Although the number of clinical trials registered annually has increased, inclusion and exclusion criteria limit the number of patients eligible for trial participation. This study aimed to identify comorbidities and other exclusion criteria that have limited the recruitment of patients with breast, colorectal, and lung cancers into early phase clinical trials.
Keywords: Clinical trials, Investigational drugs, Clinical oncology, Comorbidities, HIV infection
Abstract
Background.
Early phase clinical trials evaluate the safety and efficacy of new treatments. The exclusion/inclusion criteria in these trials are usually rigorous and may exclude many patients seen in clinical practice. Our objective was to study the comorbidities limiting the participation of patients with breast, colorectal, or lung cancer in clinical trials.
Materials and Methods.
We queried ClinicalTrials.gov on December 31, 2016. We reviewed the eligibility criteria of 1,103 trials. Logistic regression analyses were completed, and exclusion was studied as a binary variable.
Results.
Out of 1,103 trials, 70 trials (6%) excluded patients >75 years of age, and 45% made no reference to age. Eighty‐six percent of trials placed restrictions on patients with history of prior malignancies. Regarding central nervous system (CNS) metastasis, 416 trials (38%) excluded all patients with CNS metastasis, and 373 (34%) only allowed asymptomatic CNS metastasis. Regarding chronic viral infections, 347 trials (31%) excluded all patients with human immunodeficiency virus, and 228 trials (21%) excluded all patients with hepatitis B or C infection. On univariate analysis, chemotherapy trials were more likely to exclude patients with CNS metastasis and history of other malignancies than targeted therapy trials. Multivariate analysis demonstrated that industry‐sponsored trials had higher odds of excluding patients with compromised liver function.
Conclusion.
Many clinical trials excluded large segments of the population of patients with cancer. Frequent exclusion criteria included patients with CNS metastasis, history of prior malignancies, and chronic viral infections. The criteria for participation in some clinical trials may be overly restrictive and limit enrollment.
Implications for Practice.
The results of this study revealed that most early phase clinic trials contain strict exclusion criteria, potentially excluding the patients who may be more likely to represent the population treated in clinical settings, leaving patients susceptible to unintended harm from inappropriate generalization of trial results. Careful liberalization of the inclusion/exclusion criteria in clinical trials will allow investigators to understand the benefits and drawbacks of the experimental drug for a broader population, and possibly improve recruitment of patients with cancer into clinical trials.
Introduction
Cancer is the second leading cause of mortality in the U.S., and among individuals over the age of 65 it accounts for approximately 22% of all deaths [1]. The last decade has seen rapid advancement in the science of cancer care, leading to significant improvements in survival in a number of malignancies. Unfortunately, the generalizability of these findings to our elderly patients and patients with significant medical comorbidities is unclear, as these populations are generally excluded from clinical trials. Multiple studies have shown that although patients over 65 years of age account for nearly 60% of patients with cancer, less than 25% of patients who participate in clinical trials are in this age group [2].
Although the number of clinical trials registered annually is increasing [3], inclusion and exclusion criteria continue to limit the number of patients eligible for trial participation. These criteria have largely remained unchanged over the past decade despite the introduction of potentially less‐toxic therapies in the form of targeted agents and immunotherapy. This approach selects out many of our older and “sicker” patients and limits the generalizability of the evidence obtained through these clinical trials [4].
Common exclusion criteria other than age can include any number of metrics of organ dysfunction. One reason to exclude those with comorbidities is that complications from underlying comorbidities may be incorrectly attributed to the novel therapy. Despite this widely held belief, in some studies in breast and lung malignancies, older patients were not noted to have increased toxicity to the same treatment doses and regimens compared with younger patients [5], [6], [7]. The field of cancer biology has yet to establish the relationship between comorbidities and toxicity and tolerance of treatments [5], [8]. Over the last years many efforts have taken placed by national and international societies, including the American Society of Clinical Oncology (ASCO) and Friends of Cancer Research (FOCR). Task forces have been created to target common exclusion criteria. As a result, several articles have been published suggesting modifications of common exclusion criteria in order to improve the recruitment of patients into clinical trials [9]. Although these provide a landscape for future trials, the decision to follow these recommendations still rests in the hands of investigators and sponsors.
Prior studies have identified specific comorbidities (i.e., central nervous system [CNS] disease) and demographic criteria such as age as barriers to enrollment. Accordingly, we sought to characterize the most common comorbidities and demographic factors affecting clinical trial participation over the past the past 15 years. Unlike prior reports, our study included clinical trials sponsored by federal agencies, cooperative groups, and industry. We focused on early phase clinical trials because many phase I (with expansion cohorts) and phase II trials were the basis for the Food and Drug Administration (FDA) approval of new agents, including targeted therapies and immune checkpoint inhibitors [10]. Early phase trials tend to have more strict eligibility criteria (or the simple natural selection process of the patient who has been able to tolerate multiple lines of therapy) [11], [12]; as consequence of this, new treatments are approved in a more restricted patient population and produce limited safety data in patients with multiple comorbidities.
Our objective was to methodically identify the comorbidities and other exclusion criteria limiting the recruitment of patients with breast, colorectal, or lung cancers into early phase clinical trials.
Materials and Methods
We queried ClinicalTrials.gov on December 31, 2016, for all early phase therapeutic cancer clinical trials from 2000 to 2015. Primary search terms included lung, breast, and colorectal cancer. This search was further narrowed down by the secondary terms: interventional studies and phase I and phase II clinical trials. “Umbrella” (including several tumor types) trials were included, as many phase I trials have adopted this model over the past 10 years. Surgical, pediatric, and cancer prevention trials were excluded. Trials that did not include systemic anticancer therapy, those with unknown recruitment status, and those without any verification of their status in the last 12 months were also excluded. When data were incomplete or unclear, we contacted the principal investigator or trial coordinator listed on ClinicalTrials.gov to obtain a complete list of the eligibility criteria.
We decided to focus on breast, colorectal, and lung cancer as these represent the cancers with the highest prevalence among men and women (except for prostate cancer). Furthermore, despite multiple advances in the past years, lung cancer remains the number one cause of cancer mortality in the U.S. [13].
For included trials, the following data were extracted: (a) trial phase, (b) target disease, (c) anticancer therapy (chemotherapy, targeted therapy, immunotherapy, or combination therapies), (d) line of therapy (first, second, third, or any line) (e) location (U.S., Europe, Asia, or international), (f) trial sponsor (pharmaceutical industry, university or cooperative groups, or a federal agency [the National Cancer Institute]), and (g) inclusion and exclusion criteria with particular focus on age limits, comorbidities, and organ function. Inclusion criteria were defined as criteria governing entry or recruitment of individuals into trials and describing the medical condition of interest. All other criteria limiting the recruitment of patients were classified as exclusion criteria [14].
Statistical Analysis
Descriptive data are presented as counts and percentages for categorical variables. Associations between trial characteristics and exclusion criteria were evaluated using Fisher's exact test and univariate analysis. For the analysis, pre‐established cutoffs (median) were used for laboratory values, classified as “excluded” or “included,” and treated as a binary variables (Table 2). We used a multivariate logistic regression model to test the association between certain exclusion criteria and trial characteristics. Data analysis was performed using JMP version 10.0 software (SAS Institute, Cary, NC).
Table 2. Exclusion criteria I (organ function).
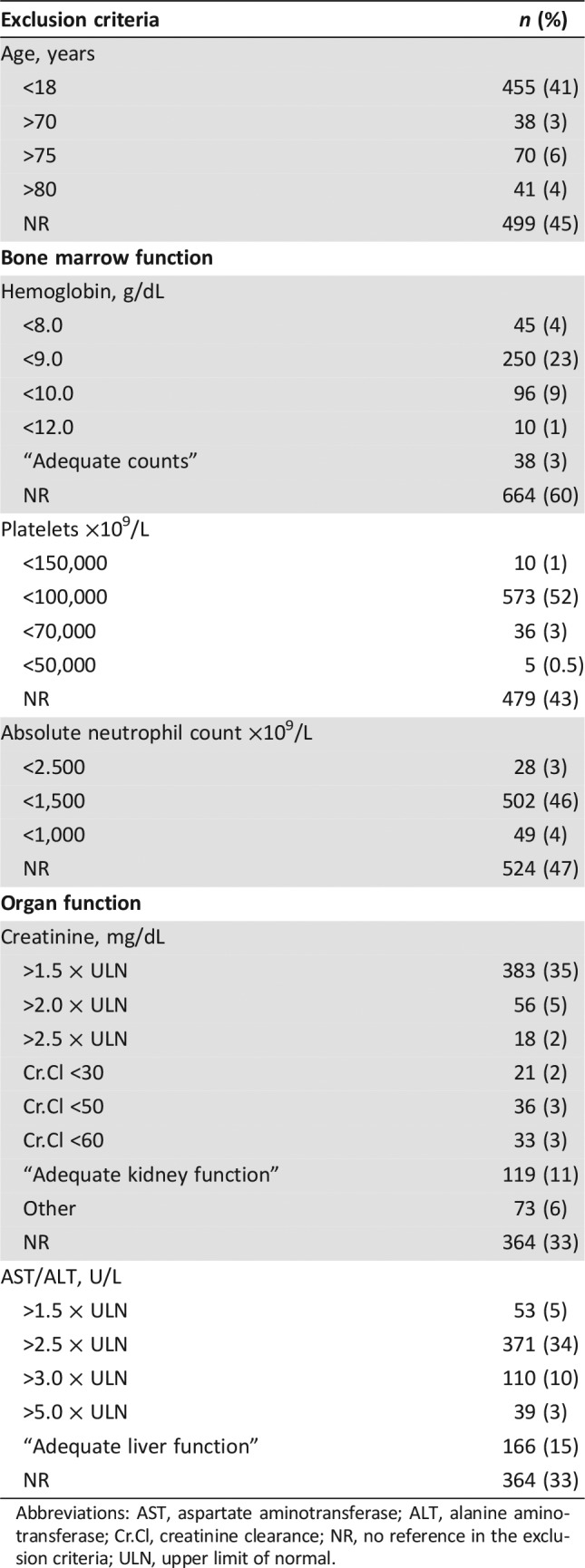
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; Cr.Cl, creatinine clearance; NR, no reference in the exclusion criteria; ULN, upper limit of normal.
Results
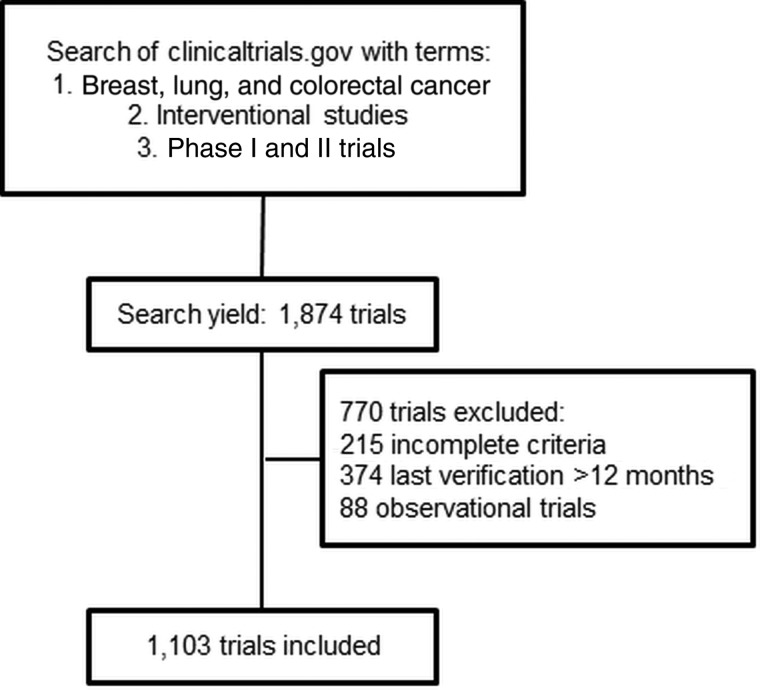
Our initial search yielded 1,874 trials, 771 of which were later excluded. The most common reason of exclusion was incomplete exclusion/inclusion criteria (n = 374, 20%) and lack of trial verification within 12 months (n = 215, 11.5%; Fig. 1). The final analysis included 1,103 trials (59%). Table 1 summarizes the characteristics of the trial population. When the trials were divided by tumor type, 451 (41%) were for breast cancer, 351 (32%) were for colorectal cancer, 228 (21%) were for non‐small cell lung cancer (NSCLC), 61 (5.5%) were for multiple cancers (“umbrella trials,” including cancers of interest), and 10 (1%) were for small cell lung cancer.
Figure 1.
Consort diagram depicting the criteria used to identify trials used in the analysis.
Table 1. Trial characteristics.
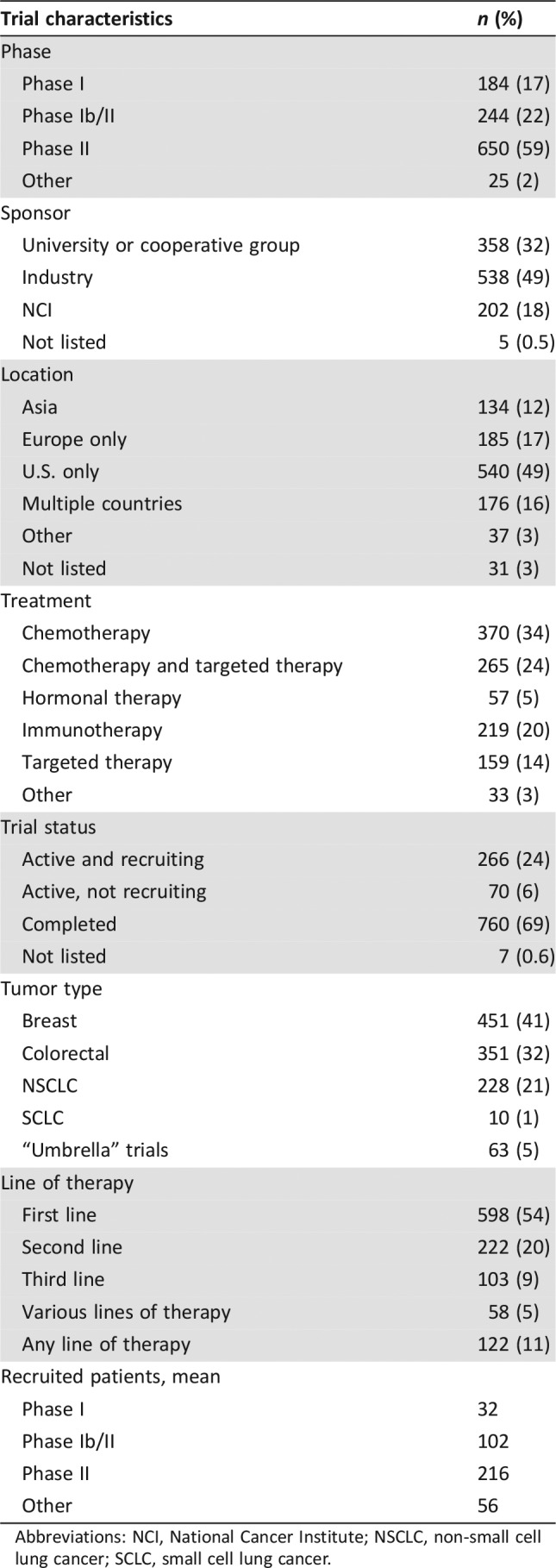
Abbreviations: NCI, National Cancer Institute; NSCLC, non‐small cell lung cancer; SCLC, small cell lung cancer.
Regarding the trial characteristics, 650 trials (59%) were phase II, 244 (22%) were phase Ib/II, 184 (17%) were phase I, and 25 (2%) were classified as “other.” The pharmaceutical industry sponsored 538 trials (49%), 358 trials (33%) were sponsored by a university or cooperative group, and 202 trials (18%) were sponsored by the National Cancer Institute (NCI) or a governmental agency. We also classified the clinical trials by recruitment sites: 540 trials (49%) were conducted in the U.S. only, 185 trials (17%) were conducted in Europe, 134 trials (12%) were conducted in Asia, 176 (16%) were conducted in multiple countries, and 37 trials (3%) were conducted in other locations (including Central and South America and Africa).
We only included trials studying a possibly active systemic anticancer therapy. Out of 1,103 trials, 370 (34) were chemotherapy trials, 265 (24%) included a combination of chemotherapy and targeted therapy, 219 (20%) were immunotherapy trials, 159 (14%) were targeted therapy only trials, 57 (5%) were hormonal therapy trials, and 32 (3%) trials included other types of therapy (Table 1).
Evaluation of Exclusion Criteria
Age.
Most trials required patients to be at least 18 years of age (41%). Only 70 trials (6%) excluded patients older than 75 years, and many (45%) made no reference to age in the exclusion criteria. Tables 2 and 3 show the most frequent exclusion criteria observed in the reviewed clinical trials.
Table 3. Exclusion criteria II (comorbidities).
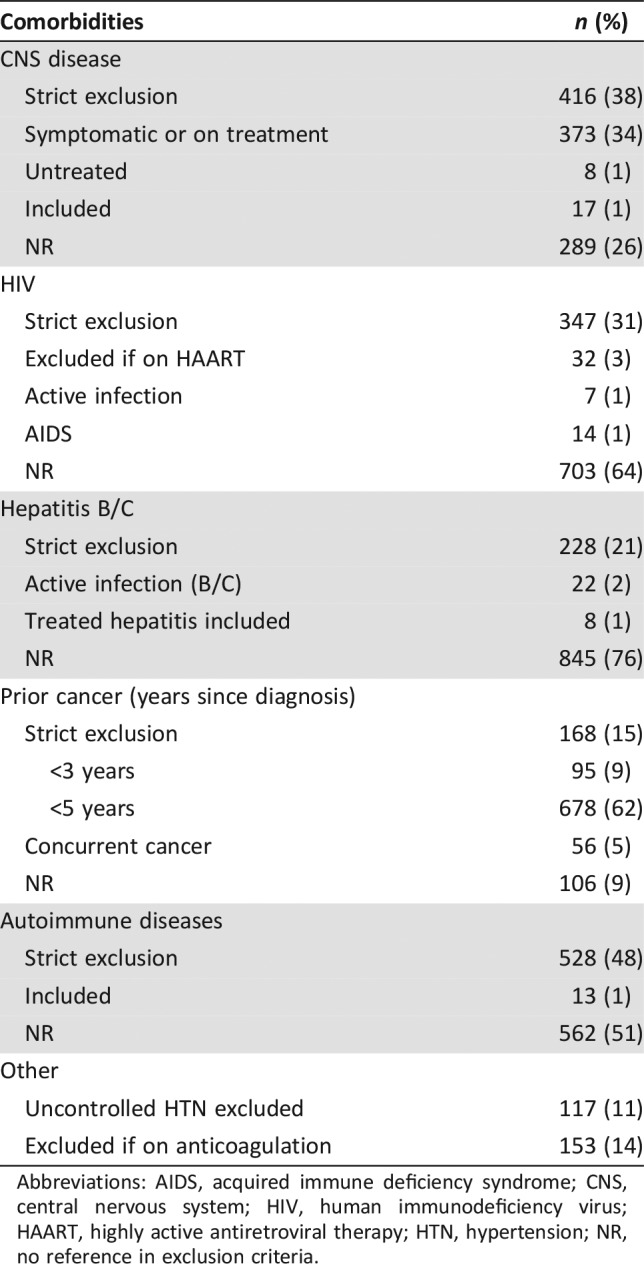
Abbreviations: AIDS, acquired immune deficiency syndrome; CNS, central nervous system; HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; HTN, hypertension; NR, no reference in exclusion criteria.
Performance Status.
Most trials (68%) allowed an Eastern Cooperative Oncology Group performance status of 0–2, but a small percentage of trials (17%) only allowed a performance status of 0–1.
History of Other Malignancies.
Eighty‐six percent of trials placed restriction on patients with history of other malignancies, 168 trials (15%) had a strict exclusion of all patients with any prior cancer history, and 678 trials (62%) excluded all patients with a history of a previous malignancy diagnosed within 5 years of enrollment except for curatively treated basal or squamous cell carcinoma of the skin or carcinoma in situ of the cervix, breast, or bladder.
Other Comorbidities.
Multiple comorbidities played a role in the exclusion of patients. CNS metastases were a frequent exclusion criterion, with 416 trials (38%) excluding all patients with CNS metastasis, 373 trials (34%) allowing for patients with CNS metastasis if asymptomatic and not requiring treatment, 17 trials (1%) including all patients with CNS disease, and 289 trials (26%) not addressing CNS metastases in their enrollment criteria. In the case of immunotherapy trials, 80% of the trials strictly excluded patients with CNS metastasis.
Exclusion of cardiovascular diseases was commonly observed: patients with atrial fibrillation were entirely excluded in 222 trials (20%) and allowed if controlled in 281 trials (25%). Patients with uncontrolled hypertension were excluded in 117 trials (11%).
In our review, 528 trials (48%) excluded all patients with autoimmune diseases except for vitiligo or autoimmune alopecia. Chronic viral infections were also significant exclusion factors, as 347 trials (31%) excluded all patients with human immunodeficiency virus (HIV), 32 trials (3%) included patients with HIV except those on highly active antiretroviral therapy (HARRT), and 703 trials (64%) made no reference to the exclusion or inclusion of patients with HIV. In regard to viral hepatitis, 228 trials (21%) excluded all patients with hepatitis B or C infection, and only 8 trials (1%) specifically allowed for patients with treated hepatitis.
Many trials (24%) did not specify the medical condition that warranted exclusion but listed terms such as “serious illness,” “limiting medical condition,” or “significant disease.”
Organ Function.
Hematologic abnormalities were frequently used as exclusion criteria. Five hundred seventy‐three trials (52%) excluded patients with platelet counts less than 100,000 mcL, 250 trials (23%) excluded patients with hemoglobin less than 9 g/dL, and 502 trials (46%) excluded patients with an absolute neutrophil count less than 1,500 per mm3. Additionally, 716 trials (65%) required “adequate counts” at the time of enrollment.
Renal and hepatic functions were also frequently evaluated as part of eligibility criteria. Creatinine less than 1.5 of the upper limit of normal (ULN) was required in 383 trials (35%), and 119 trials (11%) referred to the need for “adequate kidney function” at the time of enrollment. Hepatic function was more flexible with regard to patient inclusion, as 371 trials (34%) included patients with aspartate aminotransferase (AST) and alanine aminotransferase (ALT) less than 2.5 × ULN, and out of those 371 trials, 200 trials (54%) allowed patients with AST/ALT less than 5.0 × ULN in the presence of liver metastasis.
Temporal Trends
There was a slight trend over time toward less exclusion of patients based on chronologic age, although this was not statistically significant (p < .14). We also observed a shift away from absolute creatinine values to the use of creatinine clearance as an exclusion marker. No other temporal trends in exclusion criteria were observed during the study interval.
Univariate and Multivariate Analysis
Comorbidity exclusion was divided on permitted versus strict exclusion. We used pre‐established cutoffs for exclusion criteria regarding organ function. These were compared with other trial variables (including sponsor, phase, and location) using Pearson correlation.
In the univariate analysis, trials completed in the U.S. were more likely to exclude patients with autoimmune diseases compared with trials conducted outside of the U.S. (p < .01). Industry‐sponsored trials were more likely to exclude patients with compromised liver function compared with trials sponsored by academic institutions or the National Institutes of Health (p < .0001).
Chemotherapy trials were more likely to exclude patients with CNS metastasis (p < .001), HIV infection (p < .02), and history of other malignancies (p < .0002) than targeted therapy trials.
In multivariate analysis, industry‐sponsored trials had higher odds of excluding patients with compromised liver function (odds ratio [OR], 2.21; 95% confidence interval [CI], 1.60–3.07; p < .0001). Alternatively, U.S.‐based trials had higher odds of including patients with hemoglobin levels less than 9 g/dL (OR, 3.09; 95% CI, 1.17–9.11; p < .0001).
Discussion
Clinical trials provide the necessary data to understand the safety and efficacy of new drugs, while offering patients the opportunity to access investigational drugs. Despite this, only 3% of the U.S. population of patients with cancer was enrolled in any clinical trial [15], [16]. The goal of this study was to identify the exclusion criteria limiting the recruitment of patients with breast, lung, and colorectal cancer to cancer clinical trials. We found that most trials have strict criteria, commonly excluding the so‐called real‐life oncology patient.
In regard to organ function, more than one third of the trials excluded patients on the basis of creatinine level (>1.5 × ULN) and hepatic function (>2.5 × ULN). In addition, 11% and 15% of trials described the need for “adequate” renal and hepatic function, respectively. The use of generalized terms like “adequate function” or “adequate counts” can be counterproductive to the enrollment of patients; these can be interpreted in different ways by the research team and can contribute to the exclusion of patients with borderline organ function. These nonspecific terms should be avoided, and more objective parameters should be used. The use of organ function exclusion criteria allows investigators to avoid further organ damage and potentially toxic drug levels in patients with renal or hepatic dysfunction. However, the basis of how certain laboratory value cutoffs were selected remains unclear.
Furthermore, creatinine clearance (CrCl) has been described as a more accurate measure of renal function for adjusting drug doses in patients with chronic kidney disease [17]. The ASCO‐FOCR task force recommends using CrCl in the eligibility criteria, and if renal toxicity is not a direct treatment‐related concern, patients with lower creatinine clearance values of >30 mL/min should be included in trials [18].
In our review, 416 trials (38%) strictly excluded patients with CNS metastasis independently of their clinical status. We observed a higher exclusion of CNS metastasis in immunotherapy trials. Certainly, for programmed cell death 1 inhibitors there had been concern about inducing immune inflammation in the CNS in the presence of brain metastases [19]; however, later studies have shown these agents may be safe and have some effect in controlling CNS disease [20], indicating that the exclusion of patients with CNS metastasis may be unnecessary.
The inclusion of patients with CNS metastasis will allow investigators and regulatory agencies to understand the safety and efficacy of the experimental drug in this unique group of patients without waiting for a subgroup analysis (which is usually underpowered). Alternatively, separate clinical trials may be conducted in parallel or as a separate arm for this population. Similar to our findings, McCoach et al. reported that 14% of NSCLC clinical trials strictly excluded patients with CNS metastasis, with pharmaceutical industry‐sponsored trials having higher odds of excluding patients with brain metastasis than university or investigator‐initiated trials (OR, 2.26; p < .03) [19].
The Response Assessment in Neuro‐Oncology working group, in conjunction with ASCO‐FOCR, has recommended creating dedicated CNS substudies early in the development of a novel drug, exploring CNS pharmacokinetic variables, designing exploratory arms for patients with CNS disease, and including those with treated/stable brain metastasis in clinical trials [21], [22].
We frequently observed exclusion of patients with chronic infections. With the development of new antiviral therapies, viral hepatitis can potentially be managed with minimal side effects. Despite these advances, most trials continued to exclude patients with history of hepatitis infection (21%) [23]. The issue regarding exclusion of HIV seropositive patients is complex. First, this population is at high risk for non‐AIDS related malignancies [24]. Second, most HARRT agents are cytochrome P450 3A4 inhibitors, potentially affecting experimental drug levels and causing severe toxicity. A possible approach to this problem is the inclusion of patients with HIV in mainstream trials, following certain criteria (viral load, CD4 counts, patients with probable long‐term survival), and ongoing monitoring of these criteria during the study [25]. On the other hand, many trials do not require testing for these chronic infections, blurring the understanding of the inclusion versus exclusion of patients with hepatitis or HIV infection. There are benefits of strict eligibility criteria. Standardizing patient populations helps better identify which patients will benefit from the therapy of interest [14]. The ultimate goal is to exclude patients who may have unacceptably high risk of treatment‐related toxicity and/or insufficient efficacy based on the known characteristics of the drug [26]. Clinical practice often leads to treating patients outside of these criteria without knowing if we are exposing these patients to harm.
The advantages of stringent eligibility criteria are achieved at the risk of excluding patients who may be more likely to represent the population treated in the clinic. Strict exclusion criteria may also leave patients susceptible to unintended harm from inappropriate generalization of trial results and prevent patients from receiving beneficial treatment. Careful liberalization of the inclusion/exclusion criteria in clinical trials will allow investigators to understand the benefits and drawbacks of the experimental drug for a broader population, and in a more controlled environment than postmarketing research [27], [28], [29].
To our knowledge, our analysis is the largest review of modern clinical trial participation criteria. Compared with previous reports [14], [23], [30], [31], we analyzed a broad range of clinical trials, including combination trials, across several tumor types and trials sponsored by the NCI, academic institutions or cooperative groups, and the pharmaceutical industry. In addition, to account for the variability of inclusion/exclusion criteria over time, we queried principal investigators about updates affecting the criteria. Many patients with cancer visit ClinicalTrials.gov on a regular basis hoping to find new treatment options; our study reflects the information that is publicly available to patients and community oncologists. Having public strict exclusion criteria can reduce the referral rates of patients to institutions conducting clinical trials.
There are a number of limitations to our study. First, frequent amendments to protocols occur, and these can result in changes to inclusion/exclusion criteria that may not have been captured, as many protocols are kept confidential until the trial results are published. Next, the trials we evaluated were weighted heavily towards those from the U.S. and may not be generalizable. Additionally, there are likely additional factors and variables playing into variability of recruitment of patients into clinical trials beyond our focus on participation criteria.
In the case of first‐in‐human trials, the risks of the experimental agent are not fully known; thus it is reasonable and rational to have strict criteria to avoid unnecessary harm to patients. On the other hand, the formulations of the eligibility criteria in these trials are not entirely dependent on study investigators or sponsors. The FDA plays a significant role in the selection of these criteria. However, the inclusion and exclusion criteria should be revaluated and loosened after the toxicity patterns of the drug are better characterized. Along those lines, patients with previously malignancies should be included in first‐in‐human trials, as these typically do not have long‐term survival endpoints, and history of a previous cancer will not affect the outcome of the trial.
Finally, we want to acknowledge that participation criteria are crucial for the safe conduct of clinical trials; however, these should have a scientific reason and resemble the real‐life patient suffering from the cancer being studied as much as possible. The importance of streamlining clinical trial eligibility has been recognized as a priority by ASCO [32], with the creation of several working groups seeking solutions to the issue [26].
Our results lead to several suggestions to investigators and sponsors: (a) exclusion criteria should be based on scientific and safety reasons; (b) criteria should be re‐evaluated every time an experimental drug moves from phase I to phase II trials, and all unnecessary criteria should be removed; (c) inclusion of “high‐risk” populations in expansion cohorts or the creation of dedicated arms will allow better understanding of the experimental drug; (d) trial exclusion and inclusion criteria should be listed within the trial publication in order to offer a more comprehensive understanding of the study population; and (e) investigators and sponsors should design clinical trials with the goal of including all types of patients.
Conclusion
In this review, we found that most clinical trials excluded large segments of the population of patients with cancer from the benefits of participation in clinical trials. We observed frequent exclusion of patients with CNS metastasis, history of other malignancies, and HIV and hepatitis infection. Future trials should aim to define participation criteria based on scientific data and intermittently re‐evaluate these criteria at all phases of drug development with a goal of including as many patients as are considered safe.
Acknowledgments
Dr. Gonzalez Velez is now affiliated with the Division of Medical Oncology, Mayo Clinic, Scottsdale, Arizona.
Author Contributions
Conception/design: Narjust Duma, Sejal M. Kothadia, Jonas Paludo, Miguel Gonzalez Velez, Julian R. Molina, Ronald S. Go, Aaron S. Mansfield, Alex A. Adjei
Collection and/or assembly of data: Narjust Duma, Sejal M. Kothadia, Miguel Gonzalez Velez
Data analysis and interpretation: Narjust Duma, Sejal M. Kothadia, Tariq U. Azam, Siddhartha Yadav, Jonas Paludo, Jesus Vera Aguilera, Miguel Gonzalez Velez, Thorvardur Ragnar Halfdanarson, Joleen M. Hubbard, Ronald S. Go, Aaron S. Mansfield, Alex A. Adjei
Manuscript writing: Narjust Duma, Sejal M. Kothadia, Tariq U. Azam, Siddhartha Yadav, Jesus Vera Aguilera, Thorvardur Ragnar Halfdanarson, Julian R. Molina, Joleen M. Hubbard, Ronald S. Go, Aaron S. Mansfield, Alex A. Adjei
Final approval of manuscript: Narjust Duma, Sejal M. Kothadia, Tariq U. Azam, Siddhartha Yadav, Jonas Paludo, Jesus Vera Aguilera, Miguel Gonzalez Velez, Thorvardur Ragnar Halfdanarson, Julian R. Molina, Joleen M. Hubbard, Ronald S. Go, Aaron S. Mansfield, Alex A. Adjei
Disclosures
Aaron S. Mansfield: AbbVie, Genentech, Bristol‐Myers Squibb, Trovagene (C/A), Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Weir HK, Anderson RN, Coleman King SM et al. Heart disease and cancer deaths ‐ Trends and projections in the United States, 1969‐2020. Prev Chronic Dis 2016;13:E157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger NA, Savvides P, Koroukian SM et al. Cancer in the elderly. Trans Am Clin Climat Assoc 2006;117:147–156. [PMC free article] [PubMed] [Google Scholar]
- 3.Trends, charts, and maps . ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/resources/trends#typersofregisteredstudies. Accessed August 21, 2017.
- 4.Herrera AP, Snipes SA, King DW et al. Disparate inclusion of older adults in clinical trials: Priorities and opportunities for policy and practice change. Am J Public Health 2010;100(suppl 1):S105–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klepin HD, Pitcher BN, Ballman KV et al. Comorbidity, chemotherapy toxicity, and outcomes among older women receiving adjuvant chemotherapy for breast cancer on a clinical trial: CALGB 49907 and CALGB 361004 (Alliance). J Oncol Pract 2014;10:e285–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard EM, Moon J, Hesketh PJ et al. Comparison of platinum‐based chemotherapy in patients older and younger than 70 years of age: An analysis of Southwest Oncology Group trials 9308 and 9509. J Thorac Oncol 2011;6:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 2005;23:3112–3124. [DOI] [PubMed] [Google Scholar]
- 8.Townsley CA, Chan KK, Pond GR et al. Understanding the attitudes of the elderly towards enrolment into cancer clinical trials. BMC Cancer 2006;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim ES, Bruinooge SS, Roberts S et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol 2017;35:3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garon EB, Rizvi NA, Hui R et al.; KEYNOTE Investigators. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 11.Massett HA, Mishkin G, Rubinstein L et al. Challenges facing early phase trials sponsored by the National Cancer Institute: An analysis of corrective action plans to improve accrual. Clin Cancer Res 2016;22:5408–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomblyn MR, Rizzo JD. Are there circumstances in which phase 2 study results should be practice‐changing? Hematology am Soc Hematol Educ Program 2007:489–492. [DOI] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Fedewa SA et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 14.Van Spall HG, Toren A, Kiss A et al. Eligibility criteria of randomized controlled trials published in high‐impact general medical journals: A systematic sampling review. JAMA 2007;297:1233–1240. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine Forum on Drug Discovery , Development, and Translation. Transforming Clinical Research in the United States: Challenges and Opportunities. Workshop Summary. Washington, D.C.: National Academies Press; 2010. [PubMed] [Google Scholar]
- 16.Yaman A, Chakrabarti S, Sen A et al. How have cancer clinical trial eligibility criteria evolved over time? AMIA Jt Summits Transl Sci Proc 2016;2016:269–278. [PMC free article] [PubMed] [Google Scholar]
- 17.Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. American Fam Physician 2007;75:1487–1796. [PubMed] [Google Scholar]
- 18.Lichtman SM, Harvey RD, Damiette Smit MA et al. Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology‐Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol 2017;35:3753–3759. [DOI] [PubMed] [Google Scholar]
- 19.McCoach CE, Berge EM, Lu X et al. A brief report of the status of central nervous system metastasis enrollment criteria for advanced non‐small cell lung cancer clinical trials: A review of the ClinicalTrials.gov trial registry. J Thorac Oncol 2016;11:407–413. [DOI] [PubMed] [Google Scholar]
- 20.Kluger HM, Goldberg SB, Sznol M et al. Safety and activity of pembrolizumab in melanoma patients with untreated brain metastases. J Clin Oncol 2015;33(suppl 15):9009A. [Google Scholar]
- 21.Lin NU, Lee EQ, Aoyama H et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncology 2015;16:e270–e278. [DOI] [PubMed] [Google Scholar]
- 22.Lin NU, Prowell T, Tan AR et al. Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology‐Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol 2017;35:3760–3773. [DOI] [PubMed] [Google Scholar]
- 23.Beaver JA, Ison G, Pazdur R. Reevaluating eligibility criteria ‐ balancing patient protection and participation in oncology trials. N Engl J Med 2017;376:1504–1505. [DOI] [PubMed] [Google Scholar]
- 24.Deeks SG, Phillips AN. Clinical review: HIV infection, antiretroviral treatment, ageing, and non‐AIDS related morbidity. BMJ 2009;338:288–292. [DOI] [PubMed] [Google Scholar]
- 25.Uldrick TS, Ison G, Rudek MA et al. Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology‐Friends of Cancer Research HIV Working Group. J Clin Oncol 2017;35:3774–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim ES, Bernstein D, Hilsenbeck SG et al. Modernizing eligibility criteria for molecularly driven trials. J Clin Oncol 2015;33:2815–2820. [DOI] [PubMed] [Google Scholar]
- 27.Lara PN Jr, Higdon R, Lim N et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol 2001;19:1728–1733. [DOI] [PubMed] [Google Scholar]
- 28.Hamaker ME, Stauder R, van Munster BC. Exclusion of older patients from ongoing clinical trials for hematological malignancies: An evaluation of the national institutes of health clinical trial registry. The Oncologist 2014;19:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin EH, Scroggins MJ, Goldberg KB et al. Strategies to maximize patient participation in clinical trials. Am Soc Clin Oncol Educ Book 2017;37:216–221. [DOI] [PubMed] [Google Scholar]
- 30.Jin S, Pazdur R, Sridhara R. Re‐evaluating eligibility criteria for oncology clinical trials: Analysis of investigational new drug applications in 2015. J Clin Oncol 2017;35:3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia S, Bisen A, Yan J et al. Thoracic oncology clinical trial eligibility criteria and requirements continue to increase in number and complexity. J Thorac Oncol 2017;12:1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meropol NJ, Kris MG, Winer EP. The American Society of Clinical Oncology's blueprint for transforming clinical and translational cancer research. J Clin Oncol 2012;30:690–691. [DOI] [PubMed] [Google Scholar]