Abstract
Importance
The association between peripheral inflammatory biomarkers and Alzheimer disease (AD) is not consistent in the literature. It is possible that chronic inflammation, rather than 1 episode of inflammation, interacts with genetic vulnerability to increase the risk for AD.
Objective
To study the interaction between the apolipoprotein E (ApoE) genotype and chronic low-grade inflammation and its association with the incidence of AD.
Design, Setting, and Participants
In this cohort study, data from 2656 members of the Framingham Heart Study offspring cohort (Generation 2; August 13, 1971-November 27, 2017) were evaluated, including longitudinal measures of serum C-reactive protein (CRP), diagnoses of incident dementia including AD, and brain volume. Chronic low-grade inflammation was defined as having CRP at a high cutoff level at a minimum of 2 time points. Statistical analysis was performed from December 1, 1979, to December 31, 2015.
Main Outcomes and Measures
Development of AD and brain volumes.
Results
Of the 3130 eligible participants, 2656 (84.9%; 1227 men and 1429 women; mean [SD] age at last CRP measurement, 61.6 [9.5] years) with both ApoE status and longitudinal CRP measurements were included in this study analysis. Median (interquartile range) CRP levels increased with mean (SD) age (43.3 [9.6] years, 0.95 mg/L [0.40-2.35 mg/L] vs 59.1 [9.6] years, 2.04 mg/L [0.93-4.75 mg/L] vs 61.6 [9.5] years, 2.21 mg/L [1.05-5.12 mg/L]; P < .001), but less so among those with ApoE4 alleles, followed by ApoE3 then ApoE2 genotypes. During the 17 years of follow-up, 194 individuals (7.3%) developed dementia, 152 (78.4%) of whom had AD. ApoE4 coupled with chronic low-grade inflammation, defined as a CRP level of 8 mg/L or higher, was associated with an increased risk of AD, especially in the absence of cardiovascular diseases (hazard ratio, 6.63; 95% CI, 1.80-24.50; P = .005), as well as an increased risk of earlier disease onset compared with ApoE4 carriers without chronic inflammation (hazard ratio, 3.52; 95% CI, 1.27-9.75; P = .009). This phenomenon was not observed among ApoE3 and ApoE2 carriers with chronic low-grade inflammation. Finally, a subset of 1761 individuals (66.3%) underwent brain magnetic resonance imaging, and the interaction between ApoE4 and chronic low-grade inflammation was associated with brain atrophy in the temporal lobe (β = –0.88, SE = 0.22; P < .001) and hippocampus (β = –0.04, SE = 0.01; P = .005), after adjusting for confounders.
Conclusions and Relevance
In this study, peripheral chronic low-grade inflammation in participants with ApoE4 was associated with shortened latency for onset of AD. Rigorously treating chronic systemic inflammation based on genetic risk could be effective for the prevention and intervention of AD.
This population-based cohort study uses data from the Framingham Heart Study offspring cohort to study the interaction between the apolipoprotein E genotype and chronic low-grade inflammation and its association with the incidence of Alzheimer disease.
Key Points
Question
Is interaction of chronic low-grade inflammation and the apolipoprotein E genotype associated with development of Alzheimer disease?
Findings
In this population-based cohort study, chronic low-grade inflammation, assessed by longitudinal C-reactive protein measurements, was associated with an increased risk of Alzheimer disease only in ApoE4 carriers.
Meaning
Clinical follow-up and treatment of high levels of C-reactive protein may be beneficial for prevention of Alzheimer disease in ApoE4 carriers.
Introduction
The apolipoprotein E4 (ApoE4 [OMIM 107741]) allele is the major genetic risk factor for late-onset Alzheimer disease (AD).1 However, not all ApoE4 carriers develop AD, even among those older than 90 years.2 It is likely that a complex interaction of genetic vulnerabilities with environmental risk factors lead to AD and identifying such factors could be beneficial for the prevention of AD. One such interacting factor could be sustained or frequent systemic inflammations, as infections of the respiratory, gastrointestinal, and urinary tract systems are common in elderly individuals.
C-reactive protein (CRP) is an immune system response to toxins or injuries in systemic inflammation, while CRP levels increase with age.3 Although multiple AD-related genes are associated with the level of CRP,4 the association between blood CRP levels and risk of AD are not conclusive in the literature,5,6,7 with studies showing both low and high levels of CRP in patients with AD. Since AD is a chronic disease characterized by neurodegeneration in the brain, chronic low-grade inflammation, either sustained or frequently episodic, may be a risk factor for AD. However, most studies to date rely on one-time measurements of CRP and thus do not distinguish between a condition of acute inflammatory reaction followed by recovery and a condition of chronic inflammation without complete recovery or frequent episodic inflammation. Since preclinical studies suggest that CRP plays a role in ApoE4 leading to AD,4,8 we thus hypothesized that the association of chronic elevated CRP levels with the risk of AD would be different across ApoE genotypes. This hypothesis prompted our study that includes longitudinal measures of high levels of CRP as a biomarker of chronic low-grade inflammation to determine the risk for development of AD.
The Framingham Heart Study is a large population-based, multigeneration cohort with long and intensive follow-up that includes multiple measurements of serum CRP taken during a 2-decade period.9 The purpose of this study was to determine if and how peripheral CRP levels are associated with the onset of AD in the context of ApoE genotypes. Included in this study is the Framingham Heart Study Generation 2 cohort enrolled in 1971, who had up to 3 CRP measurements between 1979 and 2001. Chronic low-grade inflammation was defined as meeting specified cutoff levels of plasma CRP in at least 2 measurements taken years apart. We examined the association between chronic low-grade inflammation, and risk of a diagnosis of dementia ,including AD, and brain volumes, stratified by ApoE genotype.
Methods
Study Design and Participants
The Framingham Heart Study is a single-site, community-based, prospective cohort study in Framingham, Massachusetts. The design and selection criteria of the Framingham Heart Study offspring cohort (Generation 2) have been previously described.10 The source population is 3130 participants, who were 20 years or older at the second health examination (1979-1983), had baseline CRP measured during that examination, and consented to use of their genetic information (ie, ApoE genotype). Excluded were 404 individuals who did not have another CRP measurement at the sixth (1995-1998) or seventh (1998-2001) health examinations and 46 individuals with the ApoE2/4 genotype. In addition, 24 individuals with prevalent dementia at the time of each CRP measurement were excluded. Thus, the final study sample consisted of 2656 individuals (eFigure 1A in the Supplement), whose data were used for the primary analyses to examine the association between ApoE, CRP level, and risk of AD. Data on a subset of 1785 individuals, who also underwent brain magnetic resonance imaging (MRI) after the seventh examination (1999-2011) (eFigure 1B in the Supplement), were used for secondary analyses to examine the association between CRP, ApoE, and AD-related changes in brain structure. Cardiovascular disease status at examination 7 was used as a covariate and was represented as a dichotomous variable (yes or no), determined by the presence of the following conditions: myocardial infarction, angina pectoris, coronary insufficiency, congestive heart failure, and intermittent claudication. Written informed consent was obtained from all study participants and the study protocol was approved by the Institutional Review Board of Boston University Medical Campus. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. This study was monitored by a National Heart, Lung, and Blood Institute Observational Study Monitoring Board and followed their guidelines.
CRP Measurement
During the clinic visits, blood samples were drawn, under fasting condition, from the antecubital vein while participants were supine. Serum aliquots were frozen at –20°C after the initial phlebotomy and subsequently thawed for measurement of high-sensitivity CRP. Both CRP concentrations at examinations 2 and 6 were performed in the Framingham Heart Study laboratory using a previously described enzymatic immunoassay11 (Hemagen Diagnostics Inc). The CRP measurement at examination 7 was conducted by a Dade Behring BN100 nephelometer. The Pearson product moment correlation between both techniques was 0.98 and the CRP quartile assignment was identical.9
To define chronic low-grade inflammation, we used the following 2 criteria: (1) since a CRP level lower than 3 mg/L (to convert to nanomoles per liter, multiply by 9.524) is considered normal in a clinical setting, we defined low-grade inflammation as any CRP measurement above 3 mg/L and used different cutoff levels to indicate severity; and (2) chronic inflammatory status was defined as having at least 2 longitudinal CRP measurements above the stipulated cutoff levels. Participants with only 1 CRP measurement equal to or higher than 3 mg/L were not considered as having chronic low-grade inflammation.
Diagnoses of Dementia Including AD
Beginning in 1979, all Generation 2 participants have been followed up for incident dementia. The Mini-Mental State Examination (MMSE) was administered beginning at the fifth health examination (ie, 1991-1995) to monitor change in cognitive status. A performance drop in Mini-Mental State Examination score of 3 or more points from the immediately preceding examination or 5 or more points across all examinations would indicate a change in cognitive status that warranted review by a dementia diagnostic panel consisting of at least 1 neurologist and 1 neuropsychologist. Furthermore, from 1999 to 2005, all surviving Generation 2 participants were invited for an in-depth cognitive examination, which also screened for incident cognitive impairment that warranted review by the dementia diagnostic panel. Consensus diagnostic procedures have been previously described.12 Incidence of dementia, AD, and person-time accruement after the last longitudinal CRP measurement was used for analyses.
Brain Measurements
The brain MRI was conducted beginning March 1999; only data acquired from brain MRI scans after the measurement of the last CRP (eg, examinations 6 or 7) were used. The brain MRI protocol has been reported in detail elsewhere.13,14 A Siemens 1-T MR machine (Siemens Medical) with a T2-weighted double spin-echo coronal imaging sequence was used. A central laboratory blinded to demographic and clinical information processed the digital information on brain images and quantified the brain data with a custom-written computer program operating on a UNIX, Solaris platform (Sun Microsystems).
The semiautomated segmentation protocol for quantifying total cranial volume, total cerebral brain volume, frontal lobar brain volume, parietal lobe brain volume, temporal lobe brain volume, and hippocampal volume has been described elsewhere,15 as have the interrater reliabilities for these methods. For segmentation of white matter hyperintensities from other brain tissues, the first and second images from T2 sequences were summed and a log-normal distribution was fitted to the summed data. A segmentation threshold for white matter hyperintensities was determined as 1 SD in pixel intensity greater than the mean of the fitted distribution of brain parenchyma. The units for the brain volumes, including total cerebral brain volume, frontal lobar brain volume, temporal lobe brain volume, hippocampal volume, and white matter hyperintensities, were computed as the percentage of total cranial volume. Each image set underwent rigorous quality control assessment that includes assessment of the original acquisition quality as well as the quality of the image processing. Moreover, each of the analysts was highly trained to maintain rigorous precision, with intraclass (analyst) coefficients above 90% for all analyses.
Statistical Analysis
Statistical analysis was performed from December 1, 1979, to December 31, 2015. Analyses were performed using SAS software, version 9.3 (SAS Institute) and the R statistical environment (The R Foundation for Statistical Computing Platform 2017). We performed univariate analyses to describe baseline characteristics of the final sample population, stratified by ApoE genotype (ApoE2-2/2 or 2/3; ApoE3-3/3; ApoE4-3/4 or 4/4). Means and SDs were determined and analysis of variance tests were conducted on variables with normal distribution, Mann-Whitney tests were performed on variables with a skewed distribution using the median (interquartile range), and χ2 tests were used for categorical variables using number and percentage.
To establish the temporal sequence between chronic low-grade inflammation and the development of AD, we excluded individuals who did not have ApoE genotype measured, had an ApoE2/4 genotype, did not have longitudinal CRP measurements, and/or had dementia at the time of or prior to the last measurement of CRP, leaving a total sample of 2656 (eFigure 1 in the Supplement). Cox proportional hazards regression models were used to examine chronic low-grade inflammation and the development of AD, dementia, or mortality after adjusting for age, sex, educational level, and cardiovascular disease; the interaction between ApoE4 and chronic low-grade inflammation was examined as well. Kaplan-Meier survival analyses were performed to compare the onset of AD, dementia, or death among ApoE genotypes.
The secondary analysis focused on 1761 individuals who underwent a brain MRI after their last longitudinal CRP measurement (eFigure 1 in the Supplement). Multivariate linear regression was also used to study the association between chronic low-grade inflammation and total and regional brain volume measures, controlling for total brain volume, after adjusting for age (age groups, 20-29 years; 30-39 years, 40-49 years, and ≥50 years), sex, educational level, ApoE4 status, and the time between the last CRP measurement and brain MRI scan. The interaction of the CRP status and ApoE4 allele was also examined in these regression models for each brain measure. Given multiple testing and the need to minimize the rate of false positives, P values were adjusted using a conventional Bonferroni correction threshold of .005 in 2-sided t tests (eTable in the Supplement).
Results
Association of Median Levels of CRP With Age in the Context of ApoE Genotypes
The 2656 individuals who completed at least 2 CRP measurements and did not have dementia at the time of their last CRP measurement were a mean (SD) age of 61.4 (9.4) years (eFigure 1 in the Supplement). Participants were further categorized into the following 3 groups: ApoE2 (n = 364), ApoE3 (n = 1729), and ApoE4 (n = 532) (Table 1). There were no differences in age, sex, educational levels, and prevalence of cardiovascular diseases among the ApoE subgroups.
Table 1. Demographic, Longitudinal CRP Measures, and Incident AD in ApoE Genotypes in the Framingham Heart Study Population.
| Characteristic |
ApoE2 Carriers (n = 367) |
ApoE3 Carriers (n = 1746) |
ApoE4 Carriers (n = 542) |
df | F or χ2a | P Valueb |
|---|---|---|---|---|---|---|
| Age after CRP measurements, y | ||||||
| Mean (SD) | 61.9 (9.3) | 61.6 (9.7) | 61.5 (9.0) | 2 | 0.26c | .77 |
| Range | 40-82 | 37-88 | 39-87 | 2 | 0.44d | .80 |
| Female, No. (%) | 212 (57.8) | 915 (52.4) | 302 (55.7) | 2 | 4.55e | .10 |
| Education, mean (SD), y | 14.1 (2.8) | 14.1 (2.6) | 14.1 (2.6) | 2 | 0.10c | .90 |
| Cardiovascular diseases, No. (%) | 40 (10.9) | 208 (11.9) | 74 (13.7) | 2 | 1.78e | .41 |
| Follow-up after CRP measurement, mean (SD), y | 14.5 (3.9) | 14.3 (3.9) | 14.3 (4.0) | 2 | 0.37c | .69 |
| Examination 2 | ||||||
| CRP, median (IQR), mg/L | 1.1 (0.5-2.6) | 1.0 (0.4-2.4) | 0.8 (0.3-2.0) | 2 | 22.25d | <.001 |
| CRP ≥3 mg/L, No. (%) | 79 (21.5) | 351 (20.1) | 84 (15.5) | 2 | 6.88e | .03 |
| Examination 6f | ||||||
| CRP, median (IQR), mg/L | 2.1 (0.9-5.0)g | 2.2 (1.0-5.0)g | 1.8 (0.8-4.0)g | 2 | 14.61d | <.001 |
| CRP ≥3 mg/L, No./Total No. (%) | 136/339 (40.1) | 661/1635 (40.4) | 163/499 (32.7) | 2 | 7.10e | .03 |
| Examination 7f | ||||||
| CRP, median (IQR), mg/L | 2.6 (1.2-5.7)g | 2.3 (1.1-5.2)g | 1.8 (0.9-4.3)g | 2 | 20.97d | <.001 |
| CRP ≥3 mg/L, No./Total No. (%) | 156/340 (45.9) | 700/1633 (42.9) | 180/501 (35.9) | 2 | 10.18e | .006 |
| CRP levels greater than or equal to the cutoff value in 2 measurements | ||||||
| CRP ≥3 mg/L, No. (%) | 122 (33.2) | 573 (32.8) | 132 (24.4) | 2 | 14.64e | .001 |
| Age for the subgroup, mean (SD), y | 63.7 (9.1) | 63.2 (9.1) | 64.2 (8.3) | 2 | 0.66c | .51 |
| CRP ≥8 mg/L, No. (%) | 42 (11.4) | 132 (7.6) | 27 (5.0) | 2 | 13.07e | .001 |
| Age for the subgroup, mean (SD), y | 62.5 (9.0) | 62.8 (9.2) | 66.6 (8.9) | 2 | 2.07c | .13 |
| Outcomes, No. (%) | ||||||
| Mortality | 92 (25.1) | 487 (27.9) | 151 (27.9) | 2 | 1.25e | .54 |
| Cognitive outcomes | ||||||
| Incident cases of dementia | 21 (5.7) | 105 (6.0) | 65 (12.0) | 2 | 21.04e | <.001 |
| Incident cases of AD | 14 (3.8) | 83 (4.8) | 55 (10.1) | 2 | 22.20e | <.001 |
Abbreviations: AD, Alzheimer disease; CRP, C-reactive protein; IQR, interquartile range.
SI conversion factor: To convert CRP to nanomoles per liter, multiply by 9.524.
Mean (SD) with 1-way analysis of variance was used to test differences in CRP level and other variables among ApoE subgroups; median (IQR) with Kruskal-Wallis test with a χ2 value was applied when a concentration distribution was skewed. χ2 test was used to compare counts, No./Total (%).
P values for statistical significance are shown for ApoE2, ApoE3, and ApoE4 comparisons.
F value.
χ2 Value in Kruskal-Wallis test.
χ2 Value in χ2 test.
Mann-Whitney test was for comparisons of CRP level at either examination 6 or 7 with CRP levels at examination 2 within each ApoE subgroup.
P < .05.
There was a positive association between increasing mean (SD) age and higher median (interquartile range) CRP levels (43.3 [9.6] years, 0.95 mg/L [0.40-2.35 mg/L] vs 59.1 [9.6] years, 2.04 mg/L [0.93-4.75 mg/L] vs 61.6 [9.5] years, 2.21 mg/L [1.05-5.12 mg/L]; P < .001) and a higher proportion of older participants had CRP levels of 3 mg/L or higher across all ApoE genotypes (Table 1). ApoE4 carriers consistently had lower levels of CRP than did ApoE2 and ApoE3 carriers at each of the examinations. Overall, age was positively associated with chronic low-grade systemic inflammation, while ApoE4 was negatively associated (Table 1).
Association of the Interaction of ApoE4 and Chronic Low-Grade Inflammation With Increased Risk of AD
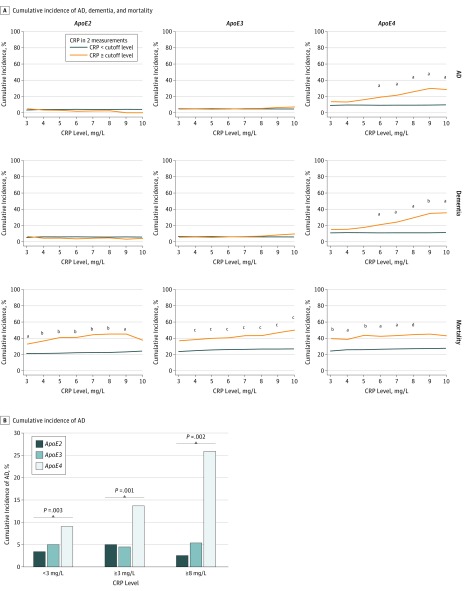
The mean (SD) follow-up from the last CRP measurement was approximately 14.1 (4.2) years and there were no significant differences in follow-up time among the 3 ApoE groups (Table 1). Of the 194 individuals (7.3%) who developed dementia, 152 (78.4%) were diagnosed with AD. Using χ2 analyses, we tested whether the association between ApoE genotype and chronic low-grade inflammation status with the risk for incident dementia including AD and mortality changed when using different CRP cutoff levels (Figure 1). Those with both the ApoE4 allele and chronic low-grade inflammation demonstrated a CRP level–dependent pattern that was linked to increased risk of AD and dementia (Figure 1A). This phenomenon was not observed in ApoE2 and ApoE3 carriers. ApoE4 carriers with CRP levels of 3 mg/L or less had a onefold to twofold increase of risk of AD compared with ApoE3 and ApoE2 carriers with similar CRP levels (37 of 410 [9.0%] vs 58 of 1174 [4.9%] vs 8 of 245 [3.3%]; P = .003; Table 1 and Figure 1B). For CRP levels of 3 mg/L or higher, ApoE4 carriers had a twofold to threefold increase of risk of AD compared with ApoE3 and ApoE2 carriers (18 of 132 [13.6%] vs 25 of 573 [4.4%] vs 6 of 122 [4.9%]; P = .001). For CRP levels of 8 mg/L or higher, ApoE4 carriers had a fivefold to 10-fold increase of risk of AD risk compared with ApoE3 and ApoE2 carriers (7 of 27 [25.9%] vs 7 of 132 [5.3%] vs 1 of 42 [2.4%]; P = .002). In contrast, as expected, chronic low-grade inflammation was positively associated with or showed a positive trend with mortality rates across all ApoE genotypes (Figure 1).
Figure 1. Cumulative Rates of Dementia, Alzheimer Disease (AD), and Mortality Based on ApoE Alleles and Chronic Low-grade Inflammation.
A, Cumulative incidence of AD, dementia, and mortality. Individuals were divided into ApoE2, ApoE3, and ApoE4 genotypes. To define the absence and presence of chronic low-grade inflammation, C-reactive protein (CRP) cutoff levels at 2 measurement points are used to represent severity. The incident rates of AD, dementia, and mortality between those without and with different severity levels of chronic low-grade inflammation were compared by using the χ2 test. To convert CRP to nanomoles per liter, multiply by 9.524. aP < .05. bP < .01. cP < .001. dP = .08. B, Cumulative incidence of AD. Individuals were divided into ApoE2, ApoE3, and ApoE4 genotypes for CRP concentrations of 3 mg/L or lower, 3 mg/L or higher, and 8 mg/L or higher. The AD incident rates were compared among the 3 ApoE subgroups by using the χ2 test for each level of CRP concentration.
We next used Cox proportional hazards regression analyses, adjusted for age, sex, educational level, cardiovascular disease, and ApoE4 status. Although chronic low-grade inflammation, based on a CRP level of 8 mg/L or higher, 9 mg/L or higher, or 10 mg/L or higher, was not found to be associated with AD or dementia, the interaction between ApoE4 and chronic low-grade inflammation was associated with AD or dementia (CRP level ≥9 mg/L: hazard ratio, 3.47; 95% CI, 1.10-10.94; P = .03) or tended to be positively associated with AD. To determine if cardiovascular diseases may account for this association, we excluded individuals with cardiovascular diseases and found that, in the absence of cardiovascular diseases, the association was significantly stronger (hazard ratio, 6.89; 95% CI, 1.74-27.30; P = .006) (Table 2). In addition, after stratifying participants by ApoE4 status, we found that chronic low-grade inflammation was significantly associated with AD risk only in ApoE4 carriers (hazard ratio, 4.70; 95% CI, 1.83-12.04; P = .001), but not in ApoE2 or ApoE3 carriers. Again, chronic low-grade inflammation was positively associated with mortality across all ApoE genotypes, but the interaction between ApoE4 and chronic low-grade inflammation was not associated with mortality (Table 2).
Table 2. Cox Proportional Hazards Regression Models for the Risk of Chronic Low-grade Inflammation on the Incidence of Dementia, Alzheimer Disease, and Mortality.
| CRP Cutoff Levela | No. (%) | Modelb | Alzheimer Disease | Dementia | Mortality | |||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| All Participants (N = 2656 [100.0%]) | ||||||||
| 8 mg/L | 201 (7.6) | 1 | 1.22 (0.71-2.10) | .47 | 1.33 (0.82-2.15) | .25 | 1.78 (1.42-2.23) | <.001 |
| With ApoE4 | 2 | 2.64 (0.89-7.80) | .08 | 2.44 (0.92-6.45) | .07 | 0.81 (0.43-1.53) | .52 | |
| 9 mg/L | 160 (6.0) | 1 | 1.43 (0.80-2.55) | .22 | 1.59 (0.96-2.64) | .07 | 1.88 (1.47-2.41) | <.001 |
| With ApoE4 | 2 | 3.47 (1.10-10.94) | .03 | 2.98 (1.08-8.27) | .04 | 0.84 (0.41-1.74) | .65 | |
| 10 mg/L | 122 (4.6) | 1 | 1.39 (0.73-2.66) | .32 | 1.66 (0.96-2.89) | .07 | 1.79 (1.36-2.35) | <.001 |
| With ApoE4 | 2 | 2.76 (0.74-10.30) | .13 | 2.46 (0.87-7.71) | .12 | 0.77 (0.32-1.83) | .55 | |
| Individuals Without CVD (n = 2334 [87.9%]) | ||||||||
| 8 mg/L | 158 (6.8) | 1 | 1.23 (0.64-2.37) | .54 | 1.33 (0.75-2.37) | .33 | 1.71 (1.28-2.27) | <.001 |
| With ApoE4 | 2 | 6.63 (1.80-24.5) | .005 | 4.14 (1.28-13.44) | .02 | 0.80 (0.31-2.07) | .65 | |
| 9 mg/L | 125 (5.4) | 1 | 1.42 (0.72-2.83) | .31 | 1.60 (0.88-2.92) | .12 | 1.86 (1.36-2.54) | <.001 |
| With ApoE4 | 2 | 6.89 (1.74-27.30) | .006 | 4.07 (1.22-13.59) | .02 | 0.88 (0.34-2.29) | .80 | |
| 10 mg/L | 95 (4.1) | 1 | 1.21 (0.53-2.78) | .65 | 1.56 (0.79-3.09) | .20 | 1.78 (1.24-2.54) | .002 |
| With ApoE4 | 2 | 6.82 (1.31-35.6) | .02 | 3.61 (0.86-15.16) | .08 | 0.99 (0.30-3.30) | .98 | |
Abbreviations: CRP, C-reactive protein; CVD, cardiovascular diseases; HR, hazard ratio.
The CRP levels greater than or equal to a given cutoff at least in 2 measurements to define chronic low-grade inflammation.
Model 1: HR of chronic low-grade inflammation after adjusting for age, sex, educational level, ApoE, and CVD. Model 2: HR of the interaction effects between chronic low-grade inflammation and ApoE4, adjusting for all covariates in model 1.
Interaction of ApoE4 and Chronic Low-grade Inflammation and Latency of Onset of AD
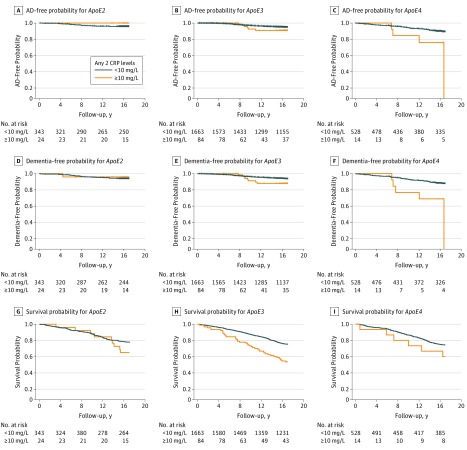
Using Kaplan-Meier analysis, we found that individuals with ApoE4 and chronic low-grade inflammation, defined as having a CRP cutoff level of 10 mg/L or more in at least 2 examinations, was more strongly associated with onset of dementia as well as AD compared with ApoE4 carriers without this level of inflammation. In comparison, chronic low-grade inflammation status did not affect risk of dementia including AD in ApoE2 carriers. Results for ApoE3 carriers fell between findings for ApoE2 and ApoE4 carriers, showing marginal influences of chronic low-grade inflammation on risk of AD. We also tested cutoff CRP levels of 8 mg/L or higher and 9 mg/L or higher to examine severity of chronic low-grade inflammation and found that they were all linked to onset of AD in the Kaplan-Meier analysis for carriers of ApoE4, but not for carriers of ApoE2 and ApoE3 (eFigure 2 in the Supplement). Again, chronic low-grade inflammation was significantly associated with a low survival rate in ApoE3 carriers and showed this same trend in ApoE2 and ApoE4 carriers (Figure 2).
Figure 2. Kaplan-Meier Analysis for Survival Free of Alzheimer Disease (AD), Dementia, and Mortality in the Context of ApoE Alleles and Chronic Low-grade Inflammation.
A, AD-free probability for ApoE2 (P = .32). B, AD-free probability for ApoE3 (P = .14). C, AD-free probability for ApoE4 (P = .009). D, Dementia-free probability for ApoE2 (P = .74). E, Dementia-free probability for ApoE3 (P = .06). F, Dementia-free probability for ApoE4 (P = .001). G, Survival probability for ApoE2 (P = .18). H, Survival probability for ApoE3 (P < .001). I, Survival probability for ApoE4 (P = .19). C-reactive protein (CRP) cutoff level of 10 mg/L or higher at a minimum of 2 time points was used to define chronic low-grade inflammation.
Association of the Interaction of ApoE4 and Chronic Low-grade Inflammation With Brain Atrophy
The above findings suggest that chronic systemic inflammation may make the brain more vulnerable to AD. To test this hypothesis, we used brain volumetric measures acquired after the last CRP measurement from 1761 participants. The mean (SD) time from the first CRP measurement to the brain MRI scan was 24.0 (3.1) years, mean (SD) time from the second CRP measurement to the brain MRI scan was 8.5 (3.1) years, and mean (SD) time from the third CRP measurement to the brain MRI scan was 5.6 (3.0) years. The brain volumes of those without (n = 1647) and with (n = 114) chronic low-grade inflammation, defined by cutoff CRP levels of 8 mg/L or higher at a minimum of 2 examinations, were compared (eTable in the Supplement). With 1 exception, we found no differences in brain regions between those without and with chronic low-grade inflammation after Bonferroni corrections. There was a significant difference between the 2 groups for total white matter volume. These associations held for multivariate linear regressions that adjusted for age, sex, educational level, ApoE4 status, and the time between CRP and brain MRI measures (Table 3). The interaction of ApoE4 and chronic low-grade inflammation, however, was negatively associated with the regions associated with AD pathologic characteristics (eg, temporal lobe brain volume: β = –0.78, SE = 0.25; P = .01) and persisted after adjusting for age and sex (temporal lobe brain volume: β = –0.85, SE = 0.22; P < .001) as well as after adjusting for age, sex, time to brain MRI, educational level, and cardiovascular disease (temporal lobe brain volume: β = –0.88, SE = 0.22; P < .001). This same association was found for hippocampal volume in the model adjusted for age, sex, time to brain MRI, educational level, and cardiovascular disease (β = –0.04, SE = 0.01; P = .005). No other significant associations were found for other brain regions.
Table 3. General Linear Regression Analyses of Chronic Low-grade Inflammation Effect and the Interaction Effect Between ApoE4 and Chronic Low-grade Inflammation on Brain Volumes.
| Outcomes (n = 1785) | Modela | Chronic Low-grade Inflammation Alone, High CRP Level (≥8 mg/L Twice) | Interaction Effect, ApoE4 × High CRP Level (≥8 mg/L Twice) | ||
|---|---|---|---|---|---|
| β Estimate (SE) | P Valueb | β Estimate (SE) | P Valueb | ||
| TCBV | 1 | −18.06 (12.97) | .82 | −10.16 (35.59) | >.99 |
| 2 | −14.07 (12.92) | >.99 | −16.52 (35.45) | >.99 | |
| FBV/TCBV%c | 1 | −0.20 (0.13) | .60 | −0.08 (0.35) | >.99 |
| 2 | −0.19 (0.13) | .74 | −0.13 (0.35) | >.99 | |
| PBV/TCBV%c | 1 | −0.03 (0.08) | >.99 | −0.42 (0.25) | .10 |
| 2 | −0.05 (0.08) | >.99 | −0.52 (0.24) | .10 | |
| TBV/TCBV%c | 1 | −0.10 (0.08) | >.99 | −0.85 (0.20) | <.001 |
| 2 | −0.09 (0.09) | >.99 | −0.88 (0.22) | <.001 | |
| HPV/TCBV%c | 1 | −0.004 (0.005) | >.99 | −0.04 (0.02) | .01 |
| 2 | −0.004 (0.005) | >.99 | −0.04 (0.02) | .01 | |
Abbreviations: CRP, C-reactive protein; FBV, frontal lobe brain volume; HPV, hippocampal volume; PBV, parietal lobe brain volume; TBV, temporal lobe brain volume; TCBV, total cerebral brain volume.
aModel 1: adjusted for age and sex. Model 2: model 1 plus time to magnetic resonance imaging, educational level, and ApoE4.
bWith Bonferroni correction.
cPercentage of TCBV indicates brain atrophy.
Discussion
C-reactive protein is a biomarker of low-grade inflammation. To our knowledge, this is the first study to use longitudinal measurements of CRP to define a chronic condition of low-grade inflammation at baseline and demonstrate that ApoE4 interacting with chronic low-grade inflammation increased the risk of AD and shortened the latency for developing AD (Figure 1 and Figure 2). Since it is well documented that infection and inflammation are common in elderly individuals, and preclinical studies have reported that inflammation induced AD pathologic characteristics in mice who only carried ApoE4,16,17 our findings may explain why ApoE4 carriers have increased risk for AD at an old age and suggest that treating chronic low-grade inflammation may delay the onset of AD in ApoE4 carriers.
The strength of this study was its longitudinal follow-up for incident cases of dementia that was preceded by multiple measurements of CRP with an interval between CRP measurements of 6 to 16 years (Table 1). This study offsets the limitation of earlier studies that relied on onetime measurement of CRP to study the development of AD that has resulted in reports of positive,5 negative,6,18 and no association7,19,20,21 between CRP and AD. Although these studies cannot distinguish between those who had an elevated CRP level and then recovered vs those who had a sustained or multiple episodically elevated CRP levels, based on our data analyses we hypothesized that genetic vulnerability for AD might be associated with a long-term low-grade inflammatory condition, albeit either sustained or episodic.
Although genetic risk factors such as ApoE4 for AD are present across the lifespan, disease onset does not occur until later in life.2 Our findings suggest that chronic low-grade inflammation interacts with ApoE4 to accelerate the onset of AD in a pattern dependent on the CRP level (Figure 1 and Figure 2; eFigure 2 in the Supplement). Although ApoE2 carriers had higher levels of CRP with increasing age than did ApoE3 and ApoE4 carriers (Table 1), high CRP levels were not associated with risk of AD among ApoE2 carriers (Figure 1 and Figure 2). It is probable that chronic low-grade inflammation linked with ApoE4 puts the brain into a vulnerable state for the development of AD (Table 3), but the brain of ApoE2 carriers is resilient to the influence of chronic low-grade inflammation on the development of AD. This possibility is consistent with other studies that report other proinflammatory factors associated with brain atrophy.22,23 Another study found that periodontal disease, another common inflammatory condition in elderly individuals, is associated with a higher brain amyloid load detected by amyloid positron emission tomographic scan in healthy elderly individuals.24 Together, these studies suggest a possible link between systemic infection and AD pathologic characteristics in the brain in humans. We propose that if chronic low-grade inflammation is detected through follow-up CRP measurements and is treated among elderly individuals who are ApoE4 carriers, the onset of AD can be delayed or even prevented, since studies have reported that delaying onset by 5 years can reduce the risk for AD by nearly 50%.25
The association between chronic low-grade inflammation and risk of AD for ApoE4 carriers became even more significant in the absence of cardiovascular diseases (Table 2). Since CRP levels are linked to cardiovascular disease,26 which is also a risk factor for AD, our results indicate that chronic low-grade inflammation may play an early role leading to AD in ApoE4 carriers4,8 independent from cardiovascular diseases. Acute inflammatory reaction to infection or injury is a physiological process that is a defense mechanism of the body and is marked by an elevation of CRP levels; however, chronic low-grade inflammation is a pathologic process that may lead to chronic diseases such as cardiovascular disease.26 Although chronic low-grade inflammation was linked to high rates of mortality across all ApoE genotypes, an increased risk of AD was found only in ApoE4 carriers (Table 2, Figure 1, and Figure 2).
The mechanism for the interaction between ApoE4 and a high sustained level of CRP that leads to an increased risk of AD is unknown. Both ApoE and CRP are produced mainly by the liver, implying a liver-brain inflammation axis for the pathogenesis of AD. A liver-brain inflammation axis has been proposed to cause abnormal clinical symptoms in brain diseases, including mood diseases, cognition diseases, and neurovegetative signs,27 but its association with AD remains unclear. Gram-negative bacteria often cause infection in elderly individuals, including in the gastrointestinal, respiratory, and urinary systems. Injection of the gram-negative bacterial cell wall component lipopolysaccharides can increase levels of CRP in the blood,28,29,30 implying that the level of CRP could be a biomarker after attacks from bacteria endotoxin lipopolysaccharides. Systemic administration of lipopolysaccharides into AD mouse models induced AD pathologic characteristics in the brain.16 It has been shown that lipopolysaccharide challenge is linked to cerebrovascular pathologic findings and increased amyloid burden in an ApoE4 AD mouse model, but not in a non-ApoE4 AD mouse model.17 One recent study found that the amount of lipopolysaccharides in the brains of humans with AD are twofold higher compared with control brains.31
Limitations
Although a strength of this study is the longitudinal measurements of CRP, a limitation is that there were not more frequent, preferably annual, CRP measures; thus, it is possible that some cases of sustained inflammatory status may have been misclassified or missed. It is also possible that we have underestimated the interactive association of ApoE4 and chronic low-grade inflammation with AD. Furthermore, the Framingham Heart Study cohort lacks ethnic diversity and thus these findings lack generalizability to nonwhite populations.
Conclusions
As systemic infection and inflammatory attacks are common in elderly individuals, recovery of the immune system to baseline could be critical for certain genotypes such as ApoE4 for the sequela of AD development. Evidence of chronic low-grade inflammation stage could be targeted for personalized treatment. Our findings provide initial evidence of the importance of ApoE genotype in clinical trial studies of anti-inflammatory drugs for AD. Although previous clinical trials of anti-inflammatory drugs for AD have failed,32 specifically targeting a subset of patients based on ApoE genotypes and inflammation status may be an important consideration for future clinical trial study design. Additional studies are warranted to determine whether rigorous treatment of infection and inflammation that lower CRP levels to a normal level will attenuate the risk of AD for ApoE4 carriers.
eTable. Brain Volume Comparisions Between Those With Low vs High Chronic Low-grade Inflammation: CRP ≥ 8 mg/dL at a Minimum of 2 Time Points
eFigure 1. Sample Selection Flowchart
eFigure 2. Kaplan-Meier Analysis for AD Dementia-Free Survival in the context of ApoE Alleles and Chronic Low-grade Inflammation
References
- 1.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):-. doi: 10.1073/pnas.90.5.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanzi RE. A genetic dichotomy model for the inheritance of Alzheimer’s disease and common age-related disorders. J Clin Invest. 1999;104(9):1175-1179. doi: 10.1172/JCI8593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephensen CB, Gildengorin G. Serum retinol, the acute phase response, and the apparent misclassification of vitamin A status in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2000;72(5):1170-1178. doi: 10.1093/ajcn/72.5.1170 [DOI] [PubMed] [Google Scholar]
- 4.Desikan RS, Schork AJ, Wang Y, et al. ; Inflammation working group, IGAP and DemGene Investigators . Polygenic overlap between C-reactive protein, plasma lipids, and Alzheimer disease. Circulation. 2015;131(23):2061-2069. doi: 10.1161/CIRCULATIONAHA.115.015489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song IU, Chung SW, Kim YD, Maeng LS. Relationship between the hs-CRP as non-specific biomarker and Alzheimer’s disease according to aging process. Int J Med Sci. 2015;12(8):613-617. doi: 10.7150/ijms.12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Bryant SE, Waring SC, Hobson V, et al. Decreased C-reactive protein levels in Alzheimer disease. J Geriatr Psychiatry Neurol. 2010;23(1):49-53. doi: 10.1177/0891988709351832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundelöf J, Kilander L, Helmersson J, et al. Systemic inflammation and the risk of Alzheimer’s disease and dementia: a prospective population-based study. J Alzheimers Dis. 2009;18(1):79-87. doi: 10.3233/JAD-2009-1126 [DOI] [PubMed] [Google Scholar]
- 8.Royall DR, Al-Rubaye S, Bishnoi R, Palmer RF. Few serum proteins mediate APOE’s association with dementia. PLoS One. 2017;12(3):e0172268. doi: 10.1371/journal.pone.0172268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson PW, Nam BH, Pencina M, D’Agostino RB Sr, Benjamin EJ, O’Donnell CJ. C-reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med. 2005;165(21):2473-2478. doi: 10.1001/archinte.165.21.2473 [DOI] [PubMed] [Google Scholar]
- 10.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham offspring study. Am J Epidemiol. 1979;110(3):281-290. doi: 10.1093/oxfordjournals.aje.a112813 [DOI] [PubMed] [Google Scholar]
- 11.Rost NS, Wolf PA, Kase CS, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32(11):2575-2579. doi: 10.1161/hs1101.098151 [DOI] [PubMed] [Google Scholar]
- 12.Seshadri S. Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and Alzheimer’s disease? J Alzheimers Dis. 2006;9(4):393-398. doi: 10.3233/JAD-2006-9404 [DOI] [PubMed] [Google Scholar]
- 13.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging. 2005;26(4):491-510. doi: 10.1016/j.neurobiolaging.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 14.Jefferson AL, Himali JJ, Beiser AS, et al. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010;122(7):690-697. doi: 10.1161/CIRCULATIONAHA.109.905091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeCarli C, Reed T, Miller BL, Wolf PA, Swan GE, Carmelli D. Impact of apolipoprotein E epsilon4 and vascular disease on brain morphology in men from the NHLBI twin study. Stroke. 1999;30(8):1548-1553. doi: 10.1161/01.STR.30.8.1548 [DOI] [PubMed] [Google Scholar]
- 16.Miklossy J. Chronic inflammation and amyloidogenesis in Alzheimer’s disease—role of spirochetes. J Alzheimers Dis. 2008;13(4):381-391. doi: 10.3233/JAD-2008-13404 [DOI] [PubMed] [Google Scholar]
- 17.Marottoli FM, Katsumata Y, Koster KP, Thomas R, Fardo DW, Tai LM. Peripheral inflammation, apolipoprotein E4, and amyloid-β interact to induce cognitive and cerebrovascular dysfunction. ASN Neuro. 2017;9(4):1759091417719201. doi: 10.1177/1759091417719201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yarchoan M, Louneva N, Xie SX, et al. Association of plasma C-reactive protein levels with the diagnosis of Alzheimer’s disease. J Neurol Sci. 2013;333(1-2):9-12. doi: 10.1016/j.jns.2013.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuitemaker A, Dik MG, Veerhuis R, et al. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2009;30(11):1885-1889. doi: 10.1016/j.neurobiolaging.2008.01.014 [DOI] [PubMed] [Google Scholar]
- 20.Licastro F, Pedrini S, Davis LJ, et al. Alpha-1-antichymotrypsin and oxidative stress in the peripheral blood from patients with probable Alzheimer disease: a short-term longitudinal study. Alzheimer Dis Assoc Disord. 2001;15(1):51-55. doi: 10.1097/00002093-200101000-00007 [DOI] [PubMed] [Google Scholar]
- 21.Dik MG, Jonker C, Comijs HC, et al. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57(12):2217-2222. doi: 10.1212/WNL.57.12.2217 [DOI] [PubMed] [Google Scholar]
- 22.Jefferson AL, Massaro JM, Wolf PA, et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68(13):1032-1038. doi: 10.1212/01.wnl.0000257815.20548.df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu Y, Manly JJ, Mayeux RP, Brickman AM. An inflammation-related nutrient pattern is associated with both brain and cognitive measures in a multiethnic elderly population. Curr Alzheimer Res. 2018;15(5):493-501. doi: 10.2174/1567205015666180101145619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamer AR, Pirraglia E, Tsui W, et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol Aging. 2015;36(2):627-633. doi: 10.1016/j.neurobiolaging.2014.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson D, Peters R, Ritchie K, Ritchie CW. Latest advances on interventions that may prevent, delay or ameliorate dementia. Ther Adv Chronic Dis. 2011;2(3):161-173. doi: 10.1177/2040622310397636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang TJ, Nam BH, Wilson PW, et al. Association of C-reactive protein with carotid atherosclerosis in men and women: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2002;22(10):1662-1667. doi: 10.1161/01.ATV.0000034543.78801.69 [DOI] [PubMed] [Google Scholar]
- 27.D’Mello C, Swain MG. Liver-brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain Behav Immun. 2014;35:9-20. doi: 10.1016/j.bbi.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 28.Jeukendrup AE, Vet-Joop K, Sturk A, et al. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin Sci (Lond). 2000;98(1):47-55. [PubMed] [Google Scholar]
- 29.Gordienko AI. Levels of serum antibodies to enterobacterial lipopolysaccharides and their relationship with concentration of C-reactive protein in diabetes mellitus patients [in Ukrainian]. Ukr Biochem J. 2015;87(3):98-106. doi: 10.15407/ubj87.03.098 [DOI] [PubMed] [Google Scholar]
- 30.Monnet E, Lapeyre G, Poelgeest EV, et al. Evidence of NI-0101 pharmacological activity, an anti-TLR4 antibody, in a randomized phase I dose escalation study in healthy volunteers receiving LPS. Clin Pharmacol Ther. 2017;101(2):200-208. doi: 10.1002/cpt.522 [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y, Cong L, Lukiw WJ. Lipopolysaccharide (LPS) accumulates in neocortical neurons of Alzheimer’s disease (AD) brain and impairs transcription in human neuronal-glial primary co-cultures. Front Aging Neurosci. 2017;9:407. doi: 10.3389/fnagi.2017.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ADAPT-FS Research Group Follow-up evaluation of cognitive function in the randomized Alzheimer’s Disease Anti-inflammatory Prevention Trial and its follow-up study. Alzheimers Dement. 2015;11(2):216-225. doi: 10.1016/j.jalz.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Brain Volume Comparisions Between Those With Low vs High Chronic Low-grade Inflammation: CRP ≥ 8 mg/dL at a Minimum of 2 Time Points
eFigure 1. Sample Selection Flowchart
eFigure 2. Kaplan-Meier Analysis for AD Dementia-Free Survival in the context of ApoE Alleles and Chronic Low-grade Inflammation