This report presents evidence for FDA approval of pembrolizumab for the treatment of adult and pediatric patients with either unresectable or metastatic, microsatellite instability‐high (MSI‐H) or mismatch repair deficient (dMMR) solid tumors that have progressed following prior treatment, and who have no satisfactory alternative treatment options, or who have metastatic, MSI‐H or dMMR colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
Keywords: GEJ adenocarcinoma, Accelerated approval, PD‐L1
Abstract
On September 22, 2017, the U.S. Food and Drug Administration (FDA) granted accelerated approval for pembrolizumab (Keytruda, Merck & Co., Inc., Whitehouse Station, NJ) for the treatment of patients with recurrent, locally advanced or metastatic, gastric or gastroesophageal junction (GEJ) adenocarcinoma with disease progression on or after two or more systemic therapies, including fluoropyrimidine‐ and platinum‐containing chemotherapy and, if appropriate, HER2/neu‐targeted therapy, and whose tumors express programmed death‐ligand 1 (PD‐L1), as determined by an FDA‐approved test. Approval was based on demonstration of durable overall response rate (ORR) in a multicenter, open‐label, multicohort trial (KEYNOTE‐059/Cohort 1) that enrolled 259 patients with locally advanced or metastatic gastric or GEJ adenocarcinoma. Among the 55% (n = 143) of patients whose tumors expressed PD‐L1 based on a combined positive score ≥1 and either were microsatellite stable or had undetermined microsatellite instability or mismatch repair status, the confirmed ORR as determined by blinded independent central review was 13.3% (95% CI, 8.2–20.0); 1.4% had complete responses. Response durations ranged from 2.8+ to 19.4+ months; 11 patients (58%) had response durations of 6 months or longer, and 5 patients (26%) had response durations of 12 months or longer. The most common (≥20%) adverse reactions of pembrolizumab observed in KEYNOTE‐059/Cohort 1 were fatigue, decreased appetite, nausea, and constipation. The most frequent (≥2%) serious adverse drug reactions were pleural effusion, pneumonia, dyspnea, pulmonary embolism, and pneumonitis. Pembrolizumab was approved concurrently with the PD‐L1 immunohistochemistry 22C3 pharmDx test (Dako, Agilent, Santa Clara, CA) for selection of patients with gastric cancer for treatment with pembrolizumab based on PD‐L1 tumor expression.
Implications for Practice.
This report presents key information on the basis for Food and Drug Administration approval of pembrolizumab for the treatment of patients with locally advanced or metastatic gastric or GEJ adenocarcinoma whose tumors express PD‐L1. The report discusses the basis for limiting the indication to patients with PD‐L1‐expressing tumors and the basis for recommending that PD‐L1 status be assessed using a fresh tumor specimen if PD‐L1 expression is not detected in an archival gastric or GEJ cancer specimen.
摘要
2017 年 9 月 22 日,美国食品和药品管理局 (FDA) 对用于治疗患有复发性局部晚期或转移性胃或胃食管连接部 (GEJ) 腺癌且在两次或多次系统治疗(包括含有氟尿嘧啶和含铂化疗以及在适当情况下的 HER2/neu 靶向治疗)之时或之后出现疾病进展以及其肿瘤由经 FDA 批准的试验确定为程序性死亡配体 1 (PD‐L1) 表达的患者的派姆单抗(Keytruda,默克公司,新泽西州,怀特豪斯站)授予快速批准。此项批准以多中心、开放标签、多队列试验(KEYNOTE‐059/队列 1)中持久的总缓解率 (ORR) 实证为依据,该试验招募了 259 名局部晚期或转移性胃或 GEJ 腺瘤患者。在 55% (n =143) 的肿瘤表达 PD‐L1 以综合阳性评分 ≥1 为依据以及肿瘤处于微卫星稳定状态或者未经确定的微卫星不稳定状态或错配修复状态的患者中,经盲性独立中央评审确定的确认 ORR 为 13.3%(95% CI,8.2–20.0);1.4% 的患者出现完全缓解。缓解持续时间的范围介于 2.8+ 个月至 19.4+ 个月之间;11 名患者 (58%) 的缓解持续时间为 6 个月或以上,5 名患者 (26%) 的缓解持续时间为 12 个月或以上。在 KEYNOTE‐059/队列 1 中观察到派姆单抗的最常见 (≥20%) 的不良反应为疲劳、食欲下降、恶心以及便秘。最常见 (≥2%) 的重度药物不良反应为胸腔积液、肺炎、呼吸困难、肺栓塞以及肺炎。派姆单抗已被批准与用于经选胃癌患者的 PD‐L1 免疫组织化学 22C3 pharmDx 试验(Dako,安捷伦,加利福尼亚州,圣克拉拉)一同用于实施基于 PD‐L1 肿瘤表达的派姆单抗治疗。
对临床实践的提示
本报告介绍了美国食品和药品管理局批准将派姆单抗用于治疗局部晚期或转移性胃或 GEJ 腺癌(肿瘤表达 PD‐L1)患者所依据的重要信息。本报告讨论了将适应症限制于 PD‐L1 表达肿瘤患者的依据以及建议在存档的胃或 GEJ 癌样本中未检测到 PD‐L1 表达的情况下使用新鲜的肿瘤样本评估 PD‐L1 状态的依据。
Introduction
Although the incidence of gastric cancer has been declining over the past two decades, it is the fifth most common malignancy in the world after cancers of the lung, breast, colon/rectum, and prostate [1], [2]. Gastric cancer occurs more commonly in men than in women, and incidence varies across geographic regions; approximately half of all cases occur in East Asia (predominantly in China), where the highest estimated mortality rates are observed (24 per 100,000 in men and 9.8 per 100,000 in women in East Asia, compared with 2.8 in men and 1.5 in women in North America). In the U.S., an estimated 28,000 patients were diagnosed in 2017, and approximately 10,960 patients were expected to die from the disease [3]. When diagnosed in the localized stages, gastric cancer is curable, with an approximate 5‐year survival rate of 70% [4]. Most patients in the U.S. present with symptomatic, incurable disease; in this setting, prognosis is poor, with a 5‐year survival rate of less than 10% [5].
Platinum‐ and fluoropyrimidine‐based combination chemotherapy regimens are used worldwide as the initial treatment of unresectable or metastatic gastric and gastroesophageal junction (GEJ) adenocarcinoma. Reported median survival with these regimens ranges from 8 to 10 months [6], [7], [8], [9], [10], [11]. Platinum‐fluoropyrimidine doublet regimens are preferred in the U.S., because the toxicity rate generally is lower than that observed with three‐drug regimens, which are generally reserved for medically fit patients [12]. Docetaxel in combination with cisplatin and fluorouracil is approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with advanced gastric and GEJ adenocarcinoma who have not received prior chemotherapy for advanced disease. In addition, trastuzumab in combination with cisplatin and capecitabine or 5‐fluorouracil is approved for the treatment of patients with HER2‐overexpressing metastatic gastric or GEJ adenocarcinoma in the first‐line setting.
After failure of first‐line therapy, options for treatment include ramucirumab, which is FDA‐approved as a single agent or in combination with paclitaxel. FDA approval of ramucirumab was based on improved survival in two randomized trials. In the first trial, patients who received ramucirumab monotherapy experienced improved survival (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.60–0.998; p = .047) compared with patients who received placebo, corresponding to an increase in median progression‐free survival from 3.8 to 5.2 months [13]. In the second trial, patients who received ramucirumab with paclitaxel experienced superior survival (HR, 0.81; 95% CI, 0.68–0.96; p = .017) compared with patients who received paclitaxel alone, corresponding to an increase in median overall survival [OS] from 7.4 to 9.6 months, as well as a significant improvement in overall response rate (ORR; 28% vs. 16%; p < .001). The median duration of response was 4.4 months in the ramucirumab plus paclitaxel arm and to 2.9 months in the placebo plus paclitaxel arm [14]. Although not FDA‐approved, single‐agent irinotecan and docetaxel are additional treatment options, based upon the results of the results of three randomized trials, which demonstrated improvement in OS in patients who received these agents compared with patients who received best supportive care [15], [16], [17]. In these trials, the median OS ranged from 4 to 5 months.
On May 23, 2017, the FDA granted accelerated approval for pembrolizumab for the treatment of adult and pediatric patients who have either unresectable or metastatic, microsatellite instability‐high (MSI‐H) or mismatch repair deficient (dMMR) solid tumors that have progressed after prior treatment and who have no satisfactory alternative treatment options, or who have metastatic, MSI‐H or dMMR colorectal cancer that has progressed after treatment with a fluoropyrimidine, oxaliplatin, and irinotecan [18]. The approval was based upon pooled data from single‐arm studies in which the observed ORR was 33% (95% CI, 23.7–44.1). The majority (78%) of responding patients had response durations of 6 months or longer.
Pembrolizumab (Keytruda, Merck & Co., Inc., Whitehouse Station, NJ) is a humanized monoclonal IgG4‐κ isotype antibody that binds to PD‐1, blocking its interaction with programmed death‐ligands 1 (PD‐L1) and 2 and releasing PD‐1 pathway‐mediated inhibition of antitumor immune response. The upregulation of PD‐1 ligands occurs in various tumor types, and signaling through this pathway can contribute to inhibition of active T‐cell immune surveillance of tumors.
Trial Design
KEYNOTE‐059 is an ongoing, open‐label, multicenter, and multicohort trial entitled “Phase II Clinical Trial of Pembrolizumab as Monotherapy and in Combination with Cisplatin+5‐Fluorouracil in Subjects with Recurrent or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma” (NCT02335411). KEYNOTE‐059 was designed to evaluate the tolerability, safety, and antitumor activity of pembrolizumab. Patients were assigned in a nonrandom fashion to one of three cohorts to evaluate pembrolizumab as a single agent (Cohorts 1 and 3) or in combination with chemotherapy (cisplatin in combination with fluorouracil; Cohort 2). Data provided to support this application were limited to Cohort 1. Key eligibility criteria for Cohort 1 included the presence of histologically or cytologically confirmed recurrent or metastatic gastric or GEJ adenocarcinoma tumors considered incurable by local therapies, evidence of disease progression on at least two prior chemotherapy regimens, presence of measurable disease based on RECIST version 1.1 as determined by blinded independent central review, and Eastern Cooperative Oncology Group performance score 0 to 1 and submission of tumor tissue exploratory testing for PD‐L1 expression. Patients with active autoimmune disease, a medical condition that required immunosuppression, or evidence of interstitial lung disease were ineligible.
The protocol enrolled patients without regard to tumor PD‐L1 status; however, analysis of PD‐L1 testing was performed in all patients. PD‐L1 status was determined using an investigational device exemption‐approved immunohistochemistry (IHC) assay. In this assay, PD‐L1 expression was measured on formalin‐fixed paraffin‐embedded tumor samples. PD‐L1 protein expression level was determined using the Combined Positive Score (CPS), defined as ratio of PD‐L1 membrane‐stained cells at any intensity (tumor cells, macrophages, lymphocytes) in the tumor microenvironment to the total tumor cells present, multiplied by 100. In KEYNOTE‐059, a tumor sample was characterized as PD‐L1 positive if the CPS score was greater than 1. Microsatellite instability expression was retrospectively tested in patients who had matching tissue and blood samples available.
Patients received pembrolizumab 200 mg administered as a 30‐minute intravenous infusion on Day 1 of every 3‐week cycle for up to 2 years until disease progression, unacceptable toxicity, withdrawal of consent, or investigator discretion. Patients with progressive disease (PD) in noncritical anatomic sites and who did not require urgent intervention were permitted to continue study treatment if they did not exhibit decline in clinical signs or symptoms. Such patients were reimaged 4–6 weeks after initial progression and were permitted to remain on therapy and resume regularly scheduled imaging if the follow‐up scan did not confirm PD.
In Cohort 1, the co‐primary efficacy endpoints were ORR per RECIST 1.1, as assessed by central radiologic review, in all patients and in patients with PD‐L1‐positive tumors using descriptive statistics. Cohort 1 was designed to demonstrate that among patients who received pembrolizumab, the lower bound of the 95% confidence interval for ORR would be 10% or higher. The protocol's analysis plan proposed a sample size of 180 patients in Cohort 1 comprising 90 patients with PD‐L1‐positive and 90 patients with PD‐L1‐negative tumors. The protocol also included a plan to conduct a futility interim analysis when the study had enrolled approximately 40 patients who were evaluable for response, with an estimated 25 patients with PD‐L1‐negative tumors. During the interim analysis for futility, enrollment of patients with PD‐L1‐negative tumors was suspended while enrollment of patients with PD‐L1‐positive tumors continued. Enrollment of patients with PD‐L1‐negative tumors resumed when the futility criterion was not met. Based on the interim futility analysis results, the protocol was amended to increase the sample size for Cohort 1 to 210 patients so as to have at least 120 patients with PD‐L1‐positive tumors enrolled. The statistical hypothesis test for the primary endpoint was removed from protocol; in the absence of formal hypothesis testing, no multiplicity adjustment was applied. All patients who received at least one dose of pembrolizumab were included in the efficacy and safety analysis populations.
Results
A total of 259 patients with gastric or GEJ adenocarcinoma enrolled in KEYNOTE‐059/Cohort 1. The baseline and disease characteristics for the 259 patients are provided in Table 1. The most common sites (>5%) for enrollment outside the U.S. were Japan (13%), France (5%), Israel (5%), and Australia (5%). MSI expression was retrospectively tested in 67% of the study population (n = 174); in this subgroup, 4% (n = 7) had MSI‐H and 96% (n = 167) had microsatellite stable (MSS) gastric cancers.
Table 1. Demographic and baseline characteristics.

Abbreviations: ECOG, Eastern Cooperative Oncology Group; MSI, microsatellite instability; MSS, microsatellite stable.
Efficacy
The median follow‐up for the study population was 5.8 months (range, 0.5–21.6). The efficacy results for the overall population and in exploratory subgroups based on PD‐L1 and MSI status are provided in Table 2 and Table 3. A description of the specimen characteristics is shown in Table 4.
Table 2. Overall response rate and response duration in the overall population and in subgroups based on PD‐L1 tumor status.
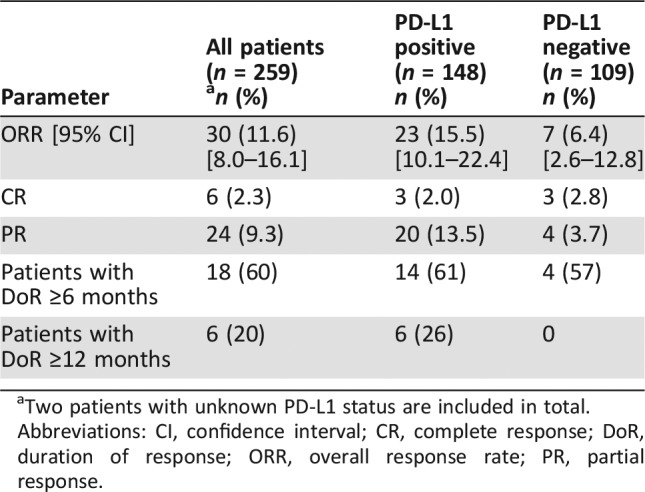
Two patients with unknown PD‐L1 status are included in total.
Abbreviations: CI, confidence interval; CR, complete response; DoR, duration of response; ORR, overall response rate; PR, partial response.
Table 3. Overall response rate in exploratory subgroups based on retrospective assessment of microsatellite tumor status.
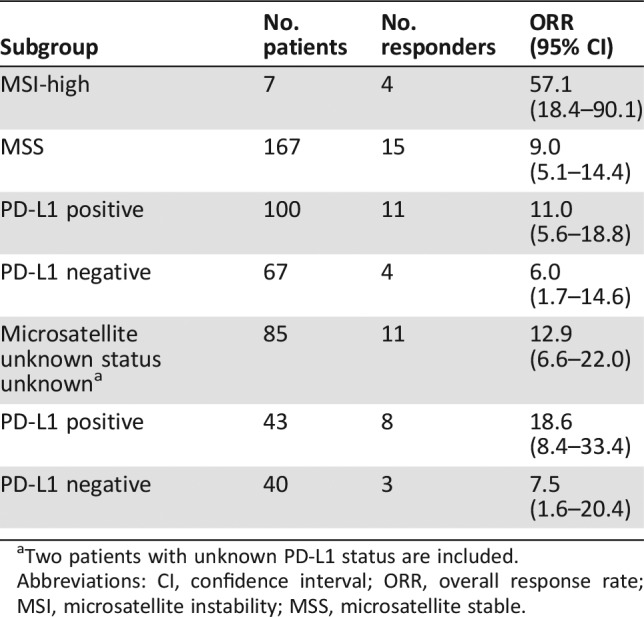
Two patients with unknown PD‐L1 status are included.
Abbreviations: CI, confidence interval; ORR, overall response rate; MSI, microsatellite instability; MSS, microsatellite stable.
Table 4. Tumor specimen characteristics.
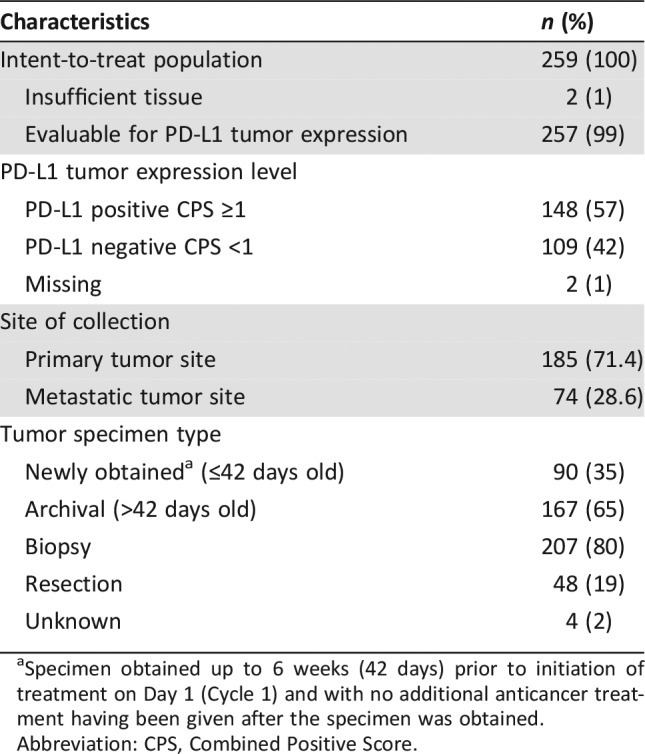
Specimen obtained up to 6 weeks (42 days) prior to initiation of treatment on Day 1 (Cycle 1) and with no additional anticancer treatment having been given after the specimen was obtained.
Abbreviation: CPS, Combined Positive Score.
Among the 143 patients whose tumors were PD‐L1 positive and MSS or had undetermined MSI or MMR status, the ORR was 13.3% (95% CI, 8.2–20.0); 1.4% had a complete response, and 11.9% had a partial response. Among the 19 responding patients, the duration of response ranged from 2.8+ to 19.4+ months, with 11 patients (58%) having responses of 6 months or longer and 5 patients (26%) having responses of 12 months or longer.
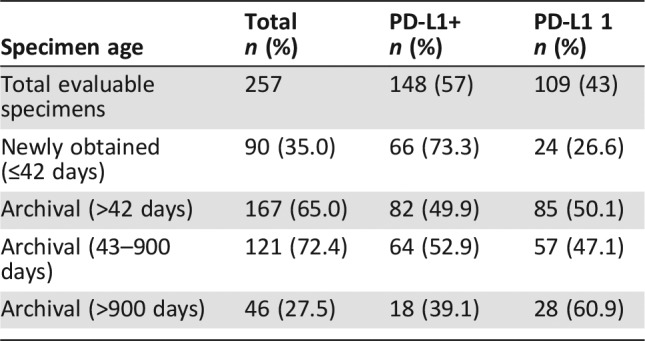
An analysis of tumor PD‐L1 expression status by age of tumor specimen revealed that the number of PD‐L1‐positive specimens was higher (73.3%) in patients who had more recently obtained (≤42 days) tissues tested with the PD‐L1 IHC 22C3 pharmDx (Dako, Agilent, Santa Clara, CA) compared with patients who had archival (≥42 days) tissue tested (49.9%). The distribution of PD‐L1 status based on age of specimen is shown in Table 5.
Table 5. Tumor specimen age by PD‐L1 expression status.
Safety
The assessment of safety for this application was based on data from the “as‐treated” population (n = 259) enrolled in KEYNOTE‐059/Cohort 1, defined as all patients receiving at least one dose of pembrolizumab. The median duration of pembrolizumab exposure was 2.1 months (range, 1 day to 21.4 months).
Serious adverse events (i.e., adverse events resulting in death or the need for hospitalization, prolonged hospitalization, or medical intervention to avoid hospitalization) occurred in 45% of patients. The most frequent serious adverse events were pleural effusion (3.5%), anemia (2.7%), intestinal obstruction, pulmonary embolism (2.3% each), nausea, vomiting, sepsis, dehydration, back pain, and acute kidney injury (1.9% each). Pembrolizumab was discontinued because of adverse events in 2.7% of patients. The most common adverse events (reported in ≥20% of patients) were fatigue, decreased appetite, nausea, and constipation.
The risks of pembrolizumab are well characterized from the results of randomized clinical trials enrolling patients with metastatic melanoma, non‐small cell lung cancer (NSCLC), and urothelial carcinoma. Adverse reactions, including immune‐mediated adverse events, occurring in patients with gastric/GEJ adenocarcinoma were generally similar to those observed in patients with melanoma or NSCLC treated with pembrolizumab (n = 2,799). Immune‐mediated adverse events were reported in 17.8% of patients. The most common immune‐mediated adverse events (reported in ≥1% of patients) were hypothyroidism (8.9%), hyperthyroidism (3.5%), colitis (2.3%), pneumonitis (1.9%), infusion reactions, severe skin reactions, and thyroiditis (1.5% each). The majority (73.9%) were grade 1 or grade 2 in severity.
Discussion
Locally advanced or metastatic gastric/GEJ adenocarcinoma is a serious and life‐threatening disease with an estimated 5‐year survival rate of less than 10%. Patients with metastatic gastric cancer who have progressed after two or more prior chemotherapy regimens (including a HER‐2 inhibitor, if indicated) have an unmet medical need, as there are no FDA‐approved therapies. Agents evaluated for the treatment of second‐ or third‐line gastric/GEJ adenocarcinoma are summarized in supplemental online Appendix 1.
The major issues addressed by the FDA review team during evaluation of this supplemental application included (a) whether data from Cohort 1 of the KEYNOTE‐059 trial provided substantial evidence of the treatment effects of pembrolizumab in patients with PD‐L1‐positive and those with PD‐L1‐negative gastric/GEJ cancer, (b) to what extent the effect observed in the subgroup of patients with PD‐L1‐positive tumors was driven in part by the presence of patients with MSI‐H tumors in either subgroup (because pembrolizumab is already approved for the subgroup of patients with MSI‐H gastric/GEJ cancers), and (c) whether testing for PD‐L1 status reliably identified patients who would benefit from treatment with pembrolizumab, considering the age of the tumor specimen.
Given that pembrolizumab was already approved for the treatment of patients with MSI‐H tumors (including MSI‐high gastric cancer), FDA's assessment of clinical benefit (see Table 6) excluded patients with tumors known to be MSI‐H. Although a substantial minority (33%) of patients in KEYNOTE‐059/Cohort 1 had unknown MSI tumor status, FDA included these patients with MSI‐H unknown tumors in the efficacy population given that only 3 or 4 of these 85 patients’ tumors would be expected to be MSI‐H tumors and unlikely to substantially alter the overall results. However, inclusion of patients with MSI‐H cancers may explain the slightly higher ORR observed in the microsatellite expression‐unknown group compared with those documented to have MSS tumors (12.9% vs. 9.0%).
Table 6. FDA benefit‐risk summary.
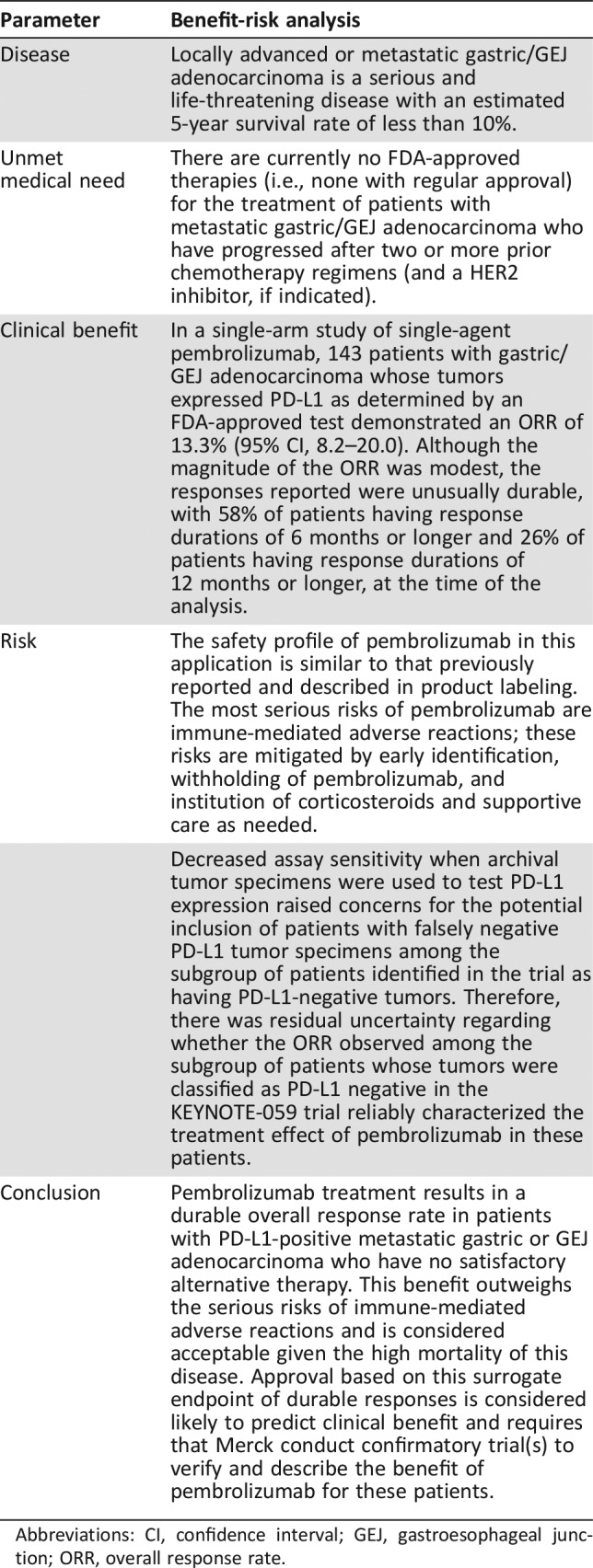
Abbreviations: CI, confidence interval; GEJ, gastroesophageal junction; ORR, overall response rate.
As shown in Table 5, the data obtained in KEYNOTE‐059/Cohort 1 demonstrated decreased assay sensitivity when archival tumor specimens were used to test for PD‐L1 tumor expression. This finding raised concerns for the potential inclusion of patients with false negative PD‐L1 tumor specimens among the subgroup of patients identified in the trial as having PD‐L1‐negative tumors and led to uncertainty regarding whether the ORR observed among the subgroup of patients whose tumors were classified as PD‐L1 negative in the KEYNOTE‐059 trial reliably characterized the treatment effect of pembrolizumab in these patients. On the basis of this uncertainty and the low ORR (6.4%) observed among the subgroup of patients with MSS or unknown microsatellite expression status who were PD‐L1 negative, FDA concluded that the KEYNOTE‐059 trial did not provide substantial evidence of a treatment effect on ORR that was likely to predict clinical benefit; thus, pembrolizumab was not approved for the treatment of patients with PD‐L1‐negative gastric/GEJ cancer.
The ability of the safety database provided in this supplemental application to identify unique adverse drug reactions occurring in patients with gastric cancer was limited by the lack of a control arm and by the relatively small number of patients enrolled in KEYNOTE‐059/Cohort 1 (n = 259), as all adverse drug reactions occurring in at least 1% of patients could not be detected. Nevertheless, the safety profile of pembrolizumab in this application did not appear to differ from the known safety profile of pembrolizumab described in product labeling.
FDA notes that the 4% of patients enrolled in KEYNOTE‐059/Cohort 1 have an approved treatment option based on the approval for pembrolizumab for treatment of patients with unresectable or metastatic, MSI‐H or dMMR solid tumors that have progressed after prior treatment and who have no satisfactory alternative treatment options. FDA did not find the ORR of 9% (95% CI, 5.1–14.4) in the remaining 96% of patients with MSS gastric/GEJ cancers to be clinically meaningful. However, FDA considered the ORR of 13.3% (95% CI, 8.2–20.0) in the 143 patients with gastric/GEJ adenocarcinoma whose tumors express PD‐L1 as determined by an FDA‐approved test but without evidence of MSI‐H or dMMR to be a clinically meaningful magnitude of response considering the durability of such responses. Specifically, the the time of the data cutoff date, 58% of such patients had response durations of 6 months or longer and 26% of patients had response durations of 12 months or longer.
Conclusion
The accelerated approval of pembrolizumab for the treatment of patients with recurrent locally advanced or metastatic, gastric or GEJ adenocarcinoma who have had disease progression on or after two or more prior systemic therapies, including fluoropyrimidine‐ and platinum‐containing chemotherapy and, if appropriate, HER2/neu‐targeted therapy, and whose tumors express PD‐L1, is concurrent with the approval of the Premarket Approval Application supplement for the Dako PD‐L1 IHC‐22C3 pharmDx assay, for the identification of patients with gastric cancer that expresses PD‐L1. The accelerated approval regulations describe approval of drugs and biologic products for serious and life‐threatening illnesses based on a surrogate endpoint likely to predict clinical benefit. Under these regulations, one or more confirmatory trials are required to verify and describe the benefit of pembrolizumab for patients with metastatic gastric or GEJ adenocarcinoma.
See http://www.TheOncologist.com for supplemental material available online.
Footnotes
For Further Reading: Sakti Chakrabarti, Haidong Dong, Harshita R. Paripati et al. First Report of Dramatic Tumor Responses with Ramucirumab and Paclitaxel After Progression on Pembrolizumab in Two Cases of Metastatic Gastroesophageal Adenocarcinoma. The Oncologist 2018;23:840–843.
Abstract: Checkpoint inhibitors targeted at programmed cell death‐1 receptor (PD‐1) and its ligand (PD‐L1) can result in significant benefit to a small proportion of patients with cancer, including those with tumors of the stomach and gastroesophageal junction. These drugs are now approved for several solid tumors, including the recent accelerated approval of pembrolizumab for gastroesophageal adenocarcinomas in the third‐line setting and beyond based on the KEYNOTE‐059 phase II trial. Data are lacking on the efficacy of chemotherapy after progression on PD‐1 blockade in metastatic gastroesophageal adenocarcinoma. This report describes the exceptional response of two patients who received ramucirumab plus paclitaxel after progressive disease on pembrolizumab. This early clinical observation suggests that the sequence of administration of PD‐1 blockade and chemotherapy may be important in this disease.
Author Contributions
Conception/design: Lola Fashoyin‐Aje, Martha Donoghue, Huanyu Chen, Kun He, Janaki Veeraraghavan, Kirsten B. Goldberg, Patricia Keegan, Amy E. McKee, Richard Pazdur
Provision of study material or patients: Lola Fashoyin‐Aje, Martha Donoghue, Huanyu Chen, Kun He, Janaki Veeraraghavan, Kirsten B. Goldberg, Patricia Keegan, Amy E. McKee, Richard Pazdur
Collection and/or assembly of data: Lola Fashoyin‐Aje, Martha Donoghue, Huanyu Chen, Kun He, Janaki Veeraraghavan, Kirsten B. Goldberg, Patricia Keegan, Amy E. McKee, Richard Pazdur
Data analysis and interpretation: Lola Fashoyin‐Aje, Martha Donoghue, Huanyu Chen, Kun He, Janaki Veeraraghavan, Kirsten B. Goldberg, Patricia Keegan, Amy E. McKee, Richard Pazdur
Manuscript writing: Lola Fashoyin‐Aje, Martha Donoghue, Huanyu Chen, Kun He, Janaki Veeraraghavan, Kirsten B. Goldberg, Patricia Keegan, Amy E. McKee, Richard Pazdur
Final approval of manuscript: Lola Fashoyin‐Aje, Martha Donoghue, Huanyu Chen, Kun He, Janaki Veeraraghavan, Kirsten B. Goldberg, Patricia Keegan, Amy E. McKee, Richard Pazdur
Disclosures
The authors indicated no financial relationships.
References
- 1.International Agency for Research on Cancer ; World Health Organization. Stomach cancer: Estimated incidence, mortality and prevalence worldwide in 2012. GLOBOCAN 2012 website. Available at http://globocan.iarc.fr/old/FactSheets/cancers/stomach‐new.asp. Accessed June 22, 2017.
- 2.Terry MB, Gaudet MM, Gammon MD. The epidemiology of gastric cancer. Semin Radiat Oncol 2002;12:111–127. [DOI] [PubMed] [Google Scholar]
- 3.Siegel, RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts and Figures: 2017 . Atlanta, GA: American Cancer Society; 2017. Available at https://www.cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐statistics/annual‐cancer‐facts‐and‐figures/2017/cancer‐facts‐and‐figures‐2017.pdf. Accessed June 22, 2017. [Google Scholar]
- 5.Power DG, Kelsen DP, Shah MA. Advanced gastric cancer–slow but steady progress. Cancer Treat Rev 2010;36:384–392. [DOI] [PubMed] [Google Scholar]
- 6.Al‐Batran SE, Hartmann JT, Probst S et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 2008;26:1435–1442. [DOI] [PubMed] [Google Scholar]
- 7.Bouché O, Raoul JL, Bonnetain F et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: A Federation Francophone de Cancerologie Digestive Group Study–FFCD 9803. J Clin Oncol 2004;22:4319–4328. [DOI] [PubMed] [Google Scholar]
- 8.Enzinger PC, Burtness BA, Niedzwiecki D et al. CALGB 80403 (Alliance)/E1206: A randomized phase II study of three chemotherapy regimens plus cetuximab in metastatic esophageal and gastroesophageal junction cancers. J Clin Oncol 2016;34:2736–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang YK, Kang WK, Shin DB et al. Capecitabine/cisplatin versus 5‐fluorouracil/cisplatin as first‐line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann Oncol 2009;20:666–673. [DOI] [PubMed] [Google Scholar]
- 10.Kim GM, Jeung HC, Rha SY et al. A randomized phase II trial of S‐1‐oxaliplatin versus capecitabine‐oxaliplatin in advanced gastric cancer. Eur J Cancer 2012;48:518–526. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzen S, Schuster T, Porschen R et al. Cetuximab plus cisplatin‐5‐fluorouracil versus cisplatin‐5‐fluorouracil alone in first‐line metastatic squamous cell carcinoma of the esophagus: A randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2009;20:1667–1673. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer. Version 2.2017 . Fort Washington, PA: National Comprehensive Cancer Institute; 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed March 6, 2018. [Google Scholar]
- 13.Cyramza (ramucirumab) injection [package insert]. Indianapolis, IN: Eli Lilly and Co.; 2015. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125477s011lbl.pdf. Accessed March 6, 2018.
- 14.Wilke H, Muro K, Van Cutsem E et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (RAINBOW): A double‐blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–1235. [DOI] [PubMed] [Google Scholar]
- 15.Ford HER, Marshall A, Bridgewater JA et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR‐02): An open‐label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78–86. [DOI] [PubMed] [Google Scholar]
- 16.Kang JH, Lee SI, Lim DH et al. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513–1518. [DOI] [PubMed] [Google Scholar]
- 17.Thuss‐Patience PC, Kretzschmar A, Bichev D et al. Survival advantage for irinotecan versus best supportive care as second‐line chemotherapy in gastric cancer–a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306–2314. [DOI] [PubMed] [Google Scholar]
- 18.Keytruda (pembrolizumab) [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2017. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s014lbl.pdf. Accessed November 1, 2017.


