Abstract
This commentary evaluates the immune‐genomic connection.
Immunotherapy and genomically targeted treatments are often considered distinct entities, with the latter recognized as a pillar of precision oncology. This distinction is in fact a false dichotomy. The fact is that the immune system is the epitome of “therapeutic” machinery that is both precise and personalized. Importantly, immunotherapy and genomics are “married” to each other.
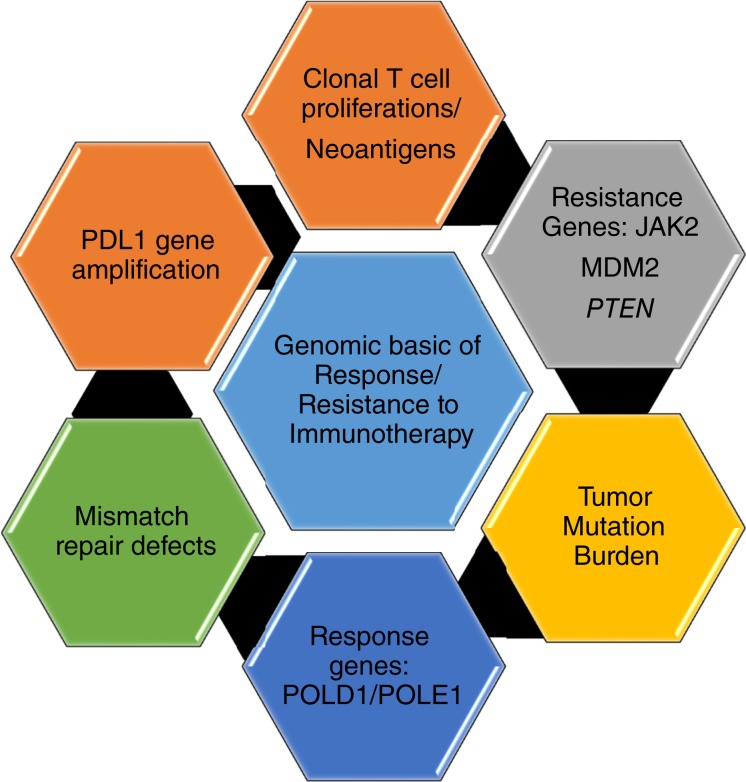
Cancer is a disease driven by aberrant genes. The interactions between the cancer genome and the host immune system are dynamic. In the last decade, massive parallel sequencing approaches entered the clinic and have been used to characterize individual patient tumors and to select therapies based on the identified mutations. Those patients that have definite driver aberrations such as the BRAFV600E were the beneficiaries of precision therapy. However, many patients have multiple genomic abnormalities, and this posed a challenge. Patients who had malignancies with unstable genomes associated with a very large number of mutations (hyper‐mutated phenotype), the so‐called “messed up genomes,” were especially problematic. Fast forward to the current era and these patients are the ones that are most clearly benefitting from immunotherapy. We already have compelling evidence for the marriage between genomics and the immune system (Fig. 1). Herein, we appraise this nuptial agreement.
Figure 1.
Genomic basis for immunotherapy response and resistance mechanisms.
Mismatch—The Perfect Match
The major proof for the immuno‐genomic connection is the story of mismatch repair (MMR) defects [1] and microsatellite instability. Microsatellite instability‐high (MSI‐H) is the hyper‐mutated genomic status arising from a deficiency of the DNA MMR process [2]. Defects in this process ensue from inactivation of one of the MMR proteins—usually MLH1, MSH2, MSH6, or PMS2. Around 80% of MSI‐H is due to somatic inactivation; 20%, from germline defects (known as Lynch syndrome, which is associated with colorectal, gastric, endometrial, and other cancers) [2].
Checkpoint inhibitor immunotherapy has transformed treatment of some solid tumors, where remarkably durable responses can be achieved, albeit in only a small subset of patients. Oncogenomics is taking center stage in this arena. The mismatch repair story is especially relevant. Checkpoint inhibitors are effective because they block the immune system deactivation that tumors exploit in order to evade the innate immune machinery and survive. Yet, immune reactivation alone is not enough for tumor eradication. The reactivated immune system must be able to recognize the tumor and differentiate it from normal elements. One of the important ways that T cells identify malignant cells for eradication is through the neo‐antigens that cancer cells produce as a result of the mutanome. Not unexpectedly, the more mutations, the easier it is for the immune system to identify the cancer cells—hence, patients whose tumors have high tumor mutational burden (TMB [and all MSI‐H tumors have high TMB]) attain the highest response rates to checkpoint inhibitors. This is a genuinely extraordinary development—the tumors whose genomes are most chaotic and previously believed to be least amenable to treatment are now the “best” tumors from the standpoint of response to checkpoint inhibitor immunotherapy.
Recently, the checkpoint inhibitor pembrolizumab was approved by the U.S. Food and Drug Administration (FDA) for all adult and pediatric solid tumors harboring MSI‐H and deficient MMR, based on high response rates in the affected tumors. This approval is a watershed event. It symbolizes the power of the precision oncology paradigm by effectively wedding genomics to checkpoint inhibitors.
TMB
TMB is a measure of the number of somatic protein‐coding base substitutions and INDELS occurring in a tumor specimen. High mutational load can be triggered by a number of causes, such as ultraviolet rays in melanomas, smoking and other environmental pollutants, food‐contained mutagenic and inflammatory compounds that pass the gastrointestinal tract, and defects in the proofreading domains of DNA polymerases encoded by the POLE and POLD1 genes. In addition, MMR deficiency and MSI‐H status are also associated with a high mutational load. The high TMB renders tumors susceptible to response by immune checkpoint inhibitors, perhaps because the more mutations, the higher the chance that some of the neo‐antigens produced will be immunogenic [3]. Alternatively, certain gene defects may also specifically produce mutations that trigger an immune response.
PD‐L1 and PD‐L2 Amplification in Hodgkin's Lymphoma
Immune checkpoint inhibitors have substantial salutary therapeutic effects in relapsed and refractory Hodgkin's lymphoma. In this disease, programmed death‐ligand 1 (PD‐L1) amplification is a hallmark genomic event. Chromosome 9p24.1/CD274 (PD‐L1)/PDCD1LG2 (PD‐L2) amplifications have been shown to increase the abundance of these programmed cell death protein 1 (PD‐1) ligands [4]. The 9p24.1 amplicon also contains Janus kinase 2 (JAK2); copy number‐dependent Janus kinase 2‐signal transducers and activators of transcription, which further increases PD‐1 ligand expression [4].
PD‐L1 Gene Amplification in Solid Tumors
The 9p24.1/PD‐L1 is seen across several lymphoma and other cancer types, and patients with diseases other than Hodgkin's lymphoma who harbor these alterations could be potential targets for immunotherapy. In a recent study from a database of 118,187 deidentified tumor samples, PD‐L1 amplifications were identified in 843 (0.7%), including more than 100 types of solid tumors [5]. Six of nine patients (66.7%) from a tertiary cancer center with PD‐L1‐amplified solid tumors achieved objective responses on immunotherapy [5]. The study showed that PD‐L1‐amplified cancers responded to checkpoint blockade, even in the absence of microsatellite instability, high PD‐L1 expression, and a high TMB [5].
Resistance Mechanisms are Genomically Driven
Although checkpoint inhibitors can confer durable benefit in some patients, a significant number of individuals do not respond (primary resistance), and some progress after an initial response (secondary resistance). Resistance mechanisms can be driven by molecular abnormalities, with defects in the interferon‐gamma effector as well as truncating mutations in JAK1/JAK2 and the antigen‐presenting protein beta‐2‐microglobulin being operative [6], [7]. Finally, accelerated tumor progression (hyper‐progression) can also occur after checkpoint blockade, and has been correlated with specific genomic alterations such as MDM2 amplification and epidermal growth factor receptor aberrations [8].
The Mutanome
The mutanome refers to the compendium of an individual patient's tumor‐specific alterations and mutations. The mutanome encodes patient‐specific antigens that are different from “shared” antigens, which are expressed in tumors from multiple patients and are typically normal, nonmutated self‐proteins [9]. In particular, mutanome‐encoded peptides may evoke a more vigorous T‐cell response due to a lack of thymic tolerance against them [9]. Understanding the specific genomic alterations that produce immunogenic neo‐antigens/peptides is now an area of intense research in the immunology field.
CAR T Cells
T cells engineered to express chimeric antigen receptor (CAR) by gene transfer technology are capable of precisely recognizing their target antigen, resulting in T‐cell activation in a major histocompatibility complex‐independent manner. This approach has yielded a paradigm shift in refractory hematologic malignancies. The FDA has approved Kymriah (tisagenlecleucel; Novartis, Basel, Switzerland) for pediatric and young adult patients with acute lymphoblastic leukemia [10] and Yescarta (axicabtagene ciloleucel; Gilead Sciences, Foster City, CA) CAR T‐cell therapy in refractory large B‐cell lymphoma [11]. These are CARs that direct the T cells to target and kill malignant cells that have a specific antigen (CD19) on the surface. It is likely that, in the near future, their utility in the management of a wide variety of cancers will be elucidated. The CAR T cell is another example of a precision therapy treatment for which genomics is a cornerstone.
Summary and Future Directions
From checkpoint inhibitors to CAR T cells, the successes of immunotherapy are grounded in the marriage between genomics and immunology. Fundamentally, the immune system recognizes the products of the mutanome, and this recognition is an important key to the basis of distinguishing cancer from self. The recent approval of the checkpoint inhibitor pembrolizumab across all cancers with MSI‐H status exemplifies the strength of the bond between immunotherapy and genomics. The knowledge of immunology of the host and genomics of tumor are poised to exponentially increase in the next decade, and it is likely that there will soon be a time when massive genomic analysis will be coupled with immune profiling in patients with cancer right from the time of diagnosis and again at relapse. The “big data” enterprise will enable this field with rapid translation to clinical applicability. The near future will exploit the marriage between genomics and immunotherapy in order to, finally, win the war against cancer.
Disclosures
Vivek Subbiah: Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, Stemcentrix, LOXO, Blueprint Medicines, Roche/Genentech (RF); Razelle Kurzrock: Incyte, Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health (RF), Sequenom, Loxo, Actuate Therapeutics (C/A), CureMatch, Inc. (OI), Roche (H).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boland CR, Thibodeau SN, Hamilton SR et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248–5257. [PubMed] [Google Scholar]
- 3.Goodman AM, Kato S, Bazhenova L et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roemer MG, Advani RH, Ligon AH et al. PD‐L1 and PD‐L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 2016;34:2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman AM, Piccioni D, Kato S et al. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA Oncol 2018;4:1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma P, Hu‐Lieskovan S, Wargo JA et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaretsky JM, Garcia‐Diaz A, Shin DS et al. Mutations associated with acquired resistance to PD‐1 blockade in melanoma. N Engl J Med 2016;375:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato S, Goodman A, Walavalkar V et al. Hyperprogressors after immunotherapy: Analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017;23:4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overwijk WW, Wang E, Marincola FM et al. Mining the mutanome: Developing highly personalized immunotherapies based on mutational analysis of tumors. J Immunother Cancer 2013;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food & Drug Adminstration . FDA News Release. FDA approval brings first gene therapy to the United States. Available at https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm574058.htm. Accessed August 1, 2018.
- 11.Neelapu SS, Locke FL, Bartlett NL et al. Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐cell lymphoma. N Engl J Med 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]