Radiotherapy is the primary treatment modality for nondisseminated nasopharyngeal carcinoma. Treatment with two‐dimensional radiotherapy transitioned to three‐dimensional conformal radiotherapy, and in particular to intensity‐modulated radiotherapy, is a step forward in the treatment of nasopharyngeal carcinoma. This article describes a long‐term follow‐up to verify ten‐year results of survival and late toxicities and to assess ultimate therapeutic ratio by intensity‐modulated radiotherapy versus two‐dimensional radiotherapy in patients with nasopharyngeal carcinoma.
Keywords: Nasopharyngeal carcinoma, Intensity‐modulated radiotherapy, Two‐dimensional radiotherapy, 10‐year results, Therapeutic ratio
Abstract
Background.
The purpose of this study was to verify 10‐year results of survival and late toxicities and assess the ultimate therapeutic ratio of intensity‐modulated radiotherapy (IMRT) versus two‐dimensional radiotherapy (2DRT) in patients with nasopharyngeal carcinoma (NPC).
Materials and Methods.
We retrospectively reviewed the data from 1,276 patients with nonmetastatic NPC who received IMRT or 2DRT from January 2003 to December 2006.
Results.
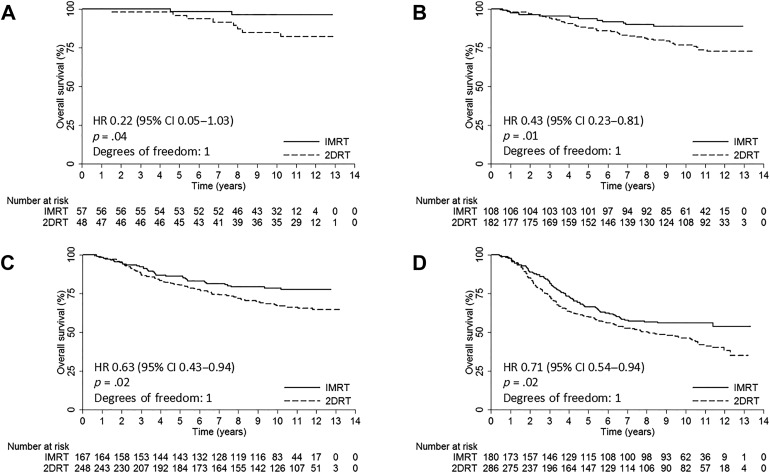
Of the 1,276 patients, 512 were treated with IMRT and 764 with 2DRT. Median follow‐up was 115 months. At 10 years, the IMRT group demonstrated significantly better results than the 2DRT group in local failure‐free survival (L‐FFS; 90% vs. 84%; hazard ratio [HR], 0.57, 95% confidence interval [CI], 0.40–0.81; p = .001), failure‐free survival (FFS; 69% vs. 58%; HR, 0.69, 95% CI, 0.57–0.83; p < .001), and overall survival (OS; 75% vs. 63%; HR, 0.62, 95% CI, 0.51–0.77; p < .001). Subgroup multivariate analyses showed that radiotherapeutic technique (IMRT vs. 2DRT) remained an independent prognostic factor for L‐FFS in the T1 subgroup (HR, 0.30; 95% CI, 0.11–0.80; p = .02); for FFS in the stage II subgroup (HR, 0.42; 95% CI, 0.24–0.73; p = .002); and for OS in the stage I (HR, 0.20; 95% CI, 0.04–0.96; p = .04), stage II (HR, 0.39; 95% CI, 0.21–0.75; p = .004), and stage IVA–B (HR, 0.74, 95% CI, 0.56–0.98; p = .04) subgroups. The incidence of grade 3–4 temporal lobe necrosis, cranial neuropathy, eye damage, ear damage, neck soft tissue damage, trismus, and dry mouth was significantly lower in the IMRT group than in the 2DRT group.
Conclusion.
IMRT demonstrated an improved ultimate therapeutic ratio compared with 2DRT in patients with NPC after a 10‐year follow‐up, with significant improvement of L‐FFS, FFS, and OS and decrease in most late toxicities.
Implications for Practice.
The ultimate therapeutic ratio of intensity‐modulated radiotherapy versus two‐dimensional radiotherapy in patients with nasopharyngeal carcinoma is unclear. In this retrospective study of 1,276 patients with nonmetastatic nasopharyngeal carcinoma with a follow‐up of 115 months, intensity‐modulated radiotherapy demonstrated an improved ultimate therapeutic ratio compared with two‐dimensional radiotherapy, with significant improvement of local failure‐free survival, failure‐free survival, and overall survival and decrease in most late toxicities and noncancer deaths. However, distant control remains insufficient with this treatment modality.
摘要
背景。本研究旨在验证鼻咽癌 (NPC) 患者调强放疗 (IMRT) 对比二维放疗 (2DRT) 的生存率和晚期毒性的 10 年结果并评估最终治疗比。
材料和方法。我们对 1 276 名在 2003 年 1 月至 2006 年 12 月期间接受 IMRT 或 2DRT 的非转移性 NPC 患者的数据进行回顾性分析。
结果。在这 1 276 名患者中,512 名患者接受 IMRT 治疗,764 名患者接受 2DRT 治疗。中位随访时间为 115 个月。到第 10 年的时候,IMRT 组比 2DRT 组在局部无失败生存率[L‐FFS;90% vs. 84%;风险比(HR),0.57,95% 置信区间(CI),0.40–0.81;p = 0.001]、无失败生存率(FFS;69% vs. 58%;HR,0.69,95% CI,0.57–0.83;p < 0.001)以及总生存率(OS;75% vs. 63%;HR,0.62,95% CI,0.51–0.77;p <0.001)等方面显示出明显更好的结果。亚组多变量分析表明, 就 T1 亚组中的 L‐FFS(HR,0.30;95% CI,0.11–0.80;p = 0.02) ;II 期亚组中的 FFS(HR,0.42;95% CI,0.24–0.73;p = 0.002);以及 I 期亚组中的 OS(HR,0.20;95% CI,0.04–0.96;p = 0.04)、II 期亚组中的 OS(HR,0.39;95% CI,0.21–0.75;p = 0.004)与 IVA–B 期亚组中的 OS(HR,0.74;95% CI,0.56–0.98;p = 0.04)而言,放疗技术(IMRT 与 2DRT)仍然是独立的预后因素。与 2DRT 组相比,IMRT 组的 3–4 级颞叶坏死、颅神经病变、眼部损伤、耳部损伤、颈部软组织损伤、牙关紧闭以及口腔干燥的发病率明显更低。
结论。在 10 年随访之后,与 2DRT 相比,IMRT 在 NPC 患者中显示出更好的最终治疗比,在 L‐FFS、FFS 和 OS 方面均有显著改进,大多数晚期毒性有所减少。
对临床实践的提示
鼻咽癌患者调强放疗对比二维放疗的最终治疗比尚不明确。在本次针对 1 276 名随访期历时 115 个月的非转移性鼻咽癌患者的回顾性研究中,与二维放疗相比,调强放疗显示出更好的最终治疗比,在局部无失败生存率、无失败生存率和总生存率方面均有显著改进,大多数晚期毒性和非肿瘤死亡均有减少。但是,远程控制对于这种治疗方式而言仍然不足。
Introduction
Radiotherapy is the primary treatment modality for nondisseminated nasopharyngeal carcinoma (NPC). Treatment with two‐dimensional radiotherapy (2DRT) transitioned to three‐dimensional conformal radiotherapy, and in particular to intensity‐modulated radiotherapy (IMRT), has represented a major step forward in the treatment of NPC [1]. Numerous studies have reported beneficial IMRT results for the treatment of NPC [1], [2], [3]; however, few comparison studies of the effects of IMRT versus 2DRT on outcomes in NPC exist.
We therefore performed a retrospective study to directly compare IMRT versus 2DRT results in patients with NPC treated in the same time period. After a median follow‐up period of 53 months, preliminary results demonstrated that the greater improvement in treatment results with IMRT than with 2DRT was primarily by achieving a higher local tumor control rate in patients with NPC, especially in the early T classification patients [4]. However, it should be noted that this follow‐up period was not long enough, especially to study overall survival (OS), and there were no data on detailed late toxicities at that time.
On the other hand, time to relapse after IMRT might be postponed compared with 2DRT. The study on IMRT showed that more than half of observed local relapses occurred after 2 years [5]. Moreover, advances in salvage treatment after initial treatment failure, including surgery, reirradiation, and chemotherapy, cannot be ignored, and this was associated with better OS than before [6], [7]. In addition, although progression‐free survival could be a valid surrogate endpoint for OS based on the results of our meta‐analysis, it does not reduce the need for long‐term follow‐up of patients, because some unexpected adverse effects that might occur later would not be captured by the surrogate endpoints [8]. Hence, the purpose of this long‐term follow‐up was to verify 10‐year results of survival and late toxicities and to further assess the ultimate therapeutic ratio of IMRT versus 2DRT in patients with NPC.
Materials and Methods
Patient Characteristics
A total of 1,276 patients with newly diagnosed, biopsy‐proven, nonmetastatic NPC presented at Sun Yat‐Sen University Cancer Center between January 2003 and December 2006 and were included in this study. All patients underwent physical examination, fiberoptic examination, chest x‐ray, abdominal ultrasonography, and magnetic resonance imaging prior to treatment. Patients with stage N2–N3 disease underwent single photon emission computed tomography for whole body bone scanning. For stage and classification, the seventh edition of the Union for International Cancer Control (UICC) staging system was used [9]. Our protocol was approved by the ethics committee of Sun Yat‐Sen University Cancer Center.
Treatment
All patients were treated with definitive‐intent radiation therapy, with 512 (40%) patients treated with IMRT and 764 (60%) patients treated with 2DRT. In cases of documented persistent disease or relapse, salvage treatments, including intracavitary brachytherapy, surgery, reirradiation, and chemotherapy, were provided, if applicable.
2DRT. Patients were immobilized in supine position with a thermoplastic mask and treated with two lateral‐opposing faciocervical portals to irradiate the nasopharynx and the upper neck in one volume, followed by application of the shrinking‐field technique to limit irradiation of the spinal cord. An anterior cervical field was used to treat the neck with a laryngeal block. The accumulated radiation doses were 68–76 Gy (median, 70 Gy), with 2 Gy per fraction applied to the primary tumor, 60–66 Gy applied to the involved areas of the neck, and 50 Gy applied to the uninvolved areas. All patients were treated with one fraction daily for 5 days per week.
A boost portal was performed if necessary. Patients with bulky parapharyngeal disease in the 2DRT group received the parapharyngeal boost technique [10]. A boost dose (8–12 Gy per four to six fractions) was delivered to the skull base in patients with NPC involving the skull base and intracranial extension. Intracavitary afterloading treatment with iridium‐192 was used to address local persistence 2–3 weeks after external radiotherapy with 12–16 Gy per three to five fractions once every 2 days in 1–2 weeks. Any palpable residual nodes presenting after external radiotherapy were boosted to 70 Gy.
IMRT. Immobilization for IMRT was the same as for 2DRT. Computed tomography after administration of intravenous contrast medium was performed by collection of 3 mm slices from the head to the level of 2 cm below the sternoclavicular joint. The primary tumor and the upper neck above the caudal edge of the cricoid cartilage were treated with IMRT. The target volumes were delineated using an institutional treatment protocol previously described [11], which was in accordance with the International Commission on Radiation Units and Measurements Reports 50 and 62. The clinical target volumes (CTVs) were individually delineated on the basis of the tumor invasion pattern [12]. The contoured image was transferred to CORVUS 3.0, an inverse IMRT planning system (Peacock) developed by Best NOMOS Corporation (Pittsburgh, PA). The radiation dose prescribed as per protocol was defined as follows: 2.27 Gy per fraction to the planning target volume (PTV) of gross tumor volume of the primary (GTV‐P), 60–66 Gy to the PTV of nodal gross tumor volume (GTV‐N), 60 Gy to the PTV of CTV‐1 (i.e., high risk regions), 54 Gy to PTV of CTV‐2 (i.e., low‐risk regions) and CTV‐N (i.e., neck nodal regions). The treatment was delivered by a dynamic, multileaf, intensity‐modulating collimator. For the lower neck, an anterior cervical field was used. All patients were treated with one fraction daily over 5 days per week. Patients with local tumor persistence 2–3 weeks after IMRT received additional intracavitary irradiation, as described for the 2DRT group.
Chemotherapy. Of the 881 patients with stage III or IVA–B disease, 699 (79%) received neoadjuvant, concomitant, and/or adjuvant chemotherapy using various regimens of cytotoxic drugs (all cisplatin‐based; supplemental online Table 1).
Follow‐Up
The duration of patient follow‐up was measured from the first day of therapy to either the day of last examination or the day of death. Patients were seen every 3 months during the first 2 years and every 6 months thereafter until death.
Statistical Analysis
The following endpoints were assessed: local failure‐free survival (L‐FFS), regional failure‐free survival (R‐FFS), distant failure‐free survival (D‐FFS), failure‐free survival (FFS), OS, and late toxic effects. We calculated L‐FFS, R‐FFS and D‐FFS from the date of commencement of therapy to the date of the first local, regional, or remote failure, respectively. FFS was calculated from therapy to the date of treatment failure or death from any cause, whichever was first, and OS was from therapy to death. We graded late toxic effects in accordance with the Late Radiation Morbidity Scoring Criteria of the Radiation Therapy Oncology Group and European Organization for Research and Treatment of Cancer [13].
Time‐to‐event data were described with the Kaplan‐Meier curves, and time‐to‐event intervals were compared using the log‐rank test. We calculated hazard ratios (HRs) with the Cox proportional hazards model. We did multivariable analyses with the Cox proportional hazards model to test the independent significance of different factors. Covariates included host factors (i.e., sex and age), tumor factors (i.e., T and N classification), radiotherapy technique (IMRT vs. 2DRT), and chemotherapy (yes vs. no). We compared toxicity rates and other categorical variables using the χ2 test (or Fisher's exact test, if indicated). All tests were two‐sided; we deemed p values of less than .05 to be significant. All analyses were done with Stata (version 10; StataCorp, College Station, TX).
Results
Patient Characteristics
The last follow‐up was December 5, 2016, and the median follow‐up for the entire cohort was 115 months (range, 2–161 months). There were no significant differences in the host factors, histological categories, tumor factors, or chemotherapy between the IMRT and 2DRT groups (Table 1, supplemental online Table )2.
Table 1. Characteristics of the patients.
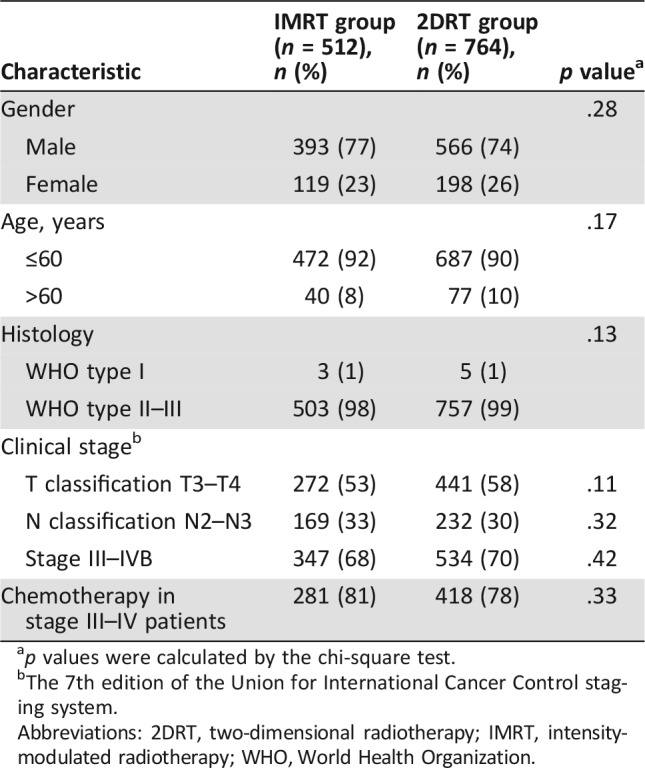
p values were calculated by the chi‐square test.
The 7th edition of the Union for International Cancer Control staging system.
Abbreviations: 2DRT, two‐dimensional radiotherapy; IMRT, intensity‐modulated radiotherapy; WHO, World Health Organization.
Overall Pattern of Failure
Overall, 422 (33%) patients experienced failure at one or more sites. Distant failure alone was the most common incidence (16%), followed by local failure alone (9%; supplemental online Table 3).
Local Control
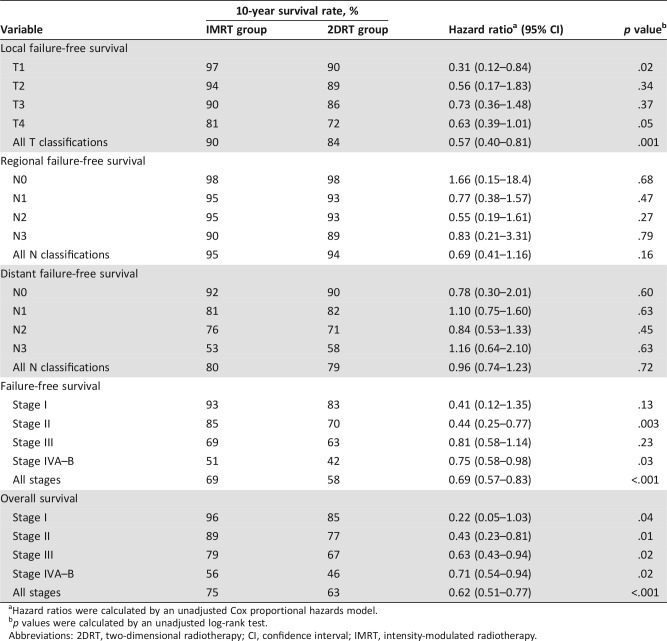
At 10 years, the IMRT group demonstrated significantly better L‐FFS than the 2DRT group (90% vs. 84%; HR, 0.57; 95% confidence interval [CI], 0.40–0.81; p = .001; Table 2). Multivariate analysis showed that the radiotherapeutic technique (IMRT vs. 2DRT) was an independent prognostic factor for L‐FFS (HR, 0.60; 95% CI, 0.42–0.85; p = .004; Table 3).
Table 2. Survival.
Hazard ratios were calculated by an unadjusted Cox proportional hazards model.
p values were calculated by an unadjusted log‐rank test.
Abbreviations: 2DRT, two‐dimensional radiotherapy; CI, confidence interval; IMRT, intensity‐modulated radiotherapy.
Table 3. Summary of prognostic factors multivariable analyses.
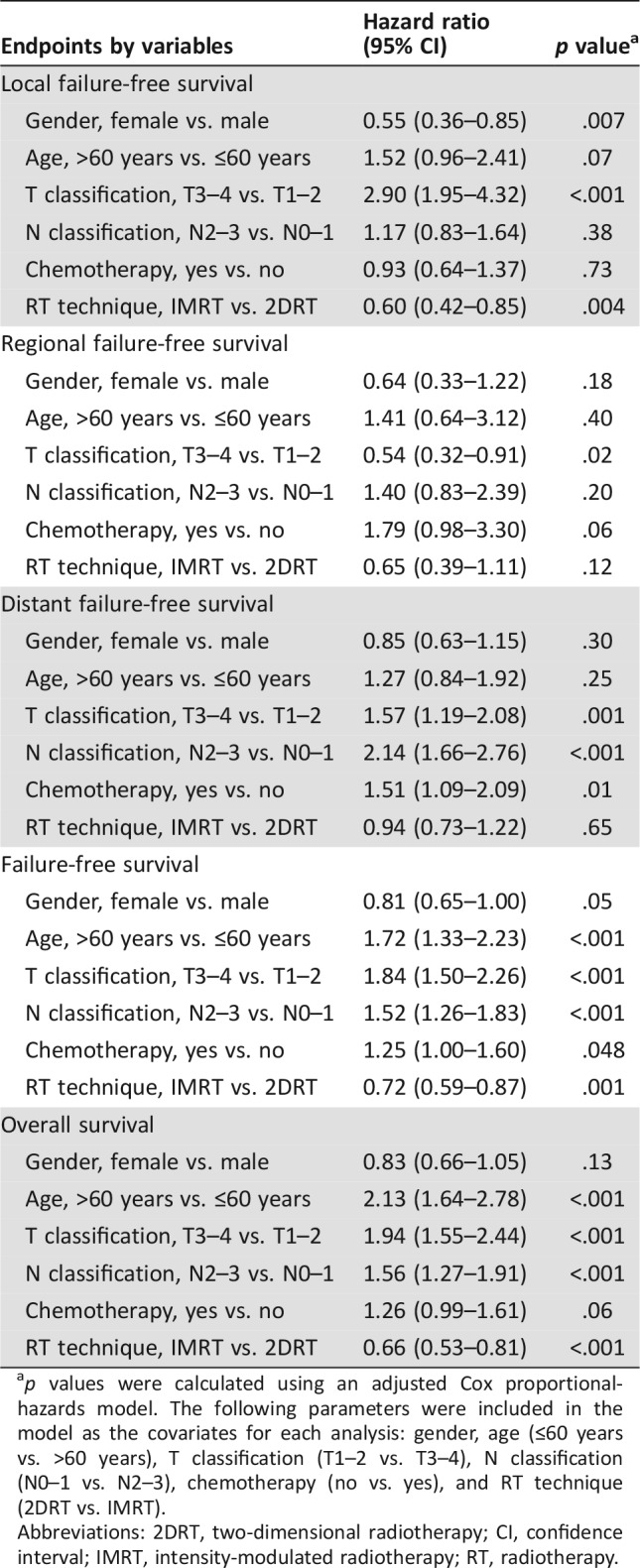
p values were calculated using an adjusted Cox proportional‐hazards model. The following parameters were included in the model as the covariates for each analysis: gender, age (≤60 years vs. >60 years), T classification (T1–2 vs. T3–4), N classification (N0–1 vs. N2–3), chemotherapy (no vs. yes), and RT technique (2DRT vs. IMRT).
Abbreviations: 2DRT, two‐dimensional radiotherapy; CI, confidence interval; IMRT, intensity‐modulated radiotherapy; RT, radiotherapy.
After stratification by T classification, 10‐year L‐FFS rates were higher in the IMRT group than the 2DRT group, but statistical significance could be reached only for the T1 classification patients, and borderline significance was observed in T4 patients (Table 2 and Fig. 1). Further subgroup multivariate analyses showed that radiotherapeutic technique (IMRT vs. 2DRT) only remained an independent prognostic factor for L‐FFS in T1 patients (HR, 0.30; 95% CI, 0.11–0.80; p = .02) and was a marginally significant predictive factor in T4 patients (HR, 0.66; 95% CI, 0.41–1.07; p = .09; supplemental online Table 4).
Figure 1.
Kaplan‐Meier local failure‐free survival curves for the IMRT group and the 2DRT group. (A): T1 classification. (B): T2 classification. (C): T3 classification. (D): T4 classification. HRs were calculated by the unadjusted Cox proportional hazards model. P values were calculated by the unadjusted log‐rank test.
Abbreviations: 2DRT, two‐dimensional radiotherapy; CI, confidence interval; HR, hazard ratio; IMRT, intensity‐modulated radiotherapy.
Regional Control
The 10‐year R‐FFS rates were 95% for the IMRT group and 94% for the 2DRT group (HR, 0.69; 95% CI, 0.41–1.16; p = .16; Table 2). Multivariate analysis failed to demonstrate that the radiotherapeutic technique (IMRT vs. 2DRT) was an independent prognostic factor for R‐FFS (Table 3). After stratification by N classification, no significant differences were observed between the IMRT and 2DRT groups in all N0–N3 classifications by univariate and multivariate analyses (Table 2 and supplemental online Table 4).
Distant Control
The 10‐year D‐FFS rate was 80% in the IMRT group and 79% in 2DRT group (HR, 0.96; 95% CI, 0.74–1.23; p = .72; Table 2). Multivariate analysis failed to demonstrate that the radiotherapeutic technique (IMRT vs. 2DRT) was an independent prognostic factor for D‐FFS (Table 3). After stratification by N classification, no significant differences were observed between the IMRT and 2DRT groups in all N0–N3 classifications with univariate and multivariate analyses (Table 2 and supplemental online Table 4).
Disease Control
The 10‐years FFS rates for the IMRT and 2DRT groups were 69% and 58%, respectively (HR, 0.69; 95% CI, 0.57–0.83; p < .001; Table 2). Multivariate analysis showed that the radiotherapeutic technique (IMRT vs. 2DRT) was an independent prognostic factor for FFS (HR, 0.72; 95% CI, 0.59–0.87; p = .001; Table 3).
After stratification by the stage, 10‐year FFS rates were higher in the IMRT group than the 2DRT group, but statistical significance could be reached only for the stage II and stage IVA–B patients (Table 2). Further subgroup multivariate analyses showed that radiotherapeutic technique (IMRT vs. 2DRT) only remained an independent prognostic factor for FFS in stage II patients (HR, 0.42; 95% CI, 0.24–0.73; p = .002) and was a marginally significant predictive factor in stage I (HR, 0.35; 95% CI, 0.10–1.19; p = .09) and stage IVA–B (HR, 0.78; 95% CI, 0.60–1.02; p = .07) patients (supplemental online Table 4).
Overall Survival
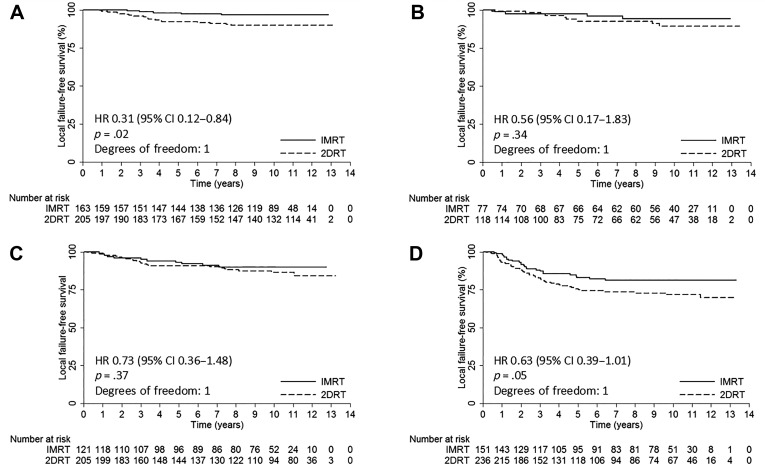
At 10 years, the IMRT group demonstrated significantly better OS than the 2DRT group (75% vs. 63%; HR, 0.62; 95% CI, 0.51–0.77; p < .001; Table 2). Multivariate analysis showed that the radiotherapeutic technique (IMRT vs. 2DRT) was an independent prognostic factor for OS (HR, 0.66; 95% CI, 0.53–0.81, p < .001; Table 3).
After stratification by the stage, 10‐year OS rates were higher in the IMRT group than the 2D‐CRT group, and statistical significance could be reached for each stage patients (Table 2 and Fig. 2). Further subgroup multivariate analyses showed that radiotherapeutic technique (IMRT vs. 2DRT) was an independent prognostic factor for OS in stage I (HR, 0.20; 95% CI, 0.04–0.96; p = .04), stage II (HR, 0.39; 95% CI, 0.21–0.75; p = .004), and stage IVA–B (HR, 0.74; 95% CI, 0.56–0.98; p = .04) patients and a marginally significant predictive factor in stage III patients (HR, 0.69; 95% CI, 0.46–1.03; p = .07; supplemental online Table 4).
Figure 2.
Kaplan‐Meier overall survival curves for the IMRT group and the 2DRT group. (A): Stage I. (B): Stage II. (C): Stage III. (D): Stage IVA–B. HRs were calculated by the unadjusted Cox proportional hazards model. P values were calculated by the unadjusted log‐rank test.
Abbreviations: 2DRT, two‐dimensional radiotherapy; CI, confidence interval; HR, hazard ratio; IMRT, intensity‐modulated radiotherapy.
The IMRT group had a significant reduction in the 10‐year actuarial incidence of death from disease progression compared with the 2DRT group (21% vs. 31%; HR, 0.64; 95% CI, 0.51–0.81; p < .001); moreover, the 10‐year actuarial incidence of death from treatment‐related toxicities, incidental causes, or unknown reasons was significantly lower in the IMRT group compared with the 2DRT group (5% vs. 8%; HR, 0.55; 95% CI, 0.34–0.90; p = .02).
Late Toxicities
Overall, 91 (18%) of 512 patients in the IMRT group and 255 (33%) of 764 patients in the 2DRT group developed one or more late grade 3–4 toxicities (p < .001). One (0.2%) of 512 patients in the IMRT group died from temporal lobe necrosis, and 8 (1%) of 764 patients in the 2DRT group died from temporal lobe necrosis, cranial neuropathy, and/or trismus (p = .08). The incidence of grade 3–4 temporal lobe necrosis, cranial neuropathy, eye damage, ear damage, neck soft tissue damage, trismus, and dry mouth was significant lower in the IMRT group compared with the 2DRT group (Table 4).
Table 4. Grade 3–4 late adverse events.
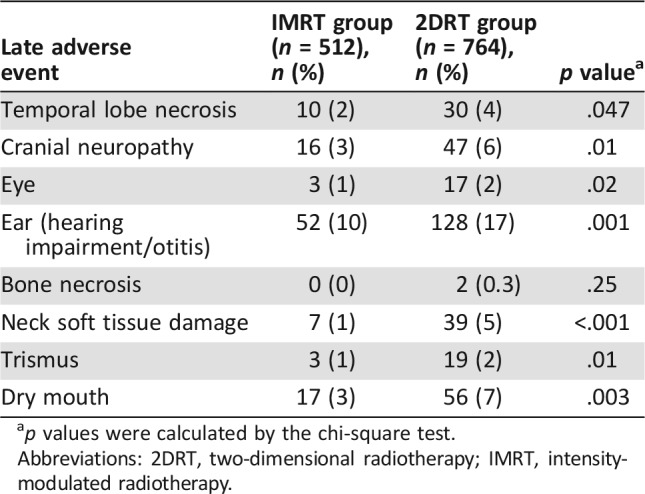
p values were calculated by the chi‐square test.
Abbreviations: 2DRT, two‐dimensional radiotherapy; IMRT, intensity‐modulated radiotherapy.
Discussion
As in our previous study, better local control with IMRT was demonstrated in patients with NPC in the current study, especially in the T1 patients. We hypothesize that this improvement was mainly due to escalation of the applied radiation dose, because there is a dose‐response relationship between tumor control and radiation dose [14]. IMRT can reach dose escalation with reasonable safety for better dose differential between tumor and normal tissues [1]. Meanwhile, IMRT showed a trend to improve local control in T4 patients, which was not shown in our previous study. With the long‐term follow‐up in the current study, we observed almost all local relapses, only half of which occurred in 2 years after treatment. According to the findings of our previous study, local control of T3–4 patients in the 2DRT group increased when they received additional boost therapy. Thus, improvement in local control of T3–4 patients in the IMRT group caused by dose escalation was diminished compared with that of the 2DRT group receiving additional boost treatment. However, it might be hard to attain satisfying local control with 2DRT plus boost therapy in patients with extensive primary tumors (T4 patients), because its dose and coverage often had to be compromised in order to avoid unacceptable complications [15]. Alternatively, IMRT could provide a high dose to target area of T4 patients while significantly sparing nearby critical normal tissues [2]. As shown in the current study, the incidence of grade 3–4 temporal lobe necrosis, cranial neuropathy, eye damage, ear damage, trismus, and dry mouth was significant higher in the 2DRT group than in the IMRT group.
IMRT did not improve regional control compared with 2DRT. Both groups reached excellent regional control because of the biological character of metastatic neck nodes [16]. However, it was remarkable that toxicity was different between these two treatments, and 2DRT was associated with more severe neck soft tissue damage. Moreover, IMRT did not improve distant control of all N classifications patients. The current study showed that distant metastasis as the only site of failure (206/1,276 patients, 16%) was much more common than distant metastasis with other sites of failure (53/1276 patients, 4%). This means that NPC has a higher probability of micrometastatic dissemination at the time of initial diagnosis. The effect of local control on D‐FFS will be small until effective methods to detect and treat subclinical distant metastasis before radiotherapy are developed.
As a result of the higher L‐FFS rate achieved by IMRT in T1 patients, FFS of stage II disease was significantly improved, and the improvement in stage I was of borderline significance. Therefore, improvement of OS in early‐stage disease with IMRT was primarily due to achieving a higher local control rate. Meanwhile, a marginal improvement in FFS was also observed in stage IVA–B patients treated with IMRT, mainly because IMRT had a trend to improve local control in T4 patients. Furthermore, IMRT demonstrated a significant effect on risk of most late toxicities compared with the 2DRT, especially in locoregionally advanced NPC. It had a significant reduction in the 10‐year actuarial incidence of noncancer death. Therefore, significantly better OS was achieved by IMRT in stage IVA–B patients, and marginal improvement was observed in stage III patients.
In a randomized trial conducted by Peng et al. with a follow‐up of only 42 months, IMRT was proved to provide improved local control, especially in patients with late‐stage NPC [17]. Unlike in our study, the improvement of local control in the IMRT group for early T classification patients in their study was not remarkable. It was hypothesized that excellent local control provided by 2DRT plus boost therapy in early T classification patients led to this result. Furthermore, different staging systems used in Peng et al.'s and our studies might also contribute to the discrepancy, because T classifications changed from the sixth to the seventh edition of the UICC staging system. In another retrospective study by Zhang et al. with a larger cohort and follow‐up of only 49 months, the results showed that IMRT significantly improved local, locoregional, and disease control compared with 2DRT for nondisseminated NPC [18]. However, no significant advantage was observed in OS when IMRT was used, which was not consistent with our results. We attributed this mainly to the relatively immature follow‐up period for OS. With changes in time to relapse and the advance of salvage treatment, a follow‐up of less than 5 years in NPC was unlikely to observe most deaths from disease progression [19]. Furthermore, a long‐term follow‐up is really needed to capture some unexpected adverse effects and noncancer deaths [8].
IMRT did not alter the NPC failure pattern, and distant metastasis remained the predominant mode of treatment failure. Intriguingly, newer drugs, molecular targeted agents, and immunotherapy have shown promising results of systemic control in patients with NPC or recurrent head and neck cancer by eradicating micrometastases [20], [21], [22]. Meanwhile, attaining favorable local control in patients with extensive primary tumors remains a significant clinical challenge. There is a great deal of interest at present in the use of more advanced radiotherapeutic technique, such as intensity‐modulated proton therapy and heavy ion radiotherapy, to treat NPC [23], [24]. Owing to the characteristics in physics and biological effect, these advanced radiotherapeutic techniques could be expected to further improve local control in patients with extensive primary tumors without increasing toxicities.
To our knowledge, this 10‐year follow‐up report is the first study to confirm the ultimate therapeutic ratio of IMRT compared with 2DRT in patients with NPC. It should be noted that our study was a nonrandomized designed study because of its retrospective nature. Nevertheless, characteristics of the patients in both groups were similar, and we also attempted to reduce any potential bias by using multivariate analyses and subgroup stratification by classification and stage. In addition, the data of late toxicities were based largely on clinical observations, and regular audiometry and/or imaging were not specified during follow‐up; thus, underestimation could not be excluded.
Conclusion
IMRT demonstrated an improved ultimate therapeutic ratio compared with 2DRT in patients with NPC after a 10‐year follow‐up, with significant improvement of L‐FFS, FFS, and OS and decrease in most late toxicities and noncancer death. However, distant control remains insufficient with this treatment modality.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This study was mainly funded by the Programme of Introducing Talents of Discipline to Universities (B14035), the National Science & Technology Pillar Program during the Twelfth Five‐Year Plan Period (2014BAI09B10), and State Scholarship Fund (201606385060). The study sponsors were not involved in the study design; the collection, analysis and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Contributed equally
Author Contributions
Conception/design: Jun Ma, Lei Chen, Yuan Zhang, Shu‐Zhen Lai
Provision of study material or patients: Jun Ma, Lei Chen, Yuan Zhang, Shu‐Zhen Lai, Wen‐Fei Li, Wei‐Han Hu, Rui Sun, Li‐Zhi Liu, Ying Sun
Collection and/or assembly of data: Lei Chen, Yuan Zhang, Shu‐Zhen Lai, Wen‐Fei Li, Fan Zhang, Hao Peng, Xiao‐Jing Du
Data analysis and interpretation: Lei Chen, Yuan Zhang, Jun Ma, Ai‐Hua Lin
Manuscript writing: Lei Chen, Yuan Zhang, Jun Ma
Final approval of manuscript: Jun Ma, Lei Chen, Yuan Zhang, Shu‐Zhen Lai, Wen‐Fei Li, Wei‐Han Hu, Rui Sun, Li‐Zhi Liu, Fan Zhang, Hao Peng, Xiao‐Jing Du, Ai‐Hua Lin, Ying Sun
Disclosures
The authors indicated no financial relationships.
References
- 1.Kam MK, Teo PM, Chau RM et al. Treatment of nasopharyngeal carcinoma with intensity‐modulated radiotherapy: The Hong Kong experience. Int J Radiat Oncol Biol Phys 2004;60:1440–1450. [DOI] [PubMed] [Google Scholar]
- 2.Lee N, Xia P, Quivey JM et al. Intensity‐modulated radiotherapy in the treatment of nasopharyngeal carcinoma: An update of the UCSF experience. Int J Radiat Oncol Biol Phys 2002;53:12–22. [DOI] [PubMed] [Google Scholar]
- 3.Wolden SL, Chen WC, Pfister DG et al. Intensity‐modulated radiation therapy (IMRT) for nasopharynx cancer: Update of the Memorial Sloan‐Kettering experience. Int J Radiat Oncol Biol Phys 2006;64:57–62. [DOI] [PubMed] [Google Scholar]
- 4.Lai SZ, Li WF, Chen L et al. How does intensity‐modulated radiotherapy versus conventional two‐dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 2011;80:661–668. [DOI] [PubMed] [Google Scholar]
- 5.Setton J, Han J, Kannarunimit D et al. Long‐term patterns of relapse and survival following definitive intensity‐modulated radiotherapy for non‐endemic nasopharyngeal carcinoma. Oral Oncol 2016;53:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou X, Han F, Ma WJ et al. Salvage endoscopic nasopharyngectomy and intensity‐modulated radiotherapy versus conventional radiotherapy in treating locally recurrent nasopharyngeal carcinoma. Head Neck 2015;37:1108–1115. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Huang Y, Hong S et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: A multicentre, randomised, open‐label, phase 3 trial. Lancet 2016;388:1883–1892. [DOI] [PubMed] [Google Scholar]
- 8.Chen YP, Sun Y, Chen L et al. Surrogate endpoints for overall survival in combined chemotherapy and radiotherapy trials in nasopharyngeal carcinoma: Meta‐analysis of randomised controlled trials. Radiother Oncol 2015;116:157–166. [DOI] [PubMed] [Google Scholar]
- 9.Sobin L, Gospodarowicz M, Wittekind C, eds. TNM Classification of Malignant Tumors. 7th ed Chichester, UK: Wiley; 2010. [Google Scholar]
- 10.Tsao SY. Technical details for radiotherapy delivery In: Van Hasselt CA, Gibb AG, eds. Nasopharyngeal carcinoma. Hong Kong: The Chinese University Press; 1991:207–208. [Google Scholar]
- 11.Zhao C, Han F, Lu LX et al. Intensity modulated radiotherapy for local‐regional advanced nasopharyngeal carcinoma [in Chinese]. Ai Zheng 2004;23(suppl 11):1532–1537. [PubMed] [Google Scholar]
- 12.Liang SB, Sun Y, Liu LZ et al. Extension of local disease in nasopharyngeal carcinoma detected by magnetic resonance imaging: Improvement of clinical target volume delineation. Int J Radiat Oncol Biol Phys 2009;75:742–750. [DOI] [PubMed] [Google Scholar]
- 13.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–1346. [DOI] [PubMed] [Google Scholar]
- 14.Marks JE, Bedwinek JM, Lee F et al. Dose‐response analysis for nasopharyngeal carcinoma: An historical perspective. Cancer 1982;50:1042–1050. [DOI] [PubMed] [Google Scholar]
- 15.Waldron J, Tin MM, Keller A et al. Limitation of conventional two dimensional radiation therapy planning in nasopharyngeal carcinoma. Radiother Oncol 2003;68:153–161. [DOI] [PubMed] [Google Scholar]
- 16.Chow E, Payne D, Keane T et al. Enhanced control by radiotherapy of cervical lymph node metastases arising from nasopharyngeal carcinoma compared with nodal metastases from other head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 1997;39:149–154. [DOI] [PubMed] [Google Scholar]
- 17.Peng G, Wang T, Yang KY et al. A prospective, randomized study comparing outcomes and toxicities of intensity‐modulated radiotherapy vs. conventional two‐dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012;104:286–293. [DOI] [PubMed] [Google Scholar]
- 18.Zhang MX, Li J, Shen GP et al. Intensity‐modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two‐dimensional radiotherapy: A 10‐year experience with a large cohort and long follow‐up. Eur J Cancer 2015;51:2587–2595. [DOI] [PubMed] [Google Scholar]
- 19.Wu LR, Liu YT, Jiang N et al. Ten‐year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity‐modulated radiotherapy: An analysis of 614 patients from a single center. Oral Oncol 2017;69:26–32. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Li WF, Chen NY et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509–1520. [DOI] [PubMed] [Google Scholar]
- 21.Lee NY, Zhang Q, Pfister DG et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): A phase 2 multi‐institutional trial. Lancet Oncol 2012;13:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferris RL, Blumenschein G Jr, Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis GD, Holliday EB, Kocak‐Uzel E et al. Intensity‐modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck 2016;38(suppl 1):E1886–E1895. [DOI] [PubMed] [Google Scholar]
- 24.Kong L, Hu J, Guan X et al. Phase I/II trial evaluating carbon ion radiotherapy for salvaging treatment of locally recurrent nasopharyngeal carcinoma. J Cancer 2016;7:774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]