A literature‐based study of prospective trials of neuroendocrine tumor (NET) treatments was performed to identify valid alternative endpoints for predicting median progression‐free survival (PFS) in clinical trials.
Keywords: Neuroendocrine tumor, Progression‐free survival, Objective response rate, Study design, Endpoint
Abstract
Background.
In phase II trials for neuroendocrine tumors (NETs), the objective response rate (ORR) is traditionally used as a primary endpoint. However, the validity of the ORR as a primary endpoint has never been systematically examined. Therefore, a literature‐based analysis of phase II trials for NETs was performed to identify valid alternative endpoints for predicting median progression‐free survival (PFS) in clinical trials for NETs.
Materials and Methods.
Phase II trials of medical treatment for advanced NETs were identified based on a systematic search using MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials.
Results.
A total of 22 trials were identified, and 1,310 patients and 27 treatment arms were included in the analysis. There was no significant relationship between the ORR and median PFS (r = .374; 95% confidence interval [CI], −0.051 to 0.800; p = .085). Conversely, 12‐month PFS rates showed very strong correlations with median PFS (r = .929; 95% CI, 0.831–1.027; p < .001).
Conclusion.
The results of the present analysis indicate that the ORR is not significantly correlated with median PFS and suggest that 12‐month PFS rates are good alternate endpoints for screening phase II trials for NETs.
Implications for Practice.
Phase II trials are screening trials that seek to identify agents with sufficient activity to continue development. Thus, earlier endpoints are preferable, and the objective response rate (ORR) has been traditionally used as a surrogate endpoint in phase II trials for neuroendocrine tumors (NETs). However, the present study showed that the ORR was not significantly correlated with median progression‐free survival (PFS). On the other hand, the 12‐month PFS rate showed very strong correlation with median PFS and is considered a good alternate endpoint for screening phase II trials for NETs.
Introduction
Neuroendocrine tumors (NETs) are epithelial neoplasms that exhibit neuroendocrine differentiation, and they can arise from neuroendocrine cells, which are distributed widely throughout the body. NETs are relatively rare, and a recent population‐based study reported that the annual age‐adjusted incidence rate of NETs was 6.98 per 100,000 persons. However, the incidence rate of NETs has been steadily rising, with a 6.4‐fold increase between 1973 and 2012 [1].
NETs are typically indolent neoplasms, but their prognosis is highly dependent on the stage. Dasari et al. reported that the median overall survival (OS) of localized NETs was >30 years, but that of metastatic disease was only 12 months [1]. The rate of such metastatic disease was reported to reach approximately 20% [2], and more effective treatment for such tumors is required. Given the dismal prognosis of this disease, it is critical to rapidly screen new treatments and move promising therapies forward for definitive results.
In oncology phase III trials, OS is considered the gold standard and is the most commonly used primary endpoint. However, in NETs, relatively long survival and variability of salvage treatments options after progression may complicate the use of OS as a primary endpoint [3], [4], [5], [6]. Thus, progression‐free survival (PFS) is frequently used as a primary endpoint in phase III trials for NETs. On the other hand, the primary goal in a phase II trial is to determine if there is sufficient evidence of antitumor activity to undertake a phase III trial. Earlier endpoints are preferable for phase II trials, and the objective response rate (ORR) is traditionally used as a primary endpoint. Somatostatin analogues (SSAs) [7], [8], and the molecular targeted drugs, sunitinib [9] and everolimus [10], [11], [12], have been shown to prolong PFS in NET patients, and they are widely used to control tumor growth. However, the ORR in these phase III trials was only <10%. These facts suggest that tumor stabilization rather than shrinkage may still result in survival benefit. This issue has been pointed out by Kulke et al. [6] and Halperin et al. [3], and alternative endpoints to ORR are needed. However, the validity of the ORR as a primary endpoint in phase II trials and the exploration of alternate endpoints for predicting PFS have never been systematically examined for NETs.
Therefore, a literature‐based study of prospective trials of NET treatments was performed to identify valid alternative endpoints for predicting median PFS in clinical trials. The primary endpoint of this study was to evaluate the correlation between the ORR and median PFS in subjects enrolled in phase II clinical trials of medical treatment for NETs. The secondary endpoint was to explore potential correlations between other possible alternative endpoints (e.g., disease control rate [DCR] and biochemical response rate) and median PFS.
Materials and Methods
Literature Search
Phase II clinical trials of medical treatment for advanced NETs published between January 1996 and December 2016 were identified based on a systematic search using MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials. The authors (H.I. and M.S.) independently screened each record for eligibility by examining the titles, abstracts, and keywords. The search terms included “neuroendocrine tumor,” “neuroendocrine neoplasm,” “neuroendocrine cancer,” “neuroendocrine carcinoma,” or “carcinoid”; “drug therapy” or “chemotherapy”; and “clinical trial,” “controlled clinical trial,” or “randomized controlled trial.” The bibliographies of the identified articles were then screened for additional eligible articles. Among the identified articles, reports on trials including ≥20 patients per arm were analyzed if they reported the ORR and median PFS. Excluded were studies in which any arm received chemoradiotherapy, arterial infusion chemotherapy, or peptide receptor radionuclide therapy; phase I clinical trials with a dose escalation design as the protocol; and studies that included patients with neuroendocrine cancer (NEC) defined by the World Health Organization (WHO) pathologic classification because the consensus report stated that NET and NEC should be studied separately [6]. NEC is regarded as a different entity genetically [13] and clinically [14], [15], and a correlation between ORR and both PFS and OS in clinical trials for NEC has been examined [16], [17]. The search was limited to articles published in English.
Data Collection
For each trial, the following data were extracted: first author's name; year of publication or report; trial design; medical treatment regimen; number of patients in each arm; median PFS; potential alternative markers (ORR, DCR, 6‐month PFS rate, 12‐month PFS rate, 12‐month OS rate, 24‐month OS rate, and biochemical response rate. The ORR was defined as the percentage of patients with a complete response (CR) or partial response (PR) using radiological evaluation criteria (the RECIST 1.0/1.1 or WHO criteria). The DCR was defined as the percentage of patients with a CR, PR, or stable disease. The biochemical response was defined as normalization or ≥50% reduction in elevated serum chromogranin A levels.
Statistical Analysis
The Pearson's correlation coefficient (r) was used to evaluate the correlations between median PFS and other potential alternative markers in each treatment arm using linear regression analysis. Accounting for the variability of the number of arms included in each model, adjusted R2 values were used to compare the goodness‐of‐fit of regression models. Values of r closer to 1 indicated a strong positive correlation between the endpoints, and those of R2 closer to 1 indicated that the variability of median PFS was predominantly explained by the alternative endpoints.
To investigate possible reasons for heterogeneity of correlations, subgroup analyses were conducted by publication year (before 2011 vs. 2011 or later), origin of the tumor (pancreas only vs. extrapancreatic organs included), study design (randomized trial vs. nonrandomized trial), progressive disease requirement as a protocol (required vs. not required), concomitant therapy with SSAs (allowed vs. not allowed), cytotoxic drugs used (used vs. not used), and molecular targeted drugs used (used vs. not used). Publication year was categorized as before 2011 versus 2011 or later, because study designs for NETs changed from 2011 based on pivotal studies [9], [10], [11] and a consensus report [6].
Bootstrap methods with 1,000 replications were used to estimate confidence intervals (CIs) for the correlation parameters. All p values <.05 were considered significant, and all p values were two‐sided. Data were analyzed using STATA version 15.1 statistical software (StataCorp, College Station, TX).
Results
Selection of Studies
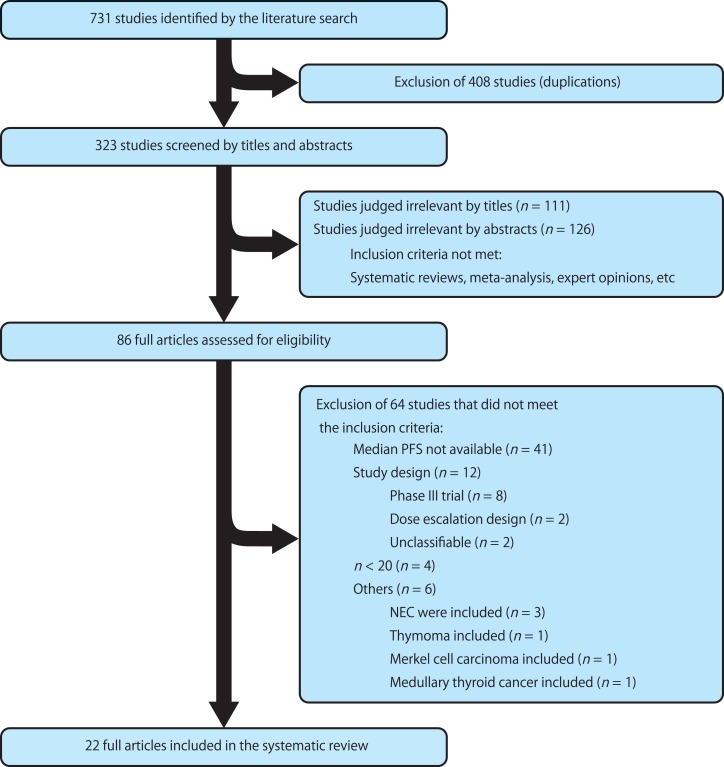
A total of 22 phase II trials [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39] with ≥20 patients per arm were identified (4 randomized trials and 18 nonrandomized trials; Fig. 1; Table 1). The primary endpoint of 14 trials (63.6%) was the ORR, and that of 7 trials (31.8%) was PFS. The radiological evaluation criteria were based on RECIST 1.0/1.1 in 20 (90.9%), and WHO criteria in 2 trials (9.1%). The median duration of enrollment and time to study completion were 20 months and 53.5 months, respectively. Fifteen trials (68.2%) included pancreatic neuroendocrine tumor (PNET): 18 of these (81.8%) also included extrapancreatic NET, but 4 (18.2%) included PNET only. The ORR, median PFS, DCR, 6‐month PFS rate, 12‐month PFS rate, 12‐month OS rate, 24‐month OS rate, and biochemical response rate were reported in 22 (100.0%), 22 (100.0%), 20 (90.9%), 9 (40.9%), 8 (36.4%), 9 (40.9%), 6 (27.3%), and 12 trials (54.5%), respectively. A total of 1,310 patients and 27 treatment arms were included in the analysis. The reported ORR and median PFS were 12% (range, 0%–56%) and 11.3 months (range, 4.5%–26.7 months), respectively.
Figure 1.
Flow chart of studies included in the systematic review of the literature. Abbreviations: NEC, neuroendocrine carcinoma; PFS, progression‐free survival.
Table 1. Characteristics of phase II trials included in the analysis.
Extrapancreatic organs: Gastrointestinal tract, lung, thymus, kidney, and larynx.
Abbreviations: ATG, autogel; CDDP, cisplatin; FU, fluorouracil; IFN, interferon; LAR, long‐acting release; ORR, objective response rate; PEG, pegylated; PFS, progression‐free survival; STZ, streptozocin.
Correlation Between the ORR and Median PFS
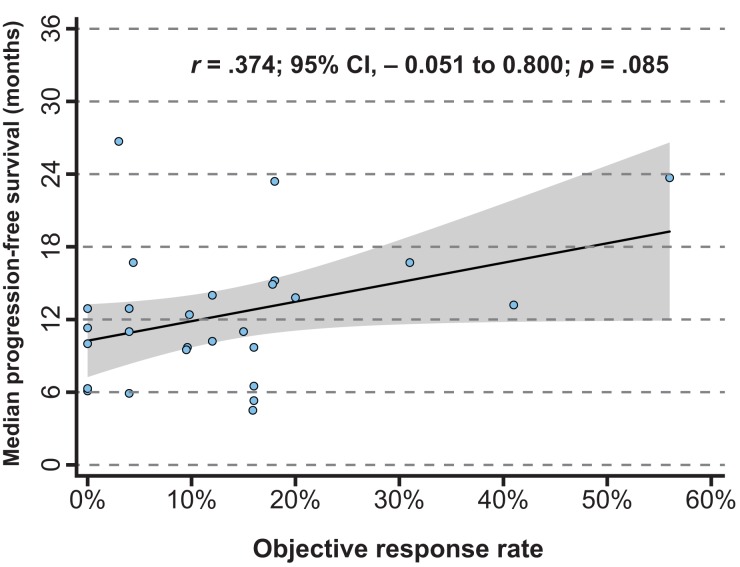
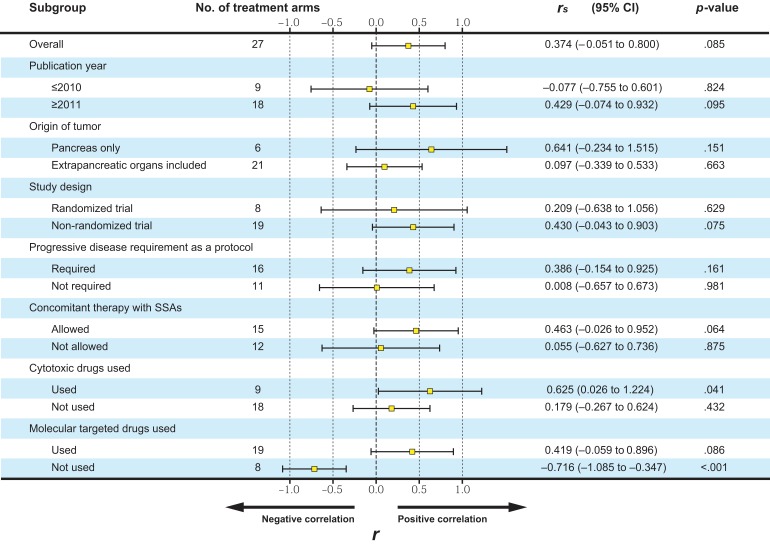
There was a nonsignificant relationship between the ORR and median PFS (p = .085). The r value for the ORR and median PFS was .374 (95% CI, −0.051 to 0.800; Fig. 2). Results of subgroup analyses are summarized in Figure 3. Although the correlation between the ORR and median PFS was significant in study arms in which cytotoxic drugs were used (r = .625; 95% CI, 0.026–1.224; p = .041), that in study arms that used molecular targeted drugs was not significant (r = .419; 95% CI, −0.059 to 0.896; p = .086).
Figure 2.
Correlations between objective response rate and median progression‐free survival. The gray area indicates the 95% confidence interval. r denotes Pearson's correlation coefficient. Abbreviation: CI, confidence interval.
Figure 3.
Correlations between objective response rate and median progression‐free survival in subgroup analyses. Abbreviations: CI, confidence interval; r, Pearson's correlation coefficient. SSAs, somatostatin analogues.
Correlations Between Potential Alternative Markers and Median PFS
Correlations between potential alternative markers and median PFS are summarized in Table 2. The DCR, 6‐month PFS rate, and 12‐month OS rate were moderately correlated with median PFS, and the 12‐month PFS rates showed a very strong correlation with median PFS (r = .929, 95% CI, 0.831–1.027; p < .001; Fig. 4). The regression equation was median PFS = −6.139 (95% CI, −13.509 to 1.231) + 0.408 (95% CI, 0.275–0.540) × 12‐month PFS rate, and the adjusted R2 was .846. Thus, this model indicated that a 10% increase in the 12‐month PFS rate corresponded to a 4.08‐month improvement of median PFS. The 12‐month PFS rate also showed a very strong correlation with median PFS in study arms that used cytotoxic drugs and those that used molecular targeted drugs (cytotoxic drug arm r= .935; 95% CI, 0.866–1.005; p < .001; molecular targeted drug arm r = .921; 95% CI, 0.802–1.041; p < .001).
Table 2. Correlation analyses between potential alternative markers and PFS.
r denotes Pearson's correlation coefficient.
Abbreviations: CI, confidence interval; DCR, disease control rate; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival.
Figure 4.
Correlations between the 12‐month progression‐free survival (PFS) rate and median PFS. The gray area indicates the 95% confidence interval. r denotes Pearson's correlation coefficient. Abbreviation: CI, confidence interval.
Discussion
The present study showed that the ORR was not significantly correlated with median PFS for subjects in NET medical treatment clinical trials. On the other hand, the 12‐month PFS rates showed a very strong correlation with median PFS. Molecular targeted drugs and SSAs were widely used for treatment of NETs. Although the ORR in phase III trials of these treatments did not reach 10%, these agents resulted in prolongation of PFS in pivotal studies. Furthermore, it has been reported that these agents preserve patients' health‐related quality of life [40]. This fact suggests that tumor stabilization rather than shrinkage may still result in clinical benefit for NETs. The ORR has been traditionally used as a primary endpoint in phase II trials for NETs. The advantage of the use of the ORR is that it requires short follow‐up periods and a small trial population. However, the present study shows that the ORR is not an appropriate endpoint for screening phase II trials.
Phase II trials are screening trials that seek to identify agents with sufficient activity to continue development. Although definitive phase III trials for NETs use PFS as a primary endpoint, it requires long follow‐up periods and a large trial population. As a result, it renders a clinical trial both time‐consuming and costly. Rapid screening phase II trials are essential for efficient and cost‐effective development of new therapeutic agents. Thus, earlier endpoints are preferable for phase II trials, and tumor shrinkage evaluated according to RECIST, the ORR, has been traditionally used as a surrogate endpoint. The basic assumption for the use of the ORR has been that a higher rate of response is predictive of improvements in survival, and that an agent would not benefit patients without resulting in significant tumor shrinkage [41], [42], [43]. This assumption generally appears to hold true for cytotoxic drugs. However, use of the ORR as a primary endpoint in phase II trials with new agents with a modest ORR (e.g., SSAs or molecular targeted drugs) could result in a potentially effective therapy being missed. Subgroup analyses showed that the ORR is correlated with median PFS in study arms that used cytotoxic drugs, but it was not correlated in study arms that used molecular targeted drugs. These facts supported our idea.
The lack of a correlation between tumor response and survival benefit is well described. Novel agents with a modest ORR, such as molecular targeted drugs, sometimes result in prolongation of median PFS or OS in phase III trials. The putative Raf kinase and antivascular agent sorafenib used for renal cell carcinoma and hepatocellular carcinoma is a known example of such novel agents. In phase II trials of sorafenib, the ORR based on RECIST was only <5% [44], [45]. However, subsequent phase III trials showed significant prolongations of both PFS and OS [46], [47]. The simple categorization of patients into responders and nonresponders based on tumor shrinkage may fail to identify potentially promising agents with antineoplastic activity. Thus, alternative or complementary endpoints for predicting PFS or OS are required. In the present analysis, the median duration of enrollment and time to study completion were 20 months and 53.5 months, respectively. Obviously, it requires a longer time to complete phase II trials for NETs. The present study showed that 12‐month PFS rate had very strong correlations with median PFS in both subgroups that used molecular targeted drugs and those that used cytotoxic drugs. It has been shown that the 12‐month PFS rate is also correlated with OS [4]. Thus, the 12‐month PFS rate is considered a good alternate endpoint for screening phase II trials in advanced NETs, and it may contribute to accelerating development of new therapeutic agents via rapid study completion.
This study had some limitations. First, the analysis relied on summary data from published trials to assess and explore alternative endpoints, so individual patient data were unavailable for analysis. It has already been reported that trial‐level surrogacy is not necessarily reflective of individual‐level outcomes [48], so the present data cannot be used to predict an individual's chance of survival on the basis of their response to treatment. The second limitation is the small number of prospective studies, especially randomized trials, available for NETs, a factor that likely contributes to the heterogeneity of the clinical trials. Finally, the study lacked optimal statistical power and should therefore be considered only an exploratory investigation. NETs are relatively rare, and they tend to exhibit indolent progression, both of which could complicate recruiting for, and completion of, clinical trials. However, the use of the 12‐month PFS rate instead of the ORR in phase II trials for NETs would allow for faster development and earlier implementation of new therapeutic agents via rapid study completion.
Conclusion
The results of the present analysis indicate that the ORR was not significantly correlated with median PFS for subjects in NET medical treatment clinical trials. On the other hand, 12‐month PFS rate showed very strong correlation with median PFS and is considered a good alternate endpoint for screening phase II trials in advanced NETs.
Author Contributions
Conception/design: Hiroshi Imaoka
Collection and/or assembly of data: Hiroshi Imaoka, Mitsuhito Sasaki
Data analysis and interpretation: Hiroshi Imaoka
Manuscript writing: Hiroshi Imaoka
Final approval of manuscript: Hiroshi Imaoka, Mitsuhito Sasaki, Hideaki Takahashi, Yusuke Hashimoto, Izumi Ohno, Shuichi Mitsunaga, Kazuo Watanabe, Kumiko Umemoto, Gen Kimura, Yuko Suzuki, Motoyasu Kan, Masafumi Ikeda
Disclosures
Masafumi Ikeda: Novartis Pharma K.K. (RF, H), Novartis Pharma K.K., Teijin Pharma, Nobel Pharma (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Dasari A, Shen C, Halperin D et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. JClin Oncol 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 3.Halperin DM, Yao JC. Clinical trial design in neuroendocrine tumors. Hematol Oncol Clin North Am 2016;30:209–217. [DOI] [PubMed] [Google Scholar]
- 4.Imaoka H, Sasaki M, Takahashi H et al. Progression‐free survival as a surrogate endpoint in advanced neuroendocrine neoplasms. Endocr Relat Cancer 2017;24:475–483. [DOI] [PubMed] [Google Scholar]
- 5.Ter‐Minassian M, Zhang S, Brooks NV et al. Association between tumor progression endpoints and overall survival in patients with advanced neuroendocrine tumors. The Oncologist 2017;22:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulke MH, Siu LL, Tepper JE et al. Future directions in the treatment of neuroendocrine tumors: Consensus report of the National Cancer Institute neuroendocrine tumor clinical trials planning meeting. JClin Oncol 2011;29:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinke A, Muller HH, Schade‐Brittinger C et al. Placebo‐controlled, double‐blind, prospective, randomized study on the effect of octreotide lar in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study group. JClin Oncol 2009;27:4656–4663. [DOI] [PubMed] [Google Scholar]
- 8.Caplin ME, Pavel M, Cwikla JB et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224–233. [DOI] [PubMed] [Google Scholar]
- 9.Raymond E, Dahan L, Raoul JL et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501–513. [DOI] [PubMed] [Google Scholar]
- 10.Pavel ME, Hainsworth JD, Baudin E et al. Everolimus plus octreotide long‐acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT‐2): A randomised, placebo‐controlled, phase 3 study. Lancet 2011;378:2005–2012. [DOI] [PubMed] [Google Scholar]
- 11.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao JC, Fazio N, Singh S et al. Everolimus for the treatment of advanced, non‐functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT‐4): A randomised, placebo‐controlled, phase 3 study. Lancet 2016;387:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yachida S, Vakiani E, White CM et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well‐differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2012;36:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorbye H, Welin S, Langer SW et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol 2013;24:152–160. [DOI] [PubMed] [Google Scholar]
- 15.Pavel M, O'Toole D, Costa F et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 2016;103:172–185. [DOI] [PubMed] [Google Scholar]
- 16.Foster NR, Qi Y, Shi Q et al. Tumor response and progression‐free survival as potential surrogate endpoints for overall survival in extensive stage small‐cell lung cancer: Findings on the basis of North Central Cancer Treatment Group trials. Cancer 2011;117:1262–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickolich M, Babakoohi S, Fu P et al. Clinical trial design in small cell lung cancer: Surrogate end points and statistical evolution. Clin Lung Cancer 2014;15:207–212. [DOI] [PubMed] [Google Scholar]
- 18.Sun W, Lipsitz S, Catalano P et al. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group study E1281. JClin Oncol 2005;23:4897–4904. [DOI] [PubMed] [Google Scholar]
- 19.Yao JC, Phan A, Hoff PM et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: A random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha‐2b. JClin Oncol 2008;26:1316–1323. [DOI] [PubMed] [Google Scholar]
- 20.Meyer T, Qian W, Caplin ME et al. Capecitabine and streptozocin +/‐ cisplatin in advanced gastroenteropancreatic neuroendocrine tumours. Eur J Cancer 2014;50:902–911. [DOI] [PubMed] [Google Scholar]
- 21.Kulke MH, Niedzwiecki D, Foster NR et al. Randomized phase II study of everolimus (E) versus everolimus plus bevacizumab (E+B) in patients (Pts) with locally advanced or metastatic pancreatic neuroendocrine tumors (pNET), CALGB 80701 (Alliance). JClin Oncol 2015;33(suppl 15):4005a. [Google Scholar]
- 22.Kulke MH, Kim H, Stuart K et al. A phase II study of docetaxel in patients with metastatic carcinoid tumors. Cancer Invest 2004;22:353–359. [DOI] [PubMed] [Google Scholar]
- 23.Yao JC, Zhang JX, Rashid A et al. Clinical and in vitro studies of imatinib in advanced carcinoid tumors. Clin Cancer Res 2007;13:234–240. [DOI] [PubMed] [Google Scholar]
- 24.Yao JC, Phan AT, Chang DZ et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low‐ to intermediate‐grade neuroendocrine tumors: Results of a phase II study. JClin Oncol 2008;26:4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao JC, Lombard‐Bohas C, Baudin E et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: A phase II trial. JClin Oncol 2010;28:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulke MH, Chan JA, Meyerhardt JA et al. A prospective phase II study of 2‐methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother Pharmacol 2011;68:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavel ME, Wiedenmann B, Capdevila J et al. RAMSETE: A single‐arm, multicenter, single‐stage phase II trial of RAD001 (everolimus) in advanced and metastatic silent neuro‐endocrine tumours in Europe. JClin Oncol 2012;30(suppl 15):4122a. [Google Scholar]
- 28.Chan JA, Stuart K, Earle CC et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. JClin Oncol 2012;30:2963–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strosberg JR, Chan JA, Ryan DP et al. A multi‐institutional, phase II open‐label study of ganitumab (AMG 479) in advanced carcinoid and pancreatic neuroendocrine tumors. Endocr Relat Cancer 2013;20:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellano D, Capdevila J, Sastre J et al. Sorafenib and bevacizumab combination targeted therapy in advanced neuroendocrine tumour: A phase II study of spanish neuroendocrine tumour group (GETNE0801). Eur J Cancer 2013;49:3780–3787. [DOI] [PubMed] [Google Scholar]
- 31.Martin‐Richard M, Massuti B, Pineda E et al. Antiproliferative effects of lanreotide autogel in patients with progressive, well‐differentiated neuroendocrine tumours: A Spanish, multicentre, open‐label, single arm phase II study. BMC Cancer 2013;13:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ducreux M, Dahan L, Smith D et al. Bevacizumab combined with 5‐FU/streptozocin in patients with progressive metastatic well‐differentiated pancreatic endocrine tumours (BETTER trial)–A phase II non‐randomised trial. Eur J Cancer 2014;50:3098–3106. [DOI] [PubMed] [Google Scholar]
- 33.Berruti A, Fazio N, Ferrero A et al. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well‐to‐moderately differentiated neuroendocrine tumors: The XELBEVOCT study. BMC Cancer 2014;14:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitry E, Walter T, Baudin E et al. Bevacizumab plus capecitabine in patients with progressive advanced well‐differentiated neuroendocrine tumors of the gastro‐intestinal (GI‐NETs) tract (BETTER trial)–A phase II non‐randomised trial. Eur J Cancer 2014;50:3107–3115. [DOI] [PubMed] [Google Scholar]
- 35.Grande E, Capdevila J, Castellano D et al. Pazopanib in pretreated advanced neuroendocrine tumors: A phase II, open‐label trial of the Spanish task force group for neuroendocrine tumors (GETNE). Ann Oncol 2015;26:1987–1993. [DOI] [PubMed] [Google Scholar]
- 36.Hobday TJ, Qin R, Reidy‐Lagunes D et al. Multicenter phase II trial of temsirolimus and bevacizumab in pancreatic neuroendocrine tumors. JClin Oncol 2015;33:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cives M, Kunz PL, Morse B et al. Phase II clinical trial of pasireotide long‐acting repeatable in patients with metastatic neuroendocrine tumors. Endocr Relat Cancer 2015;22:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bendell JC, Zakari A, Lang E et al. A phase II study of the combination of bevacizumab, pertuzumab, and octreotide LAR for patients with advanced neuroendocrine cancers. Cancer Invest 2016;34:213–219. [DOI] [PubMed] [Google Scholar]
- 39.Strosberg JR, Cives M, Hwang J et al. A phase II study of axitinib in advanced neuroendocrine tumors. Endocr Relat Cancer 2016;23:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavel ME, Singh S, Strosberg JR et al. Health‐related quality of life for everolimus versus placebo in patients with advanced, non‐functional, well‐differentiated gastrointestinal or lung neuroendocrine tumours (RADIANT‐4): A multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2017;18:1411–1422. [DOI] [PubMed] [Google Scholar]
- 41.Graf W, Pahlman L, Bergstrom R et al. The relationship between an objective response to chemotherapy and survival in advanced colorectal cancer. Br J Cancer 1994;70:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paesmans M, Sculier JP, Libert P et al. Response to chemotherapy has predictive value for further survival of patients with advanced non‐small cell lung cancer: 10 years experience of the European Lung Cancer Working Party. Eur J Cancer 1997;33:2326–2332. [DOI] [PubMed] [Google Scholar]
- 43.Torri V, Simon R, Russek‐Cohen E et al. Statistical model to determine the relationship of response and survival in patients with advanced ovarian cancer treated with chemotherapy. JNatl Cancer Inst 1992;84:407–414. [DOI] [PubMed] [Google Scholar]
- 44.Ratain MJ, Eisen T, Stadler WM et al. Phase II placebo‐controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. JClin Oncol 2006;24:2505–2512. [DOI] [PubMed] [Google Scholar]
- 45.Abou‐Alfa GK, Schwartz L, Ricci S et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. JClin Oncol 2006;24:4293–4300. [DOI] [PubMed] [Google Scholar]
- 46.Escudier B, Eisen T, Stadler WM et al. Sorafenib in advanced clear‐cell renal‐cell carcinoma. N Engl J Med 2007;356:125–134. [DOI] [PubMed] [Google Scholar]
- 47.Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 48.Berlin JA, Santanna J, Schmid CH et al. Individual patient‐ versus group‐level data meta‐regressions for the investigation of treatment effect modifiers: Ecological bias rears its ugly head. Stat Med 2002;21:371–387. [DOI] [PubMed] [Google Scholar]