The use of concurrent radiation with checkpoint inhibitors is a strategy for boosting immune responsiveness and overcoming mutual resistance. This brief communication reports patient cases in which hypo‐fractionated radiation was used concurrently with nivolumab (anti‐PD‐1 antibody) to treat resistant tumors.
Abstract
A substantial fraction of patients demonstrate resistance to immune checkpoint inhibitors, which limits their use. Use of radiation concurrently with checkpoint inhibitors has been shown to boost immune responsiveness, resulting in significant tumor regression in patients with metastatic melanoma. However, it is unknown whether radiation could play a role in reversing the inherent resistance to checkpoint inhibition in certain tumor types. Most trials testing this concurrent approach exclude such modestly responsive tumors and pursue checkpoint inhibition using anti‐cytotoxic T‐lymphocyte‐associated protein 4 antibody (anti‐CTLA‐4, ipilimumab). The efficacy of anti‐programmed‐death‐1 (anti‐PD‐1) therapy when used concurrently with radiation is less known but remains an attractive option due to less autoimmune toxicity compared with CTLA‐4 inhibition. In this first reported experience, we have safely and effectively combined anti‐PD‐1 therapy (nivolumab) concurrently with radiation to treat two patients with relapsed sarcomatoid renal carcinoma and heavily pretreated pleomorphic sarcoma. Both patients experienced a dramatic response that was durable.
Introduction
Use of concurrent radiation with checkpoint inhibitors is an emerging strategy to boost immune responsiveness and overcome mutual resistance [1], [2], [3], [4]. Ionizing radiation creates an “in‐situ vaccine phenomenon” and promotes immune‐mediated tumor rejection [2]. This strategy has been successfully employed in patients with metastatic melanoma with encouraging results [1], [3]. Although most trials testing this concurrent approach pursue checkpoint inhibition using CTLA‐4 blockade (cytotoxic T lymphocyte‐associated protein‐4, ipilimumab), there are limited data to support the synergy with anti‐programmed death‐1 (anti‐PD‐1) therapy, which is relatively less toxic and presents an attractive option especially in older patients [4], [5]. We share our experience of using hypofractionated radiation concurrently with nivolumab (anti‐PD‐1 antibody) in treating two patients with resistant tumors—recurrent sarcomatoid renal cell carcinoma and heavily pretreated undifferentiated pleomorphic sarcoma.
Patient 1
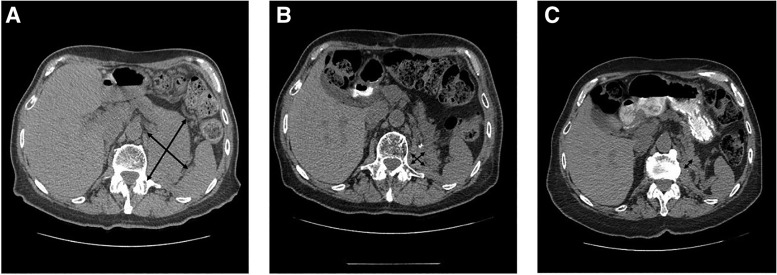
A 78‐year‐old male patient presented with hematuria, urinary retention, and weight loss. Computed tomography (CT) scan revealed a 13‐cm left renal mass. A left radical nephrectomy revealed high‐grade sarcomatoid renal cell carcinoma (sRCC) with 90% sarcomatoid component. Postoperative imaging showed no evidence of disease. A surveillance CT scan 4 months after the initial surgery revealed a 9.5‐cm mass in the left renal fossa, consistent with recurrence (Fig. 1A). At this stage, the patient was given nivolumab (3 mg/kg every 2 weeks) concurrently with radiation at a dose of 5,250 cGy in 15 daily fractions. An interim CT scan after four cycles of nivolumab showed dramatic response to treatment (Fig. 1B). Nivolumab was held after cycle 5 because of autoimmune nephritis; however, the patient continued to have an ongoing response, achieving near complete resolution of the tumor mass on the CT scan done at 6 months (Fig. 1C). Autoimmune nephritis responded well to systemic glucocorticoids, and the patient continues to be in remission more than 2 years from the initial nephrectomy.
Patient 2
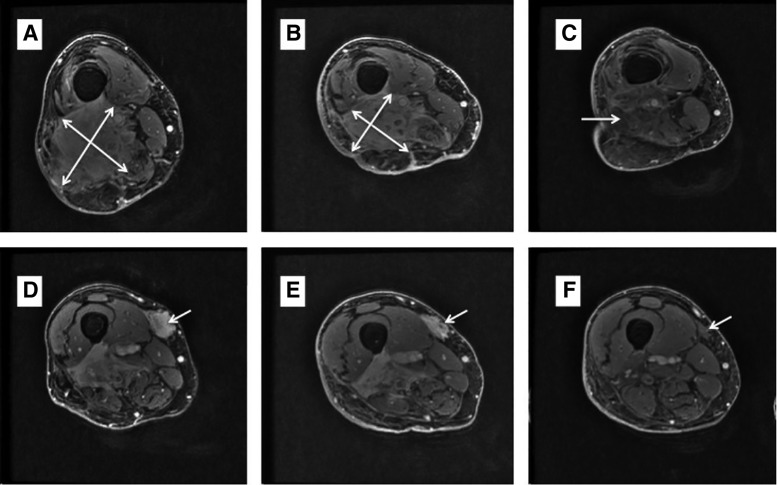
A 74‐year‐old male patient with past medical history significant for asthma and traumatic fracture of tibia presented with an enlarging right calf mass. Magnetic resonance imaging (MRI) showed a 9.2 cm × 5.8 cm × 2.8 cm mass in the right gastrocnemius muscle and adjacent subcutaneous tissue. Biopsy revealed a high‐grade undifferentiated pleomorphic sarcoma (UPS). CT scan showed no evidence of distant metastatic disease, and the patient received 5,000 cGy of neoadjuvant radiation in 25 fractions. Post‐treatment MRI showed minimal response to radiation, and the patient underwent surgical resection of the primary tumor. Pathologic analysis of the resected tumor showed gross dimensions of 10.5 cm × 7 cm × 1.5 cm with minimal necrosis (<10%), again suggesting poor response to neoadjuvant radiation. Deep margin was positive for microscopic tumor. Adjuvant chemotherapy was not pursued because of the patient's preference after a risk‐benefit discussion. The next year, the patient suffered multiple local recurrences treated with wide local excision, CyberKnife (Accuray, Sunnyvale, CA) radiosurgery (high‐dose photons delivered in a targeted fashion, 4,000 cGy in five fractions), and finally, limb‐sparing en‐bloc resection. This was followed by a brief course of pazopanib, but the patient rapidly progressed, with two large locally recurrent lesions in the right thigh, and he expressed his desire to avoid amputation and chemotherapy (Fig. 2A, D). CT scan continued to show absence of distant disease. At this stage, the patient was given nivolumab (3 mg/kg) every 2 weeks with radiation delivered concurrently at a dose of 6,000 cGy in 12 daily fractions. The treatment was given on a compassionate use basis. A restaging MRI done after five cycles showed a modest radiographic response. However, clinical improvement preceded the imaging findings with significant relief in pain and swelling within weeks of starting treatment (Fig. 2B, E). After 11 cycles of nivolumab, there was a rather dramatic radiographic response overcoming previous radioresistance with near complete resolution of the lesions in the right lateral and medial thigh (Fig. 3C, F). The response lasted for 10 months, followed by disease progression with multiple local lesions. Interestingly, unlike previous recurrences, the area within the radiation field still remains free from disease, potentially suggesting synergistic benefit from the combination treatment.
Figure 2.
Patient 2 with pleomorphic undifferentiated sarcoma. Magnetic resonance imaging showing a large, heavily pretreated, recurrent, undifferentiated pleomorphic sarcoma in the right lateral thigh (A) and right medial thigh (D). Scan after five cycles of nivolumab (and radiation) showing small radiographic response in the right lateral thigh (B) and right medial thigh (D). (C): Scan after 11 cycles of nivolumab (and radiation) showing significant tumor shrinkage and complete resolution of pain and swelling in the right lateral thigh. (F): Excellent response to the lesion in the right medial thigh with diminished enhancement. Overall, the findings are suggestive of a near‐complete response.
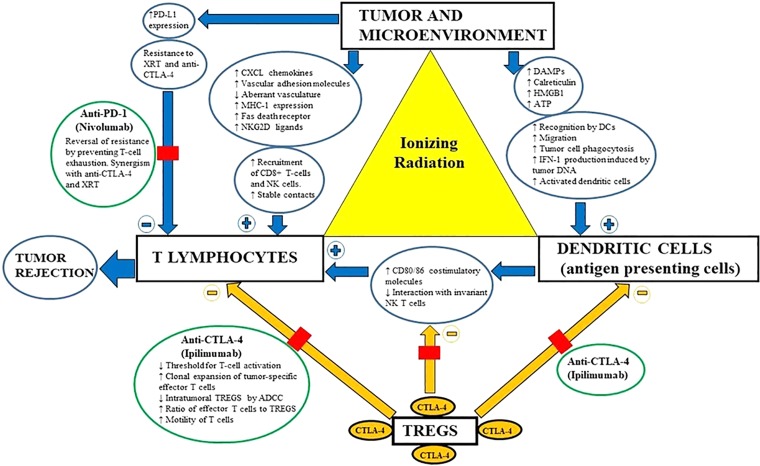
Figure 3.
Schematic representation of the in‐situ vaccine phenomenon created by radiation at all levels of immune response. Blue arrows represent direct and indirect effects of radiation (+, antitumor effects; −, protumor effects), whereas yellow arrows represent the suppressive effect of TREGs antagonized by ipilimumab. Note that anti‐PD‐1 (nivolumab) antagonizes the upregulated PD‐L1 and helps reverse the resistance and promote tumor rejection.Abbreviations: ADCC, antibody‐dependent cellular cytotoxicity; anti‐PD‐1, anti‐programmed death‐1 antibody; ATP, adenosine triphosphate; CTLA‐4, cytotoxic T‐lymphocyte antigen‐4; DAMP, damage‐associated molecular pattern; DC, dendritic cell; HMGB1, high‐mobility group protein B1; IFN‐1; interferon‐1; MHC‐1, major histocompatibility complex‐1; NK, natural killer; NKG2D, natural killer group 2, member D; PD‐L1, programmed death ligand‐1; TREG, T regulatory lymphocyte; XRT, radiation.
Figure 1.
Patient 1 with sarcomatoid renal cell carcinoma. (A): Large (9.5 cm × 6.2 cm) recurrent sarcomatoid renal cell carcinoma in the left renal fossa 4 months after radical nephrectomy. (B): Significant reduction in tumor size after concurrent radiation (5,250 cGy in 15 fractions) and nivolumab (four cycles). (C): Nivolumab was stopped after five cycles because of possible autoimmune nephritis, but response was ongoing even after stopping the drug, with near‐complete resolution of the tumor mass.
Discussion
Therapeutic strategies to boost immune responsiveness is pivotal to building on the success of checkpoint inhibitors. Systemic chemotherapeutic agents typically used for advanced UPS, such as doxorubicin, gemcitabine plus docetaxel, and high‐dose ifosfamide, yield dismal response rates ranging between 5% and 20%, with median survival of less than 1 year [6]. Similarly, relapsed sRCC is typically resistant to chemotherapy, radiation, and even targeted therapies [7]. Limited data also suggest a degree of resistance to anti‐PD‐1/programmed death‐ligand‐1 (PD‐L1) monotherapy, with response rates ranging between 15% and 20% in clinical trials using pembrolizumab and atezolizumab in UPS and sRCC, respectively [8], [9]. Radiation has been shown to exert both “on‐target” and “off‐target” (abscopal) effects when used concurrently with checkpoint inhibitors [1], [2], [3]. This synergy is demonstrated at all levels of an immune response, ranging from increased expression of class I major histocompatibility complex at the tumoral level to enhanced engagement of antigen‐presenting cells and effector T lymphocytes (Fig. 3) [2]. Upregulation of PD‐L1 is a frequent escape mechanism, imparting resistance to radiation and CTLA‐4 therapy. Hence, simultaneous blockade of the PD‐1 pathway may be necessary to overcome the resistance and derive optimal synergy [1], [2]. Despite multiple ongoing trials evaluating the combination of anti‐PD‐1 therapy with radiation, the sole published evidence comes from a recent trial of 79 patients (none with UPS or sRCC) receiving pembrolizumab with concurrent radiation [4], [5]. The trial yielded underwhelming results, with an overall response rate of 13% and a median progression‐free survival of 3 months [5]. However, this trial has provided some valuable insights. Firstly, all patients in the trial received very short courses of radiation (stereotactic body frame radiation) and it is unclear whether slightly longer fractionation schedules (similar to those of our patients) would have yielded superior outcomes. Secondly, the reported autoimmune toxicity from the combination of anti‐PD‐1 and radiation was much lower compared with historical studies involving CTLA‐4 monotherapy (grade 3, 10% vs. 25%), potentially making it useful for older and less fit patients [5], [10]. However, the occurrence of autoimmune nephritis in our patient was consistent with theoretical concerns, and trial data need to be interpreted with caution because of small study size. Finally, a predictive marker is much needed for this selectively efficacious and relatively less toxic treatment option. Although sarcomatoid histology in certain tumor types has been shown to be immunogenic, the underlying molecular‐genetic signatures are unknown and need to be elucidated [7], [11]. Despite sharing sarcomatoid lineage, a next‐generation sequencing panel (MSK‐IMPACT, Memorial Sloan Kettering Cancer Center, New York) did not identify any shared abnormalities with 9 somatic mutations in a patient with sRCC and 12 somatic mutations (including TP53) in a patient with UPS. A more comprehensive whole‐exome sequencing may help in elucidating “immune‐stimulatory” genetic signatures.
In conclusion, the combination of anti‐PD‐1 therapy and radiation proved very effective in abrogating the rapid tumor growth leading to durable responses in patients with recurrent sarcomatoid renal carcinoma and heavily pretreated undifferentiated pleomorphic sarcoma. Our cases add valuable literature in the management of these relatively resistant tumor types. Dedicated biomarker‐driven trials are needed to elucidate optimal radiation fractionation and sequencing of checkpoint blockade to derive maximal synergy and improve outcomes.
Disclosures
Dennis M. Sopka: Merck Sharpe & Dohme (E–spouse). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Twyman‐Saint Victor C, Rech AJ, Maity A et al. Radiation and dual checkpoint blockade activates non‐redundant immune mechanisms in cancer. Nature 2015;520:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanpouille‐Box C, Pilones KA, Wennerberg E et al. In situ vaccination by radiotherapy to improve responses to anti‐CTLA‐4 treatment. Vaccine 2015;33:7415–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Callahan MK, Barber CA et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 2016;4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luke JJ, Lemons JM, Karrison TG et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018;36:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.In GK, Hu JS, Tseng WW. Treatment of advanced, metastatic soft tissue sarcoma: Latest evidence and clinical considerations. Ther Adv Med Oncol 2017;9:533–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph RW, Millis SZ, Carballido EM et al. PD‐1 and PD‐L1 expression in renal cell carcinoma with sarcomatoid differentiation. Cancer Immunol Res 2015;3:1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott DF, Sosman JA, Sznol M et al. Atezolizumab, an anti‐programmed death‐ligand 1 antibody, in metastatic renal cell carcinoma: Long‐term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol 2016;34:833–842. [DOI] [PubMed] [Google Scholar]
- 9.Tawbi HAH, Burgess MA, Crowley J et al. Safety and efficacy of PD‐1 blockade using pembrolizumab in patients with advanced soft tissue (STS) and bone sarcomas (BS): Results of SARC028‐‐a multicenter phase II study. J Clin Oncol 2016;34(suppl 15):11006A. [Google Scholar]
- 10.Wolchok JD, Chiarion‐Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vieira T, Antoine M, Hamard C et al. Sarcomatoid lung carcinomas show high levels of programmed death ligand‐1 (PD‐L1) and strong immune‐cell infiltration by TCD3 cells and macrophages. Lung Cancer 2016;98:51–58. [DOI] [PubMed] [Google Scholar]