Sympathetic nerve activity (SNA) is moderately elevated in essential hypertension (EH), obstructive sleep apnea (OSA) and extremely elevated in congestive heart failure (CHF); increased SNA presumably causes or exacerbates hypertension and other signs of these diseases1, 2. The rostral ventrolateral medulla (RVLM) is a major node of the brain network that generates SNA. In this review we survey the evidence which implicates the RVLM as a contributing factor in neurogenic hypertension and heart failure. Rather than providing a catalogue of the various observations pertaining to every animal model, we organize the information by pathophysiological mechanism: altered sympatho-respiratory coupling, oxidative stress, neuro-inflammation, brainstem hypoxia/blood flow restriction, altered balance of synaptic inputs and neuronal plasticity.
RVLM: definition, cytology and normal physiology: see on-line supplement
Current understanding of the role of the RVLM in hypertension is constrained by our limited knowledge of the cellular and integrative physiology of this structure (for prior reviews see3–6). The on-line supplement provides updated information on the RVLM connectome, and describes the properties of the neurons considered of most importance to cardiovascular regulation and hypertension. The following is a brief synopsis.
The RVLM (Figure 1A) was defined over 40 years ago as the area of distribution of the C1 adrenergic neurons. The C1 cells with spinal projections (presympathetic neurons, pSNs) innervate sympathetic preganglionic neurons (SPGNs) and are clearly important for BP control but not uniquely so; the RVLM contains non-C1 pSNs and many other types of pSNs are found elsewhere (spinal cord, raphe, ventromedial medulla, pons, hypothalamus). Importantly, the C1 neurons have direct projections to both parasympathetic and sympathetic preganglionic neurons as well as a host of pontine, midbrain and diencephalic structures including the orexinergic system and the paraventricular nucleus of the hypothalamus (Figure 1B). The potential contribution of the C1 cells without spinal projections to BP control has been ignored. Although many C1 bulbospinal neurons are barosensitive, this attribute is not required for neurons to regulate the cardiovascular portion of the sympathetic system. Bulbospinal serotonergic neurons and the respiratory neurons antecedent to RVLM pSNs have minimal if any baroreceptor input and yet are important to BP control and, possibly, to hypertension7–9. Finally, current concepts regarding the way in which the RVLM operates in conscious mammals, during various behaviors or normal physiological stresses, are almost entirely extrapolations of results obtained in anesthetized or reduced preparations without a brain rostral to the colliculli. Whether these views accurately represent the function of the RVLM in intact awake animals should be at the very minimum questioned in light of recent evidence that the RVLM and, in particular the C1 cells, also control the activity of vagal efferents, the paraventricular nucleus of the hypothalamus and major brainstem structures implicated in autonomic regulations including all subgroups of noradrenergic neurons10, 11. Also considered in the supplement are the synaptic and non-synaptic factors (hypoxia, hypercapnia, gliotransmitters) that define the discharge rate of the pSNs.
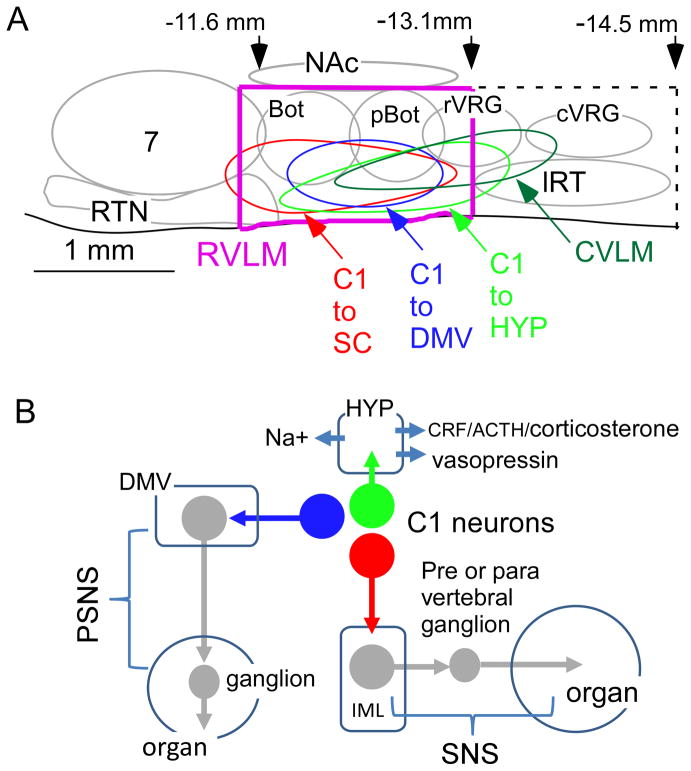
Figure 1. The RVLM and the three main clusters of C1 neurons.
A. Parasagittal schematic view of the rat RVLM. Abbreviations: Bot, pBot, rVRG, cVRG (the four subdivisions of the ventral respiratory column: Bötzinger, pre-Bötzinger, rostral ventral respiratory group and caudal VRG); RTN, retrotrapezoid nucleus (principal group of central respiratory chemoreceptors); CVLM, “caudal” VLM (location of GABAergic neurons that mediate the sympathetic baroreflex); lRT, lateral reticular nucleus. The approximate location of three subgroups of C1 cells is also shown. C1 and non-C1 RVLM pSNs are presumed to have the same general area of distribution.
B. Function of the three main groups of C1 cells located in the RVLM (color coding as in A). The C1 cells that project to the hypothalamus regulate vasopressin and ACTH release as well as sodium intake. The bulbospinal C1 cells are presympathetic for the most part. The third group of C1 cells regulate parasympathetic efferents.
In short, the RVLM connectome is far from understood. The various neuronal populations that make up this brain region remain to be identified by gene expression profiling and genetic lineage and the specific contribution of its many neuronal types to BP control has barely begun to be explored in healthy behaving mammals. Given these uncertainties, the existing pathophysiological data relative to the contribution of the RVLM to hypertension must be interpreted with caution.
Carotid body hyperactivity, RVLM and hypertension
Heightened carotid body afferent activity is now viewed as a major contributing factor to essential hypertension (modelled in rodents by the spontaneously hypertensive, SH rat), experimental renovascular hypertension (2K1C) and to hypertension caused by chronic intermittent hypoxia (CIH)12–14. Of note, CIH-induced hypertension in rats is eliminated not only by carotid body ablation but also by renal denervation, by administration of type-I angiotensin receptor (AT1R) antagonists or by adrenal gland demedullation15. This is an important lesson. The fact that a particular intervention normalizes BP does not prove that the manipulated mechanism/organ plays a unique role in the pathological process.
Because SNA is regulated by cardiopulmonary afferents and by the respiratory network, there has been a flurry of interest in the potential contribution of sympatho-respiratory coupling to SNA elevation in neurogenic hypertension. As summarized below, this mechanism accounts for many observations in reduced preparations but its contribution to SNA elevation in conscious hypertensive animals and most importantly humans is questionable. Respiration-independent mechanisms offer more plausible alternative explanations for the SNA increase and should be further examined.
The working heart-brainstem preparation (WHBP) is an arterially perfused midcollicular- decerebrated preparation from juvenile rats or mice in which brain neurons, cranial or spinal nerves and various sympathetic outflows can be recorded simultaneously. Its principal advantage is its mechanical stability, the lack of anesthesia and the ability to control the composition of the perfusion fluid. This preparation is especially useful to study respiratory-sympathetic coupling, however conclusions using this model must consider its limitations. In one study performed with this preparation16, the respiratory fluctuations of SNA were larger in SH rats than in normotensive controls whereas the respiratory outflow seemed normal, the very definition of enhanced respiratory-sympathetic coupling. However, others have since shown, also with the WHBP, that the respiratory pattern generator of the SH rat remains active at substantially lower levels of perfusate PCO2 than that of normotensive controls (online supplement in7). This suggests that central and/or peripheral chemoreceptors (Fig. 2, #4,10) and the respiratory pattern generator (Fig. 2, #7) are always more active at any given level of perfusion pressure and PCO2 in WHBPs from SH rats than normotensive controls. This is not surprising since the carotid bodies of the SH rat (Fig. 2, #4) are hypersensitive to hypoxia and carotid body afferents (Fig. 2, #5) acquire, even under normoxia, a baseline level of activity (tonicity)13, 14. Indeed, carotid body denervation attenuates hypertension considerably in the SH rat14. Also, the SH rat has a higher vascular resistance. Vascular steal is likely to reduce wash-out of metabolically produced CO2 from lower brainstem regions, that have an intact vasculature and contain the central chemoreceptors (Fig. 2, #10)17. The expected consequence is greater chemoreceptor activation, higher central respiratory drive and larger respiratory phasic fluctuations of SNA7, 16.
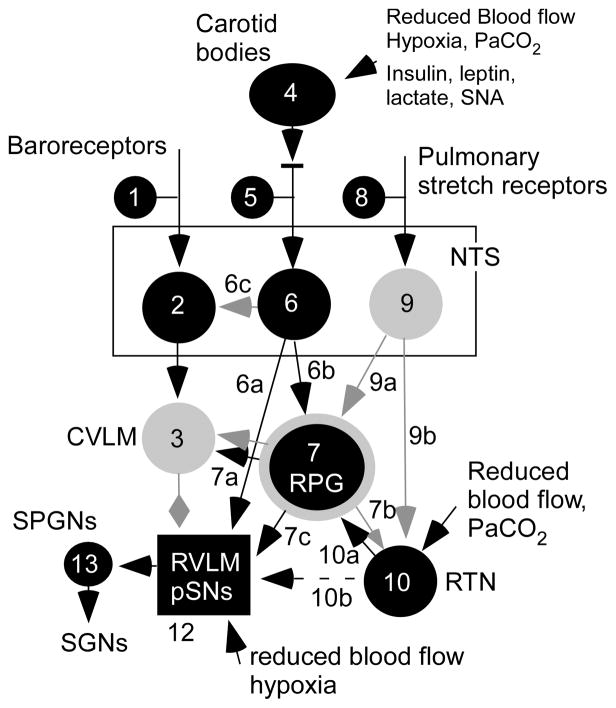
Figure 2. Major brainstem pathways implicated in sympatho-respiratory coupling.
Highly schematic representation of the pathways described in the text. In black, excitatory neurons or pathways (mono- or polysynaptic); in gray, inhibitory neurons or pathways. The RPG (respiratory pattern generator) symbolizes the entire pontomedullary circuit responsible for the generation of breathing movements; this network contains both excitatory and inhibitory neurons and resides within the medulla oblongata and the dorsolateral pons. Other abbreviations as defined in the text.
Two studies found that the SNA of SH rats is predominantly enhanced during inspiration18, 19. This pattern is attenuated by selectively silencing the C1 cells19, which follows since the activity of many C1 pSNs peaks during inspiration20. However, according to Moraes7 SNA and the discharge of RVLM pSNs are also elevated during post-inspiration and late-expiration in SH rats. Altogether, these studies suggest that, in WHBPs from SH rats, respiratory modulation of SNA, whatever its pattern, is enhanced, and that this enhancement is likely mediated through both C1 and non-C1 RVLM pSNs. The exaggerated respiratory modulation of pSNs present in SH rats may stem from enhanced central and peripheral chemoreceptor activity7, 21 and may be relayed to pSNs via pre-Bötzinger pre-inspiratory and Bötzinger post-inspiratory neurons7. In SH rats, the latter neurons had increased neuronal excitability under partial synaptic blockade which was attributed to a reduced leak and BKCa conductances, respectively7. The enhanced pre-inspiratory neuron excitability of SH rats was normalized by reducing perfusion fluid PCO2 suggesting that it could have resulted from increased central chemoreceptor activation7.
Similar types of studies have been performed in rats subjected to CIH20, 22, 23. WHBPs from CIH rats exhibit active (abdominal) expiration and a late-expiratory burst of SNA time-locked to it, unlike control preparations. Again, the enhanced respiratory modulation of SNA of the CIH-rat WHBP could result from a heightened respiratory chemoreflex since the post-CIH pattern can be replicated in a control preparation simply by raising perfusate PCO224. A priori, the carotid bodies25, 26 would seem to be the most probable cause of this enhanced chemoreceptor drive because CIH-induced hypertension is eliminated by carotid body denervation27, 28. However, acute carotid body denervation does not eliminate the exaggerated late-expiratory SNA boost in WHBPs of rats subjected to CIH22. Thus central chemoreceptors and/or a persistent brainstem network modification induced by chronic repetitive carotid body activation29 may contribute to enhanced sympatho-respiratory coupling in this preparation.
In summary, the respiratory modulation of SNA is enhanced in WHBPs from SH and CIH rats and the principal reason could be that, in this preparation, the central respiratory drive is elevated at rest. Reasons may include an increase in peripheral and/or central chemoreceptor activity and/or a form of RVLM network plasticity induced by repetitive carotid body stimulation. The key question is whether these mechanisms are a) relevant to unanesthetized freely behaving rodents and b) relevant to human hypertension.
Unfortunately, the importance of central sympatho-respiratory coupling to SNA and BP in conscious hypertensive rodents is untested and the relevance of sympatho-respiratory coupling to human hypertension is questionable. In human EH, OSA, even in mild to moderate CHF, SNA and BP are indeed elevated but, unlike CIH-exposed rats20, resting ventilation and blood gases are normal and active expiration is not reported27, 30–33. This raises two fundamental questions. First, if resting breathing is unchanged in humans with EH or OSA, why invoke sympatho-respiratory coupling to account for the increase in SNA? Secondly, why are resting breathing and blood gases unchanged in human hypertension if the carotid bodies are tonically activated?
The likely answer to the first question is that a change in breathing or sympatho-respiratory coupling are not the only mechanisms by which carotid body afferents can increase SNA. Carotid body afferents can increase SNA via at least two well-described respiration-independent mechanisms. The first is a direct connection from carotid bodies to RVLM pSNs (Fig. 2 #6a)34–36. The second is a reduction in baroreflex influence14 which also contributes to activating RVLM pSNs. This phenomenon could partly result from the synaptic summation at the level of RVLM pSNs of the baroreceptor-initiated inhibitory input from the caudal ventrolateral medulla (CVLM) (Fig. 2, #3) and the direct excitatory input from the carotid bodies (Fig.2, #6a). A second possibility is baroreflex negative biasing by carotid body afferents directly in the NTS (6c). Finally, activation of brainstem noradrenergic neurons (A5), the orexin system or the paraventricular hypothalamic nucleus could provide additional, potentially breathing-independent pathways by which carotid body hyperactivity could increase SNA37, 38.
The likely answer to the second question is that in intact and unanesthetized mammals, breathing is regulated by two interactive chemoreceptor feedback loops. Specifically, the effect of a moderate increase in carotid body activity on breathing is rapidly and fully compensated by a reduction in central chemoreceptor drive39. This process restores ventilation to normal and therefore presumably also normalizes the activity of the respiratory pattern generator. Schematically, pathway 10a (Fig. 2) is less active which compensates for an increase in pathway 4-5-6-739. This homeostatic mechanism is inoperative in open-loop systems such as anesthetized preparations or the WHBP.
In summary, carotid body hyperactivity is now a well-established contributing factor to essential hypertension and OSA. Moderate carotid body hyperactivity does not raise resting breathing in the conscious state because of the dual and interactive nature of the respiratory chemoreceptor feedback. However, carotid body hyperactivity does increase SNA via a variety of mechanisms that probably converge, at least partially, on RVLM pSNs and are likely independent of the respiratory network.
Altered ratio of excitatory to inhibitory input to RVLM pSNs in hypertension
Injection of sodium kynurenate, a broad-spectrum ionotropic glutamate receptor antagonist, into the RVLM elicits a larger BP drop in hypertensive (SH, Dahl-salt sensitive and Goldblatt 2K1C) than normotensive rats40–43, except in the Zucker obese model44. Given the complexity of the RVLM and the fact that pSNs are regulated by local interneurons45, such results could be variously interpreted. One possibility is that RVLM neurons, maybe pSNs, are more active in hypertensive strains as a result of receiving stronger glutamatergic inputs. This hypothesis is plausible because several of these models have hyperactive carotid bodies, reduced baroreflexes or elevated activity of hypothalamic vasopressin or orexin neurons, which could directly or indirectly enhance glutamate release in the RVLM25, 38, 46–50 but its validity has been experimentally verified only in a CHF model, and only under anesthesia 51. The higher effectiveness of kynurenic acid could have other explanations besides an increase in excitatory glutamatergic input to pSNs, for example increased activity of interneurons antecedent to the pSNs or simply increased excitability of downstream SPGNs52 without change in the discharge rate of RVLM pSNs.
RVLM blood flow restriction and hypertension
Lower brainstem compression by hypertrophied arteries bulging into the RVLM has been associated with cases of refractory hypertension in humans53 and a relatively small acute rise of intracerebral pressure increases respiration and blood pressure in awake mice and humans54. Thus, blood flow restriction to the lower brainstem, possibly the RVLM, could trigger hypertension via a mild form of the Cushing reflex, a concept introduced by DJ Reis in the 1980s and since rebranded as the selfish brain hypothesis55, 56. Recent data suggest that the effect of flow restriction could be mediated by tissue hypoxia and acidification. In the presence of either perturbation, brainstem astrocytes release ATP and lactate, both of which excite RVLM pSNs and therefore could contribute to SNA and BP elevation57, 58. This mechanism (see online supplement for further details) may operate in SH rats, a model of human EH, but the unresolved question is whether brainstem PO2 or pH are ever low enough in an intact unanesthetized rat to engage these glial mechanisms. Medullary PO2 is lower in the brainstem of SH rats than in normotensive controls57 but these measurements were made under anesthesia. Anesthesia disrupts cerebral blood flow autoregulation and adaptive cardiovascular responses to hypoxia that preserve blood flow to the brain. Also, a causal relationship between pSN activity and levels of medullary pO2 judged to be “physiological” remains to be established, in SH or normotensive rats.
In summary, lower brainstem blood flow restriction and/or hypoxia may activate RVLM neurons implicated in breathing and SNA generation. Cell-autonomous as well as astrocyte-dependent mechanisms may contribute to the effects of hypoxia on these neurons and these mechanisms could potentially contribute to some forms of hypertension.
RVLM, oxidative stress, angiotensin II (AngII) and increased SNA
Indices of heightened oxidative stress have been consistently detected in the RVLM of hypertensive rodent models (SH rat, high-salt diet, SHR-stroke prone, 2K1C, angiotensin perfusion, high fat diet, low dose LPS infusion, CIH)59–64. LPS may work by transendothelial signaling to RVLM C1 cells65. After CIH, radical oxygen species (ROS) elevation occurs in a regionally specific manner (RVLM and NTS but not midline medulla) and is absent in mice in which the carotid bodies have been excised64. The same phenomenon is observed in the adrenal medulla66; specifically, ROS are elevated in the adrenal medulla of animals subjected to CIH only if the carotid bodies are intact. Based on this evidence, ROS elevation within RVLM or the adrenal medulla could be elicited via neural connections. The BP of hypertensive rats is partially normalized by injecting tempol into the RVLM or by transducing unspecified RVLM cells with Cu/ZnSOD62, 67. The interpretation of these experiments is that ROS elevation in the RVLM contributes to the increase in local network activity. It is predicated on the assumption that these pharmacological treatments lack non-specific inhibitory effects on local neurons (i.e. unrelated to ROS down-regulation). Indeed, administration of non-selective agents into RVLM inevitably causes greater BP reductions in hypertensive than normotensive animals simply because RVLM pSNs are more active. Finally, since ROS elevation may be elicited via neuronal connections, in the CIH model at least, the question is whether this elevation is a pathological process or merely a physiological mechanism that enables the sustained activation of the chromaffin cells or the NTS-RVLM circuit.
A contribution of the renin-angiotensin system to the elevated oxidative stress is strongly suspected but the cells in which ROS are elevated are not precisely known. Type-I angiotensin receptors (AT1Rs) are relatively highly expressed in the RVLM and contribute to SNA regulation, partly by activating C1 neurons68. However, in the brain in general and probably in the RVLM, AT1Rs are also expressed by the vascular endothelium and by perivascular macrophages69, 70. Moreover, AT1Rs are not confined to the RVLM; they are present throughout the ventrolateral medulla, CVLM included, within the nucleus of the solitary tract and in the intermediolateral cell column71. Microinjections of AngII in these areas either decrease (NTS, CVLM) or increase BP (RVLM), i.e. produce mutually opposing effects, much like glutamate72. “Slow pressor” AngII infusion-induced hypertension in mice renders the blood brain barrier leaky, allowing circulating AngII to penetrate the brain parenchyma and reach AT1R expressed by perivascular macrophages and other cells69. This mechanism contributes to local inflammation and cerebral blood flow dysregulation but, in itself, may not have a prominent causative role in hypertension69. The BP rise elicited by acute injections of AngII into the RVLM has been attributed to NADPH oxidase-derived superoxide anion production and presynaptic enhancement of glutamate release60. However, a postsynaptic mechanism (reduced resting potassium conductance) also contributes to the depolarizing effect of AngII on the C1 cells73, 74. Whether this particular AT1R-mediated effector is activated via oxidative stress is unknown. Chronic systemic infusion of low-dose AngII in mice slowly increases BP and ROS production in the RVLM; the hypertension seems initiated in the subfornical organ and relayed to sympathetic efferents and PVN via synaptic connections75. However, this model is associated with major brain vascular dysfunction owing to the combined effect of circulating AngII, vasopressin release and the upregulation of endothelin, all of which increase ROS production in blood vessels75. Unsurprisingly, deleting AT1Rs specifically from the C1 cells reduces the magnitude of the hypertension only to a small degree76.
AngII contributes prominently to the sympathoexcitation of CHF by stimulating upregulated AT1Rs in the RVLM77. In this case, the sympathoexcitation has been attributed to the downregulation of potassium channel Kv4.3 (KCND3) via AngII-AT1R-ROS-p38 MAPK signaling78. Kv4.3 has been previously associated with AngII signaling in the heart79 and its expression in the RVLM is also down-regulated in the LPS model of hypertension61. Elsewhere, Kv2.2 and Kv3.1b have also been mentioned as possible targets of AT1Rs80.
In summary, oxidative stress is clearly elevated in the RVLM of hypertensive animals but the cells that are subject to this change remain conjectural (blood vessels, perivascular macrophages, microglia, neurons) and so are the consequences of the oxidative stress on the electrophysiological properties of the RVLM network. Finally, the root cause of the increased oxidative stress (tissue hypoxia, inflammation, tissue or circulating angiotensin, synaptically released molecules, vasopressin, endothelin) remains to be conclusively determined75.
RVLM inflammation and hypertension
Inflammation of brainstem blood vessels and/or parenchyma precedes and may contribute to hypertension in the SH rat81, 82. Vascular inflammation, vascular dysfunction/hypoperfusion and AngII activity (via AT1Rs) appear to be three pathophysiological mechanisms that reinforce each other and ultimately lead to elevated SNA81. This general scheme developed at the level of the NTS likely applies to the RVLM since the pSNs are activated by hypoxia/flow restriction and tissue PO2 is lower in the RVLM of SH rats55, 57. The pro-inflammatory state of the RVLM of the SH rat (increased IL-1, leukotriene B4 and AT1R relative to the Wistar-Kyoto control) may be caused by the up-regulation of two miRNAs (miR-135a and miR-376a)83.
Systemic inflammation, as modeled by intravenous infusion of interleukin-1β, activates the C1 neurons broadly and this effect contributes to the activation of the CRH/ACTH/corticosterone cascade84, 85. LPS-induced systemic inflammation may cause hypertension by activating RVLM microglia and increasing local production of pro-inflammatory cytokines (IL-1β, IL-6, or TNF-α protein) and ROS; these effects are reduced by intracisternal infusion of a cycloxygenase-2 (COX-2) inhibitor, by minocycline, an inhibitor of microglial activation, and by a cytokine synthesis inhibitor, pentoxifylline61. Chronic systemic inflammation therefore appears capable of causing hypertension at least in part by producing COX-2 dependent neuro-inflammation in the RVLM. AngII induced hypertension involves activation of similar mechanisms (microglia and increases in pro-inflammatory cytokines) in the PVN86.
RVLM, hypertension and sex differences
NMDA receptor subunit GluN1 is present at lower levels in the dendrites of C1 neurons in male vs. female mice; administration of a slow pressor dose of AngII produces hypertension in males only and increases membrane trafficking of GluN1 only in males, suggesting a possible causality87. Estrogens attenuate calcium current in RVLM C1 bulbospinal neurons directly through extranuclear receptors88, which could reduce their excitability in vivo. Consistent with this possibility, ovariectomy in rats elevates BP, SNA and circulating NE and increases oxidative stress in the RVLM, every parameter being normalized by administration of estrogen89. Also, sex and hormonal levels can selectively affect the expression and subcellular distribution of AT1Rs and components of the AngII signaling pathway within C1 neurons90.
Finally, although CIH produces a similar increase in BP and SNA in male and female rats, SNA increases during early inspiration in females and late expiration in males91. One interpretation is that vasoconstriction occurs predominantly in the kidneys and splanchnic region in females vs. the skeletal muscles in males92.
Summary and conclusions
The RVLM controls SNA and BP via C1 and other pSNs but its contribution to cardiovascular regulations and hypertension is unlikely to be reducible to that of a simple bulbospinal pre-sympathetic relay. The C1 cells have multiple other projections through which they control breathing, parasympathetic efferents, CNS noradrenergic neurons and multiple hypothalamic structures such as the PVN, the median preoptic region and orexinergic neurons.
RVLM anomalies that may contribute to a rise in SNA have been detected in most animal models of hypertension and in congestive heart failure. The most frequently proposed mechanism is an increase in the discharge rate of RVLM pSNs which is attributed to heightened excitatory inputs originating elsewhere (carotid body, and other hypothalamic nuclei, respiratory pattern generator). This view is generally an interpretation of pharmacological experiments performed in anesthetized preparations and awaits confirmation via recordings of defined neuronal types in conscious intact animals. Other RVLM anomalies include inflammation of the neuropil and/or blood vessels, oxidative stress and blood flow reduction. These local perturbations are suspected to also ultimately contribute to pSN hyperactivity, possibly via paracrine mechanisms relying on astrocytes, microglia or perivascular macrophages. Blood flow restriction could also increase brainstem PCO2 and activate RVLM pSNs by stimulating central chemoreceptors.
In reduced preparations from selected hypertensive models (SH rats, animals subjected to CIH), SNA enhancement appears to result predominantly from increased respiratory-phasic modulation. However, in intact conscious hypertensive mammals, including humans, breathing is normal at rest and respiratory network-independent mechanisms offer more plausible explanations of the SNA increase.
Hypertension and CHF are associated with increased oxidative stress in the RVLM. The functional consequences of the oxidative stress on the various types of RVLM cells are unclear. In CIH, ROS elevation seems confined to the regions that mediate the peripheral chemoreflex (RVLM, NTS, adrenal medulla) and, therefore, could conceivably be a normal physiological correlate of their activation rather than a pathological process. Upregulation of the local renin-angiotensin system likely contributes to the increased oxidative stress.
Finally, an animal’s sex influences its propensity to become hypertensive and, even when both sexes become hypertensive (e.g. CIH), the type of sympathetic efferents responsible for the hypertension may be sex-dependent. The RVLM seems to contribute to the sex difference in both cases.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health grants RO1 HL074011 and RO1 HL028785.
Abbreviations
- AT1R
type-I angiotensin receptor
- AngII
angiotensin II
- CIH
chronic intermittent hypoxia
- CHF
congestive heart failure
- Cu/ZnSOD
copper/zinc supraoxide dismutase
- CVLM
caudal ventrolateral medulla
- EH
essential hypertension
- HPA
hypothalamo-pituitary axis
- 2K1C
2-kidney-one clip
- LPS
lipopolysaccharide
- NTS
nucleus of the solitary tract
- OSA
obstructive sleep apnea
- pSNs
presympathetic neurons
- PVN
hypothalamic paraventricular nucleus
- ROS
radical oxygen species
- RTN
retrotrapezoid nucleus
- RVLM
rostral ventrolateral medulla
- SH rats
spontaneously hypertensive rats
- SNA
sympathetic nerve activity
- SPGNs
sympathetic preganglionic neurons
- WHBP
working heart-brainstem preparation
Footnotes
Disclosures: none.
References
- 1.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 3.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 4.Schreihofer AM, Sved AF. The ventrolateral medulla and sympathetic regulation of arterial pressure. In: Llewellyn-Smith IJ, Verberne AJ, editors. Central regulation of autonomic functions. New-York: Oxford University Press; 2011. pp. 78–97. [Google Scholar]
- 5.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 6.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: The body’s emts. Am J Physiol Regul Integr Comp Physiol. 2013;305:R187–204. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moraes DJ, Machado BH, Paton JF. Specific respiratory neuron types have increased excitability that drive presympathetic neurones in neurogenic hypertension. Hypertension. 2014;63:1309–1318. doi: 10.1161/HYPERTENSIONAHA.113.02283. [DOI] [PubMed] [Google Scholar]
- 8.McCall RB, Clement ME. Identification of serotonergic and sympathetic neurons in medullary raphe nuclei. Brain Res. 1989;477:172–182. doi: 10.1016/0006-8993(89)91405-4. [DOI] [PubMed] [Google Scholar]
- 9.McCall RB. Serotonergic excitation of sympathetic preganglionic neurons: A microiontophoretic study. Brain Res. 1983;289:121–127. doi: 10.1016/0006-8993(83)90012-4. [DOI] [PubMed] [Google Scholar]
- 10.Depuy SD, Stornetta RL, Bochorishvili G, Deisseroth K, Witten I, Coates MB, Guyenet PG. Glutamatergic neurotransmission between the c1 neurons and the parasympathetic preganglionic neurons of the dorsal motor nucleus of the vagus. J Neurosci. 2013;33:1486–1497. doi: 10.1523/JNEUROSCI.4269-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbott SB, DePuy SD, Nguyen T, Coates MB, Stornetta RL, Guyenet PG. Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice. J Neurosci. 2013;33:3164–3177. doi: 10.1523/JNEUROSCI.1046-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar P, Prabhakar NR. Peripheral chemoreceptors: Function and plasticity of the carotid body. Compr Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pijacka W, McBryde FD, Marvar PJ, Lincevicius GS, Abdala AP, Woodward L, Li D, Paterson DJ, Paton JF. Carotid sinus denervation ameliorates renovascular hypertension in adult wistar rats. J Physiol. 2016;594:6255–6266. doi: 10.1113/JP272708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA, Paton JF. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun. 2013;4:2395. doi: 10.1038/ncomms3395. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher EC. Effect of episodic hypoxia on sympathetic activity and blood pressure. Resp Physiol. 2000;119:189–197. doi: 10.1016/s0034-5687(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 16.Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: Does it contribute to hypertension? J Physiol. 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie A, Skatrud JB, Morgan BJ, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol. 2006;577:319–329. doi: 10.1113/jphysiol.2006.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czyzyk-Krzeska MF, Trzebski A. Respiratory-related discharge pattern of sympathetic nerve activity in the spontaneously hypertensive rat. J Physiol. 1990;426:355–368. doi: 10.1113/jphysiol.1990.sp018142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menuet C, Le S, Dempsey B, Connelly AA, Kamar JL, Jancovski N, Bassi JK, Walters K, Simms AE, Hammond A, Fong AY, Goodchild AK, McMullan S, Allen AM. Excessive respiratory modulation of blood pressure triggers hypertension. Cell Metab. 2017;25:739–748. doi: 10.1016/j.cmet.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Moraes DJ, da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci. 2013;33:19223–19237. doi: 10.1523/JNEUROSCI.3041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoccal DB. Peripheral chemoreceptors and cardiorespiratory coupling: A link to sympatho-excitation. Exp Physiol. 2015;100:143–148. doi: 10.1113/expphysiol.2014.079558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol. 2011;105:3080–3091. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prabhakar NR. Sensing hypoxia: Physiology, genetics and epigenetics. J Physiol. 2013;591:2245–2257. doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanduri J, Semenza GL, Prabhakar NR. Epigenetic changes by DNA methylation in chronic and intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. 2017;313:L1096–L1100. doi: 10.1152/ajplung.00325.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Rio R, Andrade DC, Lucero C, Arias P, Iturriaga R. Carotid body ablation abrogates hypertension and autonomic alterations induced by intermittent hypoxia in rats. Hypertension. 2016;68:436–445. doi: 10.1161/HYPERTENSIONAHA.116.07255. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher EC. Invited review: Physiological consequences of intermittent hypoxia: Systemic blood pressure. J Appl Physiol (1985) 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 29.Garcia AJ, 3rd, Dashevskiy T, Khuu MA, Ramirez JM. Chronic intermittent hypoxia differentially impacts different states of inspiratory activity at the level of the preBötzinger complex. Front Physiol. 2017;8:571. doi: 10.3389/fphys.2017.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinski M, Lewandowski J, Przybylski J, Bidiuk J, Abramczyk P, Ciarka A, Gaciong Z. Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens Res. 2012;35:487–491. doi: 10.1038/hr.2011.209. [DOI] [PubMed] [Google Scholar]
- 31.Guardiola J, Yu J, Hasan N, Fletcher EC. Evening and morning blood gases in patients with obstructive sleep apnea. Sleep Med. 2004;5:489–493. doi: 10.1016/j.sleep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 33.Narkiewicz K, Pesek CA, van de Borne PJ, Kato M, Somers VK. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation. 1999;100:262–267. doi: 10.1161/01.cir.100.3.262. [DOI] [PubMed] [Google Scholar]
- 34.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol. 2014;4:1511–1562. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. J Physiol. 1996;491:859–869. doi: 10.1113/jphysiol.1996.sp021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aicher SA, Saravay RH, Cravo S, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: Comparison with input from the caudal ventrolateral medulla. J Comp Neurol. 1996;373:62–75. doi: 10.1002/(SICI)1096-9861(19960909)373:1<62::AID-CNE6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 37.Li AH, Hindmarch CCT, Nattie EE, Paton JFR. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol. 2013;591:4237–4248. doi: 10.1113/jphysiol.2013.256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geraldes V, Goncalves-Rosa N, Liu B, Paton JF, Rocha I. Chronic depression of hypothalamic paraventricular neuronal activity produces sustained hypotension in hypertensive rats. Exp Physiol. 2014;99:89–100. doi: 10.1113/expphysiol.2013.074823. [DOI] [PubMed] [Google Scholar]
- 39.Guyenet PG, Bayliss DA, Stornetta RL, Kanbar R, Shi Y, Holloway BB, Souza G, Basting TM, Abbott SBG, Wenker IC. Interdependent feedback regulation of breathing by the carotid bodies and the retrotrapezoid nucleus. J Physiol. 2017 doi: 10.1113/JP274357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension. 2000;35:413–417. doi: 10.1161/01.hyp.35.1.413. [DOI] [PubMed] [Google Scholar]
- 41.Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension. 2001;37:687–691. [PubMed] [Google Scholar]
- 42.Colombari E, Sato MA, Cravo SL, Bergamaschi CT, Campos RR, Jr, Lopes OU. Role of the medulla oblongata in hypertension. Hypertension. 2001;38:549–554. doi: 10.1161/01.hyp.38.3.549. [DOI] [PubMed] [Google Scholar]
- 43.Bergamaschi C, Campos RR, Jr, Schor N, Lopes OU. Importance of rostral ventrolateral medulla in rats with Goldblatt hypertension. Fundam Clin Pharmacol. 1997;11:92S–93S. [Google Scholar]
- 44.Huber DA, Schreihofer AM. Altered regulation of the rostral ventrolateral medulla in hypertensive obese Zucker rats. Am J Physiol Heart Circ Physiol. 2011;301:H230–240. doi: 10.1152/ajpheart.00075.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dempsey B, Le S, Turner A, Bokiniec P, Ramadas R, Bjaalie JG, Menuet C, Neve R, Allen AM, Goodchild AK, McMullan S. Mapping and analysis of the connectome of sympathetic premotor neurons in the rostral ventrolateral medulla of the rat using a volumetric brain atlas. Front Neural Circuits. 2017;11:9. doi: 10.3389/fncir.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell BB, Harlan SM, Morgan DA, Guo DF, Rahmouni K. Differential contribution of POMC and AGRP neurons to the regulation of regional autonomic nerve activity by leptin. Mol Metab. 2017 doi: 10.1016/j.molmet.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huber MJ, Chen QH, Shan Z. The orexin system and hypertension. Cell Mol Neurobiol. 2017 doi: 10.1007/s10571-017-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pijacka W, Katayama PL, Salgado HC, Lincevicius GS, Campos RR, McBryde FD, Paton JFR. Variable role of carotid bodies in cardiovascular responses to exercise, hypoxia and hypercapnia in spontaneously hypertensive rats. J Physiol. 2018 doi: 10.1113/JP275487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shell B, Faulk K, Cunningham JT. Neural control of blood pressure in chronic intermittent hypoxia. Curr Hypertens Rep. 2016;18:19. doi: 10.1007/s11906-016-0627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: Axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Tonic glutamatergic input in the rostral ventrolateral medulla is increased in rats with chronic heart failure. Hypertension. 2009;53:370–374. doi: 10.1161/HYPERTENSIONAHA.108.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Briant LJ, Stalbovskiy AO, Nolan MF, Champneys AR, Pickering AE. Increased intrinsic excitability of muscle vasoconstrictor preganglionic neurons may contribute to the elevated sympathetic activity in hypertensive rats. J Neurophysiol. 2014;112:2756–2778. doi: 10.1152/jn.00350.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geiger H, Naraghi R, Schobel HP, Frank H, Sterzel RB, Fahlbusch R. Decrease of blood pressure by ventrolateral medullary decompression in essential hypertension. The Lancet. 1998;352:446–449. doi: 10.1016/s0140-6736(97)11343-5. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt EA, Despas F, Pavy-Le Traon A, Czosnyka Z, Pickard JD, Rahmouni K, Pathak A, Senard JM. Intracranial pressure is a determinant of sympathetic activity. Front Physiol. 2018;9:11. doi: 10.3389/fphys.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cates MJ, Dickinson CJ, Hart EC, Paton JF. Neurogenic hypertension and elevated vertebrobasilar arterial resistance: Is there a causative link? Curr Hypertens Rep. 2012;14:261–269. doi: 10.1007/s11906-012-0267-6. [DOI] [PubMed] [Google Scholar]
- 56.McBryde FD, Malpas SC, Paton JF. Intracranial mechanisms for preserving brain blood flow in health and disease. Acta Physiol (Oxf) 2017;219:274–287. doi: 10.1111/apha.12706. [DOI] [PubMed] [Google Scholar]
- 57.Marina N, Ang R, Machhada A, Kasymov V, Karagiannis A, Hosford PS, Mosienko V, Teschemacher AG, Vihko P, Paton JF, Kasparov S, Gourine AV. Brainstem hypoxia contributes to the development of hypertension in the spontaneously hypertensive rat. Hypertension. 2015;65:775–783. doi: 10.1161/HYPERTENSIONAHA.114.04683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koganezawa T, Paton JF. Intrinsic chemosensitivity of rostral ventrolateral medullary sympathetic premotor neurons in the in situ arterially perfused preparation of rats. Exp Physiol. 2014;99:1453–1466. doi: 10.1113/expphysiol.2014.080069. [DOI] [PubMed] [Google Scholar]
- 59.Chan SH, Chan JY. Brain stem oxidative stress and its associated signaling in the regulation of sympathetic vasomotor tone. J Appl Physiol (1985) 2012;113:1921–1928. doi: 10.1152/japplphysiol.00610.2012. [DOI] [PubMed] [Google Scholar]
- 60.Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- 61.Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation. 2012;9:212. doi: 10.1186/1742-2094-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirooka Y. Oxidative stress in the cardiovascular center has a pivotal role in the sympathetic activation in hypertension. Hypertens Res. 2011;34:407–412. doi: 10.1038/hr.2011.14. [DOI] [PubMed] [Google Scholar]
- 63.de Morais SDB, Shanks J, Zucker IH. Integrative physiological aspects of brain RAS in hypertension. Curr Hypertens Rep. 2018;20:10. doi: 10.1007/s11906-018-0810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nanduri J, Peng YJ, Wang N, Khan SA, Semenza GL, Prabhakar NR. DNA methylation in the central and efferent limbs of the chemoreflex requires carotid body neural activity. J Physiol. 2017 doi: 10.1113/JP274833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schiltz JC, Sawchenko PE. Specificity and generality of the involvement of catecholaminergic afferents in hypothalamic responses to immune insults. J Comp Neurol. 2007;502:455–467. doi: 10.1002/cne.21329. [DOI] [PubMed] [Google Scholar]
- 66.Kumar GK, Peng YJ, Nanduri J, Prabhakar NR. Carotid body chemoreflex mediates intermittent hypoxia-induced oxidative stress in the adrenal medulla. Adv Exp Med and Biol. 2015;860:195–199. doi: 10.1007/978-3-319-18440-1_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campos RR, Oliveira-Sales EB, Nishi EE, Paton JF, Bergamaschi CT. Mechanisms of renal sympathetic activation in renovascular hypertension. Exp Physiol. 2015;100:496–501. doi: 10.1113/expphysiol.2014.079855. [DOI] [PubMed] [Google Scholar]
- 68.Allen AM. Role of angiotensin in the rostral ventrolateral medulla in the development and maintenance of hypertension. Curr Opin Pharmacol. 2011;11:117–123. doi: 10.1016/j.coph.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, Racchumi G, Murphy M, Van Rooijen N, Anrather J, Iadecola C. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126:4674–4689. doi: 10.1172/JCI86950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: The role of perivascular cells. Front Biosci. 2003;8:s1321–s1329. doi: 10.2741/1211. [DOI] [PubMed] [Google Scholar]
- 71.Allen AM, Moeller I, Jenkins TA, Zhuo J, Aldred GP, Chai SY, Mendelsohn FAO. Angiotensin receptors in the nervous system. Brain Res Bull. 1998;47:17–28. doi: 10.1016/s0361-9230(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 72.Chai CY, Chen SY, Lin AMY, Tseng CJ. Angiotensin II activates pressor and depressor sites of the pontomedulla that react to glutamate. Clin Exp Pharmacol Physiol. 1996;23:415–423. doi: 10.1111/j.1440-1681.1996.tb02751.x. [DOI] [PubMed] [Google Scholar]
- 73.Li YW, Guyenet PG. Angiotensin II decreases a resting K+ conductance in rat bulbospinal neurons of the C1 area. Circ Res. 1996;78:274–282. doi: 10.1161/01.res.78.2.274. [DOI] [PubMed] [Google Scholar]
- 74.Chen D, Bassi JK, Walther T, Thomas WG, Allen AM. Expression of angiotensin type 1a receptors in C1 neurons restores the sympathoexcitation to angiotensin in the rostral ventrolateral medulla of angiotensin type 1a knockout mice. Hypertension. 2010;56:143–150. doi: 10.1161/HYPERTENSIONAHA.110.151704. [DOI] [PubMed] [Google Scholar]
- 75.Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, Iadecola C. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin ii hypertension. J Neurosci. 2012;32:4878–4886. doi: 10.1523/JNEUROSCI.6262-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jancovski N, Carter DA, Connelly AA, Stevens E, Bassi JK, Menuet C, Allen AM. Angiotensin type 1a receptor expression in C1 neurons of the rostral ventrolateral medulla contributes to the development of angiotensin-dependent hypertension. Exp Physiol. 2014;99:1597–1610. doi: 10.1113/expphysiol.2014.082073. [DOI] [PubMed] [Google Scholar]
- 77.Zucker IH, Schultz HD, Patel KP, Wang W, Gao L. Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am J Physiol Heart Circ Physiol. 2009;297:H1557–1566. doi: 10.1152/ajpheart.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao L, Li Y, Schultz HD, Wang WZ, Wang W, Finch M, Smith LM, Zucker IH. Downregulated kv4.3 expression in the RVLM as a potential mechanism for sympathoexcitation in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H945–955. doi: 10.1152/ajpheart.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Potapova IA, Cohen IS, Doronin SV. Voltage-gated ion channel kv4.3 is associated with rap guanine nucleotide exchange factors and regulates angiotensin receptor type 1 signaling to small g-protein rap. FEBS J. 2007;274:4375–4384. doi: 10.1111/j.1742-4658.2007.05966.x. [DOI] [PubMed] [Google Scholar]
- 80.Gelband CH, Warth JD, Mason HS, Zhu M, Moore JM, Kenyon JL, Horowitz B, Sumners C. Angiotensin II type 1 receptor-mediated inhibition of K+ channel subunit kv2.2 in brain stem and hypothalamic neurons. Circ Res. 1999;84:352–359. doi: 10.1161/01.res.84.3.352. [DOI] [PubMed] [Google Scholar]
- 81.Waki H, Gouraud SS, Maeda M, Raizada MK, Paton JF. Contributions of vascular inflammation in the brainstem for neurogenic hypertension. Resp Physiol Neurobiol. 2011;178:422–428. doi: 10.1016/j.resp.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Walas D, Nowicki-Osuch K, Alibhai D, von Linstow Roloff E, Coghill J, Waterfall C, Paton JF. Inflammatory pathways are central to posterior cerebrovascular artery remodelling prior to the onset of congenital hypertension. J Cereb Blood Flow Metab. 2018 doi: 10.1177/0271678X18769180. 271678X18769180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeCicco D, Zhu H, Brureau A, Schwaber JS, Vadigepalli R. MicroRNA network changes in the brain stem underlie the development of hypertension. Physiol Genomics. 2015;47:388–399. doi: 10.1152/physiolgenomics.00047.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Kempen TA, Dodos M, Woods C, Marques-Lopes J, Justice NJ, Iadecola C, Pickel VM, Glass MJ, Milner TA. Sex differences in NMDA GluN1 plasticity in rostral ventrolateral medulla neurons containing corticotropin-releasing factor type 1 receptor following slow-pressor angiotensin II hypertension. Neuroscience. 2015;307:83–97. doi: 10.1016/j.neuroscience.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res. 2006;1094:163–178. doi: 10.1016/j.brainres.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 89.Hao F, Gu Y, Tan X, Deng Y, Wu ZT, Xu MJ, Wang WZ. Estrogen replacement reduces oxidative stress in the rostral ventrolateral medulla of ovariectomized rats. Oxid Med Cell Longev. 2016;2016:2158971. doi: 10.1155/2016/2158971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pierce JP, Kievits J, Graustein B, Speth RC, Iadecola C, Milner TA. Sex differences in the subcellular distribution of angiotensin type 1 receptors and NADPH oxidase subunits in the dendrites of C1 neurons in the rat rostral ventrolateral medulla. Neuroscience. 2009;163:329–338. doi: 10.1016/j.neuroscience.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Souza GM, Bonagamba LG, Amorim MR, Moraes DJ, Machado BH. Inspiratory modulation of sympathetic activity is increased in female rats exposed to chronic intermittent hypoxia. Exp Physiol. 2016;101:1345–1358. doi: 10.1113/EP085850. [DOI] [PubMed] [Google Scholar]
- 92.Numao Y, Koshiya N, Gilbey MP, Spyer KM. Central respiratory drive-related activity in sympathetic nerves of the rat: The regional differences. Neurosci Lett. 1987;81:279–284. doi: 10.1016/0304-3940(87)90396-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.