Myeloperoxidase (MPO) was first described as a highly abundant protein in neutrophils in 19411. In 1958, MPO was purified2, but the function remained unknown. Over the next decade, studies revealed the mechanisms of degranulation3 and reactive oxygen species production4 by neutrophils during phagocytosis. A series of pivotal studies in 1967 and 1968 by Klebanoff proposed the classical role of MPO in phagocytosis, suggesting that the MPO-halide-H2O2 system is a powerful anti-microbial mechanism5–7. Highly reactive products of this system, including HOCl, are short-lived and react rapidly with any oxidizable group to kill pathogens during phagocytosis. Less reactive products, including H2O2 and some chloroamines, can travel to be toxic at a distant site. Since MPO is a strongly basic protein and can bind to surface of a negatively charged cell, this potentially allows for continuous propagation of the anti-microbial response.
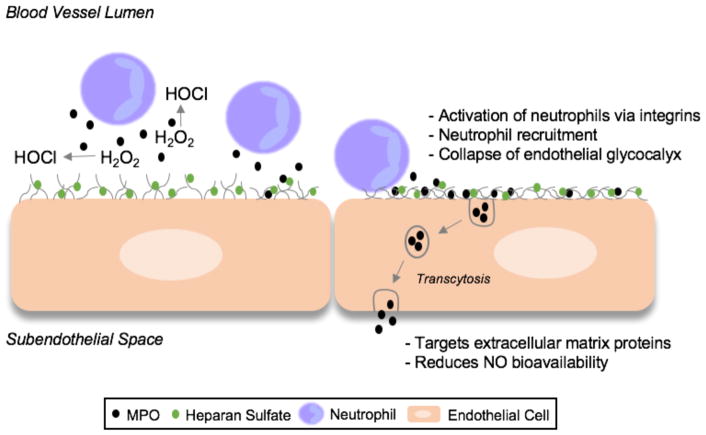
In contrast to the classical role of MPO, there has been significant evidence that MPO can damage host tissue and contribute to human disease, specifically those that involve damage to the endothelium in the vasculature. Leukocyte-endothelial interactions are critically regulated to maintain macro and microvascular health. Leukocyte MPO traditionally has been considered as a robust oxidation system that potentially has deleterious effects at the blood-endothelial interface, as well as the subendothelial space. Leukocyte interaction with the endothelium first involves an encounter with the endothelial glycocalyx. MPO has been shown to be released at or near the glycocalyx, accumulate along the endothelium, and be transported across endothelial cells8. MPO targets extracellular matrix proteins9, reduces NO availability10,11, and mediates neutrophil recruitment and activation12,13 (Figure). MPO plays a role in renal disease14, sickle cell disease15, ischemia/reperfusion injury16, atherosclerosis17, and sepsis18,19.
Figure.
The impact of MPO on the endothelium. Classically, MPO produces HOCl near the neutrophil-endothelial interface. Apart from the catalytic activity, MPO can also recruit and activate neutrophils. This novel paper demonstrates a physical interaction between MPO and heparan sulfate, leading to glycocalyx collapse. After transcytosis into the subendothelial space, MPO can also target extracellular matrix proteins and reduce NO bioavailability.
In this issue of ATVB, Manchanda et al reveal yet another deleterious role of MPO on the endothelium. Their cell culture and in vivo mouse studies elegantly demonstrate that MPO leads to the collapse of the endothelial glycocalyx. The authors revealed a novel mechanism in which MPO forms a physical interaction with glycocalyx heparan sulfate glycosaminoglycan residues leading glycocalyx collapse, independent of the classic catalytic function of MPO. The cationic charge of MPO destabilizes the negatively-charged endothelial glycocalyx, allowing for neutrophil recruitment and subsequent activation. Furthermore, MPO also stimulated the shedding of syndecan-1, a marker of endothelial glycocalyx breakdown. This is significant because these studies uncover another deleterious role of MPO on the endothelium.
Although this report by Manchanda et al reveals a novel function of MPO, it also raises multiple questions. The exact mechanism by which MPO binds to heparan sulfate remains to be elucidated. Does MPO have specific binding sites or rather is MPO binding non-specific for heparan sulfate? Heparan sulfate binds to proteins using a small number of cationic surface amino acids. The authors suggest that the binding is non-specific since MPO has more than 70 of these amino acids. Positive control experiments also showed positively charged polylysine binds to the glycocalyx reducing its negative charge. It is also important to note that neutrophil primary granules contain other cationic proteins, and these results do not exclude the possibility of their impact on the endothelial glycocalyx.
Overall, it is clear that MPO has many roles in changes in the endothelium. Studies examining the impact of MPO on altering endothelial function traditionally have focused on downstream products and actions of MPO catalytic activity, including tyrosine chlorination20, HOCl production21, MMP activation by cysteine oxidation22, and chlorinated lipid production19. The current study by Manchanda et al provides an important new mechanism for MPO elicited endothelial dysfunction. This is potentially an important and novel paradigm, but as is with all new models, important questions remain to further elucidate this model. Future studies should establish the role of the non-catalytic function of MPO in glycocalyx alterations in human disease.
Acknowledgments
Sources of Funding
This work was supported (in part) by research funding from the National Institutes of Health RO1 GM-115553 to D.A.F.
Footnotes
Disclosures
None.
References
- 1.Agner K. A ferment isolated from leucocytes. Acta Chemica Scandinavica. 1941;2:1–62. [Google Scholar]
- 2.Agner K. Crystalline Myeloperoxidase. Acta Chemica Scandinavica. 1958;12:89–94. [Google Scholar]
- 3.Hirsch JG, Cohn ZA. Degranulation of Polymorphonuclear Leucocytes following Phagocytosis of Microorganisms. The Journal of Experimental Medicine. 1960;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer GYN, Islam MF, Quastel JH. Biochemical Aspects of Phagocytosis. Nature. 1961;192:535. [Google Scholar]
- 5.Klebanoff SJ. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968;95:2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klebanoff SJ. Iodination of Bacteria: A Bactericidal Mechanism. The Journal of Experimental Medicine. 1967;126:1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klebanoff SJ. A peroxidase-mediated antimicrobial system in leukocytes. J Clin Invest. 1967;46:1078A. [Google Scholar]
- 8.Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7699–7704. doi: 10.1073/pnas.0401712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, Ma W, Tousson A, White CR, Bullard DC, Brennan ML, Lusis AJ, Moore KP, Freeman BA. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 11.Baldus S, Heitzer T, Eiserich JP, Lau D, Mollnau H, Ortak M, Petri S, Goldmann B, Duchstein HJ, Berger J, Helmchen U, Freeman BA, Meinertz T, Munzel T. Myeloperoxidase enhances nitric oxide catabolism during myocardial ischemia and reperfusion. Free Radic Biol Med. 2004;37:902–911. doi: 10.1016/j.freeradbiomed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brummer J, Rudolph V, Munzel T, Heitzer T, Meinertz T, Baldus S. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci U S A. 2005;102:431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinke A, Nussbaum C, Kubala L, et al. Myeloperoxidase attracts neutrophils by physical forces. Blood. 2011;117:1350–1358. doi: 10.1182/blood-2010-05-284513. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RJ, Couser WG, Chi EY, Adler S, Klebanoff SJ. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. The Journal of Clinical Investigation. 1987;79:1379–1387. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Xu H, Weihrauch D, Jones DW, Jing X, Shi Y, Gourlay D, Oldham KT, Hillery CA, Pritchard KA., Jr Inhibition of myeloperoxidase decreases vascular oxidative stress and increases vasodilatation in sickle cell disease mice. J Lipid Res. 2013;54:3009–3015. doi: 10.1194/jlr.M038281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthijsen RA, Huugen D, Hoebers NT, de Vries B, Peutz-Kootstra CJ, Aratani Y, Daha MR, Tervaert JWC, Buurman WA, Heeringa P. Myeloperoxidase Is Critically Involved in the Induction of Organ Damage after Renal Ischemia Reperfusion. The American Journal of Pathology. 2007;171:1743–1752. doi: 10.2353/ajpath.2007.070184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 18.Kothari N, Keshari RS, Bogra J, Kohli M, Abbas H, Malik A, Dikshit M, Barthwal MK. Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J Crit Care. 2011;26:435.e431–437. doi: 10.1016/j.jcrc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Meyer NJ, Reilly JP, Feng R, Christie JD, Hazen SL, Albert CJ, Franke JD, Hartman CL, McHowat J, Ford DA. Myeloperoxidase-derived 2-chlorofatty acids contribute to human sepsis mortality via acute respiratory distress syndrome. JCI Insight. 2017:2. doi: 10.1172/jci.insight.96432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stocker R, Huang A, Jeranian E, Hou JY, Wu TT, Thomas SR, Keaney JF., Jr Hypochlorous acid impairs endothelium-derived nitric oxide bioactivity through a superoxide-dependent mechanism. Arterioscler Thromb Vasc Biol. 2004;24:2028–2033. doi: 10.1161/01.ATV.0000143388.20994.fa. [DOI] [PubMed] [Google Scholar]
- 22.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]