Abstract
Lessons Learned.
Irinotecan could not be proven noninferior to paclitaxel as a second‐line treatment for patients with metastatic or recurrent gastric cancer.
The failure to demonstrate noninferiority may have been a result of insufficient patient enrollment.
Both agents were tolerable but showed different toxicity profiles.
Background.
This phase III study compared the efficacy and safety of paclitaxel versus irinotecan in patients with metastatic or recurrent gastric cancer (MRGC) who had experienced disease progression following first‐line chemotherapy.
Methods.
Patients were randomized to receive either paclitaxel (70 mg/m2; days 1, 8, 15, every 4 weeks) or irinotecan (150 mg/m2 every other week). The primary endpoint was progression‐free survival (PFS).
Results.
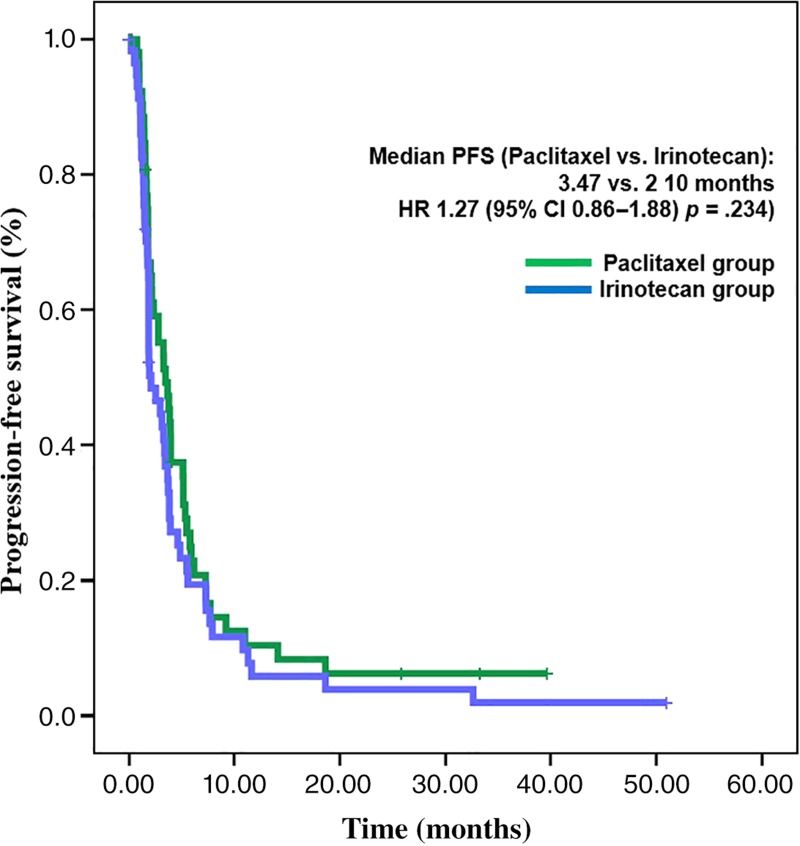
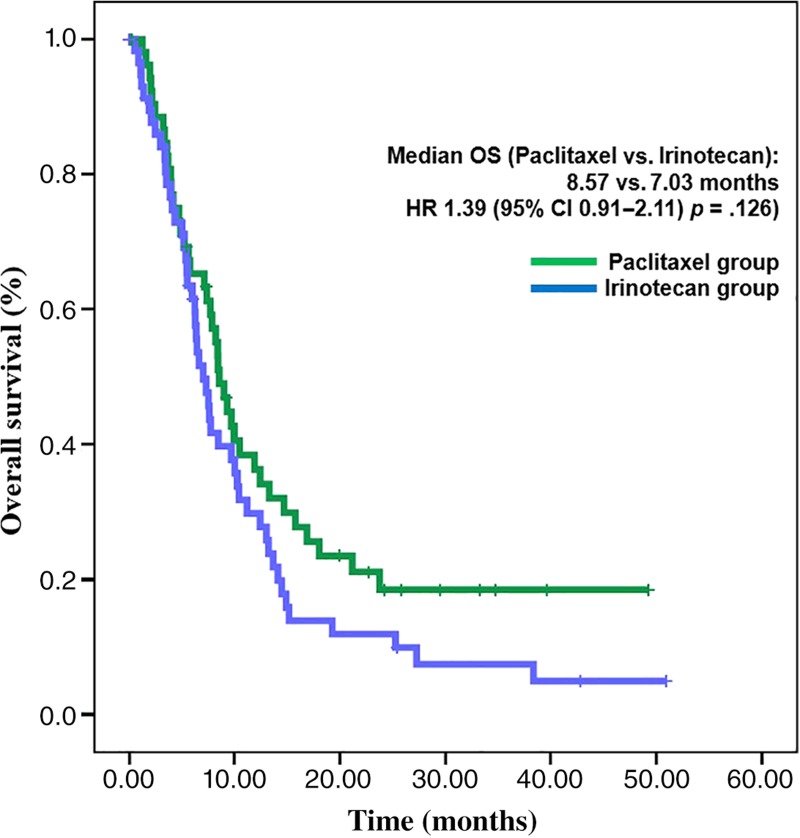
This study was stopped early due to low accrual rate. A total of 112 patients were enrolled; 54 were allocated to paclitaxel and 58 to irinotecan. Median PFS for the paclitaxel and irinotecan groups was 3.5 and 2.1 months, respectively (hazard ratio [HR], 1.27; 95% confidence interval [CI], 0.86–1.88; p = .234). Noninferiority of irinotecan to paclitaxel was not proved because the upper boundary of the 95% CI (1.88) exceeded the predefined upper margin of noninferiority (1.32). Median overall survival (OS) was 8.6 months in the paclitaxel group and 7.0 months in the irinotecan group (HR, 1.39; 95% CI, 0.91–2.11; p = .126). Among toxicities greater than or equal to grade 3, neutropenia (11.5%) was the most common, followed by peripheral neuropathy (7.7%) in the paclitaxel group, and neutropenia (34.5%) followed by nausea, vomiting, and anemia (8.6%, respectively) in the irinotecan group.
Conclusion.
Although paclitaxel showed numerically longer PFS and OS compared with irinotecan, this was statistically insignificant. Both irinotecan and paclitaxel are valid second‐line treatment options in MRGC.
Abstract
经验获取
• 就作为转移性或复发性胃癌患者的二线治疗而言,无法证明伊立替康不劣于紫杉醇。
• 未能证明非劣性可能是由于患者招募人数不足所导致的结果。
• 虽然这两种药物都具有耐受性,但是,它们显示出了不同的毒性特征。
摘要
背景。本次 III 期研究比较了紫杉醇与伊立替康在经过一线治疗之后出现疾病进展的转移性或复发性胃癌 (MRGC) 患者中的有效性和安全性。
方法。患者随机接受紫杉醇(70 mg/m2;在每 4 周的第 1 天、第 8 天、第 15 天给药)或伊立替康(150 mg/m2,每隔一周给药)治疗。主要终点是无进展生存期 (PFS)。
结果。本研究因招募率低而提前终止。一共入组了 112 名患者;54 名患者分配至紫杉醇组,58 名患者分配至伊立替康组。紫杉醇组和伊立替康组的中位 PFS 分别为 3.5 个月和 2.1 个月 [风险比 (HR),1.27;95% 置信区间 (CI),0.86–1.88;p = 0.234]。由于 95% CI 的上限 (1.88) 超过了非劣性的预定义上限 (1.32),因此,未能证明伊立替康不劣于紫杉醇。紫杉醇组和伊立替康组的中位总生存期 (OS) 分别为 8.6 个月和 7.0 个月(HR,1.39;95% CI,0.91–2.11;p = 0.126)。在 3 级或以上的毒性中,紫杉醇组最常见的是嗜中性粒细胞减少症 (11.5%), 其次是周围神经病变 (7.7%);伊立替康组最常见的是嗜中性粒细胞减少症 (34.5%), 其次是恶心、呕吐和贫血(分别为 8.6%)。
结论。尽管紫杉醇在数字上显示出比伊立替康更长的 PFS 和 OS,但是,这在统计上并不显著。伊立替康和紫杉醇都是 MRGC 的有效二线治疗选择。
Discussion
Second‐line chemotherapy for metastatic or recurrent gastric cancer (MRGC) has been shown to improve survival in previous clinical trials. Although taxane or irinotecan are commonly used as second‐line chemotherapy for MRGC, few previous studies have directly compared the efficacy between taxane and irinotecan. In our study, therefore, we compared the efficacy and safety of irinotecan and paclitaxel as second‐line therapy in MRGC. PFS and OS were not statistically different (Figs. 1 and 2). However, noninferiority of irinotecan compared with paclitaxel could not be confirmed because of low patient enrollment. Toxicity profiles of irinotecan and paclitaxel were different.
Figure 1.
Progression‐free survival.
Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression‐free survival.
Figure 2.
Overall survival.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival.
Like our study, a previous Japanese phase III trial (WJOG 4007) compared the efficacy of paclitaxel versus irinotecan. The WJOG 4007 study was conducted to verify the hypothesis that irinotecan has superior OS to paclitaxel, and the authors concluded that both irinotecan and paclitaxel are reasonable second‐line treatment options for MRGC because no significant difference in OS was observed. However, strictly speaking, the noninferiority of one agent to the other was also not proved in the WJOG 4007, considering the study design. When results of WJOG 4007 and our studies are compared, an interesting finding is observed simultaneously; paclitaxel showed numerically longer PFS (3.6 vs. 2.3 months [WJOG 4007; p = .33]; 3.5 vs. 2.1 months [our study; p = .234]) and OS (9.5 vs. 8.4 months [WJOG 4007; p = .38]; 8.6 vs. 7.0 months [our study; p = .126]) compared with irinotecan. As the difference in survival outcomes was not statistically significant in both WJOG 4007 and our studies, the observation of possible superiority of paclitaxel over irinotecan can only be considered hypothesis‐generating.
Patient enrollment was less than expected and is the most important limitation in interpreting study results. During the study period, results of WJOG 4007 were reported, and this drove investigators to be less interested in our study and thus to enroll patients less often.
In conclusion, noninferiority of irinotecan compared with paclitaxel could not be proven. The hazard ratio of PFS crossed the boundary needed to prove noninferiority. Although paclitaxel showed numerically longer PFS and OS compared with irinotecan, this was statistically insignificant. Therefore, both irinotecan and paclitaxel are thought to be valid second‐line treatment options in MRGC. Considering different toxicity profiles and treatment schedule, the choice of chemotherapeutic agents must be based on medical condition and compliance of patients.
Trial Information
- Disease
Gastric cancer
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
1 prior regimen
- Type of Study ‐ 1
Phase III
- Type of Study ‐ 2
Randomized
- Primary Endpoint
Progression‐free survival
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Overall response rate
- Secondary Endpoint
Safety
- Additional Details of Endpoints or Study Design
- For sample size calculation, median PFS of the paclitaxel group was assumed to be 3.5 months based on literature review [1], [2], [3]. The efficacy of irinotecan was hypothesized to be noninferior to that of paclitaxel, which means median PFS of irinotecan would be at least longer than 2.65 months. Accordingly we decided noninferiority would be claimed if the upper boundary of the 95% CI of the hazard ratio did not exceed 1.32.
- Using a one‐sided test with 2.5% alpha and 20% beta errors, planned accrual and follow‐up periods of 24 and 12 months, and an expected dropout rate of 10%, 260 patients per group were needed. That is, a total of 520 patients were planned.
- The primary endpoint was PFS defined as time from the randomization to disease progression or death from any cause, whichever came earlier. Secondary endpoints were OS, overall response rate (ORR), and safety profiles. OS was defined as time from the randomization to death from any cause. PFS and OS were analyzed using the Kaplan‐Meier method in intention‐to‐treat population. The HR of irinotecan compared with paclitaxel in PFS and OS analyses was calculated using the Cox proportional hazards regression model. ORR was the proportion of patients with complete response (CR) plus partial response (PR) among patients with one or more measurable lesions in baseline computed tomography. Patients with nontarget lesion only were excluded from the ORR analysis.
- Investigator's Analysis
Active but results overtaken by other developments
Drug Information for Phase III Control
- Drug 1
- Generic/Working Name
Paclitaxel
- Trade Name
Taxol
- Company Name
Boryung Pharmacetutical
- Drug Type
Small molecule
- Drug Class
Microtubule‐targeting agent
- Dose
70 mg/m2
- Route
IV
- Schedule of Administration
Paclitaxel (Taxol; 70 mg/m2) mixed with 100–250 mL of normal saline was administered intravenously over 1 hour on days 1, 8, and 15, every 4 weeks. The 4‐week schedule was considered one cycle. Premedication for paclitaxel using corticosteroid and antihistamine was conducted following each institution's policy.
Drug Information for Phase III Experimental
- Drug 1
- Generic/Working Name
Irinotecan
- Trade Name
Campto
- Company Name
CJ HealthCare Corp.
- Drug Type
Small molecule
- Drug Class
Topoisomerase I
- Dose
150 mg/m2
- Route
IV
- Schedule of Administration
Irinotecan (Campto; 150 mg/m2) mixed with 100–250 mL of 5% dextrose water was administered intravenously over 1 hour on days 1 and 15, every 4 weeks. The 4‐week schedule was considered one cycle. In patients developing irinotecan‐induced cholinergic symptoms (abdominal pain and diarrhea), atropine was used as premedication.
Patient Characteristics for Phase III Control
- Number of Patients, Male
38
- Number of Patients, Female
16
- Stage
All patients had metastatic or recurrent disease.
- Age
Median (range): 58.5 (38–82)
- Number of Prior Systemic Therapies
Median (range): 1 (1)
- Performance Status: ECOG
-
0 — 0
1 — 52
2 — 2
3 — 0
Unknown — 0
- Other
Additional information is shown in Table 1. During the period from February 2011 through January 2015, a total of 116 patients were screened, and 112 patients were enrolled at 16 sites in Korea. During the enrollment period, patient accrual rate was low, and we decided to stop this study early even though the target number of patients was 520.
- Cancer Types or Histologic Subtypes
-
Well differentiated 4
Moderately differentiated 10
Poorly differentiated 38
Undifferentiated 1
Cannot be assessed 1
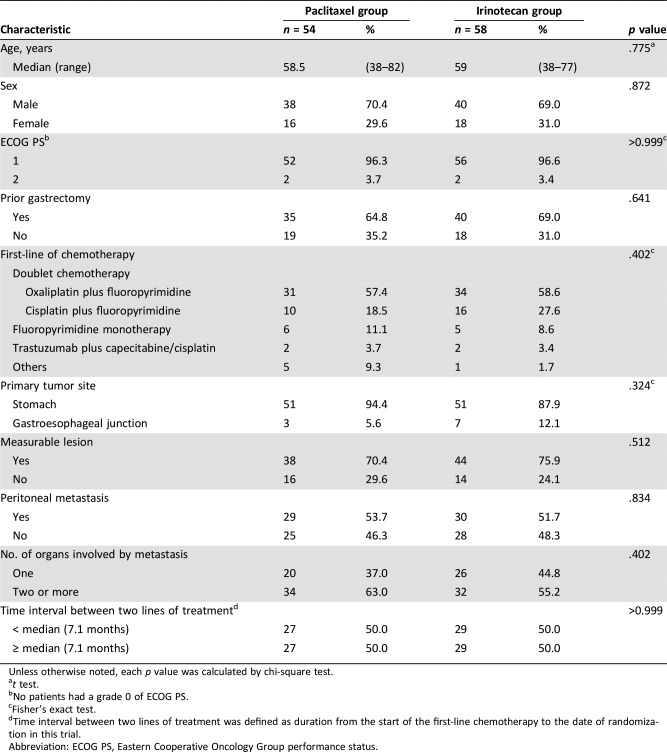
Table 1. Baseline characteristics.
Unless otherwise noted, each p value was calculated by chi‐square test.
t test.
No patients had a grade 0 of ECOG PS.
Fisher's exact test.
Time interval between two lines of treatment was defined as duration from the start of the first‐line chemotherapy to the date of randomization in this trial.
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
Patient Characteristics for Phase III Experimental
- Number of Patients, Male
40
- Number of Patients, Female
18
- Stage
All patients had metastatic or recurrent disease.
- Age
Median (range): 59 (38–77)
- Number of Prior Systemic Therapies
Median (range): 1 (1)
- Performance Status: ECOG
-
0 — 0
1 — 56
2 — 2
3 — 0
Unknown — 0
- Other
Additional information is shown in Table 1.
- Cancer Types or Histologic Subtypes
-
Well differentiated 34
Moderately differentiated 16
Poorly differentiated 5
Undifferentiated 2
Cannot be assessed 1
Primary Assessment Method for Phase III Control
- Title
Paclitaxel arm
- Number of Patients Screened
54
- Number of Patients Enrolled
54
- Number of Patients Evaluable for Toxicity
52
- Number of Patients Evaluated for Efficacy
54
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 1 (2.6%)
- Response Assessment PR
n = 5 (13.2%)
- Response Assessment SD
n = 16 (42.1%)
- Response Assessment PD
n = 14 (36.8%)
- Response Assessment OTHER
n = 2 (5.3%)
- (Median) Duration Assessments PFS
3.5 months, CI: 2.2–4.7
- (Median) Duration Assessments OS
8.6 months, CI: 7.1–10.0
- Outcome Notes
- Between February 2011 and January 2015, a total of 116 patients were screened, and 112 patients were enrolled at 16 sites in Korea. Among 112 patients, 54 were allocated to paclitaxel and 58 to irinotecan. There were three patients who did not meet the eligibility criteria; the three patients were randomized to paclitaxel and received the study treatment. Although the enrollment of these three patients was a major protocol violation, these patients were included in the intention‐to‐treat analysis per study protocol. Thus, the full analysis set consisted of 54 patients in the paclitaxel group and 58 in the irinotecan group (n = 112). Of these patients, two in the paclitaxel group and one in the irinotecan group did not receive the allocated treatment because of consent withdrawal before the first dose. Therefore, the safety analysis set included 52 patients in the paclitaxel group and 57 in the irinotecan group. Response evaluation was conducted on patients with at least one measurable lesion; 38 of 54 in the paclitaxel group and 44 of 58 in the irinotecan group.
Primary Assessment Method for Phase III Experimental
- Title
Irinotecan arm
- Number of Patients Screened
58
- Number of Patients Enrolled
58
- Number of Patients Evaluable for Toxicity
57
- Number of Patients Evaluated for Efficacy
58
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 1 (2.3%)
- Response Assessment PR
n = 5 (11.4%)
- Response Assessment SD
n = 15 (34.1%)
- Response Assessment PD
n = 16 (36.4%)
- Response Assessment OTHER
n = 7 (15.9%)
- (Median) Duration Assessments PFS
2.1 months, CI: 1.4–2.8
- (Median) Duration Assessments OS
7.0 months, CI: 5.6–8.4
Adverse Events
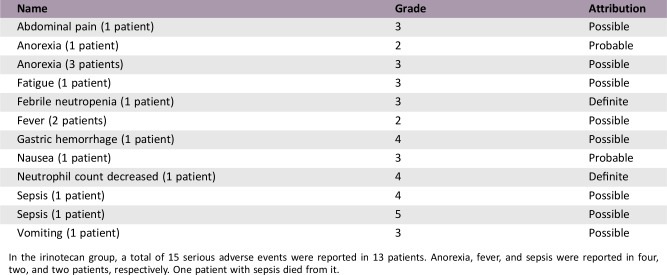
- Adverse events are shown in Table 2.
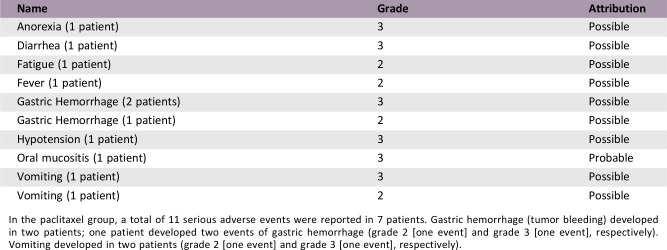
Serious Adverse Events (Paclitaxel)
In the paclitaxel group, a total of 11 serious adverse events were reported in 7 patients. Gastric hemorrhage (tumor bleeding) developed in two patients; one patient developed two events of gastric hemorrhage (grade 2 [one event] and grade 3 [one event], respectively). Vomiting developed in two patients (grade 2 [one event] and grade 3 [one event], respectively).
Serious Adverse Events (Irinotecan)
In the irinotecan group, a total of 15 serious adverse events were reported in 13 patients. Anorexia, fever, and sepsis were reported in four, two, and two patients, respectively. One patient with sepsis died from it.
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated Reason
Did not fully accrue
- Investigator's Assessment
Active but results overtaken by other developments
Gastric cancer (GC) is a major cause of cancer‐related death, with more than 720,000 deaths worldwide [4]. In Korea, where GC is one of the most common types of cancer, GC is estimated to be the fourth and fifth most common cause of death in male and female cancer patients, respectively [5]. In general, fluoropyrimidine‐based doublet chemotherapy, or triplet chemotherapy for highly selected patients, is widely used as palliative first‐line therapy in patients with metastatic or recurrent GC (MRGC) [6], [7]. If the tumor shows human epidermal growth factor 2 amplification, addition of trastuzumab to fluoropyrimidine plus platinum is now the standard first‐line treatment [8]. After failure of first‐line chemotherapy, second‐line therapy with docetaxel or irinotecan showed improved overall survival (OS) compared with best supportive care in previous phase III trials [9], [10], [11].
Paclitaxel, a microtubule stabilizing agent that inhibits depolymerization during cell division, has been tested as a second‐line chemotherapy for MRGC in previous single‐arm studies [12]. Overall response rate (ORR) was 16%–24%, with median OS of 5–8 months [1], [2], [3], [13], [14], [15]. Irinotecan inhibits topoisomerase I from unwinding DNA strands during DNA replication and has also been examined as a second‐line therapy in previous single‐arm studies, which showed 12%–20% of ORR and about 5 months of OS [16], [17]. These efficacy data seemed to be comparable between irinotecan and paclitaxel in the second‐line setting for MRGC, and both agents have been widely used in clinics in Korea. As there were no data from randomized clinical trials that directly compared the efficacy and safety of paclitaxel and irinotecan as second‐line therapy in MRGC, we designed and conducted this phase III trial.
In this phase III trial, patients with histologically confirmed metastatic or recurrent gastric adenocarcinoma were eligible if they were older than 18 years and had disease progression to the palliative first‐line chemotherapy. If patients experienced recurrence during or within 6 months after the completion of adjuvant chemotherapy following curative surgery, they were allowed to be enrolled to this trial. Other eligibility criteria were Eastern Cooperative Oncology Group performance status of 0–2; evaluable tumor lesions with or without measurable target lesions based on RECIST, version 1.1; adequate organ functions including bone marrow, kidney, and liver; and more than 16 weeks of expected survival. Patients were excluded if they had received more than one line of prior chemotherapy or had been exposed to taxane or irinotecan prior to this trial.
This was an investigator‐initiated, multicenter, randomized, phase III trial conducted at 16 centers in Korea. The study protocol was approved by the institutional review board of each participating institution and the Korean Cancer Study Group (KCSG; trial number, KCSG ST10‐01). This trial was registered with ClinicalTrials.gov (NCT01224652). The randomization was performed centrally at the KCSG data center using the method of permuted block randomization. Patients were randomly assigned in a ratio of 1:1 to either paclitaxel or irinotecan.
Paclitaxel (Taxol; 70 mg/m2) was administered intravenously on days 1, 8, and 15, every 4 weeks. Irinotecan (Campto; 150 mg/m2) was administered intravenously on days 1 and 15, every 4 weeks. For both agents, the 4‐week schedule was considered one cycle. Predefined dose reduction or delay were conducted to manage treatment‐related toxicity. If neutropenia or thrombocytopenia of greater than or equal to grade 3 or clinically significant nonhematologic toxicities developed, dose reduction of paclitaxel (60 mg/m2) and irinotecan (120 mg/m2) was performed. If clinically significant hematologic or nonhematologic toxicities developed again despite dose reduction, no more dose reduction was conducted and the study treatment was permanently withdrawn. If the administration of study treatment was delayed more than 4 weeks due to delayed recovery from toxicities, the study treatment was also permanently withdrawn. The study treatment was continued until disease progression, death, development of unacceptable toxicity, or a patient's refusal of further therapy.
Tumor response evaluation using computed tomography was performed every 8 weeks (windows, ±7 days) based on RECIST (version 1.1). Hematologic laboratory test (complete blood count with differentiation) was repeated every week during the first cycle and then checked before the administration of study drugs (day 1, 8, and 15 for paclitaxel; day 1 and 15 for irinotecan). Chemistry was performed on the first day of each cycle. Adverse event was assessed at every visit according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
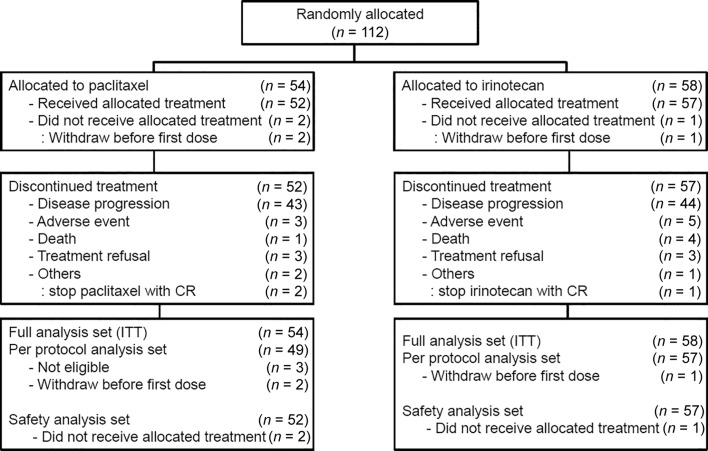
Between February 2011 and January 2015, a total of 116 patients were screened, and 112 patients were enrolled at 16 sites in Korea. During the enrollment period, the accrual rate was low, and the result of a randomized phase III trial (WJOG4007), which had a similar design to our study, was reported in 2013 [18]. Therefore, we decided to stop this study early even though the target number of patients was 520. Among 112 patients, 54 were allocated to paclitaxel and 58 to irinotecan. Thus, full analysis set consisted of 54 in the paclitaxel group and 58 in the irinotecan group (n = 112). Of these patients, two in the paclitaxel group and one in the irinotecan group did not receive the allocated treatment because of consent withdrawal before the first dose. Therefore, safety analysis set included 52 in the paclitaxel group and 57 in the irinotecan group (Fig. 3; CONSORT diagram).
Figure 3.
Consolidated Standards of Reporting Trials diagram. Abbreviations: CR, complete response; ITT, intention‐to‐treat.
Table 1 summarized baseline characteristics across two treatment groups. The median time interval from the start of the first‐line chemotherapy to the date of randomization for the study treatment in all 112 patients was 7.1 months (range, 1.0–37.7). Overall, each variable was well balanced between two groups. All patients had received fluoropyrimidine‐based first‐line chemotherapy prior to the enrollment into this study. Although there seemed to be a little imbalance in first‐line chemotherapy regimens between the two groups, this was not statistically significant. In both groups, oxaliplatin‐based doublet chemotherapy was the most common treatment used as the first‐line therapy.
The data cutoff date was December 4, 2015. Median treatment duration in paclitaxel and irinotecan groups were 10.5 (range, 0–90.3) and 6.2 (range, 0–138.3) weeks, respectively. Mean dose intensities (± standard deviation) were 46.2 (±10.8) mg/m2/week in the paclitaxel group and 60.9 (±15.3) mg/m2/week in the irinotecan group, respectively.
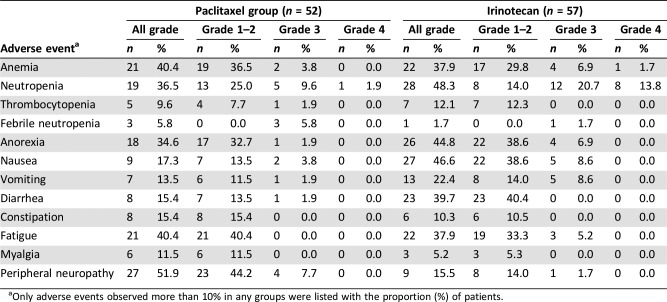
Adverse events were observed in 96.3% of all the study population. Severe adverse events (grade ≥3) occurred in 32.7% of patients in the paclitaxel group and 45.6% in the irinotecan group (p = .177). Table 2 summarized adverse events according to the grade in each group. In the paclitaxel group, the most common adverse event was peripheral neuropathy (51.9%), followed by anemia (40.4%) and fatigue (40.4%). Neutropenia and anorexia were also frequent. Among adverse events greater than grade 3, neutropenia was the most common (11.5%). Peripheral neuropathy of greater than or equal to grade 3 was observed in 7.7% of the patients. In the irinotecan group, the most common adverse event was neutropenia (48.3%), followed by nausea (46.6%) and anorexia (44.8%). Diarrhea, anemia, and fatigue were also frequent. The most common severe adverse event (greater than or equal to grade 3) was neutropenia (34.5%). Peripheral neuropathy was more common in the paclitaxel group compared with the irinotecan group, whereas diarrhea was more prevalent in the irinotecan group. The irinotecan group also showed more frequent grade 3 or 4 neutropenia compared with the paclitaxel group.
Table 2. Adverse events.
Only adverse events observed more than 10% in any groups were listed with the proportion (%) of patients.
Irinotecan did not show statistically different progression‐free survival (PFS) compared with paclitaxel. Median PFS was 2.1 months in the irinotecan group and 3.5 months in the paclitaxel group, respectively (hazard ratio [HR], 1.27; 95% confidence interval [CI], 0.86–1.88; p = .234; Fig. 1). Noninferiority of irinotecan could not be confirmed because the CI exceeded the limit of predefined noninferiority margin of 1.32. Median OS was 7.0 months in the irinotecan group and 8.6 months in the paclitaxel group. The difference was not statistically significant (HR, 1.39; 95% CI, 0.91–2.11; p = .126; Fig. 2). Of 54 patients in the paclitaxel group, 30 (56%) received poststudy treatment, and irinotecan‐containing regimens were most commonly used (83%; 25/30). Of 58 patients in the irinotecan group, 36 (62%) received poststudy chemotherapy, and these patients received taxane‐based regimens most commonly (81%; 29/36). Thus, a total of 66 patients (59%) received at least one line of treatment after end of the study treatment. Response evaluation was conducted on patients with at least one measurable lesion (38 of 54 in the paclitaxel group and 44 of 58 in the irinotecan group). Response rate was also similar between two treatment groups. ORRs of paclitaxel group and irinotecan group were 15.8% and 13.6%, respectively (p = .355; Table 3).
Table 3. Response rate.
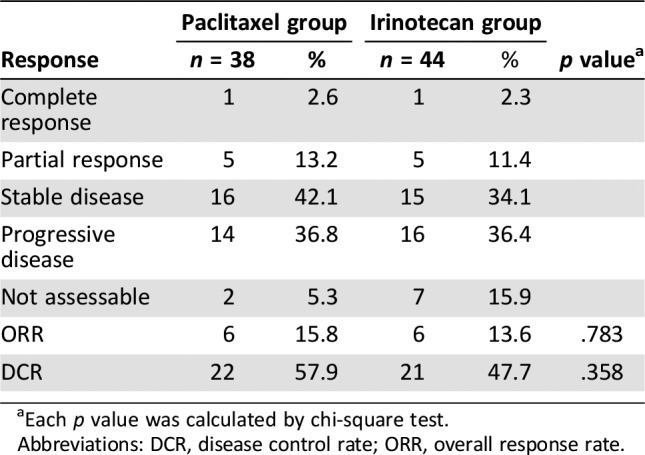
Each p value was calculated by chi‐square test.
Abbreviations: DCR, disease control rate; ORR, overall response rate.
The role of second‐line chemotherapy for MRGC has been shown in recent phase III trials. Irinotecan was found to be effective as a second‐line chemotherapy in German AIO trial despite low accrual [10]. A large phase III trial from Korea established the role of second‐line chemotherapy, where irinotecan or docetaxel was chosen according to physician's discretion. In this trial, second‐line chemotherapy showed superior OS compared with best supportive care alone (5.3 vs. 3.8 months [median]; HR, 0.657; p = .007) [9]. In the COUGAR‐02 trial, second‐line docetaxel showed improved OS compared with active symptom control group (5.2 vs. 3.6 months [median]; p = .01), although docetaxel was associated with more toxicity [11].
In our study (KCSG ST10‐01), we compared the efficacy and safety of irinotecan and paclitaxel as second‐line therapy in MRGC. However, noninferiority of irinotecan compared with paclitaxel could not be confirmed in our study. The most crucial reason for this is low patient enrollment, which was translated into lower power to test the hypothesis. Like our study, a previous Japanese phase III trial (WJOG 4007) compared the efficacy of paclitaxel versus irinotecan as a second‐line chemotherapy in MRGC [18]. The WJOG 4007 study was conducted to verify the hypothesis that irinotecan has superior OS to paclitaxel, and the authors concluded that both irinotecan and paclitaxel are reasonable second‐line treatment options for MRGC because no statistically significant difference in OS was observed between paclitaxel and irinotecan. However, strictly speaking, the noninferiority of one agent to the other was not proved in the WJOG 4007, considering the study design. When results of KCSG ST10‐01 and WJOG 4007 studies are compared, an interesting finding is observed simultaneously in both studies: Paclitaxel showed numerically longer PFS (3.6 vs. 2.3 months in WJOG 4007 [p = .33]; 3.5 vs. 2.1 months in our study [p = .234]) and OS (9.5 vs. 8.4 months in WJOG 4007 [p = .38]; 8.6 vs. 7.0 months in our study [p = .126]) compared with irinotecan, although statistically insignificant. In our study, paclitaxel showed comparable PFS and OS to those reported in recent phase III studies (2.9–3.6 months of PFS and 6.9–9.5 months of OS) [18], [19], [20]. As the difference in survival outcomes was not statistically significant in both WJOG 4007 and our studies, the observation of possible superiority of paclitaxel over irinotecan is just hypothesis‐generating. All toxicity profiles of irinotecan and paclitaxel were different. Among toxicities of greater than or equal to grade 3, neutropenia (11.5%) was the most common, followed by peripheral neuropathy (7.7%) in the paclitaxel group, and neutropenia (34.5%) followed by nausea, vomiting, and anemia (8.6%, respectively) in the irinotecan group. These toxicity profiles were consistent with previous reports and manageable [9], [10], [14], [17], [18]. Taken together, we authors agree that both irinotecan and paclitaxel are reasonable second‐line treatment options in MRGC.
During our study period, ramucirumab, a monoclonal antibody inhibiting vascular endothelial growth factor receptor 2, was approved for second‐line treatment as monotherapy or in combination with paclitaxel based on two pivotal phase III trials [19], [21]. In the REGARD trial, ramucirumab monotherapy showed longer OS compared with placebo (5.2 vs. 3.8 months [median]; HR, 0.776; p = .047). Median PFS was also improved with ramucirumab (2.1 vs. 1.3 months [median]; HR, 0.483; p < .0001) [21]. Likewise, in the RAINBOW trial, the combination of ramucirumab and paclitaxel showed superior PFS (4.4 vs. 2.9 months [median]; HR, 0.635; p < .0001) and OS (9.6 vs. 7.4 months [median]; HR, 0.807; p = .017) compared with paclitaxel alone [19]. However, in the preplanned subgroup analysis in the both studies, the OS in Asian patients were not statistically different between each treatment group [19], [21], although the subgroup analysis in the RAINBOW trial showed the superiority of PFS in the ramucirumab plus paclitaxel group compared with paclitaxel plus placebo group even in Asian patients [19]. Furthermore, ramucirumab‐related adverse events such as gastrointestinal perforation or proteinuria should not be ignored, although the incidence was not frequent. Thus, cytotoxic chemotherapy such as paclitaxel or irinotecan is still a viable option to treat patients with MRGC in the second‐line setting.
Clinical trials comparing ramucirumab plus irinotecan versus irinotecan or comparing ramucirumab plus irinotecan versus ramucirumab plus paclitaxel are not expected to be conducted in the future, considering the similar efficacy of paclitaxel and irinotecan and proven superior efficacy of ramucirumab plus paclitaxel to paclitaxel alone. Instead, comparison between ramucirumab with FOLFIRI (irinotecan, leucovorin, and 5‐fluorouracil) and ramucirumab with paclitaxel is now ongoing (NCT03081143). This study would be another indirect indicator to see if irinotecan has a different efficacy over paclitaxel when combined with ramucirumab.
Far fewer patients enrolled than expected, limiting our interpretation of study results. During the study period, the outcome of WJOG 4007 was reported, and this drove investigators to be less interested in this study and thus to enroll patients much less. Furthermore, confirmative landmark trials established the evident role of ramucirumab as a second‐line treatment in MRGC [19], [21]. In Korea, many kinds of clinical trials are conducted for MRGC. This often results in enrollment competition between various trials that have similar eligibility criteria. It is estimated that many investigators allocated patients to other clinical trials because neither paclitaxel nor irinotecan were novel investigational drugs.
Figures and Tables
Acknowledgments
This study was partly funded by Boryung Pharmaceutical and CJ HealthCare Corp. The research was supported in part by the Korean Cancer Study Group (KCSG) and KCSG data center (study number: KCSG ST10‐01). We would like to express our love and gratitude to the late Prof. Hong Suk Song, who contributed greatly to this study.
Contributed equally
Deceased
Footnotes
ClinicalTrials.gov Identifier: NCT01224652
Sponsor(s): Investigator‐initiated trial
Principal Investigator: Jae Yong Cho
IRB Approved: Yes
Disclosures
The authors indicated no financial relationships.
References
- 1.Koizumi W, Akiya T, Sato A et al. Second‐line chemotherapy with biweekly paclitaxel after failure of fluoropyrimidine‐based treatment in patients with advanced or recurrent gastric cancer: A report from the gastrointestinal oncology group of the Tokyo cooperative oncology group, TCOG GC‐0501 trial. Jpn J Clin Oncol 2009;39:713–719. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda G, Kunisaki C, Makino H et al. Phase II study of weekly paclitaxel as a second‐line treatment for S‐1‐refractory advanced gastric cancer. Anticancer Res 2009;29:2863–2867. [PubMed] [Google Scholar]
- 3.Shimoyama R, Yasui H, Boku N et al. Weekly paclitaxel for heavily treated advanced or recurrent gastric cancer refractory to fluorouracil, irinotecan, and cisplatin. Gastric Cancer 2009;12:206–211. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 5.Jung KW, Won YJ, Oh CM et al. Prediction of cancer incidence and mortality in Korea, 2017. Cancer Res Treat 2017;49:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner AD, Grothe W, Haerting J et al. Chemotherapy in advanced gastric cancer: A systematic review and meta‐analysis based on aggregate data. J Clin Oncol 2006;24:2903–2909. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Moiseyenko VM, Tjulandin S et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first‐line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol 2006;24:4991–4998. [DOI] [PubMed] [Google Scholar]
- 8.Bang YJ, Van Cutsem E, Feyereislova A et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): A phase 3, open‐label, randomised controlled trial. Lancet 2010;376:687–697. [DOI] [PubMed] [Google Scholar]
- 9.Kang JH, Lee SI, Lim DH et al. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513–1518. [DOI] [PubMed] [Google Scholar]
- 10.Thuss‐Patience PC, Kretzschmar A, Bichev D et al. Survival advantage for irinotecan versus best supportive care as second‐line chemotherapy in gastric cancer – A randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306–2314. [DOI] [PubMed] [Google Scholar]
- 11.Ford HE, Marshall A, Bridgewater JA et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR‐02): An open‐label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78–86. [DOI] [PubMed] [Google Scholar]
- 12.Wesolowski R, Lee C, Kim R. Is there a role for second‐line chemotherapy in advanced gastric cancer? Lancet Oncol 2009;10:903–912. [DOI] [PubMed] [Google Scholar]
- 13.Cascinu S, Graziano F, Cardarelli N et al. Phase II study of paclitaxel in pretreated advanced gastric cancer. Anticancer Drugs 1998;9:307–310. [DOI] [PubMed] [Google Scholar]
- 14.Kodera Y, Ito S, Mochizuki Y et al. A phase II study of weekly paclitaxel as second‐line chemotherapy for advanced gastric cancer (CCOG0302 study). Anticancer Res 2007;27:2667–2671. [PubMed] [Google Scholar]
- 15.Hironaka S, Zenda S, Boku N et al. Weekly paclitaxel as second‐line chemotherapy for advanced or recurrent gastric cancer. Gastric Cancer 2006;9:14–8. [DOI] [PubMed] [Google Scholar]
- 16.Chun JH, Kim HK, Lee JS et al. Weekly irinotecan in patients with metastatic gastric cancer failing cisplatin‐based chemotherapy. Jpn J Clin Oncol 2004;34:8–13. [DOI] [PubMed] [Google Scholar]
- 17.Kanat O, Evrensel T, Manavoglu O et al. Single‐agent irinotecan as second‐line treatment for advanced gastric cancer. Tumori 2003;89:405–407. [DOI] [PubMed] [Google Scholar]
- 18.Hironaka S, Ueda S, Yasui H et al. Randomized, open‐label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438–4444. [DOI] [PubMed] [Google Scholar]
- 19.Wilke H, Muro K, Van Cutsem E et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (RAINBOW): A double‐blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–1235. [DOI] [PubMed] [Google Scholar]
- 20.Bang YJ, Xu RH, Chin K et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first‐line therapy (GOLD): A double‐blind, randomised, placebo‐controlled, phase 3 trial. Lancet Oncol 2017;18:1637–1651. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs CS, Tomasek J, Yong CJ et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo‐controlled, phase 3 trial. Lancet 2014;383:31–39. [DOI] [PubMed] [Google Scholar]