This systematic review provides an overview of the role of radiation therapy in the management of vaginal carcinoma and summarizes data on treatment outcome and toxicity.
Keywords: Radiotherapy, Vaginal neoplasms, Systematic review, Squamous cell carcinoma
Abstract
Aim.
This study systematically reviews the recent literature on the role of definitive radiotherapy (RT) in the management of vaginal cancer (VC) and presents comprehensive data on clinical outcomes and toxicity.
Methods.
The authors performed a literature search using PubMed (2007–2016) to identify all prospective and retrospective studies that have been published on RT in invasive VC.
Results.
Of the 199 identified studies, 13 met the inclusion criteria. All studies had a retrospective design. Overall, 793 patients (median, 45; range, 26–138) were included. A high heterogeneity was found across studies in terms of RT techniques, assessment criteria, and reported outcomes. The majority of the patients were treated with a combination of external beam RT and brachytherapy (74.2%). Acute and late grade ≥3 toxicity rates ranged from 0.0% to 24.4% (median, 8.7%) and from 0.0% to 22.5% (median, 12.8%), respectively. The 5‐year local control rates ranged between 39% and 79%. The 5‐year overall survival ranged between 34% and 71.0% (median, 63.5%). Early stage of the disease (International Federation of Gynecology and Obstetrics stages I–II vs. III–IV), small tumor size (<4 cm), previous hysterectomy, high pretreatment/treatment hemoglobin levels (≥12/12.5 mg/dL), and patients’ age <70 or <64 years were correlated with better clinical outcomes.
Conclusion.
Only retrospective studies, in a limited number, have been published on RT in VC in the past decade, with significant heterogeneity in terms of treatment characteristic and evaluation criteria. Clinical results were strongly influenced by tumor stage. Prospective randomized studies are needed to improve patients’ outcomes, especially in advanced‐stage disease.
Implications for Practice.
This study systematically reviews the recent literature on the role of definitive radiotherapy in the management of vaginal cancer and presents comprehensive data on clinical outcome and toxicity. The prognosis of patients is dismal, with a 5‐year overall survival of approximately 50%. Early stage of the disease, small tumor size, previous hysterectomy, high pretreatment/treatment hemoglobin levels, and patients’ age were correlated with a better clinical outcome. A brachytherapy boost should be delivered, especially in patients with higher‐stage disease. The addition of concurrent weekly cisplatin should be considered in most patients, and transfusion should be used to maintain high hemoglobin levels.
Introduction
Vaginal carcinoma (VC) is a rare malignancy accounting for 1% to 2% of all gynecological cancers [1]. The most common histology is squamous cell carcinoma (SCC; 85%) followed by adenocarcinoma (AC; 10%–15%) [2]. Because of its anatomical location and its extensive lymphatic interconnections, the vagina is prone to metastases from other gynecologic malignancies or tumor infiltration from adjacent sites. Favorable prognostic factors, which significantly correlate with better outcome, include early clinical stage, young age, small tumor size, high hemoglobin (Hgb) levels, and previous hysterectomy (PH) [2]. Although surgery yields good local control (LC) and overall survival (OS) in selected cases of vaginal intraepithelial neoplasms and early (I–II) stages of VC [3], definitive radiation therapy (RT) based on external beam RT (EBRT) and/or brachytherapy (BT) is considered a standard treatment option [4], [5], [6]. Data available on the role of RT in the management of VC show several limitations: small sample size, a retrospective design with the inclusion of patients treated decades ago, and large heterogeneity in terms of radiation dose and technique. Currently, no prospective trials and no systematic reviews based on modern RT technology are available, to the best of our knowledge. This study systematically reviews the recent medical literature, with the aim to provide an accurate overview on the role of RT in management of VC and to evaluate and summarize data on treatment outcomes and toxicity.
Materials and Methods
To identify studies to include in our review, we conducted a systematic search of the PubMed database, using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses methodology with the following search criteria: vaginal neoplasms [MeSH Terms] OR (vaginal [All Fields] AND neoplasms [All Fields]) OR vaginal neoplasms [All Fields] OR (vaginal [All Fields] AND cancer [All Fields]) OR vaginal cancer [All Fields] AND (radiotherapy [Subheading] OR radiotherapy [All Fields] OR radiotherapy [MeSH Terms]). All full text English‐language articles related to RT for treatment of primary VC were identified and reviewed. The articles that met the following inclusion criteria were retained in the final analysis: (a) clinical prospective or retrospective studies on patients with histological confirmation of primary VC; (b) sample sizes of ≥25 patients; (c) RT based on EBRT and/or BT; (d) studies published in English in the last 10 years (2007–2016). Studies on adjuvant RT, planning studies, case reports, review articles, published conference abstracts, and studies on vaginal recurrences from other tumors or including patients with previous pelvic irradiation were excluded (Tables 1 and 2).
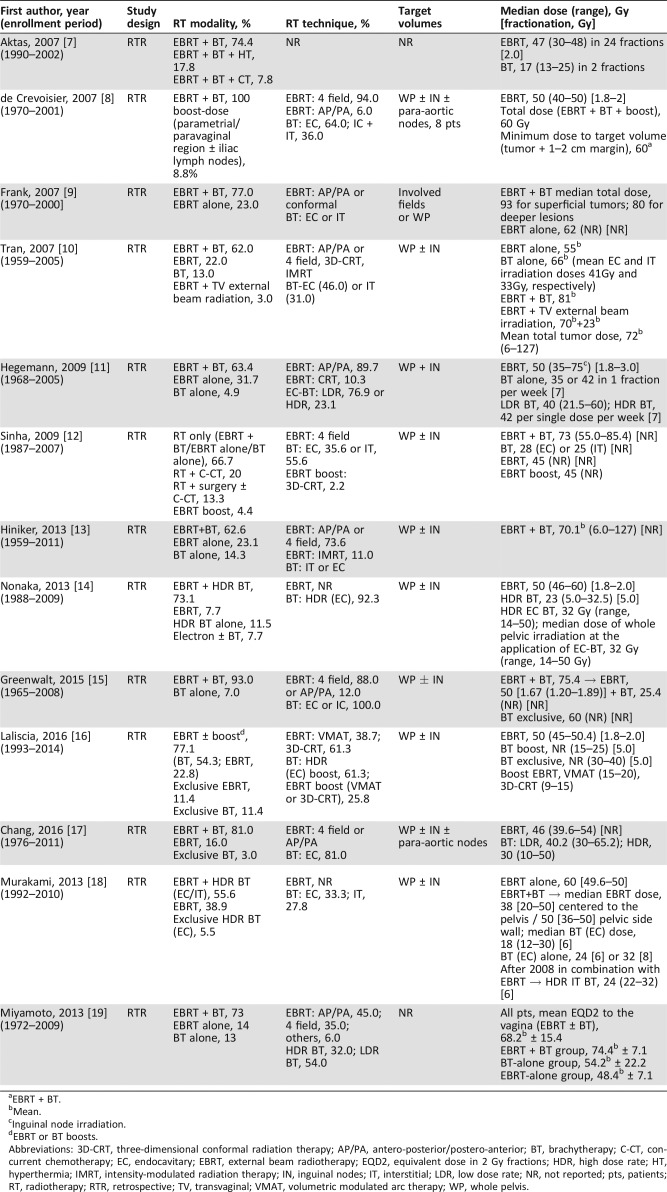
Table 1. Study and radiotherapy characteristics.
EBRT + BT.
Mean.
Inguinal node irradiation.
EBRT or BT boosts.
Abbreviations: 3D‐CRT, three‐dimensional conformal radiation therapy; AP/PA, antero‐posterior/postero‐anterior; BT, brachytherapy; C‐CT, concurrent chemotherapy; EC, endocavitary; EBRT, external beam radiotherapy; EQD2, equivalent dose in 2 Gy fractions; HDR, high dose rate; HT, hyperthermia; IMRT, intensity‐modulated radiation therapy; IN, inguinal nodes; IT, interstitial; LDR, low dose rate; NR, not reported; pts, patients; RT, radiotherapy; RTR, retrospective; TV, transvaginal; VMAT, volumetric modulated arc therapy; WP, whole pelvis.
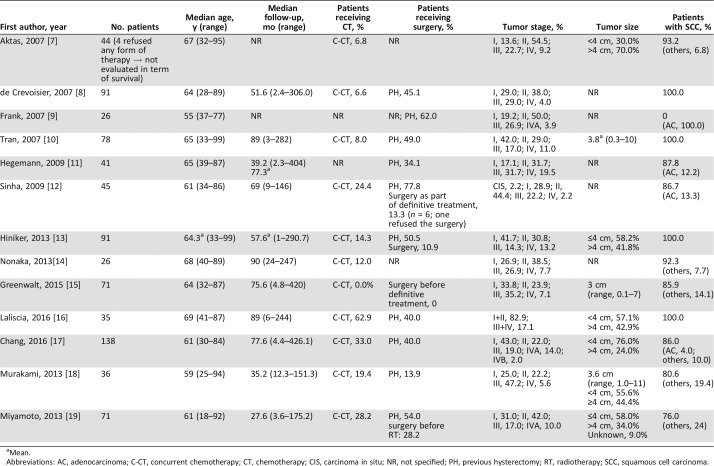
Table 2. Patients and tumor characteristics.
Mean.
Abbreviations: AC, adenocarcinoma; C‐CT, concurrent chemotherapy; CT, chemotherapy; CIS, carcinoma in situ; NR, not specified; PH, previous hysterectomy; RT, radiotherapy; SCC, squamous cell carcinoma.
As a supplementary method, the citation lists of all the included articles were screened independently and in duplicate by two independent authors (M.B., M.F.) at the title and abstract level to identify other potentially relevant studies without any duplication. Potentially eligible citations were retrieved for full‐text review, and any uncertainty was resolved by another member of the reviewing team (A.G.M.). For each study, we extracted the following data: first author's last name, enrollment period, study design (retrospective or prospective), number of patients, median age, median length of follow‐up, percentage of patients receiving concurrent chemotherapy (C‐CT) or surgery, RT modality and technique, target volumes, median dose and fractionation schedule, characteristic of the tumor, International Federation of Gynecology and Obstetrics (FIGO) stage, tumor size, percentage of SCC histological subtype, toxicity, LC, progression‐free survival (PFS), OS, and outcomes of interest. When feasible, we reported separately the results in terms of acute and late radiation‐induced toxicity. Side effects were considered as acute or late if recorded before or after the 90th day from the start of radiotherapy, respectively. No meta‐analysis was performed because of the large heterogeneity between studies in terms of reported outcomes and assessment criteria.
Results
Study Characteristics
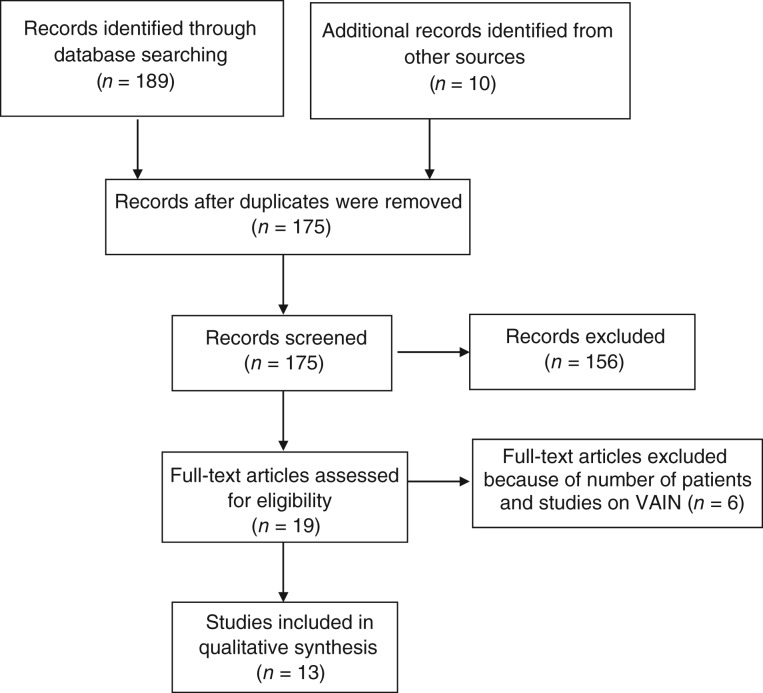
From a total of 199 retrieved studies, 13 articles reporting data on 793 patients (median, 45; range, 26–138) met our inclusion criteria [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], as shown in Figure 1. The patients included in the analyzed studies were treated between 1959 and 2014, and the median follow‐up for the whole population ranged from 27.6 to 90.0 months. In one study, follow‐up information was reported in terms of mean follow‐up [13], and in another, follow‐up information was incomplete [9]. All analyzed studies had a retrospective design. Tumor size was not reported in five studies [8], [9], [11], [12], [14]. In four studies, all treated patients had SCC VC [8], [10], [13], [16], and in one study all treated patients had AC VC [9].
Figure 1.
Process of paper selection. Abbreviation: VAIN, vaginal intraepithelial neoplasm.
The treatment strategy was different in each study and involved combinations of EBRT, BT (endocavitary [EC] and/or interstitial [IT]), C‐CT, and surgical resection. Different treatment combinations of radiation therapy were used (EBRT + BT and/or EBRT alone and/or BT alone); a majority of the patients were treated with a combination of EBRT and BT (74.2%) [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. In ten studies, a minority of patients (20.9%) received a combination of RT and chemotherapy [7], [8], [10], [12], [13], [14], [16], [17], [18], [19]. In four studies, RT was combined with up‐front surgery in 14.7% of patients [10], [12], [13], [19], and in one study, hyperthermia was administered to 15.9% of the patients [7]. In ten studies, 46.3% of the patients underwent PH [8], [9], [10], [11], [12], [13], [16], [17], [18], [19].
Concerning radiotherapy technique, a large variety of beam arrangements (antero‐posterior/postero‐anterior [AP/PA], 4‐fields, three‐dimensional conformal RT [3D‐CRT], intensity‐modulated RT [IMRT], volumetric modulated arc therapy [VMAT]) were reported across studies, and these data were incomplete [14], [18] or lacking [7] in three papers. The whole pelvis was irradiated alone, with or without inguinal nodes [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], or with or without para‐aortic nodes [8], [17]. Target volume data were lacking in two studies [7], [19].
Toxicity
The Common Terminology Criteria for Adverse Events scale [9], [10], [13], [14], [15], [17], the Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer scale [11], [12], [16], or the Franco‐Italian glossary [8] were used to grade and report toxicity. Two papers failed to report the grading scale [7], [18], and toxicity data were totally unreported in one paper [19]. Short‐term adverse effects data were lacking in a majority of studies [8], [9], [10], [11], [13], [15], [19]; the most frequently reported short‐term side effects were genitourinary [7], [16], [17] and gastrointestinal [7], [12], [17], whereas hematological [12] and skin [17] toxicities were less frequently reported, as detailed in Table 3.
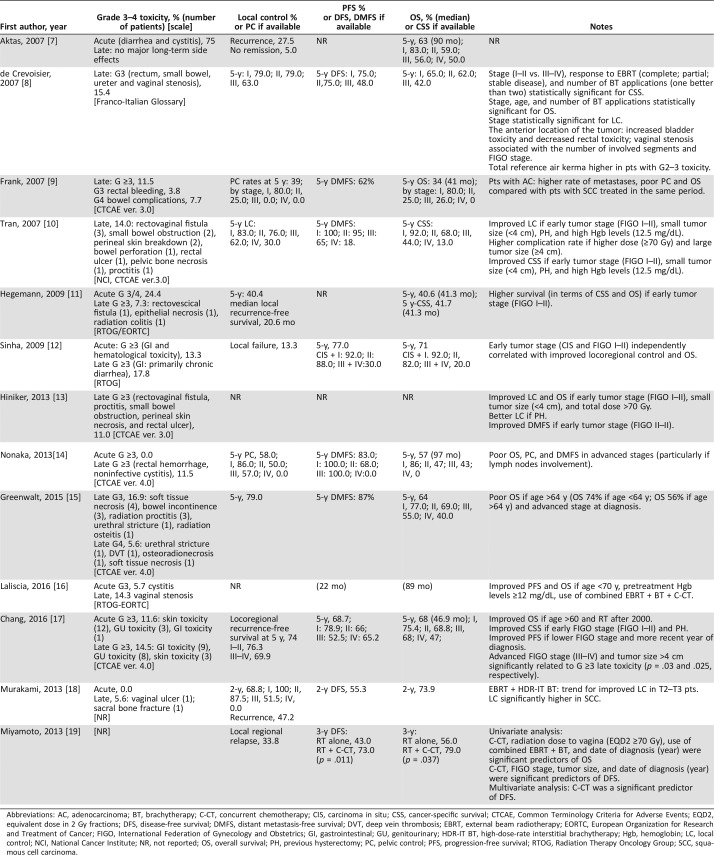
Table 3. Toxicity and outcome.
Abbreviations: AC, adenocarcinoma; BT, brachytherapy; C‐CT, concurrent chemotherapy; CIS, carcinoma in situ; CSS, cancer‐specific survival; CTCAE, Common Terminology Criteria for Adverse Events; EQD2, equivalent dose in 2 Gy fractions; DFS, disease‐free survival; DMFS, distant metastasis‐free survival; DVT, deep vein thrombosis; EBRT, external beam radiotherapy; EORTC, European Organization for Research and Treatment of Cancer; FIGO, International Federation of Gynecology and Obstetrics; GI, gastrointestinal; GU, genitourinary; HDR‐IT BT, high‐dose‐rate interstitial brachytherapy; Hgb, hemoglobin; LC, local control; NCI, National Cancer Institute; NR, not reported; OS, overall survival; PH, previous hysterectomy; PC, pelvic control; PFS, progression‐free survival; RTOG, Radiation Therapy Oncology Group; SCC, squamous cell carcinoma.
The severe late toxicity rates (grade ≥3) ranged from 0.0% to 22.5% (median: 12.8%) and were reported in 12 studies [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]. The most frequently reported severe late side effects were gastrointestinal and genitourinary, as detailed in Table 3.
Outcome
LC data were lacking in five studies [7], [12], [13], [16], [19], and the crude rate of locoregional relapse or no remission reported were 13.3% [12], 32.5% [7], and 33.8% [19], respectively. Five studies reported 5‐year LC rates ranging from 39% to 79% [9], [11], [14], [15], [17]. One study reported a 2‐year LC rate of 68.8% [18]. When specified, the 5‐year LC according to the stages ranged as follows: stage I, 79.0%–86.0%; stage II, 25.0%–79.0%; stage III, 0.0%–63.0%; stage IV, 0.0%–30.0% [8], [9], [10], [14].
Concerning the survival analysis, different types of survival rates were analyzed, including OS, cancer‐specific survival (CSS), PFS, distant metastasis‐free survival (DMFS), and disease‐free survival (DFS), as detailed in Table 3. Among these, OS was the most frequently used. OS data were lacking in two studies [13], [16], and in seven studies the 5‐year OS ranged between 34% and 71.0% (median, 63.5%) [7], [9], [11], [12], [14], [15], [17]. Six studies reported median OS ranging between 41 and 97 months (median, 68 months) [7], [9], [11], [14], [16], [17]. One study reported 2‐year OS of 73.9% [18]. One study reported 3‐year OS for patients treated with RT alone and with RT plus C‐CT of 56.0% and 79.0%, respectively [19]. When specified, the 5‐year OS ranged according to the stages as follows: stage I, 65.0–92.0%; stage II, 25.0–82.0%; stage III, 26.0–68.0%; stage IV, 0.0–50.0% [7], [8], [9], [10], [12], [14], [15], [17].
Early stage of the disease (FIGO stages II–II vs. III–IV), small tumor size (<4 cm), PH, and high pretreatment/treatment Hgb levels (≥12/12.5 mg/dL) were correlated with a better outcome (in terms of improved OS/LC/CSS/DMFS/DFS).
Several parameters (including patient characteristics, tumor characteristics, and treatment modalities) were collected and correlated with the different survival indices used in the various studies to test their impact on clinical outcome. In particular, early stage of the disease (FIGO stages II–II vs. III–IV), small tumor size (<4 cm), PH, and high pretreatment/treatment Hgb levels (≥12/12.5 mg/dL) were correlated with a better outcome (in terms of improved OS/LC/CSS/DMFS/DFS) [8], [10], [11], [12], [13], [14], [15], [16], [17]. In three studies, the use of a combined treatment strategy (EBRT + BT ± C‐CT/high‐dose‐rate [HDR] IT BT + EBRT) was associated with a better outcome. In the study by Laliscia et al., the combination of EBRT + BT and the use of C‐CT were significantly associated with an improved OS (p = .009 and .009, respectively) and PFS (p = .007 and .02, respectively) [16]. Murakami et al. documented a marginally favorable LC (p = .064) with the use of EBRT + HDR IT BT in the subgroup of patients with T2–T3 disease [18]. In the study by Miyamoto et al., the use of C‐CT was a significant predictor of DFS (p = .04) [19]. In the study by Hiniker et al., a high radiation dose (≥70 Gy) was associated with an improved OS and LC [13]; in the study by Miyamoto et al., an equivalent dose in 2 Gy fractions ≥70 Gy was a significant predictor of OS on univariate analysis (but not on multivariate analysis) [19]. However, Tran et al. reported a statistically significant higher likelihood of late severe complications with high radiation dose (≥70 Gy) and large tumor size (≥4 cm) [10], and de Crevoisier et al. documented a high rate of late grade 2–3 urinary and digestive complications with the use of high total reference air kerma (p = .03) [8].
The correlation of age with outcomes was contradictory. Greenwalt et al. and Laliscia et al. reported improved OS and PFS in younger patients (patients aged <64 and <70 years, respectively) [15], [16] whereas Chang et al. reported an improved OS in patients over 60 years of age [17].
In the study by Frank et al. [9], which included only patients with AC VC, the authors recorded a higher incidence of metastases and poor LC and OS compared with patients with SCC VC treated in the same period [4]. Murakami et al. reported poor LC for patients with a non‐SCC histologic subtype of VC (p < .001) [18].
Impact of Concurrent Chemotherapy
In trying to evaluate more specifically the role of C‐CT, it should be emphasized that a separate analysis of the results of concurrent chemoradiation is particularly difficult. In fact, only five [12], [16], [17], [18], [19] of the ten studies in which some patients received C‐CT reported the specific results in this subgroup of patients or presented a comparison with RT alone. In four studies, C‐CT was based on weekly cisplatin in most patients [12], [16], [18], [19], and in another study the chemotherapy drugs were not specified [17]. Only one study reported toxicity in patients undergoing C‐CT (grade ≥3 acute, 20%; late, 20%) [12]. Three studies reported LC rates as follows: 5‐year, 63.7% [17]; 2‐year, 64.3% [18]; and a crude rate of locoregional failures, 15% [19]. Also, survival was reported in only three studies and was described in an inhomogeneous way: 2‐year OS, 71.4% [18]; 3‐year OS, 79% [19]; and 5‐year OS, 47.8% [17]. In two studies, a similar incidence of toxicity was observed among patients undergoing C‐CT and RT alone [12], [16]. In terms of outcomes, two studies showed improved DFS and OS in patients undergoing C‐CT [16], [19]. In three other studies, there were no significant differences in terms of LC and OS [12], OS alone [17], and LC, DFS, and OS [18]. However, in these three studies, C‐CT patients had a more advanced stage of disease [12], [17], [18] and/or bulky disease in a higher percentage of cases [17], [18].
Discussion
This systematic literature review presents a qualitative analysis on the role of definitive RT in invasive VC. Over decades, despite modern technology and techniques, no reports on either prospective or randomized controlled trials exist on VC. We described 13 publications reporting on any form of RT protocol, clinical results, and outcomes.
The main limit of this analysis is represented by the retrospective design of all included studies, as well as the long enrollment period. This issue could introduce obvious risks in terms of bias, particularly for toxicity evaluation, which was inevitably performed retrospectively (e.g., at the time of treatment of most included patients, neither RTOG nor CTC scales were yet published). Furthermore, reported series are probably inhomogeneous in terms of staging procedures as well as treatment techniques, resulting in heterogeneous treatment even in the same study. However, a strong point of our analysis is the prolonged follow‐up time in most series, with 7 of 11 studies reporting median follow‐up time >5 years.
In terms of toxicity, some series reported non‐negligible rates of serious late‐term complications such as rectovaginal fistulas, vaginal stenosis, urethral stricture, bowel injuries (ulceration, obstruction, or perforation), and skin and bone necrosis [8], [9], [10], [12], [13], [14], [15], [16], [17]. Going into details of these latter studies, the reasons for this relatively poor tolerance can vary. Follow‐ups longer than 5 years as well as the use, in some subgroups, of an old‐fashioned RT technique (AP/PA opposed fields) could justify severe late toxicity reporting. In particular, Tran et al. and de Crevoisier et al. documented a statistically significant higher likelihood of late severe complications with the use of high radiation dose (≥70 Gy) and high total reference air kerma, respectively [8], [10]. However, the use of concurrent chemoradiation does not seem to be related to severe late toxicity. In the study of Laliscia et al., in which C‐CT was prescribed in 62.9% of patients (the highest rate of the included studies), the only reported late toxicity was vaginal stenosis. However, we cannot exclude that the positive results of the latter series were also produced by use of modern RT techniques (3D‐CRT, VMAT) in all patients and the relatively low total dose delivered (in most cases, ERBT up to a total dose of 45–50.4 Gy followed by BT boost of 15–25 Gy were prescribed) [16].
In terms of LC, we found a large discrepancy between two studies reporting 5‐year LC of 40.4% [11] and 79.0% [15], respectively. This issue could be explained by a larger number of low‐stage patients (stage I–II: 57.7% vs. 48.8%) and the higher percentage of patients treated with BT boost (93.0% vs. 63.4%) in the Greenwalt et al. study. Four studies reported actuarial LC according to stage [8], [9], [10], [14]. Three series had similar results (stage I, 79.0%, 83.0%, 86%; stage II, 79.0%, 76.0%, 50%; stage III, 63.0%, 62.0%, 57%, respectively) [8], [10], [14], whereas another reported clearly worse outcomes (stage II, 25.0%; stage III, 0.0%) [9]. It could easily be argued that the different VC histology of enrolled patients (100% SCC in [8], [10], 92.3% in [14], and 100% AC in [9]) could have played an important role in reported outcomes.
Among the different types of survival rates analyzed, OS was the most frequently used. Analyzing in detail the OS data, seven studies reported 5‐year actuarial results [7], [9], [11], [12], [14], [15], [17]. Frank et al. [9] and Hegemann et al. [11] reported the worst results: 34% and 40.6%, respectively. Indeed, the Frank et al. series, as previously mentioned, included all patients with AC histology [9], and Hegemann et al. [11] reported on a population of patients with relatively low rates of early‐stage disease (stage I–II, 48.8%) treated with combined EBRT + BT in 63.4% of cases; moreover, the paper does not state the proportion of patients treated with C‐CT [11]. However, the best results in terms of OS were reported by Sinha et al. [12] and by Chang et al. [17] (5‐year OS, 71% and 68%, respectively). It should be noted that in the Sinha et al. series, even patients with carcinoma in situ were included and that a high percentage of patients received combined modality treatment (RT + C‐CT, 20%; RT + surgery ± C‐CT, 13.3%) [12]; in the Chang et al. series, a high percentage of patients with early‐stage disease (stage I–II, 65%) and small tumors (<4 cm, 76.0%) were enrolled [17].
In terms of prognostic factors, reviewed papers recognize the role of several parameters. Early FIGO stage was correlated with improved LC [8], [10], [12], [13], [14], CSS [8], [10], [11], [17], DMFS [13], [14], PFS [17], DFS [19], and OS [8], [11], [12], [13], [14], [15]. Patients receiving higher RT doses showed improved LC [13] and OS [13], [19], although sometimes at the cost of increased toxicity [8], [10]. The use of combined modalities of treatment (EBRT + BT ± C‐CT) was to be correlated with improved LC [18], PFS [16], OS [16], [19], and DFS [19]. PH was associated with improved LC [10], [13] and CSS [10], [17]; high pretreatment/treatment Hgb levels were significantly associated with improved LC [10], [16], CSS [10], OS, and PFS [16]. However, the correlation of age with outcomes was contradictory in different studies: two papers reported improved OS in younger patients [15], [16], and one paper reported better OS in older patients [17].
Conclusion
Although we included in our review only publications of the past decade, the clinical outcomes of VC after RT remain disappointing based on the recorded low 5‐year OS rates (median stage I, 80%; stage II, 62%; stage III, 49%; stage IV, 40 %). Given some evidence emerging from our analysis and based on some guidelines on VC treatment [20], [21], the following indications for RT can be suggested. In stage I patients, BT alone should be reserved only for highly selected cases with superficial and low‐grade disease. In patients with infiltrating and/or high‐grade VC and/or VC of the lower third of the vagina, BT should be used as a boost after pelvic EBRT, possibly associated with C‐CT. In stage II and in patients with locally advanced disease (stage III–IVa), a combined modality approach based on EBRT + C‐CT followed by BT boost is recommended. BT technique (EC or IT) should be chosen based on the residual disease after pelvic irradiation, with EC‐BT reserved only for patients with minimal residual disease (<5 mm thick). In more infiltrating VC, the BT technique of choice should be IT. EBRT should be performed using a conformal technique, although the use of IMRT [13] or VMAT [16] seems to reduce acute toxicity. The initial dose of EBRT should be 45–50 Gy, and the overall tumor dose (EBRT + BT) should be at least 70 Gy (75–80 Gy in patients with bulky disease). In patients with stage III–IV disease, an EBRT boost on the lateral pelvic wall up to 55–60 Gy is also indicated.
In stage I patients, BT alone should be reserved only for highly selected cases with superficial and low‐grade disease. In patients with infiltrating and/or high‐grade VC and/or VC of the lower third of the vagina, BT should be used as a boost after pelvic EBRT, possibly associated with C‐CT.
Our review shows that even in recent reports, the prognosis of patients with VC is dismal, with a 5‐year OS of approximately 50%. Further trials should be addressed to improve these results by exploiting new RT techniques (image guided BT, EBRT, and/or adaptive RT), by paying more attention to supportive therapy, particularly in terms of anemia correction, and by intensified multimodal treatments, especially in patients who have a dismal prognosis because of adenocarcinoma histology or advanced FIGO stage.
Author Contributions
Conception/design: Pierandrea De Iaco, Alessio G. Morganti
Provision of study material or patients: Sara Guerri, Milly Buwenge, Martina Ferioli, Alessandra Arcelli
Collection and/or assembly of data: Sara Guerri, Anna M. Perrone, Gabriella Macchia, Rezarta Frakulli
Data analysis and interpretation: Luca Tagliaferri, Gabriella Ferrandina, Andrea Galuppi, Angela D. Andrulli, Silvia Cammelli
Manuscript writing: Sara Guerri, Anna M. Perrone, Gabriella Macchia, Alessio G. Morganti
Final approval of manuscript: Sara Guerri, Anna M. Perrone, Pierandrea De Iaco, Alessio G. Morganti
Disclosures
The authors indicated no financial relationships.
References
- 1.Parkin DM, Bray F, Ferlay J et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 2.Beller U, Maisonneuve P, Benedet JL et al. Carcinoma of the vagina. Int J Gynaecol Obstet 2003;83(suppl 1):27–39. [DOI] [PubMed] [Google Scholar]
- 3.Jain V, Sekhon R, Giri S et al. Role of radical surgery in early stages of vaginal cancer–our experience. Int J Gynecol Cancer 2016;26:1176–1181. [DOI] [PubMed] [Google Scholar]
- 4.Frank SJ, Jhingran A, Levenback C et al. Definitive radiation therapy for squamous cell carcinoma of the vagina. Int J Radiat Oncol Biol Phys 2005;62:138–147. [DOI] [PubMed] [Google Scholar]
- 5.Kucera H, Mock U, Knocke TH et al. Radiotherapy alone for invasive vaginal cancer: Outcome with intracavitary high dose rate brachytherapy versus conventional low dose rate brachytherapy. Acta Obstet Gynecol Scand 2001;80:355–360. [PubMed] [Google Scholar]
- 6.Chyle V, Zagars GK, Wheeler JA et al. Definitive radiotherapy for carcinoma of the vagina: Outcome and prognostic factors. Int J Radiat Oncol Biol Phys 1996;35:891–905. [DOI] [PubMed] [Google Scholar]
- 7.Aktas M, de Jong D, Nuyttens JJ et al. Concomitant radiotherapy and hyperthermia for primary carcinoma of the vagina: A cohort study. Eur J Obstet Gynecol Reprod Biol 2007;133:100–104. [DOI] [PubMed] [Google Scholar]
- 8.de Crevoisier R, Sanfilippo N, Gerbaulet A et al. Exclusive radiotherapy for primary squamous cell carcinoma of the vagina. Radiother Oncol 2007;85:362–370. [DOI] [PubMed] [Google Scholar]
- 9.Frank SJ, Deavers MT, Jhingran A et al. Primary adenocarcinoma of the vagina not associated with diethylstilbestrol (DES) exposure. Gynecol Oncol 2007;105:470–474. [DOI] [PubMed] [Google Scholar]
- 10.Tran PT, Su Z, Lee P, Lavori P et al. Prognostic factors for outcomes and complications for primary squamous cell carcinoma of the vagina treated with radiation. Gynecol Oncol 2007;105:641–649. [DOI] [PubMed] [Google Scholar]
- 11.Hegemann S, Schäfer U, Lellé R et al. Long‐term results of radiotherapy in primary carcinoma of the vagina. Strahlenther Onkol 2009;185:184–189. [DOI] [PubMed] [Google Scholar]
- 12.Sinha B, Stehman F, Schilder J et al. Indiana University experience in the management of vaginal cancer. Int J Gynecol Cancer 2009;19:686–693. [DOI] [PubMed] [Google Scholar]
- 13.Hiniker SM, Roux A, Murphy JD et al. Primary squamous cell carcinoma of the vagina: Prognostic factors, treatment patterns and outcomes. Gynecol Oncol 2013;131:380–385. [DOI] [PubMed] [Google Scholar]
- 14.Nonaka T, Nakayama Y, Mizoguchi N et al. Definitive radiation therapy for invasive carcinoma of the vagina: Impact of high‐dose rate intracavitary brachytherapy. Int J Clin Oncol 2013;18:314–320. [DOI] [PubMed] [Google Scholar]
- 15.Greenwalt JC, Amdur RJ, Morris GC et al. Outcomes of definitive radiation therapy for primary vaginal carcinoma. Am J Clin Oncol 2015;38:583–587. [DOI] [PubMed] [Google Scholar]
- 16.Laliscia C, Fabrini MG, Delishaj D et al. Concomitant external‐beam irradiation and chemotherapy followed by high‐dose rate brachytherapy boost in the treatment of squamous cell carcinoma of the vagina: A single‐center retrospective study. Anticancer Res 2016;36:1885–1890. [PubMed] [Google Scholar]
- 17.Chang JH, Jang WI, Kim YB et al. Definitive treatment of primary vaginal cancer with radiotherapy: Multi‐institutional retrospective study of the Korean Radiation Oncology Group (KROG 12‐09). J Gynecol Oncol 2016;27:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami N, Kasamatsu T, Sumi M et al. Radiation therapy for primary vaginal carcinoma. J Radiat Res 2013;54:931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto DT, Viswanathan AN. Concurrent chemoradiation for vaginal cancer. PLoS One 2013;8:e65048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee LJ, Jhngran A, Kidd E et al.; Expert Panel on Radiation Oncology. American College of Radiology ACR Appropriateness Criteria: Management of Vaginal Cancer. Reston, VA: American College of Radiology; 2013. Available at https://acsearch.acr.org/docs/3082701/Narrative/. Accessed on April 28, 2018.
- 21.PDQ Adult Treatment Editorial Board, National Cancer Institute . Treatment option overview: Roles of radiation, surgery, and chemotherapy. In: Vaginal Cancer Treatment (PDQ) ‐ Health Professional Version. Bethesda, MD: National Cancer Institute; updated February 6, 2018. Available at https://www.cancer.gov/types/vaginal/hp/vaginal-treatment-pdq#section/_33. Accessed on April 28, 2018.