Cancer and cancer treatments could accelerate the cognitive aging process. This article describes pre‐ and post‐treatment objective cognitive changes in older adults with early stage breast cancer after adjuvant treatment compared with matched healthy controls.
Keywords: Cognition disorders, Breast neoplasms, Aging, Chemotherapy, Cognitive changes
Abstract
Background.
Group‐based trajectory modeling is particularly important to identify subgroups of patients with pathological cognitive changes after cancer treatment. To date, only one study has explored cognitive trajectories in older patients with cancer. The present article describes objective cognitive changes before to after adjuvant treatment in older adults with early‐stage breast cancer (EBC) after adjuvant treatment compared with healthy controls.
Patients and Methods.
Participants were patients ≥65 years of age with newly diagnosed EBC and healthy controls (age‐, sex‐, and education‐matched). The pretreatment assessment was conducted before adjuvant therapy, and the post‐treatment assessment after the end of the first adjuvant treatment. Objective cognitive changes before to after treatment were evaluated based on the Reliable Change Index for cognitive decline accounting for cognitive impairment status.
Results.
The sample consisted of women newly diagnosed with EBC (n = 118) and healthy controls (n = 62). Five patterns of changes before to after treatment were identified based on the presence of cognitive decline and cognitive impairment. The distribution of these five change patterns was statistically significant (p = .0001). Thirty‐six percent of patients had phase shift changes, 31% without initial objective cognitive impairment developed impairment, 15% had a normal aging, 12% had a nonpathological decline, and 6% experienced accelerated cognitive decline.
Conclusion.
This study described for the first time objective cognitive changes before to after treatment of older adults with EBC immediately after the end of adjuvant treatment. A longer‐term remote follow‐up of adjuvant treatment is needed to better understand the cognitive trajectories of older patients with EBC.
Implications for Practice.
After the end of adjuvant treatment, 31% of older adults with early‐stage breast cancer without initial objective cognitive impairment developed impairment, and 6% experienced accelerated cognitive decline. Initial cognitive functioning should be included in the balance of benefits and harms of systemic therapy for patients who are likely to be at highest risk for cognitive decline after cancer treatments. Regular cognitive follow‐up of patients who had cognitive impairment before cancer treatment should monitor symptoms suggestive of neurodegenerative disease and avert the effect of cognitive disorders on patients’ autonomy.
Introduction
Cancer and cancer treatments might accelerate the cognitive aging process because of the potential relation between aging, neurodegeneration, biologic processes underlying cancer, and the effect of cancer treatments on cognition [1], [2], [3]. Group‐based trajectory modeling is particularly important to identify subgroups of patients with pathological cognitive changes after cancer treatment. Based on longitudinal assessments, in reference to the model proposed by Ahles, it is possible to evaluate whether age‐associated cognitive declines in these patients parallel those of older adults with no cancer history (the phase shift hypothesis) or follow a steeper slope of decline (the accelerated aging hypothesis) [2].
To date, only one study has explored cognitive trajectories in older patients with cancer. This study concerned the long‐term trajectories of subjective cognitive function in older survivors of breast cancer and showed that, if the majority of survivors maintained good long‐term self‐reported cognition, a small subset of survivors manifested accelerated cognitive decline [4].
The aim of this article is to describe the objective cognitive changes in older adults with early‐stage breast cancer (EBC) after adjuvant treatment compared with matched healthy controls. This study was based on post hoc secondary analysis of previously published data [5].
Materials and Methods
Participants
Inclusion criteria were EBC and age of more than 65 years [5], [6]. Exclusion criteria included prior exposure to chemotherapy or radiotherapy, neurological comorbidities, known psychiatric comorbidities that might affect ability to participate, major cognitive disorders, and documented alcohol or drug abuse [5], [6]. Participants with a Mini‐Mental State Examination (MMSE) score of less than 25 out of 30, indicative of possible pathological aging, were excluded from the study [7], [8], [9]. Similarly, participants who reported a period of formal education of less than 5 years (end of primary school) were not eligible because of the lack of normative data for these individuals.
A sample of healthy controls who met the same inclusion (except cancer diagnosis) and exclusion criteria were recruited by community advertisements. Healthy controls were age‐, sex‐, and education‐matched to patients.
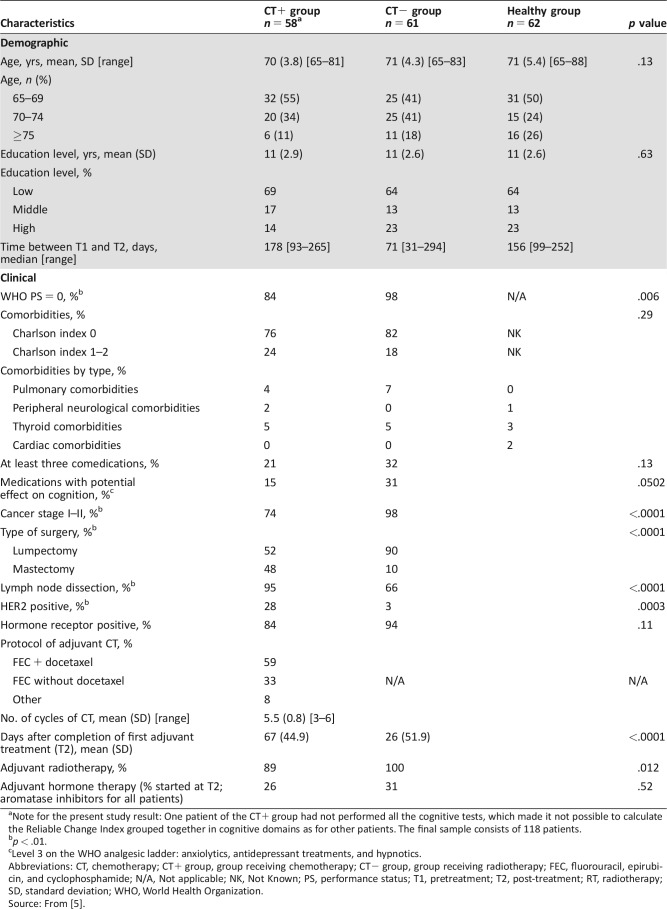
The pretreatment assessment (T1) was conducted after surgery and before adjuvant therapy. The post‐treatment assessment (T2) was conducted after the end of the first adjuvant treatment. Adjuvant treatment was chemotherapy (CT+ group: n = 58; median 178 days) or radiotherapy (CT− group: n = 61; median 71 days). The interval for healthy controls was about the mean of that of the two patient groups (Table 1).
Table 1. Demographic and clinical characteristics of patients and healthy controls.
Note for the present study result: One patient of the CT+ group had not performed all the cognitive tests, which made it not possible to calculate the Reliable Change Index grouped together in cognitive domains as for other patients. The final sample consists of 118 patients.
p < .01.
Level 3 on the WHO analgesic ladder: anxiolytics, antidepressant treatments, and hypnotics.
Abbreviations: CT, chemotherapy; CT+ group, group receiving chemotherapy; CT− group, group receiving radiotherapy; FEC, fluorouracil, epirubicin, and cyclophosphamide; N/A, Not applicable; NK, Not Known; PS, performance status; T1, pretreatment; T2, post‐treatment; RT, radiotherapy; SD, standard deviation; WHO, World Health Organization.
Source: From [5].
All participants provided written informed consent for the study, which was approved by the local ethics committee and registered at ClinicalTrials.gov (NCT01333735).
Measures
Objective cognitive functioning (episodic memory, working memory, processing speed, and executive functions) was assessed with standardized and recommended neuropsychological tests [5]: the Grober and Buschke procedure; the Rey complex figure test; arithmetic, digit‐span and letter‐number sequencing of the Wechsler Adult Intelligence Scale‐III; the trail making test, (parts A and B); and the verbal fluency test [8], [10], [11], [12], [13], [14].
Sample Characteristics: Data Previously Published
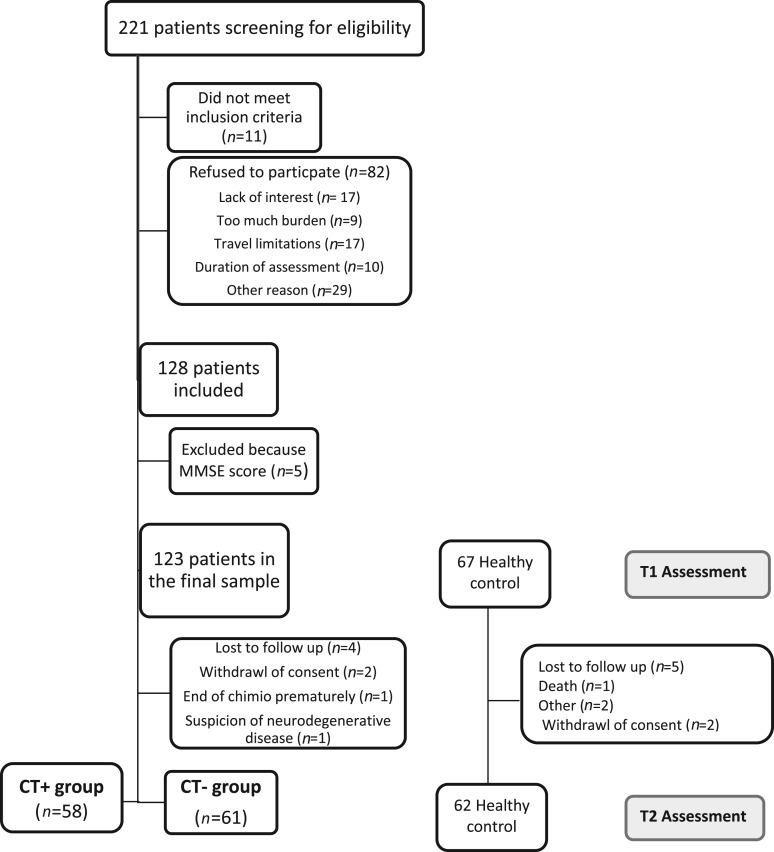
A summary of sample characteristics and main previous results [5] is presented here to place the methods and results of the present study in context. Newly diagnosed women with EBC (n = 119) aged more than 65 years and a sample of healthy controls (n = 62) were included. As described in Figure 1, the final sample for T1–T2 analysis was 58 CT+ patients, 61 CT− patients, and 62 healthy controls. Participants’ demographic and medical information is summarized in Table 1. No significant difference in age and education was observed between the three groups at baseline [5]. No significant difference between the patient groups was observed at baseline regarding objective cognitive scores, subjective cognitive complaints, anxiety, depression, fatigue, and geriatric and biological measures [5].
Figure 1.
Flow diagram of participant follow‐up.
Abbreviations: CT+, chemotherapy; CT−, radiotherapy; MMSE, Mini‐Mental State Examination; T1, pretreatment; T2, post‐treatment.
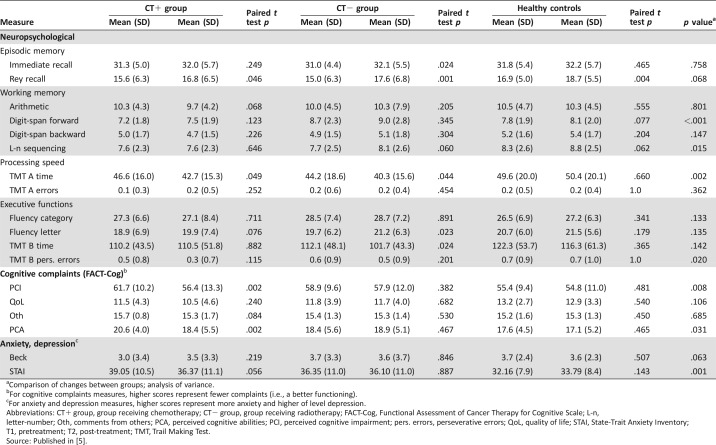
Raw neuropsychological scores, cognitive complaints, and anxiety and depression scores for T1 and T2 are presented in Table 2.
Table 2. Neuropsychological measures, cognitive complaints, and anxiety and depression scores.
Comparison of changes between groups; analysis of variance.
For cognitive complaints measures, higher scores represent fewer complaints (i.e., a better functioning).
For anxiety and depression measures, higher scores represent more anxiety and higher of level depression.
Abbreviations: CT+ group, group receiving chemotherapy; CT− group, group receiving radiotherapy; FACT‐Cog, Functional Assessment of Cancer Therapy for Cognitive Scale; L‐n, letter‐number; Oth, comments from others; PCA, perceived cognitive abilities; PCI, perceived cognitive impairment; pers. errors, perseverative errors; QoL, quality of life; STAI, State‐Trait Anxiety Inventory; T1, pretreatment; T2, post‐treatment; TMT, Trail Making Test.
Source: Published in [5].
According to the T1 results, 41% of patients had objective cognitive impairment (46% in the CT+ group, 26/57; 38% in the CT− group, 23/61) [5].
Based on Reliable Change Index (RCI) analysis (T1 and T2 data), overall, no significant difference was observed between the two patient groups on objective change in at least one domain or in any cognitive domain [5]. According to the mixed model analysis (groups × time analysis), there was no significant effect of group and time on any of the objective or subjective cognitive scores [5].
Statistical Analysis
This study was a post hoc secondary analysis of previously published data [5]. The primary goal of this study was to assess cognitive change before to after treatment, considering changes of each patient individually. To identify objective cognitive changes before to after treatment, the first parameter taken into account was cognitive decline, based on the RCI (as explained below). Cognitive impairment status was the second parameter taken into account, based on normative data and according to International Cognition and Cancer Task Force (ICCTF) definition [15], a two‐part criterion: if patients performed at a z‐score of ≤−1.5 on two or more tests, or if they performed ≤−2.0 on a single test, they were classified as impaired.
The RCI was used to determine standardized change scores for each patient on every neuropsychological score in order to compare T2 with T1 scores [5], [16], [17]. The calculation included the practice effect based on the healthy group scores (change from T1 to T2 in the healthy group). The Iverson formula includes an adapted standard error of the difference that incorporates T2's variability. The recommended decline threshold of RCI score was −1.645 [16]. RCI scores were grouped together in cognitive domains. A decline in one domain was considered significant when at least one of the domain scores declined significantly.
Based on previous published results—which overall showed there was no significant difference between patient groups receiving or not receiving chemotherapy in objective cognitive decline in any cognitive domain or in at least one domain [5]—cognitive changes before to after treatment are presented independently of received treatment (chemotherapy and/or radiotherapy) and were elaborated from a decline in at least one domain.
Results
The final sample consisted of 118 patients.
Cognitive Changes Before to After Treatment
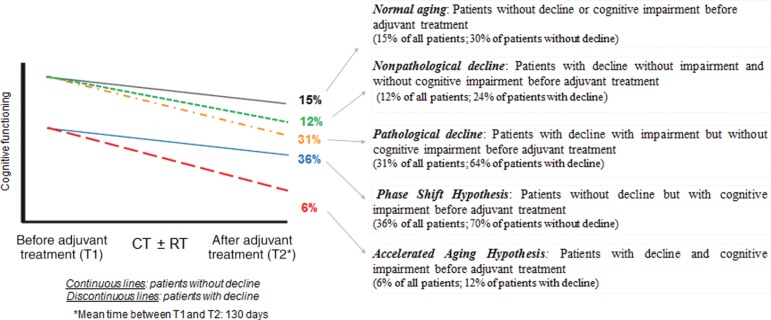
Five patterns of changes before to after treatment were identified based on the presence of cognitive decline and cognitive impairment (as shown in Fig. 2, where continuous lines indicate no decline and discontinuous lines indicate decline). The distribution of these five change patterns was statistically significant (p = .0001).
Figure 2.
Cognitive trajectories in older patients with early‐stage breast cancer after adjuvant treatment.
Two parameters were taken into account to identify objective cognitive trajectories: cognitive decline (based on the Reliable Change Index, which takes into account the healthy group scores) and cognitive impairment (based on normative data). The first percentage indicates among all patients (without distinction of decline status) the proportion of patients identified on each trajectory. The second percentage indicates among the subgroup “patients without decline” (represented by continuous lines) or “patients with decline” (represented by discontinuous lines) the proportion of patients identified on each trajectory.
Abbreviations: CT, chemotherapy; RT, radiotherapy.
Patients Without Decline
Fifty‐one percent of patients with EBC (n = 60/118) had no cognitive decline after cancer treatment: cognitive changes of these patients were not significantly different from changes of the healthy control group. Among these patients, 30% (n = 18/60; 15% of all patients) had normal aging: the same changes as healthy controls. Seventy percent of patients with no cognitive decline after treatment (n = 42/60; 36% of all patients) had cognitive impairment before adjuvant treatment (based on normative data) but no increase in impairment after treatment: cognitive changes of these patients were not significantly different from changes of the healthy control group. For this subgroup of patients, change may correspond to the phase shift hypothesis, which states that patients’ pretreatment cognitive functioning was below normal aging cognitive functioning (this subgroup had pretreatment impairment) and stayed below normal aging cognitive functioning after cancer treatment but without significant decline. This change parallels that of normal aging.
Patients with Decline
Forty‐nine percent of patients with EBC had a cognitive decline (n = 58/118). Among them, 24% (n = 14/58; 12% of all patients) had normal aging before treatment and presented a decline after treatment but did not develop impairment: although these patients had cognitive decline, their cognitive performances remained above the threshold of impairment (nonpathological decline).
Furthermore, among patients with decline, 64% (n = 37/58; 31% of all patients) had normal aging before adjuvant treatment and developed impairment after treatment (i.e., a pathological decline).
The remaining 12% (n = 7/58; 6% of all patients) had cognitive impairment before adjuvant treatment with an increase after treatment. This change may correspond to the accelerated aging hypothesis, which states that the cognitive decline of patients treated for cancer is accelerated in comparison with normal aging (healthy controls).
Discussion
The results of this study suggest that in the short term after adjuvant treatment, 36% of older adults with EBC had phase shift changes, 31% without initial objective cognitive impairment developed impairment, 15% had a normal aging, 12% had a nonpathological decline, and a small subset experienced accelerated cognitive decline.
Group‐based trajectory modeling is particularly important to identify subgroups of patients with pathological cognitive changes after cancer treatment, particularly in older patients, because of potential initial cognitive impairment that could be increased after treatment. Nevertheless, few studies have assessed cognitive functioning in older patients with cancer, and few have used an individual analysis of cognitive performances to measure changes of cognitive aging before to after treatment. To the best of our knowledge, only one study has described individual cognitive change in older patients with cancer. However, this study assessed only self‐reported cognitive function (based on two items of the European Organization for Research and Treatment of Cancer's Quality of Life Questionnaire‐C30) of older breast cancer survivors and showed that only 7.6% of the cohort had accelerated decline [4].
Individual cognitive change analyses complement mixed model analysis and add meaningful clinical information. Further investigations are needed to characterize subgroups of patients who are particularly at risk of developing cognitive impairment after adjuvant treatment and who could have accelerated aging, so that therapeutic management can be adapted. This article presents cognitive changes before to after adjuvant treatment in reference to the model proposed by Ahles [2]. We observed a subgroup of patients (6%) who experienced accelerated cognitive decline. Furthermore, 31% of patients developed cognitive impairment after treatment, and 36% of older adults with EBC had phase shift changes according to the definition of Ahles [2]. This last subgroup of patients had initial cognitive impairment, but their cognitive changes paralleled those of normal aging with no cognitive decline. Overall, these results suggest that it is important not only to detect cognitive impairment before treatment but also to initiate regular cognitive follow‐up, particularly in patients who had cognitive impairment before cancer treatment, to monitor the occurrence of symptoms suggestive of a neurodegenerative disease and to avert the effect of cognitive disorder on patients’ autonomy. For the majority of young patients, cognitive impairment abates within 6–12 months after treatment [18], [19]. In older patients, the recovery of cognitive impairment can be longer. Cognitive changes in the current study were obtained just after the end of adjuvant treatment and suggested acute treatment effects. A longer follow‐up is necessary to confirm the results and follow patients who developed cognitive impairment after treatment or who had an accelerated decline.
Compared with the sample in this study, in real life, older patients constitute a more heterogeneous population (with altered general state altered, geriatric frailties, or neurodegenerative disease). Our results have probably underestimated cognitive decline because recruited patients had little comorbidity or geriatric frailty and were not shown to be impaired by a cognitive screening test [6].
Insofar as cognitive impairment can interfere with care and influence prognosis, a diagnosis of cognitive impairment may influence clinical decision making [20]. Geriatric assessment at the initiation of adjuvant treatment is therefore crucial to identify patients at risk of cognitive vulnerability and to propose appropriate therapeutic follow‐up and management. For subgroups of patients who are likely to be at highest risk of cognitive decline after cancer treatments, initial cognitive functioning should be included in the balance of benefits and harms of systemic therapy [3]. The comprehensive geriatric assessment often includes only a cognitive screening test, mainly the MMSE [9]. However, the mild cognitive impairments that patients with cancer may experience may not be measured by this test. Several studies have shown with various patient populations that the Montreal Cognitive Assessment (MoCA) [21] is more sensitive in detecting these subtle disorders [22], [23], [24]. In the absence of neuropsychological testing prior to starting adjuvant treatment, which may not be achievable in clinical practice, MoCA could be recommended as screening tool [1].
Cognitive deficits could be related to adherence difficulties and treatment discontinuation [25], [26]. Indeed, a relationship has been observed between cognition (prospective memory, executive functioning, working memory, and attention) and adherence, particularly in older patients [27], [28]. Cognitive assessment and follow‐up are very important to avert potential effect on adherence to hormone therapy for breast cancer.
The definition of cognitive decline, inclusion of a healthy control group, RCI formula (taking into account the practice effect based on the healthy group scores, or not) and cognitive tools could influence the construction of cognitive trajectories. Hence it is important to develop a study that uses the tests and cognitive impairment criteria recommended by the ICCTF [15], uses the recommended threshold of RCI [16], and has a healthy control group to facilitate cross‐study comparison.
Conclusion
This study described for the first time objective cognitive changes of older adults with EBC before to after treatment and showed that immediately after the end of adjuvant treatment, 36% had phase shift changes, 31% without initial cognitive impairment developed impairment, and a small subset experienced accelerated cognitive decline. A longer‐term remote follow‐up of adjuvant treatment is needed to better understand the cognitive trajectories of older patients with EBC.
Acknowledgments
We thank all the subjects for their participation, the clinicians in the breast cancer department, the Cancéropôle Nord‐Ouest, Lucie Laroche, Sophie Aumont, Marion Delarue, and Maxime Feutry. This work was supported by a national grant (Programme Hospitalier de Recherche Clinique, grant APN 2008 n°06–08) and Sanofi. The Northwest Data Center (CTD‐CNO) is acknowledged for managing the data. It is supported by grants from the French National League Against Cancer (LNC) and the French National Cancer Institute (INCa).
Author Contributions
Conception/design: Sabine Noal, Francis Eustache, Bénédicte Giffard, Florence Joly
Provision of study material or patients: Sabine Noal, Olivier Rigal, Jean‐Emmanuel Kurtz, Christelle Lévy, Djelila Allouache, Corinne Veyret, Philippe Barthélémy
Collection and/or assembly of data: Marie Lange, Natacha Heutte, Chantal Rieux, Johan Lefel, Nadine Longato, Héléne Castel
Data analysis and interpretation: Marie Lange, Natacha Heutte, Francis Eustache, Bénédicte Giffard, Florence Joly
Manuscript writing: Marie Lange, Natacha Heutte, Olivier Rigal, Sabine Noal, Jean‐Emmanuel Kurtz, Christelle Lévy, Djelila Allouache, Chantal Rieux, Johan Lefel, Bénédicte Clarisse, Alexandra Leconte, Corinne Veyret, Philippe Barthélémy, Nadine Longato, Laure Tron, Hélène Castel, Francis Eustache, Bénédicte Giffard, Florence Joly
Final approval of manuscript: Marie Lange, Natacha Heutte, Olivier Rigal, Sabine Noal, Jean‐Emmanuel Kurtz, Christelle Lévy, Djelila Allouache, Chantal Rieux, Johan Lefel, Bénédicte Clarisse, Alexandra Leconte, Corinne Veyret, Philippe Barthélémy, Nadine Longato, Laure Tron, Hélène Castel, Francis Eustache, Bénédicte Giffard, Florence Joly
Disclosures
Philippe Barthélémy: Bristol‐Myers Squibb, Pfizer, Novartis, Ipsen, Roche, Sanofi, Janssen Cilag (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Lange M, Rigal O, Clarisse B et al. Cognitive dysfunctions in elderly cancer patients: A new challenge for oncologists. Cancer Treat Rev 2014;40:810–817. [DOI] [PubMed] [Google Scholar]
- 2. Ahles TA. Brain vulnerability to chemotherapy toxicities. Psychooncology 2012;21:1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mandelblatt JS, Hurria A, McDonald BC et al. Cognitive effects of cancer and its treatments at the intersection of aging: What do we know; what do we need to know? Semin Oncol 2013;40:709–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mandelblatt JS, Clapp JD, Luta G et al. Long‐term trajectories of self‐reported cognitive function in a cohort of older survivors of breast cancer: CALGB 369901 (Alliance). Cancer 2016;122:3555–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lange M, Heutte N, Rigal O et al. Decline in cognitive function in older adults with early‐stage breast cancer after adjuvant treatment. The Oncologist 2016;21:1337–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lange M, Giffard B, Noal S et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer 2014;50:2181–2189. [DOI] [PubMed] [Google Scholar]
- 7. Crum RM, Anthony JC, Bassett SS et al. Population‐based norms for the Mini‐Mental State Examination by age and educational level. JAMA 1993;269:2386–2391. [PubMed] [Google Scholar]
- 8. Kalafat M, Hugonot‐Diener L, Poitrenaudl J. French standardization of the “Mini Mental State” (MMS) GRECO version [in French]. Rev Neuropsych 2003;13:209–236. [Google Scholar]
- 9. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 10. Cardebat D, Doyon B, Puel M et al. Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level [in French]. Acta Neurol Belg 1990;90:207–217. [PubMed] [Google Scholar]
- 11. Rey A. Manual of Copy and Memory Reproduction Test of Complex Geometric Figures [in French]. Paris: Éditions Centre de psychologie appliquée, 1959. [Google Scholar]
- 12. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Motor Skills 1958;8:271–276. [Google Scholar]
- 13. Van der Linden M, Adam S, Agniel A et al. Assessment of Memory Impairment [in French]. Marseille: Solal, 2004. [Google Scholar]
- 14. Wechsler D. Wechsler Adult Intelligence Scale‐III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 15. Wefel JS, Vardy J, Ahles T et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 2011;12:703–708. [DOI] [PubMed] [Google Scholar]
- 16. Duff K. Evidence‐based indicators of neuropsychological change in the individual patient: Relevant concepts and methods. Arch Clin Neuropsychol 2012;27:248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iverson GL. Interpreting change on the WAIS‐III/WMS‐III in clinical samples. Arch Clin Neuropsychol 2001;16:183–191. [PubMed] [Google Scholar]
- 18. Vardy JL, Bray VJ, Dhillon HM. Cancer‐induced cognitive impairment: Practical solutions to reduce and manage the challenge. Future Oncol 2017;13:767–771. [DOI] [PubMed] [Google Scholar]
- 19. Lange M, Joly F. How to identify and manage cognitive dysfunction after breast cancer treatment. J Oncol Pract 2017;13:784–790. [DOI] [PubMed] [Google Scholar]
- 20. Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: Understanding frailty and the geriatric assessment. CA Cancer J Clin 2010;60:120–132. [DOI] [PubMed] [Google Scholar]
- 21. Nasreddine ZS, Phillips NA, Bédirian V et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 22. Zadikoff C, Fox SH, Tang‐Wai DF et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson's disease. Mov Disord 2008;23:297–299. [DOI] [PubMed] [Google Scholar]
- 23. Pendlebury ST, Cuthbertson FC, Welch SJ et al. Underestimation of cognitive impairment by Mini‐Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: A population‐based study . Stroke 2010;41:1290–1293. [DOI] [PubMed] [Google Scholar]
- 24. Olson R, Tyldesley S, Carolan H et al. Prospective comparison of the prognostic utility of the Mini Mental State Examination and the Montreal Cognitive Assessment in patients with brain metastases. Support Care Cancer 2011;19:1849–1855. [DOI] [PubMed] [Google Scholar]
- 25. Hayes TL, Larimer N, Adami A et al. Medication adherence in healthy elders: Small cognitive changes make a big difference. J Aging Health 2009;21:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bluethmann SM, Alfano CM, Clapp JD et al. Cognitive function and discontinuation of adjuvant hormonal therapy in older breast cancer survivors: CALGB 369901 (Alliance). Breast Cancer Res Treat 2017;165:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ettenhofer ML, Hinkin CH, Castellon SA et al. Aging, neurocognition, and medication adherence in HIV infection. Am J Geriatr Psychiatry 2009;17:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zogg JB, Woods SP, Sauceda JA et al. The role of prospective memory in medication adherence: A review of an emerging literature. J Behav Med 2012;35:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]