The EVERLAR study was conducted to assess the anti‐tumor activity of the everolimus and the somatostatin analog octreotide combination in progressive nonfunctioning gastrointestinal neuroendocrine tumors and to understand the relationship between the activation of the translation of IGFR‐PI3K‐mTOR signal and response to treatment.
Keywords: Neuroendocrine tumors, Everolimus, Octreotide, Nonfunctioning
Abstract
Background.
Antitumor activity of the combination of somatostatin analogues (SSAs) and the mammalian target of rapamycin (mTOR) inhibitor everolimus in patients with neuroendocrine tumors (NETs) has been reported but not confirmed in prospective trials.
Materials and Methods.
This prospective, multicenter, single‐arm phase II EVERLAR study evaluated everolimus 10 mg/day and the SSA octreotide 30 mg every 28 days in patients with advanced nonfunctioning well‐differentiated gastrointestinal NETs (GI‐NETs) that progressed in the last 12 months (ClinicalTrials.gov NCT01567488). Prior treatment with SSAs and any systemic or locoregional therapy was allowed except for mTOR inhibitors. Patients continued treatment until disease progression or unacceptable adverse events (AEs). The primary endpoint was progression‐free survival (PFS) at 12 months; secondary endpoints included early biochemical response, objective response rate (ORR) by RECIST v1.0, overall survival (OS), AEs, activation of mTOR pathway (insulin‐like growth factor 1 receptor [IGF1R] and phosphoS6 [pS6] expression).
Results.
Forty‐three patients were included in the intent‐to‐treat analyses. After 12 months of treatment, 62.3% (95% confidence interval [CI] 48%–77%) of patients had not progressed or died. The 24‐month PFS rate was 43.6% (95% CI 29%–58%). The confirmed ORR was 2.3%, and stable disease was 58.1%. Median OS was not reached after 24 months of median follow‐up. Dose reductions and temporary interruptions due to AEs were required in 14 (33%) and 33 (77%) patients, respectively. The most frequent AEs were diarrhea, asthenia, mucositis, rash, and hyperglycemia. No correlation was observed between IGFR1 and pS6 expression and PFS/OS.
Conclusion.
The everolimus‐octreotide combination provided clinically relevant efficacy in nonfunctioning GI‐NETs, similar to the results of RADIANT‐2 in functioning setting.
Implications for Practice.
The EVERLAR study reports prospective data of somatostatin analogue in combination with everolimus in nonfunctioning gastrointestinal neuroendocrine tumors suggesting meaningful activity and favorable toxicity profile that supports drug combination in this setting.
Introduction
Neuroendocrine tumors (NETs) comprise a broad family of tumors arising from the diffuse endocrine system found most often in the bronchial or gastrointestinal systems. Patients with advanced NETs who have clinically significant tumor burden or progressive disease can be therapeutically managed with the somatostatin analogues (SSAs) octreotide or lanreotide to control tumor growth [1].
The mammalian target of rapamycin (mTOR) regulates growth, proliferation, cellular metabolism, and angiogenesis with activated downstream signaling of the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) pathway in gastroenteropancreatic NETs (GEP‐NETs) [2], [3], [4]. Everolimus, an mTOR inhibitor, has shown robust antitumor activity in patients with advanced NETs in the last 5 years, with significant improvements in progression‐free survival (PFS) compared with placebo in three phase III RAD001 in Advanced Neuroendocrine Tumors (RADIANT) studies in over 1,000 patients, supporting the use of everolimus as part of the NET management strategies [5].
The mechanism of action of SSAs and mTOR inhibitors suggests that combining the two agents could have potential synergy and be an effective treatment option for patients with NETs [6]. Although there are no randomized data comparing the outcome of patients who received an mTOR inhibitor agent alone versus the combination with an SSA, there are several indications that the combined use of octreotide and everolimus could increase efficacy compared with either agent alone. In the phase II RADIANT‐1 study, patients with pancreatic NETs were stratified according to concomitant octreotide at the time of study enrollment. There were fewer patients in the subgroup of patients who received everolimus plus octreotide (n = 45) than everolimus alone (n = 115); however, the median PFS was 15.2 months and the median overall survival (OS) had not been reached at the time of data cutoff among patients receiving the combination, compared with 8.5 months and 24.9 months, respectively, in the group of patients receiving everolimus alone [7]. In clinical practice, the combination of everolimus with SSAs has also shown efficacy in patients with advanced NETs, with a median time to progression (TTP) of 25.8 months (95% confidence interval [CI] 11.3–40.3) and a 78.5% probability of being progression‐free after 6 months of combined treatment [8].
The EVERLAR study was conducted to assess the antitumor activity of the everolimus and the SSA octreotide combination in progressive nonfunctioning gastrointestinal NETs (GI‐NETs) and to understand the relationship between the activation of the translation of IGFR‐PI3K‐mTOR signal and the response to treatment.
Materials and Methods
Study Design
EVELAR was a prospective, multicenter, single‐arm phase II study of the mTOR inhibitor everolimus (Afinitor; Novartis Farmaceutica, SA, Spain) 10 mg/day taken orally in combination with the SSA octreotide (Sandostatin LAR; Novartis Farmaceutica, SA, Spain) 30 mg injected intramuscularly every 28 days in patients with advanced nonfunctioning well‐differentiated GI‐NETs that progressed in the last 12 months (ClinicalTrials.gov NCT01567488). In case of adverse events (AEs), two dose reductions of everolimus were allowed to 5 mg/day and to 5 g every other day. Patients continued treatment until progression or unacceptable AEs.
Patients
The study population included adult (≥18 years of age) patients with a life expectancy >3 months, a World Health Organization functional status 0–2, adequate bone marrow, liver and renal function, normal baseline levels of serum cholesterol and triglycerides, and with measurable cytological or histological confirmation of advanced inoperable or metastatic low‐ to intermediate‐grade GI‐NET that had progressed in the 12 months prior to the inclusion in the study. If patients received antitumor treatment in the 12 months prior to inclusion in the study, disease progression was to be radiologically documented. Patients with NETs of unknown primary origin were also included if they presented with hepatic metastases. Patients who had undergone surgery were required to provide paraffin‐embedded primary tumor or metastases samples. Prior treatment with SSAs and any systemic or locoregional therapy was allowed except for mTOR inhibitors.
Exclusion criteria included NETs of low differentiation, high grade, chemotherapy, immunotherapy or radiotherapy <4 weeks prior to study entry, hepatic arterial embolization <6 months prior to study entry, or cryoablation/radiofrequency ablation of hepatic metastases <2 months prior to study entry, diabetes mellitus, any serious disease and/or an uncontrolled clinical condition, chronic treatment with corticosteroids or any other immunosuppressive agent, human immunodeficiency virus seropositive, intolerance or hypersensitivity to octreotide or other SSAs and to everolimus or other mTOR inhibitors, and previous cancer (except in the case of successfully treated squamous or epithelial skin carcinoma or any in situ carcinoma).
Objectives
The primary endpoint was PFS, evaluated after 12 months of treatment in the intent‐to‐treat (ITT) population. The secondary endpoints included PFS at 24 months in the ITT and per‐protocol (PP) populations; early biochemical response: the rate of patients reporting normalization or decrease (≥30% decrease at week 4 in chromogranin A [CgA] levels); objective response rate (ORR), defined as patients reporting a complete response (CR) or a partial response (PR), and disease control rate (DCR), defined as patients reporting CR, PR, or stable disease (SD) according to RECIST v1.0, including duration of objective response and SD; and OS at 24 months. AEs were collected and graded for severity according to the Common Terminology Criteria for Adverse Events v4.0.
A biological study evaluated the activation of mTOR pathway (insulin‐like growth factor 1 receptor [IGF1R], phosphorylated mTOR [pmTOR], and phosphoS6 [pS6]). Tumor expression was centrally evaluated, with validated monoclonal antibodies for IGFR (3C8B1 Abcam ab54274), phospho‐mTOR (Ser‐2448, 49F9 Cell Signaling 2976), and for pS6 (Phospho‐S6 Ribosomal Protein [Ser235/236; 91B2] Rabbit mAb 4857 [Cell Signaling Technology, Danvers, MA]) according to manufacturer's instructions and as has been previously published [9], [10]. IGF1R expression was graded on a 0–2 scale (0, no staining; 1, light‐intensity membrane staining; 2, strong‐intensity membrane staining). pmTOR and pS6 expression was also graded on a 0–2 scale (0, no staining; 1, light‐intensity cytoplasmic staining; 2, strong‐intensity cytoplasmic staining). Samples were considered positive if they were 1 or 2.
Statistical Analyses
Sample size estimation was based on the data from a preliminary two‐arm phase II study evaluating 12 months of treatment with 5 mg/day or 10 mg/day of everolimus in patients with advanced well‐differentiated gastroenteropancreatic NETs [11]; the PFS rate was 49% after 12 months of treatment, and the 6‐month PFS rate was 68% in patients with progressive disease at study inclusion. To estimate a 12‐month PFS rate ≥50% versus a 12‐month PFS rate ≥75%, with 75% potency and 10% alpha risk, 44 patients were estimated to be included in order to have at least 40 evaluable patients. The data cutoff occurred 24 months after treatment initiation of the last patient included in the study. The level of significance in the statistical analyses was .05 (two‐sided alpha).
The ITT population comprised all patients included in the study who received at least one dose of study medication and had at least a baseline and a post‐treatment value. The PP population comprised the patients of the ITT population who fulfill all study selection criteria and completed the treatment per protocol without interruption due to early disease progression (<12 weeks). The safety population comprised all patients included in the study who received at least one dose of the study drug.
PFS was defined as the time from treatment initiation to the first event of documented progression or death from any cause. Patients without an event at the time of analysis were censured at the time of last radiological result indicating an absence of progression, or if unavailable, at the last visit. PFS was expressed as the percentage of patients without signs of progressive disease after 12 months of treatment, including 95% CIs. As predefined in the study protocol, a sensitivity analysis of the PFS variable was performed with a Kaplan‐Meier survival model, estimating the median.
OS was defined as the time from study inclusion until death from any cause, and patients without an event were censored at the time of the last visit. A log‐rank test was performed between PFS, OS, and all variables analyzed. To study the correlation between activation of the mTOR pathway and outcome, a Cox regression between PFS or OS and mTOR pathway activation adjusted by age, tumor size, number of prior treatment lines, baseline CgA and 5‐HIAA levels, cumulative drug dose, and the following covariates: presence or absence of distant metastases, prior SSA therapy, and treatment compliance.
The protocol was approved by the Ethics Committee of the participating hospitals. The study followed International Conference on Harmonization Good Clinical Practice guidelines, and all patients provided signed informed consent.
Results
Eleven centers within the Spanish Task Force for Neuroendocrine and Endocrine Tumors (GETNE) initially participated in the study; however, two centers were prematurely closed without recruiting any patients. Between June 21, 2011, and April 17, 2013, 44 patients were enrolled in the study; however, one patient was deemed ineligible due to a diagnosis of a pancreatic NET. The ITT population consisted of 43 patients. Two patients interrupted treatment during >21 days, one patient had creatinine levels that exceeded the upper limit specified in the protocol, and two patients initiated treatment with everolimus 5 mg/day instead of 10 mg/day. The PP included 38 patients. The safety population included all 44 patients. Twelve patients interrupted treatment before disease progression (related to an AE in eight patients and as a result of patient decision in the other four patients). At the data cutoff of June 12, 2015, four patients were still receiving treatment.
Of the 43 patients in the ITT population, 23 (53.5%) were male, 31 (72.1%) had an Eastern Cooperative Oncology Group Performance Score of 0 (Table 1). The primary tumor location was foregut (11.6%), midgut (48.8%), hindgut (11.6%), and unknown (27.9%). Most patients had received prior SSAs (37, 86.1%). Patients also received prior treatment with chemotherapy or interferon.
Table 1. Baseline demographics and disease characteristics.
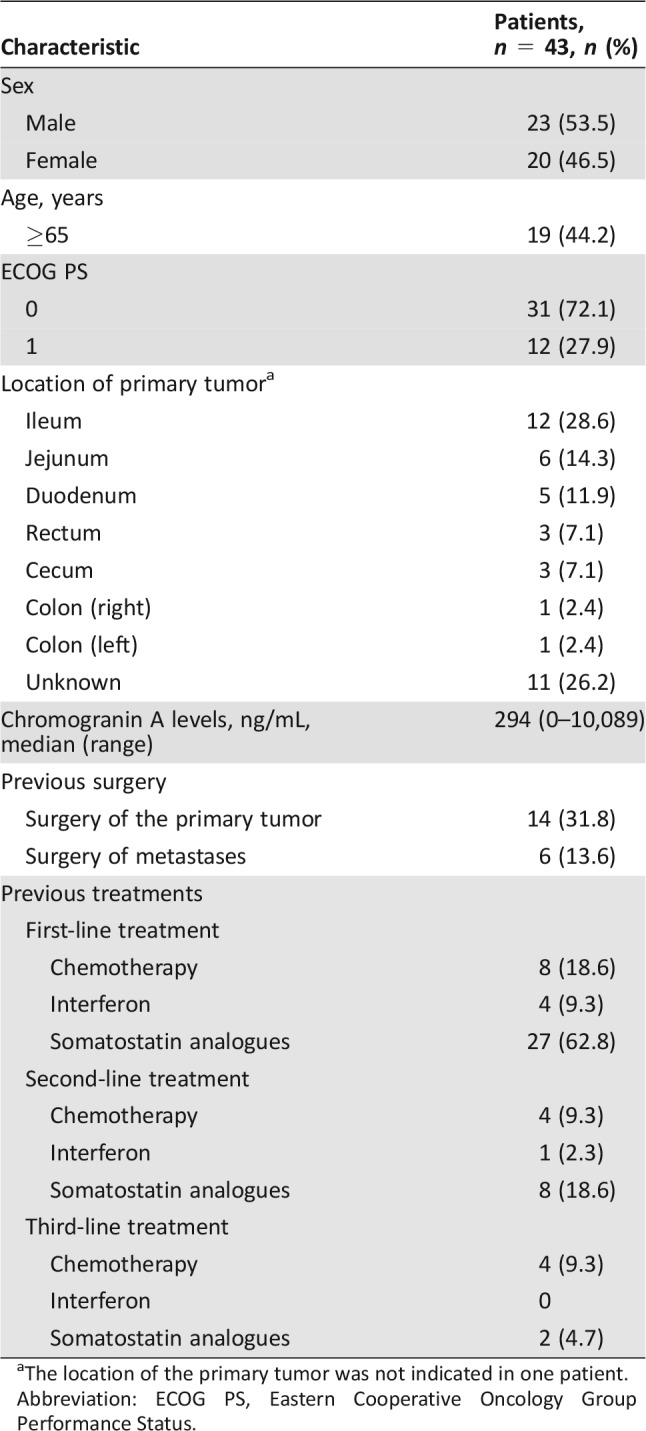
The location of the primary tumor was not indicated in one patient.
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Efficacy
Primary Endpoint.
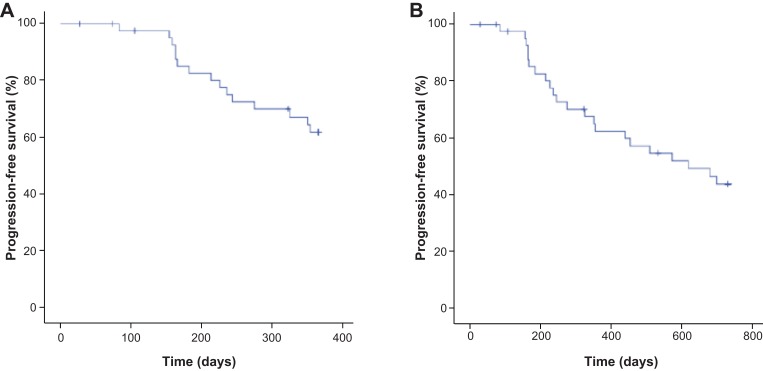
After 12 months of treatment, 15 (34.9%) patients had reported events of disease progression or death. The 12‐month PFS rate, with Kaplan‐Meier estimation accounting for the time that each patient remained on study, was 62.3% (95% CI 47.8%–76.8%) in the ITT population. The mean PFS was 10.2 months (95% CI 9.3–11.1 months), and the median was not estimable because less than 50% of patients had an event of progression or death (Fig. 1A). In the PP population, the PFS rate at 12 months was 63.7% (95% CI 49.3%–78.1%).
Figure 1.
Progression‐free survival in the intent‐to‐treat population. (A): 12 months. (B): 24 months.
Secondary Endpoints.
After 24 months of treatment, 22 (51.2%) patients had progressed or died. The 24‐month PFS rate was 43.6% (95% CI 28.8%–58.4%) in the ITT population, with a mean PFS of 16.7 months and a median of 20.3 months (95% CI 14.2–19.1 months; Fig. 1B). The 24‐month PFS rate was 48.8% (95% CI 33.9%–63.7%) in the PP population.
Early biochemical response was reported in 6 patients (13.6% [95% CI 3.5%–23.8%]).
A confirmed PR was reported in 1 (2.3%) patient, SD was reported in 25 (58.1%) patients, and progressive disease (PD) was reported in 17 (39.6%) patients in the ITT population. The ORR was 2.3% (95% CI −2% to 7%), and duration of response was 3 years and 11 months. Similarly, in the PP population, there was 1 (2.6%) patient with PR, 22 (57.9%) patients with SD, and 15 (39.5%) patients with PD as best response according to RECIST v1.0.
Disease stabilization at 12 months was reported in 34 (79.1%) patients in the ITT population. At 12 months, the mean duration of stabilization was 10.8 months (95% CI 6.2–15.3 months). At 24 months, the mean duration of stabilization was 26.0 months (95% CI 19.8–32.3 months) and the median was 28.9 months.
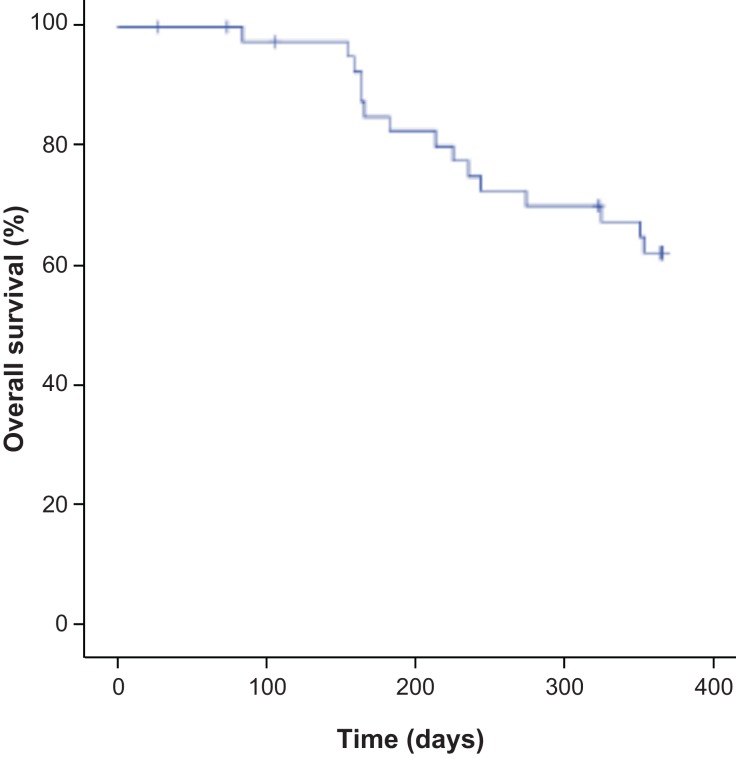
Of the 43 patients in the ITT population, 11 (25.6%) patients had died by the end of the study (24 months). Consequently, the median OS was not reached after 24 months (mean 21.1 months [95% CI 19.3–22.9 months]; Fig. 2). The 24‐month OS Kaplan‐Meier estimated rate was 71.7% (95% CI 58.2%–85.2%).
Figure 2.
Overall survival in the intent‐to‐treat population.
Safety.
An everolimus dose reduction due to toxicity occurred in 14 (31.8%) of the 44 patients in the safety population. Dose reductions were due to hematologic toxicities in 13 of 14 patients. In 13 patients, the first reduction was to 5 mg/day, and in 1 patient, it was reduced to 5 mg every other day. A second dose reduction occurred in 6 of these 14 patients, a third dose reduction in 3 of these 6 patients, and a fourth dose reduction in 1 of these 3 patients.
There were 51 everolimus treatment interruptions due to toxicity in 33 (75.0%) of the 44 patients in the safety population. There was one treatment interruption in 17 patients, two treatment interruptions in 11 patients, three treatment interruptions in 2 patients, and 1 patient had six treatment interruptions. Octreotide treatment interruptions occurred in 12 (27.3%) patients: two treatment interruptions in 7 patients and three in 5 patients.
The observed safety profile was similar to other reports of everolimus in this setting. The most frequent grade 1–2 AEs included diarrhea (70%), mucositis (66%), and fatigue (61%). Grade 3–4 AEs were reported at a frequency below 10%, with fatigue (9%), diarrhea (7%), pneumonitis (7%), hyperglycemia (4%), and mucositis (4%) being the most frequent. Table 2 lists the AEs that were related to treatment with everolimus.
Table 2. AEs related to everolimus (reported in ≥4 patients).
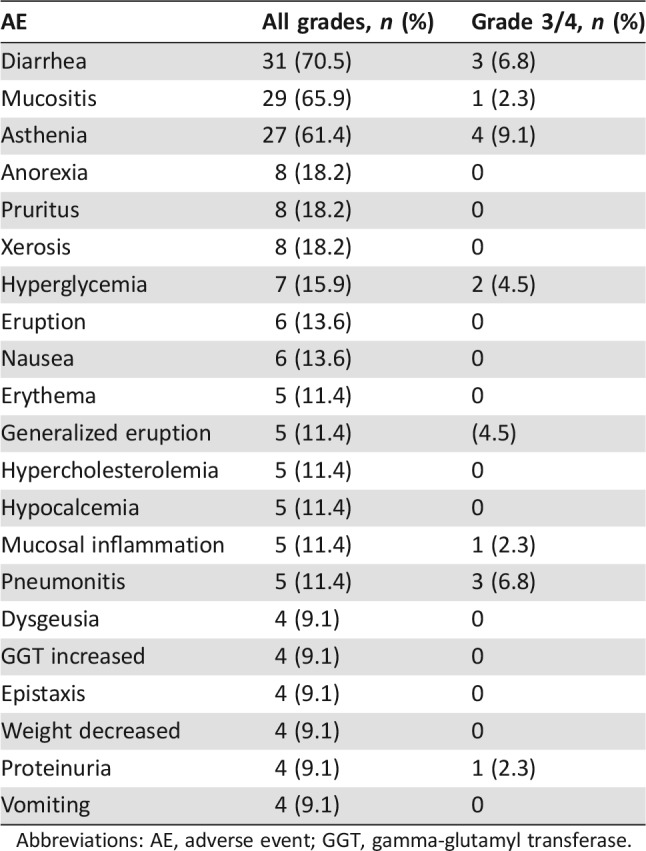
Abbreviations: AE, adverse event; GGT, gamma‐glutamyl transferase.
Twenty serious AEs (SAEs) were reported in 18 patients. In addition, there were three clinically significant conditions that were notified to Pharmacovigilance. There were 15 SAEs that were not considered to be related to study drugs (Hickman infection and uncontrolled pain in one patient, abdominal pain, pericardial effusion, ectopic adrenocorticotropic syndrome due to disease progression, dyspnea, perianal abscess, disease progression in the lung, fever, renal right colic, intestinal occlusion/abdominal pain in one patient due to disease progression, abdominal perforation due to disease progression, and intestinal perforation/lower back pain due to disease progression; all SAEs resolved except for the last two, in which the patients died due to disease progression). One SAE of septic shock with cholangitis and bacteremia due to Escherichia coli requiring hospitalization was considered to be possibly related to octreotide. Four SAEs were considered to be related to everolimus: pneumonitis (n = 2), vomiting (n = 1), and renal insufficiency (n = 1). All SAEs required hospitalization. In addition, two clinically significant AEs of decreased creatinine clearance and interstitial pneumonitis were also considered related to everolimus. A clinically significant AE of hyperglycemia was considered related to both everolimus and octreotide. All events resolved.
Biomarker Analyses.
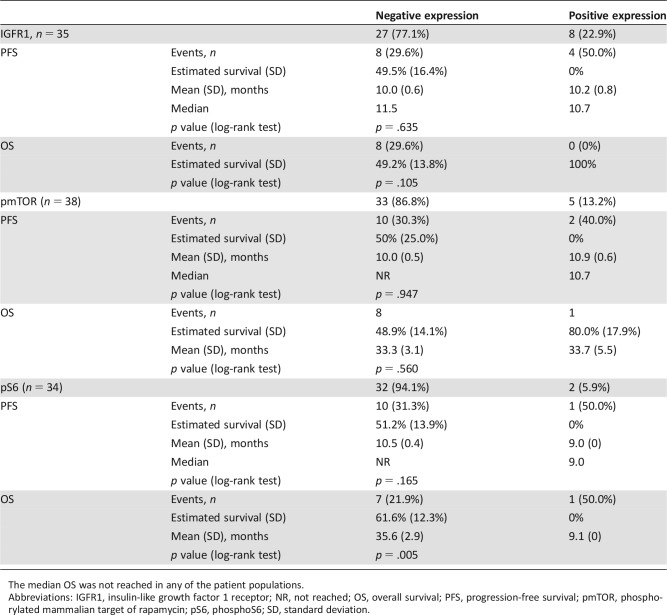
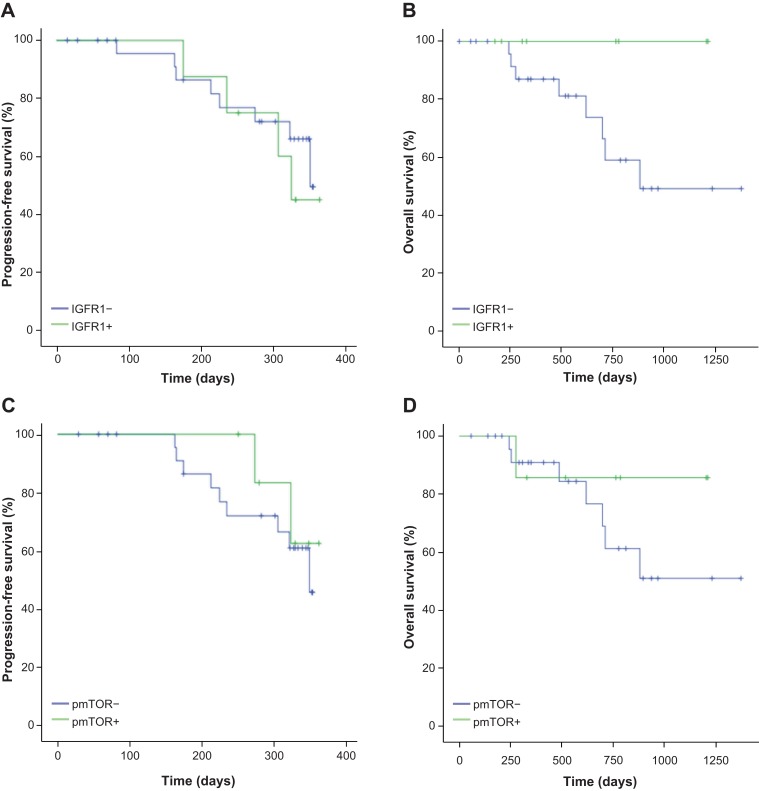
Biomarker analysis was performed to evaluate the capacity of each biomarker to discriminate for PFS or OS in this series of patients. Three markers of the IGF1R‐mTOR pathway were evaluated by immunohistochemistry. These markers were total IGF1R and phospho‐specific determinations of phospho‐mTOR and its downstream activated molecule, phospho‐S6, because they had been successfully described in the literature [9], [10] None of these biomarkers were able to discriminate patients' PFS or OS, neither in the unadjusted analyses (Table 3; Fig. 3) nor in the analyses adjusted by presence or absence of distant metastases, prior SSA therapy, and treatment compliance. In the case of phospho‐S6, the presence of only one patient with positive expression led to a misleading statistical p < .005, but it does not represent a significant difference, and no clear conclusions may be suggested.
Table 3. Biomarker evaluations.
The median OS was not reached in any of the patient populations.
Abbreviations: IGFR1, insulin‐like growth factor 1 receptor; NR, not reached; OS, overall survival; PFS, progression‐free survival; pmTOR, phosphorylated mammalian target of rapamycin; pS6, phosphoS6; SD, standard deviation.
Figure 3.
Progression‐free survival and overall survival in the intent‐to‐treat population. (A, B): According to IGFR1 expression. (C, D): According to pmTOR expression.
Abbreviations: IGFR1, insulin‐like growth factor 1 receptor; pmTOR, phosphorylated mammalian target of rapamycin.
Discussion
The combination of the mTOR inhibitor everolimus and the SSA octreotide provided clinically relevant efficacy in patients with nonfunctioning GI‐NETs in this prospective, multicenter, single‐arm phase II EVERLAR study. The 12‐ and 24‐month PFS rates were 62.3% and 43.6%, respectively, and the median PFS and OS had not yet been reached.
Treatment with SSAs, such as octreotide, can manage symptoms related to hormone secretion in patients with NETs and have direct antitumor effect, as clearly demonstrated in two large prospective studies. Firstly, the randomized, placebo‐controlled PROMID study in functioning and nonfunctioning gastrointestinal NETs reported that compared with placebo, octreotide delayed the median TTP (from 6 to 14.3 months, HR 0.34 [95% CI 0.20–0.59]; p = .000072) in 85 patients with metastatic midgut NETs, along with an increased stabilization of disease after 6 months of treatment (67% in the octreotide arm vs. 37% in the placebo arm) [12]. Long‐term follow‐up reported a median OS of 84.7 months in the octreotide arm and 83.7 months in the placebo arm, after most patients in the placebo arm received octreotide at progression [13]. More recently, in the international, randomized, double‐blind, placebo‐controlled phase III CLARINET trial of lanreotide versus placebo in 204 patients with nonfunctioning gastroenteropancreatic NETs, PFS was substantially improved in the lanreotide arm (after a median 24.0 months of lanreotide treatment, median PFS was not reached in the lanreotide arm vs. 18.0 months in the placebo arm; HR 0.47 [95% CI 0.30–0.73]; p < .001) [14]. After a median 40.0 months of lanreotide treatment with an open‐label extension of the study, the median PFS was 32.8 months in the lanreotide arm [15]. These studies confirmed the antitumor effect of SSAs in both functioning and nonfunctioning NETs of gastrointestinal tract and pancreatic origins.
On the other hand, everolimus has also demonstrated a significant impact on tumor growth control in pancreatic, gastrointestinal, and lung NETs, as reported in the RADIANT‐3 and RADIANT‐4 studies. In RADIANT‐3, patients with progressive pancreatic NETs had a statistically significant improvement in PFS associated with everolimus compared with placebo (11.0 vs. 4.6 months, HR 0.35 [95% CI 0.27–0.45]; p < .001) [16]. A median OS extension by 6.3 months in the everolimus arm did not reach statistical significance (44.0 vs 37.7 months, HR 0.94 [95% CI 0.73–1.20]; p = .30) [17]. In RADIANT‐4, treatment with everolimus led to a significant improvement in PFS in patients with progressive lung NETs or GI‐NETs (11.0 vs. 3.9 months; HR 0.48 [95% CI 0.35–0.67]; p < .00001) [18].
However, one of the open issues in the management of advanced NETs is the use of combined therapy with SSAs and targeted therapies. In patients with functioning tumors, management with combination therapy is a widely accepted practice among treating physicians, even in the absence of conclusive data from prospective clinical trials. Combination therapy in this patient population is used due to the continued need of SSA therapy in most patients and the results of the RADIANT‐2 study, in which treatment with everolimus plus octreotide led to a 5.1‐month increase in median PFS compared with placebo plus octreotide (16.4 vs. 11.3 months, HR 0.77 [95% CI 0.59–1.00]) in patients with advanced NETs with carcinoid syndrome, although the difference did not reach statistical significance [19].
Despite the clinical and molecular rationale for combining SSAs and mTOR inhibitors, there are few data supporting this combination as standard of care in nonfunctioning NETs. In a multicenter phase II trial of 50 treatment‐naive patients with advanced well‐differentiated GEP‐NETs or of lung origin, the combination of everolimus 10 mg daily with octreotide LAR 30 mg every 28 days led to an ORR of 18.0% (95% CI 6%–31%) in the ITT population and 19.6% in the PP population of 46 patients (95% CI 10%–33%) [20]. The clinical benefit rate, defined as patients reporting a CR, PR, or SD, was 92%. Median TTP and OS had not been reached after a median follow‐up of 9.1 months. There are also data coming from routine clinical practice that suggest a benefit of the combination of SSAs and targeted therapies. A Spanish cross‐sectional analysis reported that the combination of everolimus with the SSA lanreotide in patients with advanced NETs led to a median TTP of 25.8 months (95% CI 11.3–40.3) and a 78.5%, 68.6%, and 57.0% probability of being progression‐free after 6, 12, and 18 months of combined treatment, respectively [8].
Moreover, there are scarce prospective data on the combination of SSAs and mTOR inhibitors in nonfunctioning NETs. With the EVERLAR study, we report on a cohort of patients with gastrointestinal nonfunctioning NETs prospectively treated with the combination of octreotide and everolimus, most of which had already been pretreated with SSAs (86%). The combined therapy showed clinically relevant data of PFS rate at 12 months of 62%, suggesting a potential benefit for the combination therapy. Data coming from the COOPERATE‐2 (COmbination Of Pasireotide and evERolimus in Advanced Tumors of neuroEndocrine origin) trial, a randomized phase II study of everolimus in combination with the SSA pasireotide LAR compared with everolimus alone in 160 patients with advanced, well‐differentiated, progressive pancreatic neuroendocrine tumor did not report a difference in the primary endpoint of mPFS between the two treatment arms (median 16.8 months vs. 16.6 months) but clearly showed a higher ORR for the combination arm (20% vs. 6%), suggesting an additive effect of the SSA pasireotide and everolimus [21]. However, there was increased toxicity related with the combination of everolimus plus SSAs reported in both the RADIANT‐2 and COOPERATE‐2 studies. This increased toxicity could possibly explain the initial benefit observed in these studies, mPFS for RADIANT‐2 and ORR for COOPERATE‐2, that did not translate into an OS impact. In the LUNA study, a prospective, multicenter, randomized, open‐label, three‐arm phase II study evaluating everolimus, pasireotide LAR alone or in combination in 124 patients with advanced NETs of the lung and thymus, the combination of everolimus and SSA led to a higher proportion of patients progression‐free at month 9 (the primary endpoint): 58.5% compared with 39.0% for patients receiving pasireotide LAR alone or 33.3% for patients receiving everolimus alone [22]. At the time of the analyses, median mPFS was 11.8 months in the combination arm, 8.5 months in the pasireotide LAR alone arm, and 12.5 months in the everolimus arm. On the whole, the combination of SSA and everolimus is well tolerated, but further studies are needed to determine the advantage of the combination in terms of antineoplastic efficacy [23].
In our study, the lack of a comparative arm precludes any comparison in OS outcomes, but the toxicity profile was similar to prior reports of everolimus monotherapy (RADIANT‐1, −3, and −4 studies), suggesting that better management of everolimus AEs may reduce the potential toxicity of the combination with SSAs, thereby increasing the chance to impact tumor growth control.
And finally, the lack of predictive biomarkers to select the better population for combined therapy also jeopardizes the possibilities to find a significant benefit in survival. We performed a pharmacodynamics analysis for some of the main players related with the mTOR pathway, but no relationship with clinical benefit was observed. Recently, an analysis of RADIANT 2 pharmacokinetic data evaluated whether drug‐drug interactions between everolimus and octreotide could negatively impact efficacy outcomes, such as tumor response and mPFS [24]). Co‐administration of octreotide LAR with everolimus resulted in an increased octreotide Cmin; however, it was unlikely that this increase in octreotide levels resulted from a metabolism‐mediated drug interaction or a protein‐binding interaction with everolimus that could reduce the efficacy of everolimus and have impact on clinical efficacy.
Conclusion
The combination of everolimus and SSAs will likely continue to be standard of care in patients with functioning NETs who require continued administration of SSAs for symptom control and meet the criteria to be treated with everolimus. However, at the moment, there are no conclusive data to suggest combining everolimus plus SSAs for antitumoral purposes, although the data from the LUNA study support this combination. Increased toxicity reported in previous combination trials may be better managed with the current knowledge of short‐ and long‐term effects of everolimus. Improved toxicity management together with the clinically relevant data that we report in our trial for the nonfunctioning setting may reopen the potential to explore SSAs and targeted therapy combination. The search for predictive biomarkers should be performed in parallel with clinical development to select the best target population and optimize therapy.
Acknowledgments
This study was previously presented as a poster at ESMO 2016. We thank the patients, their families, and the medical and nursing staff of all the participating institutions. The Spanish Task Force for Neuroendocrine and Endocrine Tumors (GETNE) group promoted the trial. This study was supported by the GETNE group. Monitoring, Statistics, and Data Management were provided by Cristina Vidal. Financial support for this research trial was provided by Novartis Pharma, S.A, Spain. Support for third‐party writing assistance for this manuscript was provided by Novartis. We especially thank Aurora O'Brate for her medical writing support.
Footnotes
For Further Reading: Michael Michael, Rocio Garcia‐Carbonero, Matthias M. Weber et al. The Antiproliferative Role of Lanreotide in Controlling Growth of Neuroendocrine Tumors: A Systematic Review. The Oncologist 2017;22:272.285.
Implications for Practice: This review presents the current clinical evidence for the antiproliferative activity of lanreotide Autogel in patients with midgut or pancreatic neuroendocrine tumors (NETs) and shows its effectiveness, safety, and tolerability in these patient populations. By systematically presenting all the clinical evidence, the review adds to existing publications by discussing results in a broad range of settings. The review also indicates future directions for investigation of the use of lanreotide Autogel in NETs originating in other locations, in combination therapy, or as maintenance therapy in progressive disease.
Author Contributions
Conception/design: Ramon Salazar
Provision of study material or patients: Jaume Capdevila, Alexandre Teulé, Jorge Barriuso, Daniel Castellano, Carlos Lopez, Jose Luis Manzano, Vicente Alonso, Rocío García‐Carbonero, Emma Dotor, Ignacio Matos, Ana Custodio, Oriol Casanovas, Ramon Salazar
Collection and/or assembly of data: Jaume Capdevila, Alexandre Teulé, Jorge Barriuso, Daniel Castellano, Carlos Lopez, Jose Luis Manzano, Vicente Alonso, Rocío García‐Carbonero, Emma Dotor, Ignacio Matos, Ana Custodio, Oriol Casanovas, Ramon Salazar
Data analysis and interpretation: Jaume Capdevila, Jorge Barriuso, Oriol Casanovas, Ramon Salazar
Manuscript writing: Jaume Capdevila
Final approval of manuscript: Jaume Capdevila, Alexandre Teulé, Jorge Barriuso, Daniel Castellano, Carlos Lopez, Jose Luis Manzano, Vicente Alonso, Rocío García‐Carbonero, Emma Dotor, Ignacio Matos, Ana Custodio, Oriol Casanovas, Ramon Salazar
Disclosures
The authors indicated no financial relationships.
References
- 1.National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology: Neuroendocrine tumors version 3.2107. Available at https://www.nccn.org/professionals/physician_gls/default.aspx. [DOI] [PubMed]
- 2. Missiaglia E, Dalai I, Barbi S et al. Pancreatic endocrine tumors: Expression profiling evidences a role for AKT‐mTOR pathway. J Clin Oncol 2010;28:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiao Y, Shi C, Edil BH et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capurso G, Fazio N, Festa S et al. Molecular target therapy for gastroenteropancreatic endocrine tumours: Biological rationale and clinical perspectives. Crit Rev Oncol Hematol 2009;72:110–124. [DOI] [PubMed] [Google Scholar]
- 5. Pusceddu S, De Braud F, Lo Russo G et al. How do the results of the RADIANT trials impact on the management of NET patients? A systematic review of published studies. Oncotarget 2016;7:44841–44847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bousquet C, Lasfargues C, Chalabi M et al. Clinical review: Current scientific rationale for the use of somatostatin analogs and mTOR inhibitors in neuroendocrine tumor therapy. J Clin Endocrinol Metab 2012;97:727–737. [DOI] [PubMed] [Google Scholar]
- 7. Yao JC, Lombard‐Bohas C, Baudin E et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: A phase II trial. J Clin Oncol 2010;28:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Capdevila J, Sevilla I, Alonso V et al. Evaluation of the efficacy and safety of lanreotide in combination with targeted therapies in patients with neuroendocrine tumours in clinical practice: A retrospective cross‐sectional analysis. BMC Cancer 2015;15:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casanovas O, Teule A, Villabona C et al. Study on activation of the IGF‐1R mTOR pathway in neuroendocrine tumours (NETs). J Clin Oncol 2013;31(suppl 15):4139a. [Google Scholar]
- 10. Choe G, Horvath S, Cloughesy TF et al. Analysis of the phosphatidylinositol 3'‐kinase signaling pathway in glioblastoma patients in vivo. Cancer Res 2003;63:2742–2746. [PubMed] [Google Scholar]
- 11. Yao JC, Phan AT, Chang DZ et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low‐ to intermediate‐grade neuroendocrine tumors: Results of a phase II study. J Clin Oncol 2008;26:4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rinke A, Muller HH, Schade‐Brittinger C et al. Placebo‐controlled, double‐blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study group. J Clin Oncol 2009;27:4656–4663. [DOI] [PubMed] [Google Scholar]
- 13. Rinke A, Wittenberg M, Schade‐Brittinger C et al. Placebo‐controlled, double‐blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): Results of long‐term survival. Neuroendocrinology 2017;104:26–32. [DOI] [PubMed] [Google Scholar]
- 14. Caplin ME, Pavel M, Cwikla JB et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224–233. [DOI] [PubMed] [Google Scholar]
- 15. Caplin ME, Pavel M, Cwikla JB et al. Anti‐tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: The CLARINET open‐label extension study. Endocr Relat Cancer 2016;23:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao JC, Shah MH, Ito T et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yao JC, Pavel M, Lombard‐Bohas C et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: Overall survival and circulating biomarkers from the randomized, phase III RADIANT‐3 study. J Clin Oncol 2016;34:3906–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao JC, Fazio N, Singh S et al. Everolimus for the treatment of advanced, non‐functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT‐4): A randomised, placebo‐controlled, phase 3 study. Lancet 2016;387:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pavel ME, Hainsworth JD, Baudin E et al. Everolimus plus octreotide long‐acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT‐2): A randomised, placebo‐controlled, phase 3 study. Lancet 2011;378:2005–2012. [DOI] [PubMed] [Google Scholar]
- 20. Bajetta E, Catena L, Fazio N et al. Everolimus in combination with octreotide long‐acting repeatable in a first‐line setting for patients with neuroendocrine tumors: An ITMO group study. Cancer 2014;120:2457–2463. [DOI] [PubMed] [Google Scholar]
- 21. Kulke MH, Ruszniewski P, Van Cutsem E et al. A randomized, open‐label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well‐differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE‐2 trial. Ann Oncol 2017;28:1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferolla P, Brizzi MP, Meyer T et al. Efficacy and safety of long‐acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): An open‐label, multicentre, randomised, phase 2 trial. Lancet Oncol 2017;18:1652–1664. [DOI] [PubMed] [Google Scholar]
- 23. Chan DL, Segelov E, Singh S. Everolimus in the management of metastatic neuroendocrine tumours. Therap Adv Gastroenterol 2017;10:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pavel ME, Becerra C, Grosch K., Cheung W., Hasskarl J., Yao JC. Effect of everolimus on the pharmacokinetics of octreotide long-acting repeatable in patients with advanced neuroendocrine tumors: An analysis of the randomized phase III RADIANT-2 trial. Clin Pharmacol Ther 2017;101:462–468. [DOI] [PubMed] [Google Scholar]