Abstract
Lessons Learned.
Itacitinib in combination with nab‐paclitaxel plus gemcitabine demonstrated an acceptable safety profile with clinical activity in patients with advanced solid tumors including pancreatic cancer.
The results support future studies of itacitinib as a component of combination regimens with other immunologic and targeted small molecule anticancer agents.
Background.
Cytokine‐mediated signaling via JAK/STAT is central to tumor growth, survival, and systemic inflammation, which is associated with cancer cachexia, particularly in pancreatic cancer. Because of their centrality in the pathogenesis of cancer cachexia and progression, JAK isozymes have emerged as promising therapeutic targets. Preclinical studies have demonstrated antiproliferative effects of JAK/STAT pathway inhibition in both in vitro and in vivo models of cancer, including pancreatic cancer.
Methods.
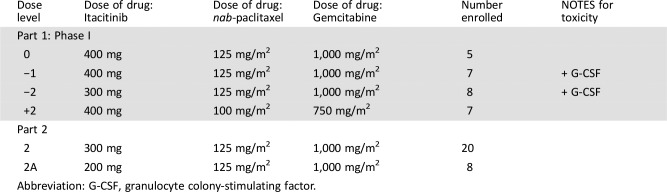
This phase Ib/II dose‐optimization study assessed itacitinib, a selective JAK1 inhibitor, combined with nab‐paclitaxel plus gemcitabine in adults with treatment‐naïve advanced/metastatic disease (Part 1) or pancreatic adenocarcinoma (Parts 2/2A; NCT01858883). Starting doses (Part 1) were itacitinib 400 mg, nab‐paclitaxel 125 mg/m2, and gemcitabine 1,000 mg/m2. Additional dose levels incorporated were granulocyte colony‐stimulating factor, de‐escalations of itacitinib to 300 mg once daily (QD), nab‐paclitaxel to 100 mg/m2, and gemcitabine to 750 mg/m2.
Results.
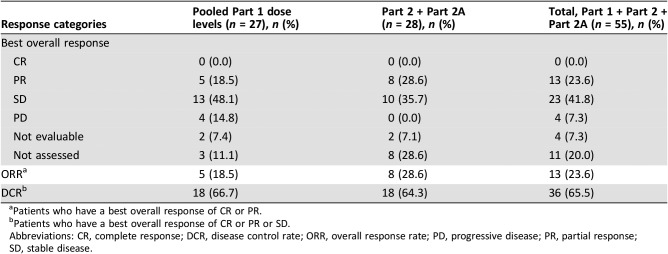
Among 55 patients in Part 1, 6 developed seven hematologic dose‐limiting toxicities (Cycle 1). Itacitinib 300 mg plus nab‐paclitaxel 125 mg/m2 and gemcitabine 1,000 mg/m2 was tolerated and expanded in Part 2. Treatment discontinuation and grade 3/4 neutropenia rates prompted itacitinib de‐escalation to 200 mg QD in Part 2A. The most common grade 3/4 toxicities were fatigue and neutropenia. Partial responses occurred across all itacitinib doses and several tumor types (overall response rate, 24%).
Conclusion.
Itacitinib plus chemotherapy demonstrated acceptable safety and clinical activity in patients with advanced solid tumors including pancreatic cancers. This study was terminated early (sponsor's decision) based on negative phase III results for a JAK1/2 inhibitor in previously treated advanced pancreatic cancer.
Abstract
经验获取
• Itacitinib 和白蛋白结合型紫杉醇及吉西他滨联合治疗在晚期实体肿瘤(包括胰腺癌)患者中显示出可接受的安全特性与临床活性。
• 研究结果支持将 Itacitinib 作为含有其他免疫和靶向小分子抗癌剂的联合治疗的组成部分开展未来研究。
摘要
背景。通过 JAK/STAT 的细胞因子介导信号传导对于肿瘤生长、生存以及与癌症恶病质(特别是胰腺癌)相关的系统性炎症而言,至关重要。由于 JAK 同工酶在癌症恶病质和癌症进展的致病机制中具有重要地位,它们现已成为有希望的治疗靶点。临床前研究已经证明 JAK/STAT 通路抑制在癌症(包括胰腺癌)的体外和体内模型中具有抗增殖作用。
方法。本次 Ib/II 期剂量优化研究对既往未接受治疗的晚期/转移性癌(第 1 部分)或胰腺癌(第 2/2A 部分;NCT01858883)成年患者的 Itacitinib(一种选择性的 JAK1 抑制剂)和白蛋白结合型紫杉醇及吉西他滨联合治疗进行了评估。初始剂量(第 1 部分)为 Itacitinib 400 mg,白蛋白结合型紫杉醇 125 mg/m2,吉西他滨 1 000 mg/m2。加入的额外剂量是粒细胞集落刺激因子,Itacitinib 降至 300 mg,每天给药一次 (QD),白蛋白结合型紫杉醇降至 100 mg/m2,吉西他滨降至 750 mg/m2。
结果。在第 1 部分中的 55 名患者中,有 6 名患者出现七次血液学剂量限制性毒性(第 1 周期)。在第 2 部分中,可以耐受和扩展 Itacitinib 300 mg 和白蛋白结合型紫杉醇 125 mg/m2 及吉西他滨 1 000 mg/m2 联合治疗。在第 2A 部分中,治疗终止以及 3/4 级嗜中性粒细胞减少症比率导致 Itacitinib 降至 200 mg QD。最常见的 3/4 级毒性为疲劳和嗜中性粒细胞减少症。部分缓解在所有 Itacitinib 剂量和几种肿瘤类型中均有出现(总缓解率为 24%)。
结论。Itacitinib 联合化疗在晚期实体肿瘤(包括胰腺癌)患者中显示出可接受的安全性与临床活性。基于 JAK1/2 抑制剂在经治的晚期胰腺癌中的阴性 III 期结果,本研究提前终止(由申办方决定)。
Discussion
This two‐part, phase Ib/II dose‐finding and dose‐expansion study assessed itacitinib (INCB039110), a novel, potent, and selective inhibitor of JAK1 [1], [2], combined with nab‐paclitaxel plus gemcitabine in patients with advanced solid tumors, including locally advanced/metastatic pancreatic cancer. For dose optimization (Part 1), the primary objectives were safety and tolerability, and defining the maximum tolerated dose (MTD) or pharmacologically active dose (PAD) of itacitinib in combination with nab‐paclitaxel and gemcitabine in patients with advanced solid tumors. For dose expansion (Parts 2 and 2A), the primary objective was further characterization of the safety and tolerability of the MTD or PAD of the itacitinib combination in patients with locally advanced or metastatic pancreatic adenocarcinoma.
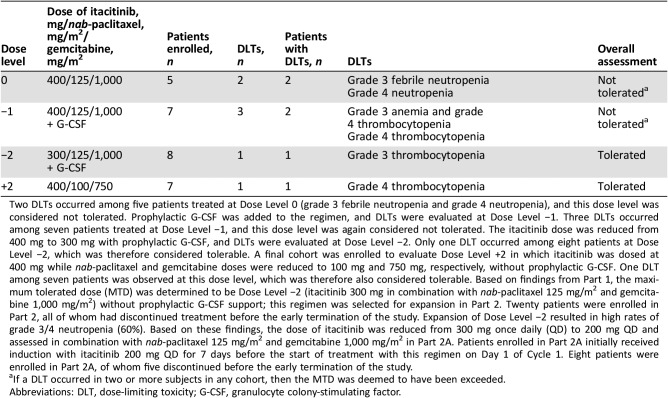
Table 1. Dose‐limiting toxicities.
Two DLTs occurred among five patients treated at Dose Level 0 (grade 3 febrile neutropenia and grade 4 neutropenia), and this dose level was considered not tolerated. Prophylactic G‐CSF was added to the regimen, and DLTs were evaluated at Dose Level −1. Three DLTs occurred among seven patients treated at Dose Level −1, and this dose level was again considered not tolerated. The itacitinib dose was reduced from 400 mg to 300 mg with prophylactic G‐CSF, and DLTs were evaluated at Dose Level −2. Only one DLT occurred among eight patients at Dose Level −2, which was therefore considered tolerable. A final cohort was enrolled to evaluate Dose Level +2 in which itacitinib was dosed at 400 mg while nab‐paclitaxel and gemcitabine doses were reduced to 100 mg and 750 mg, respectively, without prophylactic G‐CSF. One DLT among seven patients was observed at this dose level, which was therefore also considered tolerable. Based on findings from Part 1, the maximum tolerated dose (MTD) was determined to be Dose Level −2 (itacitinib 300 mg in combination with nab‐paclitaxel 125 mg/m2 and gemcitabine 1,000 mg/m2) without prophylactic G‐CSF support; this regimen was selected for expansion in Part 2. Twenty patients were enrolled in Part 2, all of whom had discontinued treatment before the early termination of the study. Expansion of Dose Level −2 resulted in high rates of grade 3/4 neutropenia (60%). Based on these findings, the dose of itacitinib was reduced from 300 mg once daily (QD) to 200 mg QD and assessed in combination with nab‐paclitaxel 125 mg/m2 and gemcitabine 1,000 mg/m2 in Part 2A. Patients enrolled in Part 2A initially received induction with itacitinib 200 mg QD for 7 days before the start of treatment with this regimen on Day 1 of Cycle 1. Eight patients were enrolled in Part 2A, of whom five discontinued before the early termination of the study.
If a DLT occurred in two or more subjects in any cohort, then the MTD was deemed to have been exceeded.
Abbreviations: DLT, dose‐limiting toxicity; G‐CSF, granulocyte colony‐stimulating factor.
The study was terminated early by the sponsor on February 11, 2016, after an interim analysis from the phase III JANUS 1 and 2 trials of ruxolitinib (JAK1/2 inhibitor) plus capecitabine showed no additional benefit over capecitabine in patients with advanced pancreatic cancer with high systemic inflammation [3].
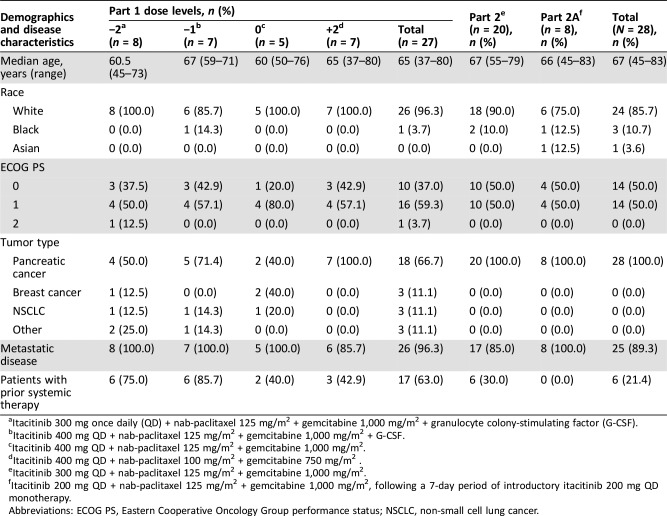
Here, data are reported from July 11, 2013 (first patient consented) to March 21, 2016 (last patient completed treatment). Among the 55 patients enrolled (Part 1, n = 27; Part 2, n = 20; Part 2A, n = 8), most (84%) had advanced pancreatic cancer. The median age was 67 years; prior systemic therapy had been administered to 63% of patients in Part 1, 30% in Part 2, and none in Part 2A.
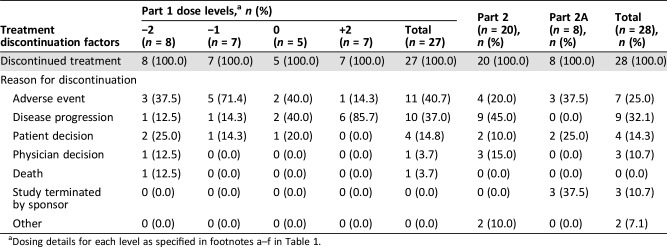
All patients had discontinued treatment before the sponsor's decision to terminate the study. The most common reasons for treatment discontinuation were adverse events (n = 18; 32.7%) and disease progression (n = 19; 34.5%).
In Part 1, six patients developed seven hematologic dose‐limiting toxicities (DLTs) in Cycle 1; itacitinib 300 mg with nab‐paclitaxel 125 mg/m2 and gemcitabine 1,000 mg/m2 was tolerated and expanded in Part 2. Although patients with chemotherapy‐naïve pancreatic cancer appeared to show better tolerability of the combination treatment regimen, in Part 2, a higher discontinuation rate within the first two cycles for reasons other than progressive disease was observed. In addition, high rates of grade 3/4 neutropenia were observed. Consequently, in Part 2A, a reduced dose of itacitinib (200 mg once daily [QD]) was administered in combination with standard nab‐paclitaxel and gemcitabine therapy in the same untreated patient population. This therapeutic combination was better tolerated.
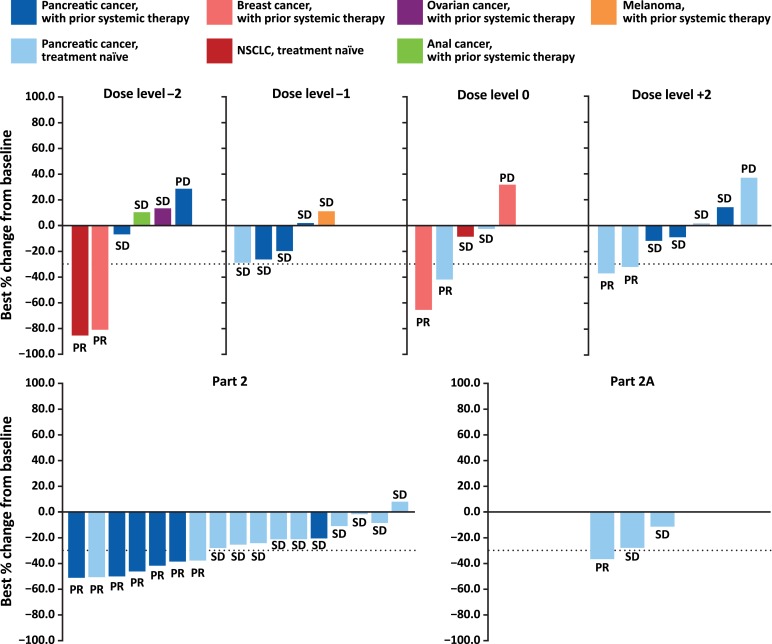
Overall response rate was 24% (13/55) with all responses occurring as partial responses (PRs). PRs were seen across all doses of itacitinib (200, 300, and 400 mg) and in patients with pancreatic cancer, breast cancer, and non‐small cell lung cancer.
Itacitinib in combination with nab‐paclitaxel plus gemcitabine demonstrated an acceptable safety profile with clinical activity seen in patients with advanced solid tumors including pancreatic cancers.
Trial Information
- Disease
Advanced cancer/solid tumor only
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
One prior regimen
- Type of Study – 1
Phase I/II
- Type of Study – 2
Dose optimization and expansion
- Primary Endpoint
Safety
- Primary Endpoint
Tolerability
- Primary Endpoint
Maximum tolerated dose
- Primary Endpoint
Pharmacologically active dose
- Secondary Endpoint
Pharmacokinetics
- Secondary Endpoint
Biomarkers of clinical activity
- Additional Details of Endpoints or Study Design
- Study Design and Objectives
- This two‐part, phase Ib/II dose‐finding and dose‐expansion study assessed itacitinib combined with nab‐paclitaxel plus gemcitabine in patients with advanced solid tumors, including locally advanced/metastatic pancreatic cancer. For dose optimization (Part 1), the primary objectives were safety, tolerability, and defining the MTD or PAD of itacitinib in combination with nab‐paclitaxel and gemcitabine in patients with advanced solid tumors. For dose expansion (Parts 2 and 2A), the primary objective was further characterization of the safety and tolerability of the MTD or PAD of the itacitinib combination in patients with locally advanced or metastatic pancreatic adenocarcinoma.
- The study was conducted in accordance with the study protocol, Declaration of Helsinki, Good Clinical Practice (U.S. Code of Federal Regulations Title 21), International Conference on Harmonisation‐Good Clinical Practice consolidated guidelines (E6), and applicable regulatory requirements. All patients provided written, informed consent before study participation. The study protocol, its amendments, and the patient informed consent were approved by local institutional review boards.
- The study was terminated early by the sponsor on February 11, 2016, after an interim analysis from the phase III JANUS 1 and 2 trials of ruxolitinib (JAK1/2 inhibitor) plus capecitabine showed no additional benefit over capecitabine in patients with advanced pancreatic cancer with high systemic inflammation [3].
- Eligibility Criteria
- Eligible patients were 18 years or older, with Eastern Cooperative Oncology Group (ECOG) performance status 0–1. Patients with solid tumors who had received no more than one prior systemic chemotherapy regimen for advanced or metastatic disease were eligible for Part 1. Patients with pancreatic ductal adenocarcinoma were eligible for Part 2 and Part 2A. Initially, patients who had received prior chemotherapy for advanced or metastatic disease were eligible for Part 2 and Part 2A; however, such patients were excluded by a subsequent protocol amendment (September 12, 2014).
- Treatments
- Starting doses (Part 1) were itacitinib 400 mg, nab‐paclitaxel 125 mg/m2, and gemcitabine 1,000 mg/m2. Additional dose levels incorporated were granulocyte colony‐stimulating factor, de‐escalations of itacitinib to 300 mg QD, nab‐paclitaxel to 100 mg/m2, and gemcitabine to 750 mg/m2.
- Dose Optimization and Expansion
- Patients were assigned to consecutive dose cohorts, and each cohort was observed for a minimum of 28 days before the next cohort opened for enrollment. If a DLT was observed in two or more patients in any cohort, the MTD was deemed to be exceeded.
- Itacitinib was self‐administered orally in a fasting state beginning on Day 1 of Cycle 1 and continued QD for 28‐day cycles. nab‐Paclitaxel 125 mg/m2 or 100 mg/m2 was administered intravenously over 30 minutes before infusion of gemcitabine on Days 1, 8, and 15 of each 28‐day cycle. Gemcitabine 1,000 mg/m2 or 750 mg/m2 was administered intravenously over 30 minutes on Days 1, 8, and 15 of each 28‐day cycle. In Part 1, the starting dose level (Dose Level 0) consisted of itacitinib 400 mg, nab‐paclitaxel 125 mg/m2, and gemcitabine 1,000 mg/m2. Additional dose levels that were evaluated incorporated (a) the use of prophylactic granulocyte colony‐stimulating factor (G‐CSF) support with filgrastim (Dose Level −1), (b) a de‐escalation of itacitinib to 300 mg QD with prophylactic G‐CSF (Dose Level −2), and (c) a de‐escalation of nab‐paclitaxel and gemcitabine to 100 mg/m2 and 750 mg/m2, respectively (Dose Level +2). Filgrastim was supplied by the institution's pharmacy and administered according to the package insert and institutional guidelines.
- The MTD was determined and further explored in Part 2 and Part 2A. Patients enrolled in Part 2A initially received induction with itacitinib 200 mg QD for 7 days before the start of treatment with itacitinib plus nab‐paclitaxel and gemcitabine on Day 1 of Cycle 1.
- Assessments
- Safety and tolerability were assessed by treatment‐emergent adverse events, vital signs, physical examinations, electrocardiograms, and laboratory blood tests. Efficacy was assessed by monitoring tumor response rates using RECIST version 1.1 [4]. Venous blood samples were collected to assess the pharmacokinetics (PK) of itacitinib, paclitaxel, and gemcitabine (e.g., area under the concentration‐response curve, maximal concentration) and to determine concentrations of cytokines and other biomarkers. As a measure of JAK1 inhibition, the capacity of interleukin‐6 to induce STAT3 phosphorylation in peripheral blood mononuclear cells collected from patients at baseline and after beginning treatment was assessed. Preliminary PK and pharmacodynamics (PD) data were determined for 14 enrolled patients; the protocol‐specified PK and PD analyses were ultimately not conducted because the study was terminated by the sponsor before they were scheduled to occur.
- All safety and efficacy analyses were performed on patients enrolled in the study who had received at least one dose of study drug. Preliminary PK/PD analyses were performed in patients who received at least one dose of itacitinib and provided at least one blood sample after the dose for PK or biomarker assessment. All statistical analyses were exploratory in nature.
- Investigator's Analysis
- Itacitinib in combination with nab‐paclitaxel and gemcitabine demonstrated an acceptable safety profile with clinical activity in patients with advanced solid tumors including pancreatic cancers.
Drug Information for Phase II Experimental
- Drug 1
- Generic/Working Name
Itacitinib
- Company Name
Incyte Corporation
- Drug Type
Small molecule
- Drug Class
JAK kinase
- Dose
200–300 milligrams (mg) per flat dose
- Route
Oral (po)
- Schedule of Administration
Once daily
- Drug 2
- Generic/Working Name
nab‐paclitaxel
- Trade Name
Abraxane
- Company Name
Celgene
- Drug Type
Drug conjugate
- Drug Class
Tubulin/microtubules targeting agent
- Dose
125 milligrams (mg) per squared meter (m2)
- Route
Intravenous (IV)
- Schedule of Administration
Days 1, 8, and 15 of each 28‐day cycle
- Drug 3
- Generic/Working Name
Gemcitabine
- Drug Type
Small molecule
- Drug Class
Antimetabolite
- Dose
1,000 milligrams (mg) per squared meter (m2)
- Route
Intravenous (IV)
- Schedule of Administration
Days 1, 8, and 15 of each 28‐day cycle
Drug Information for Phase I Experimental
- Drug 1
- Generic/Working Name
Itacitinib
- Company Name
Incyte Corporation
- Drug Type
Small molecule
- Drug Class
JAK kinase
- Dose
300–400 milligrams (mg) per flat dose
- Route
Oral (po)
- Schedule of Administration
Once daily
- Drug 2
- Generic/Working Name
nab‐paclitaxel
- Trade Name
Abraxane
- Company Name
Celgene
- Drug Type
Drug conjugate
- Drug Class
Tubulin/microtubules targeting agent
- Dose
125 milligrams (mg) per squared meter (m2)
- Route
Intravenous (IV)
- Schedule of Administration
Days 1, 8, and 15 of each 28‐day cycle
- Drug 3
- Generic/Working Name
Gemcitabine
- Drug Type
Small molecule
- Drug Class
Antimetabolite
- Dose
1,000 milligrams (mg) per squared meter (m2)
- Route
Intravenous (IV)
- Schedule of Administration
Days 1, 8, and 15 of each 28‐day cycle
Dose Escalation Table for Phase I Experimental
Patient Characteristics for Phase II Experimental
- Number of Patients, Male
18
- Number of Patients, Female
10
- Age
Median (range): 67 years (45–83 years)
- Performance Status: ECOG
-
0 — 14
1 — 14
2 — 0
3 — 0
Unknown — 0
- Cancer Types or Histologic Subtypes
Pancreatic cancer, 28 (100%)
- Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Patient Characteristics for Phase I Experimental
- Number of Patients, Male
14
- Number of Patients, Female
13
- Age
Median (range): 65 years (37–80 years)
- Performance Status: ECOG
-
0 — 10
1 — 16
2 — 1
3 — 0
Unknown — 0
- Other
See Table 2
- Cancer Types or Histologic Subtypes
-
Pancreatic cancer, 18 (66.7%)
Breast cancer, 3 (11.1%)
Non‐small cell lung cancer, 3 (11.1%)
Other, 3 (11.1%)
- Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Table 2. Demographics and disease characteristics.
Itacitinib 300 mg once daily (QD) + nab‐paclitaxel 125 mg/m2 + gemcitabine 1,000 mg/m2 + granulocyte colony‐stimulating factor (G‐CSF).
Itacitinib 400 mg QD + nab‐paclitaxel 125 mg/m2 + gemcitabine 1,000 mg/m2 + G‐CSF.
Itacitinib 400 mg QD + nab‐paclitaxel 125 mg/m2 + gemcitabine 1,000 mg/m2.
Itacitinib 400 mg QD + nab‐paclitaxel 100 mg/m2 + gemcitabine 750 mg/m2 .
Itacitinib 300 mg QD + nab‐paclitaxel 125 mg/m2 + gemcitabine 1,000 mg/m2.
Itacitinib 200 mg QD + nab‐paclitaxel 125 mg/m2 + gemcitabine 1,000 mg/m2, following a 7‐day period of introductory itacitinib 200 mg QD monotherapy.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC, non‐small cell lung cancer.
Primary Assessment Method for Phase II Experimental
- Number of Patients Evaluated for Efficacy
28
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 8 (28.6%)
- Response Assessment SD
n = 10 (35.7%)
- Response Assessment PD
n = 0 (0%)
- Response Assessment OTHER
n = 10 (35.7%)
- Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Primary Assessment Method for Phase I Experimental
- Title
Total Patient Population
- Number of Patients Evaluated for Efficacy
27
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 5 (18.5%)
- Response Assessment SD
n = 13 (48.1%)
- Response Assessment PD
n = 4 (14.8%)
- Response Assessment OTHER
n = 5 (18.5%)
- Outcome Notes
Table 6. Best overall response.
Patients who have a best overall response of CR or PR.
Patients who have a best overall response of CR or PR or SD.
Abbreviations: CR, complete response; DCR, disease control rate; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Adverse Events
- See Table 5
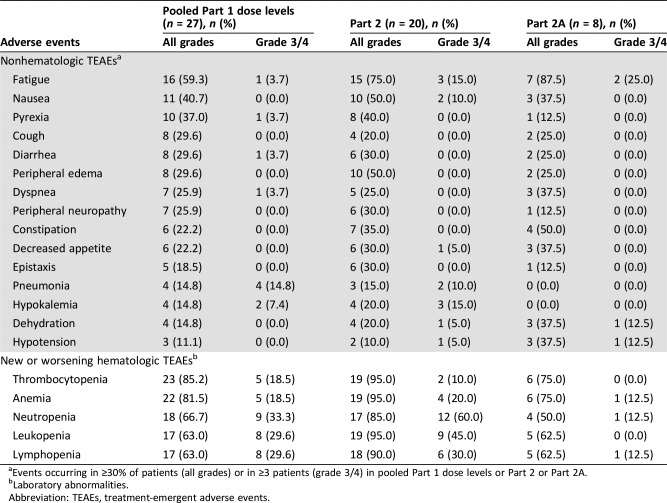
Table 5. Adverse events.
Events occurring in ≥30% of patients (all grades) or in ≥3 patients (grade 3/4) in pooled Part 1 dose levels or Part 2 or Part 2A.
Laboratory abnormalities.
Abbreviation: TEAEs, treatment‐emergent adverse events.
Serious Adverse Events
- See Table 4
- Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
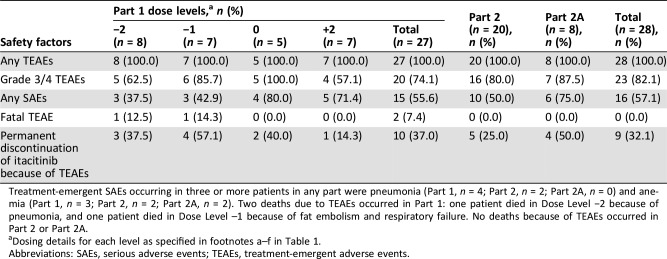
Table 4. Safety summary.
Treatment‐emergent SAEs occurring in three or more patients in any part were pneumonia (Part 1, n = 4; Part 2, n = 2; Part 2A, n = 0) and anemia (Part 1, n = 3; Part 2, n = 2; Part 2A, n = 2). Two deaths due to TEAEs occurred in Part 1: one patient died in Dose Level −2 because of pneumonia, and one patient died in Dose Level –1 because of fat embolism and respiratory failure. No deaths because of TEAEs occurred in Part 2 or Part 2A.
Dosing details for each level as specified in footnotes a–f in Table 1.
Abbreviations: SAEs, serious adverse events; TEAEs, treatment‐emergent adverse events.
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Investigator's Assessment
- Itacitinib in combination with nab‐paclitaxel and gemcitabine demonstrated an acceptable safety profile with clinical activity in patients with advanced solid tumors including pancreatic cancers.
In 2013, positive findings from the phase III MPACT trial (improved overall survival [OS], progression‐free survival, and response rate with nab‐paclitaxel plus gemcitabine vs. gemcitabine alone) [5] led to the regulatory approval of nab‐paclitaxel plus gemcitabine as a treatment option for patients with metastatic pancreatic cancer. In 2015, updated OS data confirmed and extended the primary report, supporting the superior efficacy of nab‐paclitaxel plus gemcitabine over gemcitabine alone [6]. Given the manifestations of cachexia in patients with pancreatic adenocarcinoma [7], [8] and the resulting poor tolerance of anticancer therapy, it was hypothesized that the addition of itacitinib to nab‐paclitaxel in combination with gemcitabine might confer clinical benefit, leading to the alleviation of symptoms and improvement in OS. Moreover, because of the disparate mechanisms of action of itacitinib and chemotherapy, it was predicted that the toxicity profile of the combination should be similar to that observed for each agent alone.
In this phase Ib/II open‐label study, itacitinib administered in combination with nab‐paclitaxel plus gemcitabine showed an acceptable safety profile in patients with advanced solid tumors with evidence for clinical activity. The toxicity profile of the combination therapy was similar to that reported for nab‐paclitaxel plus gemcitabine [5]. The median duration of itacitinib treatment was 84 days for each study part (i.e., Part 1 and pooled Part 2/2A). The most common grade 3/4 nonhematologic treatment‐emergent adverse events (TEAEs) were fatigue, pneumonia, and nausea, and the most frequent grade 3/4 hematologic TEAEs (new or worsening laboratory abnormalities) were neutropenia, lymphopenia, and leukopenia. Partial responses were seen across all itacitinib doses investigated and across several tumor types. Across all dose cohorts, the overall response rate and disease control rate were 24% and 65%, respectively. Forty percent of patients (8/20) with pancreatic cancer in Part 2 and Part 2A had a >50% reduction of target lesion size from baseline.
At the time this phase Ib/II study was underway, ruxolitinib, a selective inhibitor of JAK1 and JAK2, in combination with capecitabine, was also being assessed in the randomized, phase III JANUS 1 and JANUS 2 trials (NCT02117479 and NCT02119663) [9] for the treatment of patients with advanced or metastatic pancreatic cancer who had evidence of high systemic inflammation. These studies were both terminated early by the sponsor on February 11, 2016, after an interim analysis showed no additional benefit of ruxolitinib plus capecitabine versus capecitabine alone. Anemia was the most frequent toxicity in these studies, which is an on‐target toxicity of JAK2 inhibitors, including ruxolitinib, and reflects the essential role of JAK2 in normal hematopoiesis [10]. Itacitinib, which spares JAK2, was thus expected to have reduced myelosuppressive activity compared with broader‐specificity JAK inhibitors including ruxolitinib.
Initial results from the first four cohorts enrolled in Part 1 of the study suggested that the combination treatment regimen may be tolerated better in those patients with pancreatic cancer who were chemotherapy‐naïve. Yet a higher discontinuation rate, not relating to progressive disease, was observed within the first two cycles of Part 2, and high rates of grade 3/4 neutropenia were also observed when itacitinib 300 mg once daily (QD) was administered in combination with nab‐paclitaxel 125 mg/m2 plus gemcitabine 1,000 mg/m2. Based on these findings, the dose of itacitinib was reduced from 300 mg QD to 200 mg QD and assessed in combination with standard nab‐paclitaxel 125 mg/m2 and gemcitabine 1,000 mg/m2 therapy in Part 2A in the same untreated patient population. This therapeutic combination was better tolerated: grade 3/4 neutropenia decreased from 60% in Part 2 to 12.5% in Part 2A.
Preliminary pharmacokinetic (PK data (including area under the concentration‐response curve and maximal concentration) determined for 14 enrolled patients suggested no significant PK interactions of itacitinib with nab‐paclitaxel or gemcitabine [11]. In addition, a similar projected average interleukin (IL)‐6 signaling inhibition was observed at both 300 mg and 400 mg doses. We found that the level of pharmacodynamic activity observed at 300 mg QD was higher than expected based on what was predicted from data obtained in healthy volunteers and in patients with inflammatory diseases. This finding might be explained by altered metabolism of itacitinib in patients with advanced malignancies. Nevertheless, our preliminary data indicate that the 300 mg QD dose of itacitinib represents a pharmacologically active dose in these patients. Although higher doses may result in incrementally improved JAK/STAT pathway inhibition and possibly better intratumoral inhibition, higher doses may also not be well tolerated, especially in patients with advanced disease. Of note, we found that JAK/STAT inhibition observed with itacitinib 200 mg QD may be sufficient in advanced malignancies, particularly metastatic pancreatic adenocarcinoma, and may offer a balance between the potential for toxicity and efficacy of the JAK1 inhibitor.
Recent studies suggest that although chemotherapeutics can abrogate signaling networks that control tumor cell proliferation, they can also enhance proinflammatory signaling networks, which in turn can limit the efficacy of these agents and promote metastatic spread [12]. For example, paclitaxel activates signaling via Toll‐like receptor‐4, which promotes nuclear factor κ‐light‐chain‐enhancer of activated B‐cells expression, leading to the upregulation of inflammatory mediators including IL‐6, IL‐8, and vascular endothelial growth factor‐A [13], [14], [15], [16], [17], [18], [19]. Therefore, because of the key role of JAK/STAT in cytokine‐mediated signaling, it is possible that inhibition of JAK1 with itacitinib may sensitize tumors to chemotherapy, either directly or by inhibiting the ensuing proinflammatory response, as has been suggested in preclinical models with inhibition of the IL‐6 pathway [20].
In summary, itacitinib in combination with nab‐paclitaxel plus gemcitabine demonstrated an acceptable safety profile with clinical activity seen in patients with advanced solid tumors including pancreatic cancers. This study was terminated early by the sponsor, based on negative results from a phase III trial of a JAK1/2 inhibitor combined with chemotherapy in previously treated patients with advanced pancreatic cancer. Based on the potential for additive or synergistic effects, itacitinib is currently being explored in combination with immunotherapeutic agents including the anti‐programmed cell death protein 1 antibody, pembrolizumab (NCT02646748), and small‐molecule inhibitors including ibrutinib (NCT02760485), dabrafenib plus trametinib (NCT03272464), and osimertinib (NCT02917993).
Figures and Tables
Figure 1.
Best percentage change of target lesion size from baseline. Dotted line = best change in target lesion size from baseline required to achieve a partial response (≥30%).
Abbreviations: NSCLC, non‐small cell lung cancer; PD, progressive disease; PR, partial response; SD, stable disease.
Table 3. Reasons for treatment discontinuation.
Dosing details for each level as specified in footnotes a–f in Table 1.
Acknowledgments
The authors thank the patients and their families, investigators, and site personnel who participated in this study. Medical writing assistance was provided by Felicity Leigh, Ph.D., of Evidence Scientific Solutions (Philadelphia, PA) and funded by Incyte Corporation. Additional funding to support G.L.B. was provided by grant 2013107 from the Doris Duke Charitable Foundation.
Footnotes
ClinicalTrials.gov Identifier: NCT01858883
Sponsor(s): Incyte Corporation (Wilmington, DE)
Principal Investigator: Gregory L. Beatty
IRB Approved: Yes
Disclosures
Gregory L. Beatty: Incyte (C/A, RF); Safi Shahda: Bayer, Ipsen, AstraZeneca, Exelixis (C/A), APEX, Incyte, Array, Boston Biomedical, Boehringer Ingelheim, Merck, Regeneron, Amgen (RF); Albert Assad: Incyte (C/A); Julie Switzky: Incyte (C/A, OI); Huiling Zhen: Incyte (E, OI); Daniel D. Von Hoff: Incyte (RF: institutional support). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/ inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Bissonnette R, Luchi M, Fidelus‐Gort R et al. A randomized, double‐blind, placebo‐controlled, dose‐escalation study of the safety and efficacy of INCB039110, an oral janus kinase 1 inhibitor, in patients with stable, chronic plaque psoriasis. J Dermatolog Treat 2016;27:332–338. [DOI] [PubMed] [Google Scholar]
- 2.Luchi M, Fidelus‐Gort R, Douglas D et al. A randomized, dose‐ranging, placebo‐controlled, 84‐day study of INCB039110, a selective janus kinase‐1 inhibitor, in patients with active rheumatoid arthritis (abstract 1797). Arthritis Rheumatol 2013;65:S765–S766. [Google Scholar]
- 3.Beatty GL, Shahda S, Beck TJ et al. A phase 1b/2 study of INCB039110 + nab‐paclitaxel (N) and gemcitabine (G) in patients (pts) with advanced solid tumors and pancreatic cancer (PC). J Clin Oncol 2017;35(suppl 4):362A. [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 5.von Hoff DD, Ervin T, Arena FP et al.Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein D, El‐Maraghi RH, Hammel P et al. nab‐Paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long‐term survival from a phase III trial. J Natl Cancer Inst 2015;107:dju413. [DOI] [PubMed] [Google Scholar]
- 7.Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev 2010;38:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebrahimi B, Tucker SL, Li D et al. Cytokines in pancreatic carcinoma: Correlation with phenotypic characteristics and prognosis. Cancer 2004;101:2727–2736. [DOI] [PubMed] [Google Scholar]
- 9.Hurwitz H, Van Cutsem E, Bendell J et al. Ruxolitinib + capecitabine in advanced/metastatic pancreatic cancer after disease progression/intolerance to first‐line therapy: JANUS 1 and 2 randomized phase III studies. Invest New Drugs 2018;36:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verstovsek S. Therapeutic potential of JAK2 inhibitors. Hematology Am Soc Hematol Educ Program 2009:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Data on file. Wilmington, DE: Incyte Corporation; 2017.
- 12.Vyas D, Laput G, Vyas AK. Chemotherapy‐enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther 2014;7:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White CM, Martin BK, Lee LF et al. Effects of paclitaxel on cytokine synthesis by unprimed human monocytes, T lymphocytes, and breast cancer cells. Cancer Immunol Immunother 1998;46:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee LF, Haskill JS, Mukaida N et al. Identification of tumor‐specific paclitaxel (Taxol)‐responsive regulatory elements in the interleukin‐8 promoter. Mol Cell Biol 1997;17:5097–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pusztai L, Mendoza TR, Reuben JM et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 2004;25:94–102. [DOI] [PubMed] [Google Scholar]
- 16.Volk LD, Flister MJ, Bivens CM et al. Nab‐paclitaxel efficacy in the orthotopic model of human breast cancer is significantly enhanced by concurrent anti‐vascular endothelial growth factor A therapy. Neoplasia 2008;10:613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volk LD, Flister MJ, Chihade D et al. Synergy of nab‐paclitaxel and bevacizumab in eradicating large orthotopic breast tumors and preexisting metastases. Neoplasia 2011;13:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins TS, Lee LF, Ting JP. Paclitaxel up‐regulates interleukin‐8 synthesis in human lung carcinoma through an NF‐κB and AP‐1‐dependent mechanism. Cancer Immunol Immunother 2000;49:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajput S, Volk‐Draper LD, Ran S. TLR4 is a novel determinant of the response to paclitaxel in breast cancer. Mol Cancer Ther 2013;12:1676–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long KB, Tooker G, Tooker E et al. IL6 receptor blockade enhances chemotherapy efficacy in pancreatic ductal adenocarcinoma. Mol Cancer Ther 2017;16:1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]