This article reports the case of a patient with urothelial carcinoma who developed a rash after treatment with atezolizumab and the diagnostic workup and management of dermatologic toxicities from immune checkpoint inhibitors based on input from an immune‐related adverse events tumor board.
Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment paradigms for a broad spectrum of malignancies. Because immune checkpoint inhibitors rely on immune reactivation to eliminate cancer cells, they can also lead to the loss of immune tolerance and result in a wide range of phenomena called immune‐related adverse events (irAEs). At our institution, the management of irAEs is based on multidisciplinary input obtained at an irAE tumor board that facilitates expedited opinions from various specialties and allows for a more uniform approach to these patients. In this article, we describe a case of a patient with metastatic urothelial carcinoma who developed a maculopapular rash while being treated with a programmed death‐ligand 1 inhibitor. We then describe the approach to management of dermatologic toxicities with ICIs based on the discussion at our irAE Tumor Board.
Key Points.
Innocuous symptoms such as pruritis or a maculopapular rash may herald potentially fatal severe cutaneous adverse reactions (SCARs); therefore, close attention must be paid to the symptoms, history, and physical examination of all patients.
Consultation with dermatology should be sought for patients with grade 3 or 4 toxicity or SCARs and prior to resumption of immune checkpoint inhibitors for patients with grade 3 or higher toxicity.
A multidisciplinary immune‐related adverse events (irAE) tumor board can facilitate timely input and expertise from various specialties, thereby ensuring a streamlined approach to management of irAEs.
Introduction
Evasion of immunosurveillance by upregulation of immune checkpoint pathways is a crucial step in carcinogenesis; therefore, inhibiting these axes by utilizing monoclonal antibodies has proven to be a successful therapeutic strategy for a wide range of malignancies. Because immune checkpoints play a physiologic role in immune homeostasis, unbridled immune activation with immune checkpoint inhibitors (ICIs) can result in a broad spectrum of immune‐related adverse effects (irAEs) that resemble autoimmune disorders in their clinical presentation.
Because of the broad spectrum of organ systems that can potentially be involved, a multidisciplinary approach is key to optimal medical management of irAEs. At Cleveland Clinic, we conduct a monthly irAE tumor board at which patients with challenging irAEs are discussed among oncologists, endocrinologists, rheumatologists, pulmonologists, dermatologists, pathologists, gastroenterologists, hepatologists, neurologists, ophthalmologists, and others depending on the organ system involved. The goal of this tumor board is to not only obtain timely input from all specialties for management of complex cases but to also use the cumulative clinical experience to create a unified approach to treatment of irAEs.
In this article, we describe a case of a patient with urothelial carcinoma who developed a rash after treatment with atezolizumab and the diagnostic workup and management of dermatologic toxicities from ICIs based on input from our irAE tumor board.
Patient Story
A 52‐year‐old man presented with gross hematuria and was diagnosed with high‐grade muscle‐invasive urothelial carcinoma. He received neoadjuvant chemotherapy with gemcitabine and cisplatin followed by robotic‐assisted laparoscopic radical cystoprostatectomy and bilateral pelvic node dissection. Ten months later he was found to have metastatic disease to the bone and was started on atezolizumab. Twelve weeks into treatment, he developed a disseminated rash. He reported no fever, chills, recent infections, myalgias, arthralgias, ocular discomfort or photophobia, odynophagia, or dysuria at the time.
Pertinent medical history was notable for coronary artery disease, atrial fibrillation, and seizures. The patient reported no personal or family history of autoimmune conditions. Other medications included levetiracetam, hydromorphone, aspirin, clopidogrel, mirtazapine, and venlafaxine, which he had been taking for at least 6 months prior to the onset of the rash.
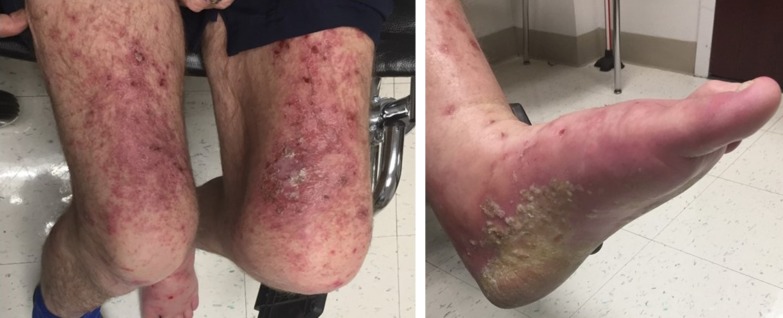
On examination, he was noted to have a grade 3 rash (per Common Terminology Criteria for Adverse Events, version 5) involving the entire back, both arms, legs, palms, and soles. The rash was pruritic and painful and consisted of erythematous well‐demarcated scaly papules and plaques, with focal erosions and crusts. On the hands and plantar aspect of the feet the plaques also had a thick adherent scale (Fig. 1). There were no visible mucosal lesions or ocular involvement on examination. The rest of the physical examination was unremarkable.
Figure 1.
Disseminated rash 12 weeks into treatment with atezolizumab.
Laboratory examination revealed a normal white blood cell count with a normal differential count. Renal and hepatic function tests were within normal limits. The patient was evaluated by a dermatologist and underwent a punch biopsy. Atezolizumab was held because of concern for a dermatologic irAE.
irAE Tumor Board
Clinical Presentation
The leading differential diagnoses based on our patient's clinical presentation were psoriasiform dermatitis or cutaneous toxicity from atezolizumab.
Dermatologic irAEs are the most common irAE reported and can be seen in 37%–70% (all grade) of patients treated with ipilimumab and 17%–37% of those treated with programmed cell death protein/ligand 1 inhibitors [1]. Of these, 1%–3% of patients have grade 3 or higher toxicity.
Time to onset of cutaneous toxicities of ICIs can vary between 2 weeks to several months into treatment [2], [3]. The most common clinical presentation is a maculopapular rash and/or pruritus [4], often starting on the trunk and spreading peripherally, usually sparing the face.
Curry et al. categorized dermatologic toxicities of ICIs into four broad groups: inflammatory, immunobullous, secondary to alteration of keratinocytes, and those due to alteration of melanocytes [4]. Inflammatory rashes are the most common and can manifest as dermal hypersensitivity reactions, acneiform, exfoliative, psoriasiform lesions, or severe cutaneous adverse reactions (SCARs) such as DRESS (drug reaction with eosinophilia and systemic symptoms), SJS/TEN (Stevens‐Johnson syndrome/ toxic epidermal necrolysis) or AGEP (acute generalized exanthematous pustulosis). Mucosal involvement may manifest in the form of oropharyngeal sores, odynophagia, painful bowel movements, ocular discomfort, photophobia, or sores in the nares or perineum. A positive Nikolsky sign, in which sloughing of the skin is observed when friction is applied parallel to the skin surface, is a harbinger of SJS/TEN and bullous pemphigoid (BP). Unlike BP, SJS/ TEN characteristically involves mucosal surfaces and may be accompanied by fever and other constitutional symptoms. Blisters in BP induced by ICIs may persist for some time despite discontinuation of immunotherapy.
Rashes from alteration of the keratinocytes can present as Grover's disease or seborrheic keratoses. Alteration of melanocytes can lead to vitiligo [5], [6], [7], repigmentation of hair [8], or tumoral melanosis. Vitiligo is most commonly seen in patients with melanoma, probably because of shared antigens among melanoma cells and melanocytes [9]. Vitiligo tends to be symmetric and bilateral in distribution and irreversible.
Other less frequently reported cutaneous toxicities include granulomatous reactions that resemble sarcoidosis [10], [11], [12], dermatomyositis [13], [14], vasculitis [15], [16], Sjogren's‐like syndrome [15], Sweet's syndrome, alopecia [17], and skin and hair textural changes [18].
Histopathology
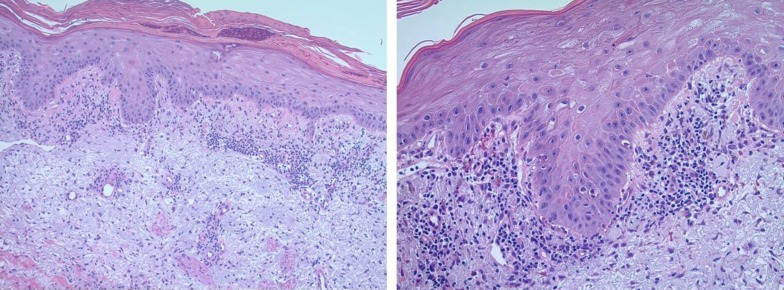
The punch biopsy from our patient's skin lesion showed superficial and deep perivascular and interstitial lymphoplasmacytic infiltrates. Interface dermatitis was present with apoptotic keratinocytes, vacuolization of the dermal‐epidermal junction, melanoderma, and reactive keratinocyte atypia (Fig. 2).
Figure 2.
Punch biopsy of a skin lesion. Biopsy revealed superficial and deep perivascular and interstitial lymphoplasmacytic infiltrate. Interface dermatitis with apoptotic keratinocytes, vacuolization of the dermo‐epidermal junction, and reactive keratinocyte atypia were also noted.
Despite the varied clinical presentation of cutaneous toxicities with ICIs, histology from these lesions often shows a lichenoid reaction or interface pattern [19]. Keratinocytes act as antigen‐presenting cells to infiltrating T cells. This interaction with cytotoxic T cells is physiologically regulated by immune checkpoint pathways. Dysregulation of immune tolerance due to ICIs results in unbridled T‐cell infiltration leading to keratinocyte injury [20]. A varying degree of infiltration by eosinophils [21] and peripheral eosinophilia [22], [23] has also been previously described.
Of the relatively less common nonlichenoid patterns, a sizeable proportion of patients have either a psoriasiform or urticarial type of reaction on histology. Bullous pemphigoid is characterized by a subepidermal cleft and linear deposits of IgG and complement 3 at the basal membrane zone evident upon direct immunofluorescence.
Diagnostic Workup and Management
Our patient was evaluated by a dermatologist, and a thorough evaluation did not reveal any other etiologies for his rash. Atezolizumab was held because of the severity of the rash (involving >70% of the body surface area). He was started on oral prednisone 1 mg/kg per day along with an antihistamine, emollients, and topical 0.1% triamcinolone acetonide ointment. He failed to respond to initial treatment over a period of 10 days and was subsequently treated with intravenous (IV) methylprednisolone at 1 mg/kg per day for 5 days followed by a gradual prednisone taper over 4 weeks. Although there are no published guidelines on how long to try oral steroids before switching to IV, we feel that 7–10 days is more than sufficient time to see improvement.
For patients who develop a cutaneous adverse reaction to ICIs, a thorough review of the history and medication list and a detailed physical examination including an evaluation of the mucosal surfaces must be performed. Other labs may be performed based on the clinical presentation: hepatic and renal function tests for patients with systemic involvement such as DRESS; antinuclear, anti‐Ro, anti‐La, ds‐DNA antibodies for those with suspected autoimmune conditions such as lupus or dermatomyositis. Serum enzyme‐linked immune sorbent assay (ELISA) for the pathognomonic antibodies to BP180 and BP230 can be performed to confirm the diagnosis of bullous pemphigoid [24], [25]. A skin biopsy can be useful in patients in whom an alternate diagnosis is being considered, for rashes that are resistant to initial treatment and for patients with suspected SCARs.
ICIs should be held for grade 3 or higher cutaneous toxicity, symptomatic bullous dermatosis, or SCAR of any severity. Pruritis can often be managed with systemic antihistamines along with alcohol‐free emollients, topical corticosteroids, cold compresses, or oatmeal baths [3]. If the above strategies are unsuccessful, doxepin or aprepitant may be useful to relieve pruritis [26]. For patients with photosensitive rashes, a broad‐spectrum sunscreen should be applied to exposed areas every 2 hours in addition to protective clothing.
The management of grade 1 or 2 inflammatory rashes with immune checkpoint inhibitors usually includes topical mild to moderate potency corticosteroids. For patients with grade 2 rash that does not improve with these measures, consider holding ICI and initiating high‐potency topical corticosteroids along with 1 mg/kg prednisone tapered over 4 weeks. Immune checkpoint inhibitors should be withheld until the rash improves to grade 1. For resistant or grade 4 rash, dermatology should be consulted, and treatment with IV methylprednisolone dosed at 1–2 mg/kg with a gradual taper should be considered. Additional immunosuppression may be considered with infliximab, mycophenolate mofetil, or cyclophosphamide [3].
For bullous dermatoses, any symptomatic bullae should be considered at least grade 2, and ICI should be held. Management consists of aggressive wound care with petroleum ointment and bandages and close monitoring and treatment of superimposed infections. In addition, high‐potency topical corticosteroids with a low threshold to start systemic steroids—prednisone 1 mg/kg for grade 2 toxicity and methylprednisolone 1–2 mg/kg for grades 3 and 4—are recommended. In some patients, rituximab has been used successfully and may allow for shorter courses of steroids [27].
SCARs are rare but potentially fatal, and ICIs must be discontinued regardless of severity. All SCARs are considered to be grade 2 or higher [1]. For grade 2 toxicity (involving 10%–30% of body surface area [BSA] with systemic symptoms, lymphadenopathy, or facial swelling), topical treatments such as emollients, medium‐ to high‐potency topical corticosteroids, and oral antihistamines should be initiated. Oral prednisone at a dose of 0.5–1 mg/kg tapered over 4 weeks may be considered for more severe or refractory cases. Any evidence of skin sloughing/blistering or mucosal involvement should be considered a grade 3 (<10% BSA) or grade 4 (≥10% BSA involved, other organ involvement as seen with DRESS) adverse event. Such patients should be admitted to a burn unit or intensive care unit. Diligent wound care of involved surfaces along with topical emollients and high dose topical corticosteroids is imperative. Supportive care should include monitoring of fluid and electrolyte balance, pain control, and monitoring for and treating superimposed infections. Dermatology should be consulted to guide therapy for these patients, and other specialties may be consulted for mucosal involvement. High‐dose IV methylprednisolone at 0.5–1 mg/kg per day (for grade 3) and 1–2 mg/kg per day (for grade 4) should be initiated and tapered gradually over 4 weeks. For patients who fail to respond to high dose steroids or those with severe reactions, treatment with IV immunoglobin or cyclosporine may be considered.
Patient Update
Our patient noticed a significant improvement in his rash with IV methylprednisolone, and his rash resolved by week 3 of his prednisone taper. Once his rash improved to grade 1, atezolizumab was resumed. He received three additional cycles of atezolizumab without any recurrent dermatologic toxicity or additional irAEs. Unfortunately, he developed progressive disease and opted against additional systemic therapy.
Implications for Clinical Practice
Dermatologic toxicities are common with immune checkpoint inhibitors and are often mild to moderate in severity. Although a majority of cutaneous reactions resolve with interruption of ICIs and topical therapies, higher‐grade toxicities or SCARs require immunosuppression. Certain dermatologic irAEs such as vitiligo may be irreversible, and patients should be informed about the associated risk prior to initiating ICIs. Emerging data suggest that the development of irAEs is associated with objective tumor responses and improved survival outcomes [28], [29]. It is therefore imperative that we treat irAEs effectively while minimizing interruption of therapy with ICIs, because patients who develop irAEs appear to be more likely to derive benefit from continued treatment.
Acknowledgments
Dr. Vamsidhar Velcheti is currently at the Laura and Isaac Perlmutter Cancer Center, New York University Langone Health, New York, New York, USA.
Author Contributions
Conception/design: Pradnya D. Patil, Nathan A. Pennell
Provision of study material or patients: Pradnya D. Patil, Mohamad Khasawneh
Collection and/or assembly of data: Pradnya D. Patil
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Disclosures
Vamsidhar Velcheti: Bristol‐Myers Squibb, Merck, AstraZeneca, Genentech, Celegene, Foundation Medicine, Novartis, Clovis Oncology, Reddy Labs, Nektar Pharmaceuticals, Fulgent Genetics (C/A); Nathan A. Pennell: AstraZeneca (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puzanov I, Diab A, Abdallah K et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman CF, Proverbs‐Singh TA, Postow MA. Treatment of the immune‐related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol 2016;2:1346–1353. [DOI] [PubMed] [Google Scholar]
- 4.Curry JL, Tetzlaff MT, Nagarajan P et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol 2017;44:158–176. [DOI] [PubMed] [Google Scholar]
- 5.Hua C, Boussemart L, Mateus C et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016;152:45–51. [DOI] [PubMed] [Google Scholar]
- 6.Hwang SJ, Carlos G, Wakade D et al. Cutaneous adverse events (AEs) of anti‐programmed cell death (PD)‐1 therapy in patients with metastatic melanoma: A single‐institution cohort. J Am Acad Dermatol 2016;74:455–461. [DOI] [PubMed] [Google Scholar]
- 7.Dai J, Belum VR, Wu S et al. Pigmentary changes in patients treated with targeted anticancer agents: A systematic review and meta‐analysis. J Am Acad Dermatol 2017;77:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera N, Boada A, Bielsa MI et al. Hair repigmentation during immunotherapy treatment with an anti‐programmed cell death 1 and anti‐programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol 2017;153:1162–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsabal M, Marti A, Jacquemin C et al. Vitiligo‐like lesions occurring in patients receiving anti‐programmed cell death‐1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol 2017;76:863–870. [DOI] [PubMed] [Google Scholar]
- 10.Tetzlaff MT, Nelson KC, Diab A et al. Granulomatous/sarcoid‐like lesions associated with checkpoint inhibitors: A marker of therapy response in a subset of melanoma patients. J Immunother Cancer 2018;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotliar J, Querfeld C, Boswell WJ et al. Pembrolizumab‐associated sarcoidosis. JAAD Case Rep 2016;2:290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danlos FX, Pages C, Baroudjian B et al. Nivolumab‐induced sarcoid‐like granulomatous reaction in a patient with advanced melanoma. Chest 2016;149:e133–136. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi Y, Abe R, Haga N et al. A case of drug associated dermatomyositis following ipilimumab therapy. Eur J Dermatol 2016;26:320–321. [DOI] [PubMed] [Google Scholar]
- 14.Sheik S, Goddard AL, Luke JJ et al. Drug‐induced dermatomyositis following ipilimumab therapy. JAMA Dermatol 2015;151:195–199. [DOI] [PubMed] [Google Scholar]
- 15.Le Burel S, Champiat S, Routier E et al. Onset of connective tissue disease following anti‐PD‐1/PD‐L1 cancer immunotherapy. Ann Rheum Dis 2017;76:43–50. [DOI] [PubMed] [Google Scholar]
- 16.Gambichler T, Strutzmann S, Tannapfel A et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer 2017;17:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber JS, Hodi FS, Wolchok JD et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–792. [DOI] [PubMed] [Google Scholar]
- 18.Sibaud V, Meyer N, Lamant L et al. Dermatologic complications of anti‐PD‐1/PD‐L1 immune checkpoint antibodies. Curr Opin Oncol 2016;28:254–263. [DOI] [PubMed] [Google Scholar]
- 19.Shi VJ, Rodic N, Gettinger S et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti‐programmed cell death 1 and anti‐programmed cell death ligand 1 immunotherapy. JAMA Dermatol 2016;152:1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okiyama N, Fujimoto M. Clinical perspectives and murine models of lichenoid tissue reaction/interface dermatitis. J Dermatol Sci 2015;78:167–172. [DOI] [PubMed] [Google Scholar]
- 21.Perret RE, Josselin N, Knol AC et al. Histopathological aspects of cutaneous erythematous‐papular eruptions induced by immune checkpoint inhibitors for the treatment of metastatic melanoma. Int J Dermatol 2017;56:527–533. [DOI] [PubMed] [Google Scholar]
- 22.Lacouture ME, Wolchok JD, Yosipovitch G et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol 2014;71:161–169. [DOI] [PubMed] [Google Scholar]
- 23.Jaber SH, Cowen EW, Haworth LR et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti‐cytotoxic T‐lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol 2006;142:166–172. [DOI] [PubMed] [Google Scholar]
- 24.Naidoo J, Schindler K, Querfeld C et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD‐1 and PD‐L1. Cancer Immunol Res 2016;4:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez AT, Khanna T, Antonov N et al. A review of bullous pemphigoid associated with PD‐1 and PD‐L1 inhibitors. Int J Dermatol 2018;57:664–669. [DOI] [PubMed] [Google Scholar]
- 26.Ito J, Fujimoto D, Nakamura A et al. Aprepitant for refractory nivolumab‐induced pruritus. Lung Cancer 2017;109:58–61. [DOI] [PubMed] [Google Scholar]
- 27.Sowerby L, Dewan AK, Granter S et al. Rituximab treatment of nivolumab‐induced bullous pemphigoid. JAMA Dermatol 2017;153:603–605. [DOI] [PubMed] [Google Scholar]
- 28.Judd J, Zibelman M, Handorf E et al. Immune‐related adverse events as a biomarker in non‐melanoma patients treated with programmed cell death 1 inhibitors. The Oncologist 2017;22:1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman‐Keller M, Kim Y, Cronin H et al. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016;22: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]