angioimmunoblastic T-cell lymphomas, and its identification helps to elucidate many of the features of that T-cell subtype.16,17
Angioimmunoblastic T-cell lymphoma and related T-cell lymphomas of TFH phenotype
Angioimmunoblastic T-Cell Lymphoma
Angioimmunoblastic T-cell lymphoma was initially proposed as an abnormal immune reaction or form of atypical lymphoid hyperplasia with a high risk of progression to malignant lymphoma. Because the majority of cases show clonal rearrangements of T-cell receptor genes, it is now regarded as a variant of T-cell lymphoma. The median survival is generally <5 years.18
Angioimmunoblastic T-cell lymphoma occurs in adults and has not been described in children. Most patients have generalized lymphadenopathy, hepatosplenomegaly, skin rash, and prominent constitutional symptoms. There is usually polyclonal hypergammaglobulinemia and other hematologic abnormalities such as Coombs-positive hemolytic anemia. Rituximab has been used in some recent clinical trials, in an attempt to control some of the effects of B-cell hyperactivity in this disease, but rituximab therapy has not had an impact on overall survival.19 Patients may also show evidence of immunodeficiency with recurrent opportunistic infections that may ultimately lead to their demise.
The nodal architecture is generally effaced, but peripheral sinuses are often open and even dilated.20 At low power, there is usually a striking proliferation of postcapillary or high endothelial venules with prominent arborization. Follicles are typically regressed, but there is a proliferation of dendritic cells around high endothelial venules. The atypical T cells have clear cytoplasm, and are associated with small lymphocytes, immunoblasts, plasma cells, and histiocytes. The abnormal cells are usually positive for CD3, CD4, CD10, and CD279 (PD-1), a phenotype characteristic of TFH. A relationship to TFH cells has been confirmed by recent gene expression profiling data.21 Strong expression of CD10 and PD-1 in perifollicular lymphocytes can be helpful in the differential diagnosis with reactive hyperplasia. However, PD-1 is more weakly expressed normally in paracortical T cells, and therefore, only strong intense staining is diagnostically useful. CXCL13, a chemokine involved in B-cell trafficking into the germinal centers, is also expressed in angioimmunoblastic T-cell lymphoma.
Expansion of B cells is a hallmark of angioimmu-noblastic T-cell lymphoma, likely due to the function of the neoplastic cells as TFH. Polyclonal hypergam-maglobulinemia and plasmacytosis were recognized early on as characteristic features, and in some series clonal B-cell populations were identified by molecular techniques.22 Because both B cells and T cells were expanded, some early reports viewed angio-immunoblastic T-cell lymphoma as a disorder of immunoregulation, with a predisposition to lymphoma.23–25 Subsequent studies identifying clonality of the T cells led to the view that angioimmunoblastic T-cell lymphoma was a form of PTCL.26 However, more recently the B-cell proliferations in angioimmunoblastic T-cell lymphoma and other PTCL of TFH derivation have received greater attention.
EBV-positive B cells comprise the most frequently identified atypical B-cell component in angioimmunoblastic T-cell lymphoma. EBV-positive B cells, often with immunoblastic features, are nearly always present in the background.27 The supposition was that there was defective immune surveillance for EBV related to the underlying T-cell lymphoma. However, the exact role of EBV in the disease remains uncertain—is it a precipitating event or just a passenger?18 The number of EBV-positive cells is variable, and progression to EBV-positive DLBCL has been reported in rare cases.28,29 In other cases, EBV-positive large B-cell lymphoma may be a presenting feature, even obscuring the underlying T-cell process (Figure 1).
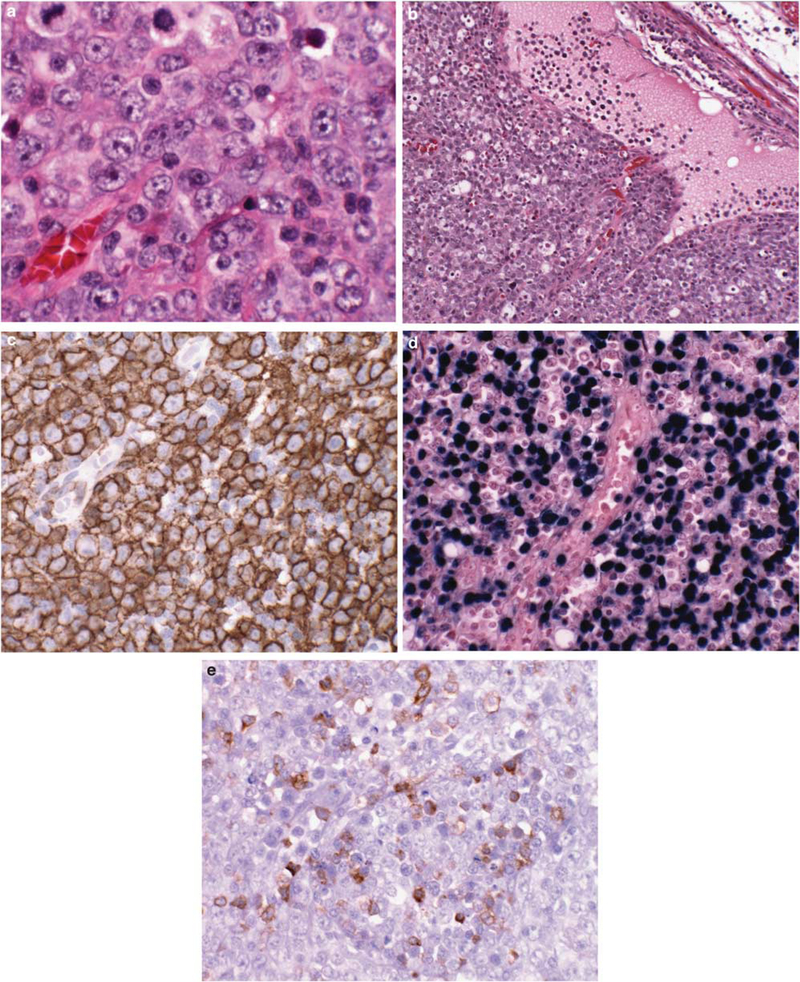
Figure 1.
Angioimmunoblastic T-cell lymphoma obscured by an EBV-positive diffuse large B-cell lymphoma, found at initial presentation. (a) Atypical large B cells with immunoblastic features diffusely efface the nodal architecture. (b) The peripheral cortical sinus is dilated, a clue to the underlying T-cell lymphoma. Immunoblastic cells are diffusely positive for CD20 (c), and EBV by EBER in situ hybridization (d). (e) Scattered CD10-positive T cells are clustered around high endothelial venules, the only indication for the underlying T-cell lymphoma. Both B-cell and T-cell clones were found, with the same T-cell clone found at relapse 3 years later with angioimmunoblastic T-cell lymphoma.
The EBV-positive B cells may resemble Hodgkin/Reed Sternberg (HRS) cells both morphologically and phenotypically.30 In some cases, this may lead to an erroneous diagnosis of classical Hodgkin’s lymphoma; the underlying angioimmunoblastic T-cell lymphoma may only become apparent over time, at relapse. A clue to the correct diagnosis may lie in the detection of the neoplastic cells through immunophenotypic means, expressing both strong PD-1 and less consistently CD10. In some cases, the HRS cells may be rosetted by the neoplastic T cells, suggesting the possibility of cooperation between these two populations of different lineages (Figure 2). Studies have shown that PD-L1, the ligand of PD-1, can be upregulated in Hodgkin’s lymphoma and EBV-positive post-transplant lymphoproliferative disease.31 The binding of PD-1-positive tumor cells to PD-L1 on abnormal B cells may lead to an immunosuppressive blockade, perhaps allowing continued survival of both populations.32
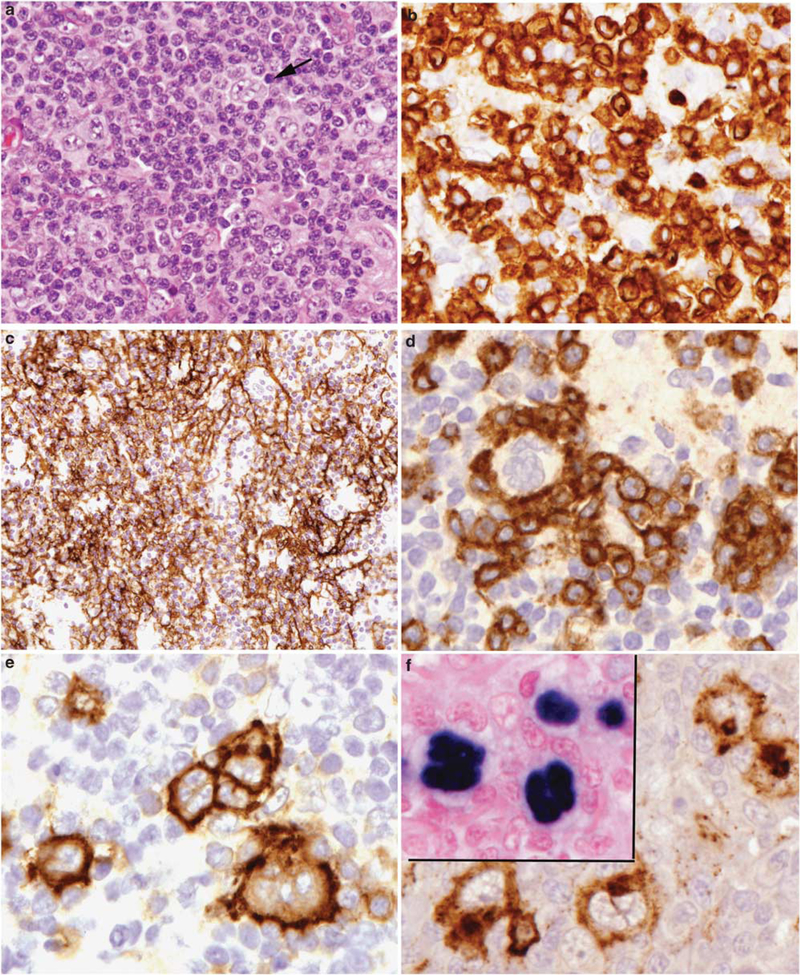
Figure 2.
Angioimmunoblastic T-cell lymphoma with Hodgkin-Reed Sternberg-like cells positive for EBV. (a) Atypical large cells, some binucleate (arrow) are seen in a background of atypical lymphocytes. (b) Cytological atypia is evident in the T cells, highlighted with a CD3 stain. (c) Follicular dendritic cell meshworks are expanded, as seen with a stain for CD21. (d) CD10-positive atypical lymphocytes rosette large atypical cells with Hodgkin-like features. The Hodgkin-like cells are positive for CD30 (e), CD15 (f), and EBER (inset).
We recently compiled our experience of cases resembling classical Hodgkin’s lymphoma and co-existent T-cell lymphoma. Fifty-seven such cases were identified.33 Differential diagnosis with classical Hodgkin’s lymphoma was the major diagnostic problem, with the majority of the Hodgkin-like cells expressing CD30 (100%), CD15 (81%), and PAX5 (100%). CD20 was negative in 33% of the cases. The majority of the T-cell lymphomas were classified as angioimmunoblastic T-cell lymphoma (32/57; 56%). Twenty-three cases (40%) were classified as PTCL, not otherwise specified, most with a demonstrable TFH phenotype; three of these had an intrafollicular growth pattern characteristic of the follicular variant, and one had a T-zone pattern. Two of the cases were classified as adult T-cell lymphoma/leukemia. Prior studies have reported EBV-positive Hodgkin’s cells in this HTLV-1 associated T-cell lymphoma characterized by marked immunosuppression.34,35
In 52 cases, as expected, the Hodgkin-like cells were EBV positive. However, in five cases EBV-negative Hodgkin’s cells were associated with a T-cell lymphoma of TFH phenotype (Figure 3). Three of the cases were classified as angioimmunoblastic T-cell lymphoma and two were considered as the follicular variant of PTCL, not otherwise specified. These observations expand the spectrum of abnormal B-cell proliferations occurring in angioimmunoblastic T-cell lymphoma and related TFH neoplasms, and confirm that the B-cell expansion is not attributable to EBV alone. Notably, in 4/5 of the T-cell lymphomas with EBV-negative Hodgkin’s cells, there was prominent rosetting of the Hodgkin’s cells by the neoplastic T cells expressing PD-1. This finding lends support to the view that the T cells may be facilitating the expansion of the abnormal B cells through an immunological blockade.32
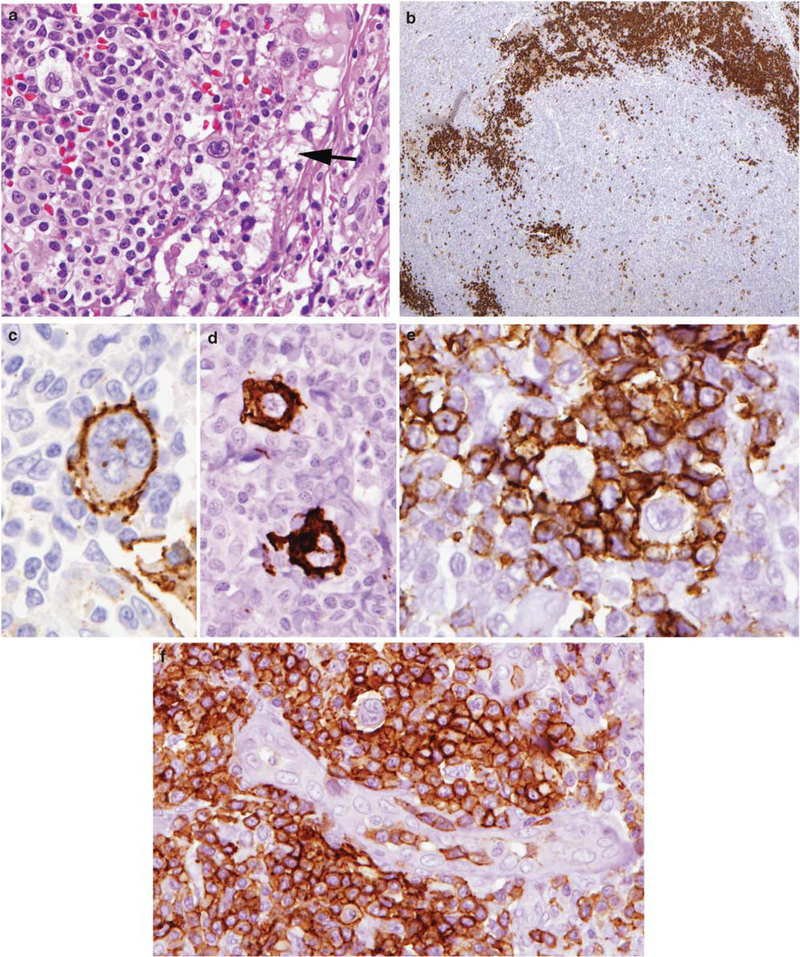
Figure 3.
Angioimmunoblastic T-cell lymphoma with Hodgkin-Reed Sternberg-like cells negative for EBV. (a) Atypical clear cells surround Hodgkin-like cells with pleomorphic nuclear features. The peripheral cortical sinus is dilated (arrow). (b) CD20 stain shows peripheralized B-cell areas in the cortex, and scattered larger atypical cells in the paracortex. (c) The Hodgkin-like cells are variably positive for CD20 (c), and CD15 (d), but negative for EBV (not shown). (e) The Hodgkin-like cells are rosetted by PD-1-positive T cells. (f) The PD-1-positive T cells cluster around high endothelial venules and surround a Hodgkin-like cell.
Clonal B-cell proliferations negative for EBV were previously reported by Balague et al.36 These too were more common in T-cell lymphomas of TFH derivation, and in most of the reported cases the atypical and clonal B cells showed evidence of plasmacytoid differentiation. There was some variation in the classification of the T-cell lymphomas with 4/15 cases classified as angioimmunoblastic T-cell lymphoma. The EBV-negative B-cell/plasma cell process may dominate the histological picture in some cases, making proper diagnosis difficult (Figure 4).37 Thus, the nature of angioimmunoblastic T-cell lymphoma is more complex than originally thought, with frequent clonal expansions of both B cells and T cells.
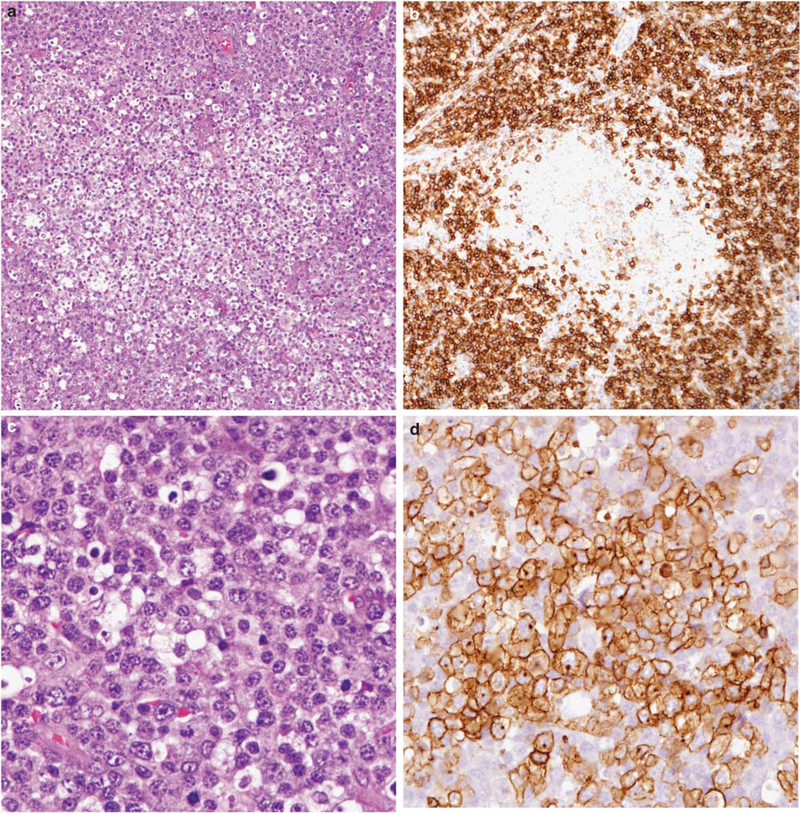
Figure 4.
Angioimmunoblastic T-cell lymphoma partially obscured by a monoclonal EBV-negative plasma cell proliferation. (a) Clusters of clear cells are surrounded by sheets of plasma cells, which occupied most of the lymph node. (b) Plasma cells are positive for CD138, and showed restricted staining for kappa light chain (not shown). Clear cell foci are negative for CD138. (c) Plasma cells show some nuclear atypia. (d) Clear cell foci stained for PD-1. CD10 and CD3 were also expressed/positive (not shown).
Clonal rearrangements of the T-cell receptor genes are found in most cases of angioimmunoblastic T-cell lymphoma. Clonal rearrangements of the immunoglobulin genes have been reported in 25–50% of cases, likely reflecting the B-cell expansion characteristic of the disease, or the role of EBV.22 The most common cytogenetic abnormalities are trisomy 3, trisomy 7, and an additional X chromosome.38 Most recently, mutations in IDH2 genes have been reported in 20–40% of cases.39 Mutations in IDH genes also have been found in glioblastoma and acute myeloid leukemia.
Peripheral T-Cell Lymphoma, Not Otherwise Specified, Follicular Variant
Peripheral T-cell lymphoma, not otherwise specified, follicular variant, is also composed of TFH cells, and may show overlap with angioimmunoblastic T-cell lymphoma, at least at the immunophenotypic level. The neoplastic cells are often confined to B-cell follicles, and may mimic follicular lymphoma.40 The cells typically express BCL6 but are usually negative for CD10. The lesions lack expansion of follicular dendritic cell meshworks outside the follicles, and usually do not have a prominent inflammatory background. However, in common with angioimmunoblastic T-cell lymphoma they may contain both EBV-positive and EBV-negative Hodgkin-like cells, as discussed above.33
Clinically, patients with the follicular variant of peripheral T-cell lymphoma more often present with localized disease, and lack the prominent constitutional symptoms associated with angioimmunoblastic T-cell lymphoma. Whether the follicular variant of peripheral T-cell lymphoma should be segregated from other TFH lymphomas requires further study.41 For example, a recent study identified TET2 mutations in angioimmunoblastic T-cell lymphoma (47%), and in 58% of cases of peripheral T-cell lymphoma, not otherwise specified, expressing TFH markers.42 Overlap among the TFH neoplasms has also been shown by gene expression profiling.21 However, genetic studies identified a novel t(5;9)(q33;q22) fusion involving the ITK and SYK genes in a proportion of cases of the follicular variant, but not in angioimmunoblastic T-cell lymphoma.43 Future studies should help to resolve these issues.
Peripheral T-cell lymphomas, not otherwise specified
As the name implies, peripheral T-cell lymphoma, not otherwise specified, is a diagnosis of exclusion and is admittedly a heterogeneous category with most cases being nodal in origin. Therefore, not unexpectedly the cytological spectrum is very broad.44 An inflammatory background is frequent, consisting of eosinophils, plasma cells, and histiocytes. If the epithelioid histiocytes are numerous and clustered, then the neoplasm fulfils the criteria for the lymphoepithelioid cell variant of PTCL. The T-zone variant is composed of small- to medium-sized cells that preferentially involve the paracortical regions of the lymph node.45,46
Clinically, peripheral T-cell lymphomas, NOS most often present in older adults. Advanced stage is common, and with poor clinical outcome. Treatment with anthracycline-containing regimens, the basis for treatment of aggressive B-cell lymphomas, has not improved long-term survival, and new treatment options are being sought.1 This category is by definition heterogeneous, and it is likely that individual clinicopathologic entities will be delineated in the future. Thus far, immunophenotypic criteria have not been helpful in delineating subtypes. Most cases have a mature T-cell phenotype, and express one of the major subset antigens: CD4˃CD8. These are not clonal markers, and antigen expression can change over time. Loss of one of the pan T-cell antigens (CD3, CD5, CD2, or CD7) is seen in 75% of cases, with CD7 most frequently being absent. Gene expression profiling studies have shown some cases with a profile resembling angioimmunoblastic T-cell lymphoma.21 Cases with a high proliferation signature appear to have a more aggressive clinical course,47 but expression profiling has not yet led to the delineation of distinctive subtypes as independent entities.48
Adult T-cell leukemia/lymphoma
Adult T-cell leukemia/lymphoma is a distinct clinicopathologic entity associated with the retrovirus HTLV-I, which is found clonally integrated in the T cells. HTLV-1 infection is endemic in Southwestern Japan, the Caribbean basin, and also encountered in Africa.49 The disease has a long latency, and affected individuals usually are exposed to the virus very early in life. The virus may be transmitted in breast milk, through sexual contact, and through exposure to blood and blood products. The cumulative incidence is estimated to be 2.5% among HTLV-1 carriers over a 70-year lifespan.
The median age of affected individuals is 45 years. Patients may present with leukemia or with generalized lymphadenopathy. Other clinical findings include lymphadenopathy, hepatosplenomegaly, lytic bone lesions, and hypercalcemia. Cutaneous involvement is seen in the major of patients. Both the acute form of the disease, which is mainly leukemic, and the lymphomatous form are associated with a poor prognosis and a median survival of o2 years.50,51 Complete remissions may be obtained but the relapse rate is nearly 100%. Chronic and smoldering forms of the disease are seen less commonly, and are associated with minimal lympha-denopathy. The predominant clinical manifestation is skin rash, with only small numbers of atypical cells in the peripheral blood.
The cytologic spectrum is extremely diverse.52 The cells are often markedly polylobated, and have been referred to as flower cells. Peripheral blood involvement is very common, but often in the absence of bone marrow disease. Immunopheno-typically, the neoplastic cells are positive for mature T-cell antigens, such as CD2, CD3, and CD5; they are typically CD4/CD25 positive, a phenotype that resembles Tregs.13 Some cases express FoxP3, but usually in a minority of tumor cells.13 Other studies have found distinguishing features between normal Tregs, and the tumor cells of adult T-cell leukemia/lymphoma.53 The HTLV-1 virus appears to have a role in inducing the Treg phenotype.54
ALCL, ALK positive
ALCL, ALK positive, is characterized by pleomorphic or monomorphic cells, which have a propensity to invade lymphoid sinuses.55 Because of the sinusoidal location of the tumor cells, and their lobulated nuclear appearance, this disease when first observed was suspected to be of histiocytic origin. A consistent feature is the strong expression of CD30 antigen, a diagnostic hallmark. However, CD30 expression is not specific and can be seen in a variety of conditions, of course, including classical Hodgkin’s lymphoma. The disease is associated with a characteristic chromosomal translocation, t(2;5)(p23;q35) involving NPM/ALK genes, respectively. A number of variant trans-locations have been identified that involve partners other than NPM. All lead to overexpression of ALK, although the cellular distribution of ALK varies according to the gene partner.56
Classical ALCL usually displays a cohesive growth pattern. The cells have large, often lobulated nuclei with small basophilic nucleoli, so-called hallmark cells. The cytoplasm is usually abundant, amphophilic. A prominent Golgi region is generally visible. Small cell and lymphohistiocytic variants constitute part of the entity, and perhaps paradoxically, are associated with a more aggressive clinical course.57
The cells exhibit an aberrant phenotype with loss of many of the T cell-associated antigens. Both CD3 and CD5 are negative in ˃50% of cases. CD2 and CD4 are positive in the majority of cases; CD8 is usually negative. The cells exhibit positivity for the cytotoxic-associated antigens TIA-1, granzyme B, and perforin. In addition, clusterin is generally present and represents another potentially useful diagnostic marker.58 By molecular studies, in most of the cases a clonal T-cell receptor rearrangement is found, confirming a T-cell origin.
ALCL, ALK positive, is most common in children and young adults, with a marked male predominance. Although most patients present with nodal disease, a high incidence of extranodal involvement has been reported (involving skin, bone, and soft tissue). Approximately 75% of patients present with advanced-stage and systemic symptoms. Although these lymphomas have an aggressive natural clinical history, they respond well to chemotherapy. Overall survival and disease-free survival are significantly better for ALK-positive vs ALK-negative cases. However, both ALK-positive and ALK-negative ALCL have a better prognosis than other T-cell lymphomas, with a plateau in the survival curve seen in both groups.1
ALCL, ALK negative (provisional entity)
It has been controversial whether anaplastic lymphoma negative for ALK is a separate entity or part of the spectrum of peripheral T-cell lymphoma, not otherwise specified. Part of the controversy relates to the lack of absolute criteria to recognize this disease. It should be morphologically and phenotypically similar to ALK anaplastic lymphoma, with strong CD30 expression and a cytotoxic phenotype. These lesions occur in an older age group than in ALK-positive cases, and as noted above, appear to have a better prognosis than other T-cell lymphoma subtypes.59 PAX5 gene amplification has been reported in a subset of ALK-negative tumors, and is associated with weak expression of PAX5 in those tumors.60 Disruptions in the DUSP22/IRF4 locus also have been reported as a recurrent abnormality in some cases of ALK-negative anaplastic lymphoma.61 As the molecular phenotypes are better delineated, the nature of ALK-negative ALCL is likely to be more clearly resolved.
There is a subset of T-cell lymphomas with anaplastic morphology, strong expression of CD30, and co-expression of CD15.62 These tumors show histological overlap with ALK-negative anaplastic lymphoma, but appear to have a more aggressive clinical course. They are generally grouped with the peripheral T-cell lymphomas, not otherwise specified, until this issue is resolved.
Seroma-associated ALCL of the breast
In recent years, a series of individual case reports and small series have described ALCL, ALK negative arising in association with breast implants placed for cosmetic purposes or as part of reconstruction following surgery for carcinoma.63–65 More than 40 cases have been reported. The median time from placement of the implant to development of ALCL is 8 years. Both silicone- and saline-filled implants have been implicated, but with a higher proportion of cases involving silicone. It has been suggested that textured implants are associated with an increased risk.66
Patients affected with this tumor most often present with a seroma, hence the designation ‘seroma-associated ALCL.’ Other common presentations include a mass in the fibrous capsule or severe contracture of the fibrous capsule surrounding the implant. All cases to date have been unilateral.
Cytologically, the neoplastic cells are large, pleomorphic cells with basophilic cytoplasm, best identified in cytological specimens obtained from the seroma fluid. In histological sections, the tumor cells are generally adherent to the fibrous capsule. The cells are non-cohesive and lack an inflammatory background. By immunohistochemistry, the cells are strongly positive for CD30, usually positive for cytotoxic granule-associated proteins, EMA, and clusterin, and negative for CD15 and CD20. The cells express T cell-associated markers, most commonly CD3 and CD2, with variable expression of other T cell-associated antigens. In situ hybridization for EBER is negative. Most cases of seroma-associated ALCL show clonal rearrangement of the T-cell receptor genes.
When confined to the seromatous fluid, these cases appear to have a good prognosis.65 The optimal therapeutic management has not yet been determined, and some patients have done well with limited local therapy, including removal of the implant with watchful waiting.67 Cases with invasion of the breast parenchyma or axillary lymph nodes require more aggressive approaches.68
Primary cutaneous ALCL
The primary cutaneous form of ALCL is closely related to lymphomatoid papulosis, and differs clinically, immunophenotypically, and at the molecular level from the systemic form. Lymphomatoid papulosis and cutaneous anaplastic lymphoma are part of the spectrum of CD30-positive cutaneous T-cell lymphoproliferative diseases. Small lesions are likely to regress. Patients with large tumor masses may develop regional lymph-node involvement. However, primary cutaneous anaplastic lymphoma is a much more indolent disease than systemic anaplastic lymphoma. Because the skin nodules may show spontaneous regression, usually a period of observation is warranted before the institution of any chemotherapy. The neoplastic cells are CD30 positive but ALK negative. CD15 is expressed in some cases, and when lymph-node involvement occurs, the lesion may simulate classical Hodgkin’s lymphoma.69 The cells lack translocations involving the ALK gene, but may shown translocations of IRF4/DUSP22 in a subset of cases.70 Mucosal presentations with a similar morphology and immunophenotype have been reported, and are likely part of the same spectrum of disease (Figure 5).71 The mucosal forms show overlap with traumatic ulcerative granulomas with stromal eosinophilia, referred to as TUGSE, which has alarming cytological features but a usually self-limited clinical course.72,73
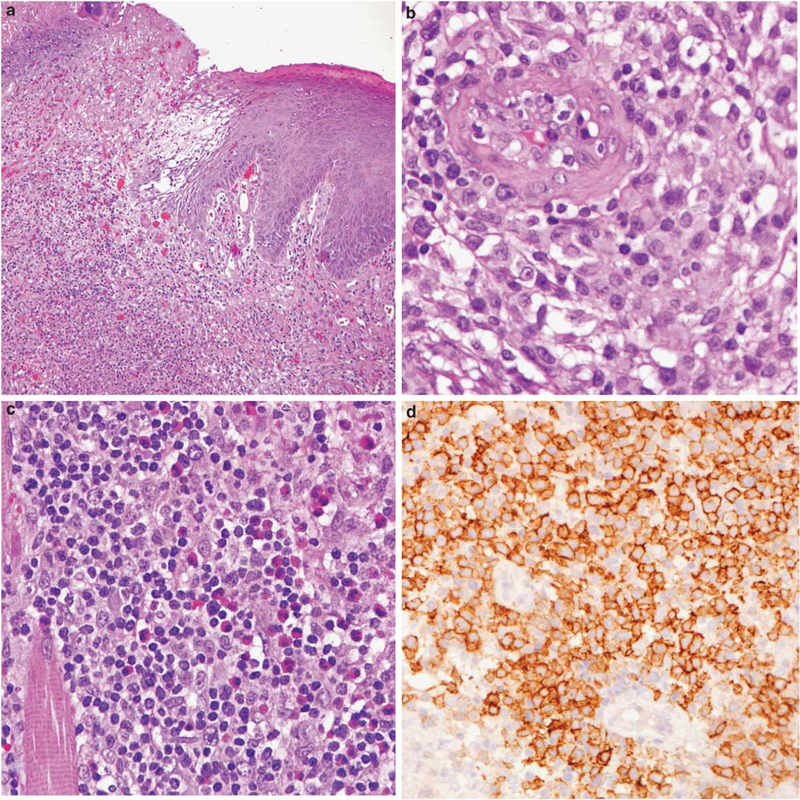
Figure 5.
CD30-positive T-cell lymphoproliferative disorder presenting with an ulcerative lesion of the tongue. (a) The mucosal surface is ulcerated, and a polymorphous lymphoid infiltrate extends deeply. (b) Atypical large T cells cluster around blood vessel.(c) Lymphocytes are admixed with numerous eosinophils. (d) Atypical lymphoid cells are CD30 positive and were clonal by T-cell receptor g gene rearrangement. Lesions with these features have been referred to in the literature as traumatic ulcerative granulomas with stromal eosinophilia, but likely represent a mucosal variant of primary cutaneous CD30-positive T-cell lymphoproliferative disease.
Mycosis fungoides/Sézary syndrome
Mycosis fungoides/Sézary syndrome are now regarded as separate diseases, but are often considered together for historical reasons and some overlap in the clinical presentation.74 Both are primary cutaneous T-cell malignancies derived from mature CD4-positive skin-homing T cells. Skin involvement may be manifested as multiple cutaneous plaques or nodules. Sézary syndrome is characterized by erythroderma and a leukemic phase.75 Lymph-adenopathy is usually not present at presentation and, when identified, is associated with a poor prognosis. The staging system for mycosis fungoides and Sézary syndrome includes an assessment of lymph-node involvement at the histological level.75 In early stages, enlarged lymph nodes may only show dermatopathic changes (NI). If malignant cells are present in significant numbers and are associated with architectural effacement (NII or NIII), then the prognosis is significantly worse.75
Cytologically, the small cells of mycosis fungoides demonstrate cerebriform nuclei with clumped chromatin, inconspicuous nucleoli, and sparse cytoplasm. Epidermotropism is a hallmark but Pautrier microabscesses are often absent in early plaque stage lesions.76,77 Sézary syndrome presents with exfoliative erythroderma and circulating cerebriform lymphocytes. Both forms of cutaneous T-cell lymphoma are CD2, CD3, CD5, CD4, and CD8. However, CD8-positive variants of mycosis fungoides have been described, and are more common in children. The absence of CD7 is a constant feature but may also be seen in reactive conditions, and therefore is of limited diagnostic value. Aberrant expression of other T-cell antigens may be seen but mainly occurs in the advanced (tumor) stages. Inactivation of p16 (CDKN2A) and PTEN has been identified in some cases and may be associated with disease progression. The molecular alterations in Sézary syndrome differ from those of mycosis fungoides, and implicate potential tumor suppressor genes,78 and upregulation of certain microRNAs.79
Subcutaneous panniculitis-like T-cell lymphoma
This form of T-cell lymphoma presents with subcutaneous nodules, primarily affecting the extremities and trunk.80,81 The nodules range in size from 0.5 cm to several centimeters in diameter. The disease has a very wide age range, and may be encountered in young children.82 The median age at presentation is ~30 years. Atypical lymphoid cells rim individual fat cells. The differential diagnosis with panniculitis is problematic in some cases, and some cytological atypia should be evident. Admixed reactive histiocytes are frequently present, particularly in areas of fat infiltration and destruction. Vascular invasion may be seen in some cases, and necrosis and karyorrhexis are common. The neoplastic cells are CD8-positive αβ T cells, with tumors composed of γδ T cells now included under primary cutaneous γδ T-cell lymphomas.83 The cells display an activated cytotoxic immunophenotype (positive for TIA-1, granzyme B, and perforin). These proteins may be responsible for the cellular destruction seen in these tumors.
A hemophagocytic syndrome is less often seen than in panniculitis-like tumors of γδ T-cell derivation, but whenever seen, is associated with an adverse prognosis.84 Patients present with fever, pancytopenia, and hepatosplenomegaly. The cause of the hemophagocytic syndrome appears related to cytokine production by the malignant cells.
The differential diagnosis includes lupus profundus. Because oligoclonal T-cell populations can sometimes be detected in lupus profundus, the differential diagnosis can be challenging. Plasma cells are usually very few in number, and a prominent plasmacytosis favors lupus profundus panniculitis. Some studies have reported a history of lupus or other autoimmune diseases in patients with panniculitis-like T-cell lymphoma.85 However, most of these cases have had a very benign course, and were successfully managed with steroids or relatively limited therapy, raising the possibility of misdiagnosis as lymphoma. A conservative approach is recommended if convincing evidence of malignancy is absent.
Primary cutaneous γδ T-cell lymphoma
Primary cutaneous γδ T-cell lymphoma is considered as a distinct entity, which can involve the subcutis, the dermis, or with epidermal infiltration.7,86 These are clinically aggressive tumors. The cells have a cytotoxic phenotype, and like normal γδ T cells, lack CD5, and express cytotoxic molecules. They may be CD8 positive or more often, double negative for CD4 and CD8. Cases with involvement of subcutaneous tissue may resemble panniculitis-like T-cell lymphoma, but have a more aggressive clinical course.84 While skin is the most common presenting site, similar lymphomas of γδ T-cell origin can present in other extranodal sites, most often gastrointestinal tract.9 A distinction from enteropathy-associated T-cell lymphoma (EATL), Type II, which is commonly of γδ T-cell derivation, may be difficult. Prominent epitheliotropism is required for the latter diagnosis. As currently defined, the cells of primary cutaneous γδ T-cell lymphoma are EBV negative, and show clonal rearrangement of T-cell receptor genes. If EBV is positive, then the diagnosis of extranodal nasal NK/T-cell lymphoma is favored.
Primary cutaneous CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma and primary cutaneous CD4-positive small/medium T-cell lymphoma
These are provisional entities listed in the 2008 WHO classification.
Primary Cutaneous CD8-Positive Aggressive Epidermotropic Cytotoxic T-Cell Lymphoma
Primary cutaneous CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma is an aggressive cutaneous neoplasm that shares many clinical features with primary cutaneous γδ T-cell lymphoma, but is derived from cytotoxic ab T cells. As the term implies, the neoplastic cells show prominent epidermotropism. Distinction from other CD8 T-cell neoplasms including a subset of mycosis fungoides and lymphomatoid papulosis type D may be difficult, and requires careful clinical assessment.87,88
Primary Cutaneous CD4-Positive Small/Medium-Cell Lymphoma
Primary cutaneous CD4-positive small/medium T-cell lymphoma most often presents with slowly growing, localized skin lesions, often involving the head or scalp. It is associated with an excellent prognosis, and requires only limited localized therapy.89,90 The presence of rapidly growing or bulky tumors should raise concern.89 Some authors have questioned whether this lymphoid proliferation should be considered as a form of ‘pseudolymphoma’, often containing T-cell clones, but having limited potential for progression.91,92 The lesions are rich in B cells, and the proliferating T cells have a TFH phenotype.93 PD-1 is the most useful marker for recognition, as CD10 is usually negative, in contrast to angioimmunoblastic T-cell lymphoma.92,93 Alternative terminologies have been suggested, such as ‘primary cutaneous CD4 T-cell lymphoproliferative disease’, or ‘cutaneous nodular proliferation of pleomorphic T lymphocytes of undetermined significance’.91
Enteropathy-associated T-cell lymphoma
Two variants of EATL were included in the 2008 WHO, referred to as Type I and Type II. Type I is associated with either overt or clinically silent gluten-sensitive enteropathy, and is largely seen in patients of European extraction, whereas the Type II form has a more worldwide distribution, and is not clearly linked to celiac disease. It should be considered as a separate entity. Patients with both types usually present with abdominal symptoms, including pain, small bowel perforation, and associated peritonitis. The clinical course is aggressive, and most patients have multifocal intestinal disease.94
In Type I, the cytological composition is somewhat varied, and more polymorphous than Type II lymphomas. The neoplastic cells show prominent invasion of the mucosa and are cytotoxic T cells most often of αβ origin. The cells also express the homing receptor CD103 (HML-1). Cells with anaplastic features positive for CD30 may be present. The adjacent small bowel usually shows villous atrophy associated with celiac disease. In Type II, the infiltrate is monomorphic, composed of medium-sized cells with clear cytoplasm showing prominent epitheliotropism. Spread of tumor cells in the epithelium distal to the main lesion may be seen, mimicking the changes of celiac disease. In Type II, the cells are CD56 positive, CD8 positive, and most often of γδ T-cell derivation. An association with celiac disease is only seen sporadically, and this form of the disease is relatively common in Asia.9,95,96 Both Type I and II share some genetic aberrations, including chromosomal gains on 9q33–34. Other T-cell lymphomas can present with intestinal disease, and should be distinguished from EATL. These include the EBV-positive extranodal T/ NK cell lymphomas of nasal type, and other γδ T-cell lymphoma lacking epitheliotropism.9,97
Hepatosplenic T-cell lymphoma
Hepatosplenic T-cell lymphoma presents with marked hepatosplenomegaly in the absence of lymphadenopathy. The great majority of cases are of γδ T-cell origin.8,98 Most patients are male, with a peak incidence in young adults. There is an association with iatrogenic immunosuppression, both in solid organ transplant recipients and in patients with Crohn’s disease receiving immunosuppressive agents, in particular purine analogs and infliximab, an inhibitor of tumor necrosis factor.8,99,100 Although patients may respond initially to chemotherapy, relapse has been seen in the majority of cases, and the median survival is <3 years. Allogeneic hematopoietic cell transplantation has led to long-term disease-free survival in some cases.
The cells of hepatosplenic T-cell lymphoma are usually moderate in size, with a rim of pale cytoplasm. The nuclear chromatin is dispersed, with small inconspicuous nucleoli. The pattern of infiltration mimics the homing pattern of γδ T cells with marked sinusoidal infiltration in liver and spleen. Abnormal cells are usually present in the sinusoids of the bone marrow but may be difficult to identify without immunohistochemical stains. The neoplastic cells also have a phenotype that resembles that of normal resting γδ T cells. They are often negative for both CD4 and CD8, although CD8 may be expressed in some cases. CD56 is typically positive. The neoplastic cells express markers associated with cytotoxic T cells, such as TIA-1. However, perforin and granzyme B are usually negative, suggesting that these cells are not activated. Isochromosome 7q is a consistent cytogenetic abnormality, and is often seen in association with trisomy 8. Cases of αβ T-cell derivation have similar immunophenotypic and genetic features, but are more common in females, with an older age distribution. Interestingly, they have a very similar gene expression profile to tumors of γδ T-cell derivation.101
Extranodal NK/T-cell lymphoma, nasal-type, and other EBV-positive T/NK cell neoplasms
Extranodal NK/T-Cell Lymphoma, Nasal Type
Extranodal NK/T-cell lymphoma, nasal type, is a distinct clinicopathologic entity highly associated with EBV.102,103 It is much more common in Asians than in Europeans.104 Clusters of the disease also have been reported in Central and South America in individuals of Native American heritage, suggesting that ethnic background, ie, genetic risk factors, may have a role in the pathogenesis of these lymphomas.105,106 It affects adults (median age 50) and the most common clinical presentation is a destructive nasal or midline facial lesion. Palatal destruction, orbital swelling, and edema may be prominent. Extranodal NK/T-cell lymphomas have been reported in other extranodal sites, including skin, soft tissue, testis, upper respiratory tract, and gastrointestinal tract. The clinical course is usually aggressive, with improved median survival for patients with localized disease, in which local radiation therapy has a role.104 A hemophagocytic syndrome is a common clinical complication, and adversely affects survival.
Extranodal NK/T-cell lymphoma, nasal type, is characterized by a broad cytologic spectrum. Tumors composed of smaller cells, with a more polymorphic composition, have been associated with a slightly improved prognosis, but clinical factors are more important than cytology in predicting outcome.107,108 Although the cells express some T cell-associated antigens, most commonly CD2, other T-cell markers, such as surface CD3, are usually absent. The cells express cytoplasmic CD3, but usually lack T-cell receptor gene rearrangement. The cells are usually CD56 positive, but do not express CD57 or CD16. EBV is positive in 100% of cases by in situ hybridization. A subset of cases is of true T-cell derivation, with clonal rearrangement of T-cell receptor genes.97
Aggressive NK-Cell Leukemia
Aggressive NK-cell leukemia is a closely related entity. It presents at a younger age than extranodal NK/T-cell lymphoma, is associated with systemic disease, and a fulminant clinical course. It has a similar phenotype, EBV association, and epidemiology. Gene expression profiling studies show similarities among all of the EBV-positive NK-cell malignancies.109 Deletions of chromosome 6q are also commonly found.110
EBV + T-Cell and NK-Cell Proliferations
There are other EBV T-cell and NK-cell proliferations that are seen mainly in children, and are often included under the broad heading of chronic active EBV infection.111,112 These include systemic EBV T-cell lymphoproliferative disease,113 hydroa vacciniforme-like lymphoma,114,115 and mosquito bite allergy,116 the latter usually being derived from NK cells. All are seen most often in Asian children but also reported in Central and South America, affecting indigenous populations. The latter two conditions affect mainly the skin and have a more indolent clinical course.
Hydroa Vacciniforme
Hydroa vacciniforme is associated with a very sparse CD3 + EBER + infiltrate with vesicle formation. The lesions are induced by sun exposure, and patients improve when exposure is avoided. It often regresses spontaneously as the children reach adulthood, but in some cases the infiltrate becomes more pronounced, meeting criteria for hydroa vacciniforme-like lymphoma. Criteria for the distinction of hydroa vacciniforme from hydroa vacciniforme-like lymphoma are not well delineated, but the former often lacks T-cell clonality at the molecular level, has a sparse infiltrate, and lacks cytological atypia.
Systemic EBV + T-Cell Lymphoproliferative Disease
Systemic EBV T-cell lymphoproliferative disease has a very aggressive clinical course with survival measured in weeks. It may arise in a background of chronic active EBV infection117 and there is overlap between what has been termed as ‘severe chronic active EBV infection’ and systemic EBV T-cell lymphoproliferative disease.111 It also show overlap in the clinical features with aggressive NK-cell leukemia/lymphoma, differing in the lineage of the tumor cells. Both conditions are associated with a hemophagocytic syndrome.
Footnotes
Disclosure/conflict of interest
The authors declare no conflict of interest.
References
- 1.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008;26:4124–4130. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe ES, Harris NL, Stein H, et al. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood 2008;112:4384–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delves PJ, Roitt IM. The immune system. First of two parts. N Engl J Med 2000;343:37–49. [DOI] [PubMed] [Google Scholar]
- 4.Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol 2009;6: 707–717. [DOI] [PubMed] [Google Scholar]
- 5.Krenacs L, Smyth MJ, Bagdi E, et al. The serine protease granzyme M is preferentially expressed in NK-cell, gamma delta T-cell, and intestinal T-cell lymphomas: evidence of origin from lymphocytes involved in innate immunity. Blood 2003;101:3590–3593. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe ES, Krenacs L, Raffeld M. Classification of cytotoxic T-cell and natural killer cell lymphomas. Semin Hematol 2003;40:175–184. [DOI] [PubMed] [Google Scholar]
- 7.Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood 2003;101:3407–3412. [DOI] [PubMed] [Google Scholar]
- 8.Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic {gamma}{delta} T-cell lymphoma is a rare clinico-pathologic entity with poor outcome: report on a series of 21 patients. Blood 2003;102:4261–4269. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Herrera A, Song JY, Chuang SS, et al. Non-hepatosplenic gammadelta T-cell lymphomas represent a spectrum of aggressive cytotoxic T-cell lymphomas with a mainly extranodal presentation. Am J Surg Pathol 2011;35:1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffe ES. Pathobiology of peripheral T-cell lymphomas. Hematology/The Education Program of the American Society of Hematology American Society of Hematology 2006;317–322. [DOI] [PubMed]
- 11.Crotty S Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011;29:621–663. [DOI] [PubMed] [Google Scholar]
- 12.Ma CS, Deenick EK, Batten M, et al. The origins, function, and regulation of T follicular helper cells. J Exp Med 2012;209:1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roncador G, Garcia JF, Garcia JF, et al. FOXP3, a selective marker for a subset of adult T-cell leukaemia/lymphoma. Leukemia 2005;19:2247–2253. [DOI] [PubMed] [Google Scholar]
- 14.Jones D Functional classification of peripheral T-cell lymphomas as an approach to improve outcome prediction and therapy selection. Semin Hematol 2010;47(Suppl 1):S1–S4. [DOI] [PubMed] [Google Scholar]
- 15.Teruya-Feldstein J, Setsuda J, Yao X, et al. MIP-1alpha expression in tissues from patients with hemophagocytic syndrome. Lab Invest 1999;79:1583–1590. [PubMed] [Google Scholar]
- 16.Grogg KL, Attygalle AD, Macon WR, et al. Angioimmunoblastic T-cell lymphoma: a neoplasm of germinal-center T-helper cells? Blood 2005;106:1501–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am J Surg Pathol 2006;30:490–494. [DOI] [PubMed] [Google Scholar]
- 18.Dunleavy K, Wilson WH, Jaffe ES. Angioimmuno-blastic T cell lymphoma: pathobiological insights and clinical implications. Curr Opin Hematol 2007;14: 348–353. [DOI] [PubMed] [Google Scholar]
- 19.Delfau-Larue MH, de Leval L, Joly B, et al. Targeting intratumoral B-cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma. A clinico-biological study of the GELA. Haematologica; advance online publication, 27 February 2012; 10.3324/haematol.2011.061507. (e-pub ahead of print) [DOI] [PMC free article] [PubMed]
- 20.Ottaviani G, Bueso-Ramos CE, Seilstad K, et al. The role of the perifollicular sinus in determining the complex immunoarchitecture of angioimmunoblastic T-cell lymphoma. Am J Surg Pathol 2004;28:1632–1640. [DOI] [PubMed] [Google Scholar]
- 21.de Leval L, Rickman DS, Thielen C, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood 2007;109:4952–4963. [DOI] [PubMed] [Google Scholar]
- 22.Tan BT, Warnke RA, Arber DA. The frequency of B- and T-cell gene rearrangements and epstein-barr virus in T-cell lymphomas: a comparison between angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified with and without associated B-cell proliferations. J Mol Diagn 2006;8: 466–475; quiz 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukes RJ, Tindle BH. Immunoblastic lymphadenopathy: a hyperimmune entity resembling Hodgkin’s disease. N Engl J Med 1975;292:1–8. [DOI] [PubMed] [Google Scholar]
- 24.Frizzera G, Moran E, Rappaport H. Angio-immunoblastic lymphadenopathy with dysproteinemia. Lancet 1974;i:1070–1073. [DOI] [PubMed] [Google Scholar]
- 25.Lipford EH, Smith HR, Pittaluga S, et al. Clonality of angioimmunoblastic lymphadenopathy and implications for its evolution to malignant lymphoma. J Clin Invest 1987;79:637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss L, Strickler J, Dorfman R, et al. Clonal T-cell populations in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Am J Pathol 1986;122:392–397. [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss LM, Jaffe ES, Liu XF, et al. Detection and localization of Epstein-Barr viral genomes in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Blood 1992;79:1789–1795. [PubMed] [Google Scholar]
- 28.Abruzzo LV, Schmidt K, Weiss LM, et al. B-cell lymphoma after angioimmunoblastic lymphadenopathy: a case with oligoclonal gene rearrangements associated with Epstein-Barr virus. Blood 1993;82: 241–246. [PubMed] [Google Scholar]
- 29.Zettl A, Lee SS, Rudiger T, et al. Epstein-Barr virus-associated B-cell lymphoproliferative disorders in angloimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J Clin Pathol 2002;117:368–379. [DOI] [PubMed] [Google Scholar]
- 30.Quintanilla-Martinez L, Fend F, Moguel LR, et al. Peripheral T-cell lymphoma with Reed-Sternberg-like cells of B-cell phenotype and genotype associated with Epstein-Barr virus infection. Am J Surg Pathol 1999;23:1233–1240. [DOI] [PubMed] [Google Scholar]
- 31.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res 2012;18:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicolae A, Plttaluga S, Venkataraman G, et al. Peripheral T-cell lymphomas of follicular T-helper cell derivation with Hodgkin/Reed-Sternberg cells of B-cell lineage: Both EBV-positive and EBV-negative variants exist. Am J Surg Pathol (in press). [DOI] [PMC free article] [PubMed]
- 34.Ohshima K, Kikuchi M, Yoshida T, et al. Lymph nodes in incipient adult T-cell leukemia-lymphoma with Hodgkin’s disease-like histologic features. Cancer 1991;67:1622–1628. [DOI] [PubMed] [Google Scholar]
- 35.Venkataraman G, Berkowitz J, Morris JC, et al. Adult T-cell leukemia/lymphoma with Epstein-Barr virus-positive Hodgkin-like cells. Hum Pathol 2011;42: 1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balague O, Martinez A, Colomo L, et al. Epstein-Barr virus negative clonal plasma cell proliferations and lymphomas in peripheral T-cell lymphomas: a phenomenon with distinctive clinicopathologic features. Am J Surg Pathol 2007;31:1310–1322. [DOI] [PubMed] [Google Scholar]
- 37.Huppmann A, Roullet M, Raffeld M, et al. Angioim-munoblastic T-cell lymphoma partially obscured by an EBV-negative clonal plasma cell proliferation. J Clin Oncol (in press) [DOI] [PMC free article] [PubMed]
- 38.Schlegelberger B, Zhang Y, Weber-Matthiesen K, et al. Detection of aberrant clones in nearly all cases of angioimmunoblastic lymphadenopathy with dysproteinemia-type T-cell lymphoma by combined interphase and metaphase cytogenetics. Blood 1994; 84:2640–2648. [PubMed] [Google Scholar]
- 39.Cairns RA, Iqbal J, Lemonnier F, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood 2012;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Leval L, Savilo E, Longtine J, et al. Peripheral T-cell lymphoma with follicular involvement and a CD4 /bcl-6 phenotype. Am J Surg Pathol 2001;25: 395–400. [DOI] [PubMed] [Google Scholar]
- 41.Agostinelli C, Hartmann S, Klapper W, et al. Peripheral T cell lymphomas with follicular T helper phenotype: a new basket or a distinct entity? Revising Karl Lennert’s personal archive. Morphologic and immunophenotypic variants of nodal T-cell lymphomas and T-cell lymphoma mimics. Histopathology 2011;59:679–691. [DOI] [PubMed] [Google Scholar]
- 42.Lemonnier F, Couronne L, Parrens M, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood 2012;120:1466–1469. [DOI] [PubMed] [Google Scholar]
- 43.Streubel B, Vinatzer U, Willheim M, et al. Novel t(5;9) (q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia 2006;20:313–318. [DOI] [PubMed] [Google Scholar]
- 44.Weisenburger DD, Savage KJ, Harris NL, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood 2011;117: 3402–3408. [DOI] [PubMed] [Google Scholar]
- 45.Rudiger T, Ichinohasama R, Ott MM, et al. Peripheral T-cell lymphoma with distinct perifollicular growth pattern: a distinct subtype of T-cell lymphoma? Am J Surg Pathol 2000;24:117–122. [DOI] [PubMed] [Google Scholar]
- 46.Warnke RA, Jones D, Hsi ED. Morphologic and immunophenotypic variants of nodal T-cell lymphomas and T-cell lymphoma mimics. Am J Clin Pathol 2007;127:511–527. [DOI] [PubMed] [Google Scholar]
- 47.Cuadros M, Dave SS, Jaffe ES, et al. Identification of a proliferation signature related to survival in nodal peripheral T-cell lymphomas. J Clin Oncol 2007;25: 3321–3329. [DOI] [PubMed] [Google Scholar]
- 48.Ballester B, Ramuz O, Gisselbrecht C, et al. Gene expression profiling identifies molecular subgroups among nodal peripheral T-cell lymphomas. Onco-gene 2006;25:1560–1570. [DOI] [PubMed] [Google Scholar]
- 49.Sonoda S, Li HC, Tajima K. Ethnoepidemiology of HTLV-1 related diseases: ethnic determinants of HTLV-1 susceptibility and its worldwide dispersal. Cancer Sci 2011;102:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tobinai K Clinical trials for human T-cell lympho-tropic virus type I-associated peripheral T-cell lymphoma in Japan. Semin Hematol 2010;47(Suppl 1): S5–S7. [DOI] [PubMed] [Google Scholar]
- 51.Takatsuki K Adult T-cell leukemia. Intern Med 1995; 34:947–952. [DOI] [PubMed] [Google Scholar]
- 52.Jaffe ES, Blattner WA, Blayney DW, et al. The pathologic spectrum of adult T-cell leukemia/lymphoma in the United States. Am J Surg Pathol 1984; 8:263–275. [DOI] [PubMed] [Google Scholar]
- 53.Abe M, Uchihashi K, Kazuto T, et al. Foxp3 expression on normal and leukemic CD4 CD25 T cells implicated in human T-cell leukemia virus type-1 is inconsistent with Treg cells. Eur J Haematol 2008;81:209–217. [DOI] [PubMed] [Google Scholar]
- 54.Satou Y, Utsunomiya A, Tanabe J, et al. HTLV-1 modulates the frequency and phenotype of FoxP3 CD4 T cells in virus-infected individuals. Retro-virology 2012;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaffe ES. Anaplastic large cell lymphoma: the shifting sands of diagnostic hematopathology. Mod Pathol 2001;14:219–228. [DOI] [PubMed] [Google Scholar]
- 56.Falini B, Pulford K, Pucciarini A, et al. Lymphomas expressing ALK fusion protein(s) other than NPM-ALK. Blood 1999;94:3509–3515. [PubMed] [Google Scholar]
- 57.Lamant L, McCarthy K, d’Amore E, et al. Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: results of the ALCL99 study. J Clin Oncol 2011;29:4669–4676. [DOI] [PubMed] [Google Scholar]
- 58.Wellmann A, Thieblemont C, Pittaluga S, et al. Detection of differentially expressed genes in lymphomas using cDNA arrays: identification of cluster-in as a new diagnostic marker for anaplastic large cell lymphomas (ALCL). Blood 2000;96:398–404. [PubMed] [Google Scholar]
- 59.Savage KJ, Harris NL, Vose JM, et al. ALK-anaplastic large-cell lymphoma is clinically and immunophe-notypically different from both ALK ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 2008;111:5496–5504. [DOI] [PubMed] [Google Scholar]
- 60.Feldman AL, Law ME, Inwards DJ, et al. PAX5-positive T-cell anaplastic large cell lymphomas associated with extra copies of the PAX5 gene locus. Mod Pathol 2010;23:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feldman AL, Dogan A, Smith DI, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood 2011;117: 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barry TS, Jaffe ES, Sorbara L, et al. Peripheral T-cell lymphomas expressing CD30 and CD15. Am J Surg Pathol 2003;27:1513–1522. [DOI] [PubMed] [Google Scholar]
- 63.Miranda RN, Lin L, Talwalkar SS, et al. Anaplastic large cell lymphoma involving the breast a clinico-pathologic study of 6 cases and review of the literature. Arch Pathol Lab Med 2009;133:1383–1390. [DOI] [PubMed] [Google Scholar]
- 64.de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA 2008;300:2030–2035. [DOI] [PubMed] [Google Scholar]
- 65.Roden AC, Macon WR, Keeney GL, et al. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: an indolent T-cell lymphoproliferative disorder. Mod Pathol 2008;21: 455–463. [DOI] [PubMed] [Google Scholar]
- 66.Brody GS. Brief recommendations for dealing with a new case of anaplastic large T-cell lymphoma. Plast Reconstr Surg 2012;129:871e–872ee. [DOI] [PubMed] [Google Scholar]
- 67.Thompson PA, Lade S, Webster H, et al. Effusion-associated anaplastic large cell lymphoma of the breast: time for it to be defined as a distinct clinico-pathological entity. Haematologica 2010;95:1977–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aladily TN, Medeiros LJ, Amin MB, et al. Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol 2012;36: 1000–1008. [DOI] [PubMed] [Google Scholar]
- 69.Eberle FC, Song JY, Xi L, et al. Nodal involvement by cutaneous CD30-positive T-cell lymphoma mimicking classical Hodgkin lymphoma. Am J Surg Pathol 2012;36:716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol 2011;24:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sciallis AP, Law ME, Inwards DJ, et al. Mucosal CD30-positive T-cell lymphoproliferations of the head and neck show a clinicopathologic spectrum similar to cutaneous CD30-positive T-cell lym-phoproliferative disorders. Mod Pathol 2012;25: 983–992. [DOI] [PubMed] [Google Scholar]
- 72.Gagari E, Stathopoulos P, Katsambas A, et al. Traumatic ulcerative granuloma with stromal eosinophilia: a lesion with alarming histopathologic presentation and benign clinical course. Am J Dermato-pathol 2011;33:192–194. [DOI] [PubMed] [Google Scholar]
- 73.Salisbury CL, Budnick SD, Li S. T-cell receptor gene rearrangement and CD30 immunoreactivity in traumatic ulcerative granuloma with stromal eosinophilia of the oral cavity. Am J Clin Pathol 2009;132:722–727. [DOI] [PubMed] [Google Scholar]
- 74.Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sezary syndrome. Lancet 2008;371:945–957. [DOI] [PubMed] [Google Scholar]
- 75.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007;110:1713–1722. [DOI] [PubMed] [Google Scholar]
- 76.Massone C, Kodama K, Kerl H, et al. Histopathologic features of early (patch) lesions of mycosis fungoides: a morphologic study on 745 biopsy specimens from 427 patients. Am J Surg Pathol 2005;29:550–560. [DOI] [PubMed] [Google Scholar]
- 77.Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol 2005; 53:1053–1063. [DOI] [PubMed] [Google Scholar]
- 78.Braun FC, Grabarczyk P, Mobs M, et al. Tumor suppressor TNFAIP3 (A20) is frequently deleted in Sezary syndrome. Leukemia 2011;25:1494–1501. [DOI] [PubMed] [Google Scholar]
- 79.Narducci MG, Arcelli D, Picchio MC, et al. Micro-RNA profiling reveals that miR-21, miR486 and miR-214 are upregulated and involved in cell survival in Sezary syndrome. Cell Death Dis 2011;2:e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar S, Krenacs L, Medeiros J, et al. Subcutaneous panniculitic T-cell lymphoma is a tumor of cytotoxic T lymphocytes. Hum Pathol 1998;29:397–403. [DOI] [PubMed] [Google Scholar]
- 81.Salhany KE, Macon WR, Choi JK, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinicopathologic, immunophenotypic, and genotypic analysis of alpha/beta and gamma/delta subtypes. Am J Surg Pathol 1998;22:881–893. [DOI] [PubMed] [Google Scholar]
- 82.Mehta N, Wayne AS, Kim YH, et al. Bexarotene is active against subcutaneous panniculitis-like T-cell lymphoma in adult and pediatric populations. Clin Lymphoma Myeloma Leuk 2012;12:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768–3785. [DOI] [PubMed] [Google Scholar]
- 84.Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood 2008;111:838–845. [DOI] [PubMed] [Google Scholar]
- 85.Pincus LB, LeBoit PE, McCalmont TH, et al. Sub-cutaneous panniculitis-like T-cell lymphoma with overlapping clinicopathologic features of lupus erythematosus: coexistence of 2 entities? Am J Dermatopathol 2009;31:520–526. [DOI] [PubMed] [Google Scholar]
- 86.Toro JR, Beaty M, Sorbara L, et al. gamma delta T-cell lymphoma of the skin: a clinical, microscopic, and molecular study. Arch Dermatol 2000;136:1024–1032. [DOI] [PubMed] [Google Scholar]
- 87.Saggini A, Gulia A, Argenyi Z, et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8 cytotoxic T-cell lymphoma. Description of 9 cases. Am J Surg Pathol 2010;34:1168–1175. [DOI] [PubMed] [Google Scholar]
- 88.Cardoso J, Duhra P, Thway Y, et al. Lymphomatoid papulosis type D: a newly described variant easily confused with cutaneous aggressive CD8-positive cytotoxic T-cell lymphoma. Am J Dermatopathol 2012;34:762–765. [DOI] [PubMed] [Google Scholar]
- 89.Garcia-Herrera A, Colomo L, Camos M, et al. Primary cutaneous small/medium CD4 þ T-cell lymphomas: a heterogeneous group of tumors with different clinicopathologic features and outcome. J Clin Oncol 2008;26:3364–3371. [DOI] [PubMed] [Google Scholar]
- 90.Grogg KL, Jung S, Erickson LA, et al. Primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma: a clonal T-cell lympho-proliferative disorder with indolent behavior. Mod Pathol 2008;21:708–715. [DOI] [PubMed] [Google Scholar]
- 91.Beltraminelli H, Leinweber B, Kerl H, et al. Primary cutaneous CD4 small-/medium-sized pleomorphic T-cell lymphoma: a cutaneous nodular proliferation of pleomorphic T lymphocytes of undetermined significance? A study of 136 cases. Am J Dermatopathol 2009;31:317–322. [DOI] [PubMed] [Google Scholar]
- 92.Cetinozman F, Jansen PM, Willemze R. Expression of programmed death-1 in primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma, cutaneous pseudo-T-cell lymphoma, and other types of cutaneous T-cell lymphoma. Am J Surg Pathol 2012;36:109–116. [DOI] [PubMed] [Google Scholar]
- 93.Rodriguez Pinilla SM, Roncador G, Rodriguez-Peralto JL, et al. Primary cutaneous CD4 small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. Am J Surg Pathol 2009;33: 81–90. [DOI] [PubMed] [Google Scholar]
- 94.Delabie J, Holte H, Vose JM, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the International Peripheral T-Cell Lymphoma Project. Blood 2011;118:148–155. [DOI] [PubMed] [Google Scholar]
- 95.Chan JK, Chan AC, Cheuk W, et al. Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent gammadelta T-cell receptor expression. Am J Surg Pathol 2011;35:1557–1569. [DOI] [PubMed] [Google Scholar]
- 96.Sun J, Lu Z, Yang D, et al. Primary intestinal T-cell and NK-cell lymphomas: a clinicopathological and molecular study from China focused on type II enteropathy-associated T-cell lymphoma and primary intestinal NK-cell lymphoma. Mod Pathol 2011;24:983–992. [DOI] [PubMed] [Google Scholar]
- 97.Pongpruttipan T, Sukpanichnant S, Assanasen T, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and alphabeta, gammadelta, and alphabeta/gammadelta T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol 2012;36:481–499. [DOI] [PubMed] [Google Scholar]
- 98.Cooke CB, Krenacs M, Stetler-Stevenson M, et al. Hepatosplenic gamma/delta T-cell lymphoma: a distinct clinicopathologic entity of cytotoxic gamma/ delta T-cell origin. Blood 1996;88:4265–4274. [PubMed] [Google Scholar]
- 99.Veres G, Baldassano RN, Mamula P. Infliximab therapy for pediatric Crohn’s disease. Expert Opin Biol Ther 2007;7:1869–1880. [DOI] [PubMed] [Google Scholar]
- 100.Herrinton LJ, Liu L, Abramson O, et al. The incidence of hepatosplenic T-cell lymphoma in a large managed care organization, with reference to anti-tumor necrosis factor therapy, Northern California, 2000–2006. Pharmacoepidemiol Drug Saf 2012;21:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Travert M, Huang Y, de Leval L, et al. Molecular features of hepatosplenic T-cell lymphoma unravels potential novel therapeutic targets. Blood 2012;119: 5795–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jaffe ES, Chan JK, Su IJ, et al. Report of the workshop on nasal and related extranodal angiocentric T/ natural killer cell lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol 1996;20:103–111. [DOI] [PubMed] [Google Scholar]
- 103.Chiang AK, Tao Q, Srivastava G, et al. Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin’s disease. Int J Cancer 1996;68:285–290. [DOI] [PubMed] [Google Scholar]
- 104.Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 2009;113:3931–3937. [DOI] [PubMed] [Google Scholar]
- 105.Arber DA, Weiss LM, Albujar PF, et al. Nasal lymphomas in Peru. High incidence of T-cell immunophenotype and Epstein-Barr virus infection. Am J Surg Pathol 1993;17:392–399. [PubMed] [Google Scholar]
- 106.Elenitoba-Johnson KSJ, Zarate-Osorno A, Meneses A, et al. Cytotoxic granular protein expression, Epstein-Barr virus strain type, and latent membrane protein-1 oncogene deletions in nasal T-lymphocyte/natural killer cell lymphomas from Mexico. Mod Pathol 1998; 11:754–761. [PubMed] [Google Scholar]
- 107.Ho FC, Choy D, Loke SL, et al. Polymorphic reticulosis and conventional lymphomas of the nose and upper aerodigestive tract: a clinicopathologic study of 70 cases, and immunophenotypic studies of 16 cases. Hum Pathol 1990;21:1041–1050. [DOI] [PubMed] [Google Scholar]
- 108.Liang R Diagnosis and management of primary nasal lymphoma of T-cell or NK-cell origin. Clin Lymphoma 2000;1:33–37; discussion 8. [DOI] [PubMed] [Google Scholar]
- 109.Iqbal J, Weisenburger DD, Chowdhury A, et al. Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic gammadelta T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro. Leukemia 2011;25:348–358. [DOI] [PubMed] [Google Scholar]
- 110.Wong N, Wong KF, Chan JK, et al. Chromosomal translocations are common in natural killer-cell lymphoma/leukemia as shown by spectral karyotyping. Hum Pathol 2000;31:771–774. [DOI] [PubMed] [Google Scholar]
- 111.Cohen JI, Kimura H, Nakamura S, et al. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8–9 September 2008. Ann Oncol 2009;20:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood 2012;119:673–686. [DOI] [PubMed] [Google Scholar]
- 113.Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV( ) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood 2000;96:443–451. [PubMed] [Google Scholar]
- 114.Gupta G, Man I, Kemmett D. Hydroa vacciniforme: a clinical and follow-up study of 17 cases. J Am Acad Dermatol 2000;42:208–213. [DOI] [PubMed] [Google Scholar]
- 115.Barrionuevo C, Anderson VM, Zevallos-Giampietri E, et al. Hydroa-like cutaneous T-cell lymphoma: a clinicopathologic and molecular genetic study of 16 pediatric cases from Peru. Appl Immunohistochem Mol Morphol 2002;10:7–14. [DOI] [PubMed] [Google Scholar]
- 116.Tokura Y, Ishihara S, Tagawa S, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of a juvenile type of Epstein-Barr virus-associated natural killer cell leukemia/lymphoma. J Am Acad Dermatol 2001;45:569–578. [DOI] [PubMed] [Google Scholar]
- 117.Kimura H Pathogenesis of chronic active Epstein-Barr virus infection: is this an infectious disease, lymphoproliferative disorder, or immunodeficiency? Rev Med Virol 2006;16:251–261. [DOI] [PubMed] [Google Scholar]