This article examines the relationship between expenditures on oncology drugs and the mortality‐to‐incidence ratio in Central and Eastern Europe compared with Western Europe, analyzing the differences in expenditures on oncology drugs.
Keywords: Cancer, Incidence, Mortality, Oncology, Central and Eastern Europe, Drug expenditures
Abstract
Background.
There is a steady decline in cancer mortality in Western Europe (WE), but this trend is not so obvious in Central and Eastern Europe (CEE). One of the largest discrepancies between WE and CEE is the level of investment in cancer care. The objective of our analysis was to examine the correlation between mortality‐to‐incidence (M/I) ratio and expenditures on oncology drugs in CEE and WE.
Materials and Methods.
This cross‐sectional analysis was done on publicly available data. Data on expenditures for oncology drugs were obtained from QuintilesIMS, and data on M/I ratio from Globocan. The main outcome was mortality‐to‐incidence ratio, and the primary analysis was performed by Spearman's rank correlation.
Results.
There is a large discrepancy in expenditure on oncology drugs per cancer case between WE and CEE, and within CEE. Average expenditure on oncology drugs per capita as well as per new cancer case was 2.5 times higher in WE than in CEE. Availability of oncology drugs was highest in Germany (100%), relatively similar in WE (average of 91%), but in CEE it ranged from 37% to 86%, with an average of 70%. Annual expenditures on all oncology drugs per new cancer case was significantly negatively correlated with the M/I ratio (Spearman's ρ = −0.90, p < .001).
Conclusion.
There is a financial threshold for oncology drugs per cancer case needed to increase survival. Based on significantly lower expenditures for oncology drugs in CEE in comparison with WE, more investment for drugs as well as better, more organized, value‐ oriented consumption is needed.
Implications for Practice.
Cancer is not treated equally successfully in Western Europe (WE) and in Central and Eastern Europe (CEE). This study showed that success in treatment of cancer is associated with the amount of money invested in oncology drugs. CEE countries spend on average 2.5 times less than WE countries for oncology drugs per new cancer case. These findings should be used by health care providers and oncologists struggling for more resources and better, more organized, evidence‐based allocation of these resources as well as better oncology outcomes.
Introduction
Over the last decades, cancer mortality in Europe has declined steadily [1], [2], [3], [4], but the pace of this decline was and still is different between particular countries. Importantly, this trend is not seen in Central and Eastern Europe (CEE), where some countries even experienced an increase in cancer mortality [2], [4]. This discrepancy between CEE and Western Europe (WE) has complex causes, probably with unequal effects in different countries. Causes may include differences in distribution of risk factors, less primary prevention, lower access to cancer screening, later diagnosis, more deadly cancer types, lower access to quality care, fewer available treatment options, lower availability of novel drugs, shortage of radiotherapy and other equipment, lack of national cancer plans, lack of multidisciplinary teams, and absence of comprehensive cancer registries [5], [6], [7]. The discrepancy between mortality trends in CEE and WE is associated with large differences in health care budgets and the absolute investment in oncology [5], [7], [8], [9], [11]. An important part of this investment is the cost of anticancer drugs. When adjusted for inflation, expenditure on cancer care per capita increased in the European Union (EU) between 1995 and 2014 by 56%, and this increase was larger by one third in CEE than in WE [12]. However, the share of cancer care in the total health expenditure has been stable, meaning that total health expenditures increased at a similar pace. It has been documented that significant reductions in cancer mortality may be attributed to pharmaceutical innovation [13], [14]. The association of mortality‐to‐incidence (M/I) ratio with different socioeconomic, general health, and lifestyle factors, expenditure on health care in general, cancer‐specific expenditure, and finally the expenditure on oncology drugs is different in particular cancers [5]. The association of M/I ratio with expenditure on oncology drugs is most visible in cancers for which effective treatment strategies emerged years ago; it is high and similar in breast and colorectal cancers, but it is markedly lower in lung cancer [5]. In simple words: The more investment in drugs for breast cancer, the better the outcome, but that is still not so true for lung cancer. The impact of the very recent introduction of successful, more efficient treatment modalities in the therapy of lung cancer (tyrosine kinase inhibitors and immunotherapy) in cancer registries is expected to be seen after certain lag time. At the same time, there are differences in incidence rates of certain cancers between CEE and WE, and different cancer types are associated with different financial consequences [11]. Self‐evidently, high costs of novel oncology drugs and their economic burden on already overstretched health care budgets may affect patient access, especially in CEE [15]. The objective of our analysis was to examine the relationship between expenditures on oncology drugs and M/I ratio in CEE and WE, and to analyze the differences in expenditure on oncology drugs.
Materials and Methods
Study Design
This cross‐sectional analysis of publicly available data was performed by a panel of oncology leaders from CEE countries. The panel was established at the 11th Central European Oncology Congress held in Croatia in 2015.
Targeted Population
The targeted populations encompasses 10 CEE countries: Bosnia and Herzegovina, Bulgaria, Croatia, the Czech Republic, Hungary, Poland, Romania, Serbia, Slovakia, and Slovenia, with a total population of 108 million. The comparator consists of seven WE countries: Austria, Germany, Italy, France, Spain, Sweden, and Switzerland, with three of them neighboring the targeted CEE region (Austria, Germany, and Italy). The total population of these seven WE countries was 277 million.
Outcome
The outcome was the M/I ratio. We obtained the mean age standardized per 100,000 inhabitants and the annual incidence and mortality rates for all cancers in each country from the study by Ferley et al. [16] and from Globocan 2012 [17]. We calculated the M/I ratio by dividing these two mean rates for all cancers in each country [18]. Higher M/I ratio indicates less favorable higher mortality. M/I ratio is a population‐based indicator of survival. It is a valid approximation of the 5‐year relative survival rate, but its validity is not equal in different tumors and is not validated in low‐resource countries [19].
Independent Variable
The independent variable was the 2015 expenditure on ATC L01 (antineoplastic agents within Anatomical Therapeutic Chemical Classification System of World Health Organization) class drugs per new cancer case. We monitored expenses for 35 drugs. Data on annual expenditures were provided by QuintilesIMS (London, United Kingdom) [20]. QuintilesIMS sales data are based on manufacturers prices and do not represent the final sales price.
Other Explanatory and Confounding Variables
The source of data on expenditures on all prescription drugs was QuintilesIMS. Population sizes and data on gross domestic products (GDP) were obtained from the Statistical Office of the European Communities [21].
Statistical Analysis
We performed the primary analysis using Spearman's rank correlation (ρ). We had no missing data. Availability of particular drugs in each country was estimated based on the existence of any sales during the monitored time period. We treated the drugs with any sales as available, and the drugs with no sales as unavailable. Coefficients of variation of expenditures per cancer case in WE and CEE countries was calculated as standard deviation of these expenditures in the region, divided by the mean expenditure within the region. We conducted a data analysis using NCSS 12 Statistical Software (2018; NCSS, LLC, Kaysville, UT).
Results
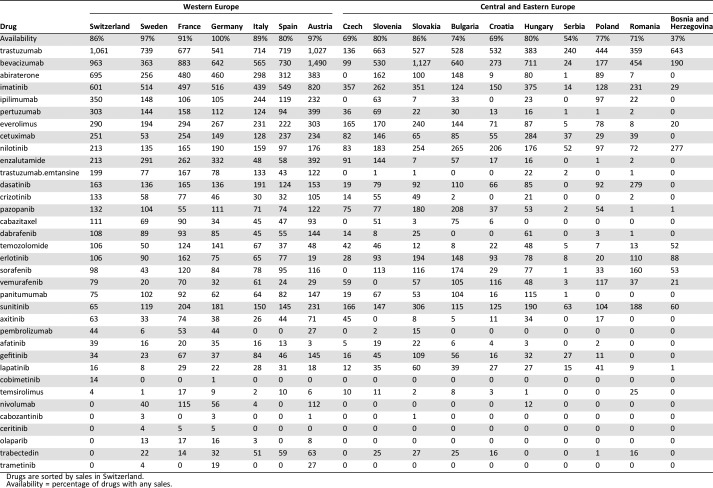
The mean percentage of GDP per capita spent on ATC L01 class drugs was similar in WE (0.25%) and in CEE (0.28%; Table 1). However, owing to large differences in GDP, absolute expenditures on oncology drugs were markedly different between WE and CEE countries, as well as within these two regions (Table 1). Average expenditure on oncology drugs per capita as well as per new cancer case was 2.5 times higher in WE than in CEE. Compared with CEE, WE was more homogeneous in regard to overall expenditure per cancer case, with coefficients of variation, calculated as standard deviation of expenditures per cancer case in all countries divided by the mean expenditure, of 56% and 13%, respectively. The expenditures per cancer case in WE ranged from €11,586 in Italy to €17,879 in Switzerland, a difference of 54%. In contrast, the difference in expenditure between Serbia (the CEE country with the smallest expenditure) and Slovakia (the one with the largest expenditure) was a staggering 907%. In CEE, the correlation between the percentage of GDP spent on oncology drugs in 2013 and relative change in expenditures from 2013 to 2015 was positive and low (Spearman's ρ = 0.20), and in WE, it was even smaller (Spearman's ρ = 0.11). Availability of oncology drugs, defined as “any sales for 35 oncology drugs” during 2015, was highest in Germany (all 35 oncology drugs were commercially available, 100%) and relatively similar in WE with an average of 91% availability (Table 2). In CEE, availability ranged from 37% in Bosnia and Herzegovina to 86% in Hungary, with an average of 70%. Overall expenditure on oncology drugs has increased from 2013 to 2015 in all CEE countries except the Czech Republic and Romania, where there has been a small decline, and in Serbia, where the expenditure has not changed. However, overall costs for all prescription drugs increased more than did the costs for oncology drugs in all countries except the Czech Republic, Romania, and Bosnia and Herzegovina. Consequently, the share of costs for oncology drugs among total costs for all prescription drugs increased in CEE from 2013 to 2015 at a slower pace than the absolute expenditure. Furthermore, differences were increased by parallel trade in several cases. We observed similar expenditures per cancer case patterns: More was spent on trastuzumab and bevacizumab than on abiraterone and imatinib. All those drugs had relatively higher sales in WE than in CEE compared with other oncology drugs, although nilotinib and sunitinib had relatively higher sales in CEE compared with other drugs. It is interesting to see that the uptake of trastuzumab emtansine was much lower in CEE compared with WE countries (Table 2).
Table 1. Expenditures on oncology drugs and mortality‐to‐incidence ratio of all cancer types in CEE and WE countries [18], [19] .
Data are sorted by mortality‐to‐incidence ratio within the region. Both incidence and mortality are age‐standardized (European standard population) rates per 100,000 inhabitants.
Abbreviations: B&H, Bosnia and Herzegovina; CEE, Central and Eastern Europe; GDP, gross domestic product per capita 2015 [20]; M/I, mortality‐to‐incidence ratio; Rx, prescription; WEE, Western Europe.
Table 2. Oncology drug sales per new cancer cases during 2015 (EUR) [16], [17] .
Drugs are sorted by sales in Switzerland.
Availability = percentage of drugs with any sales.
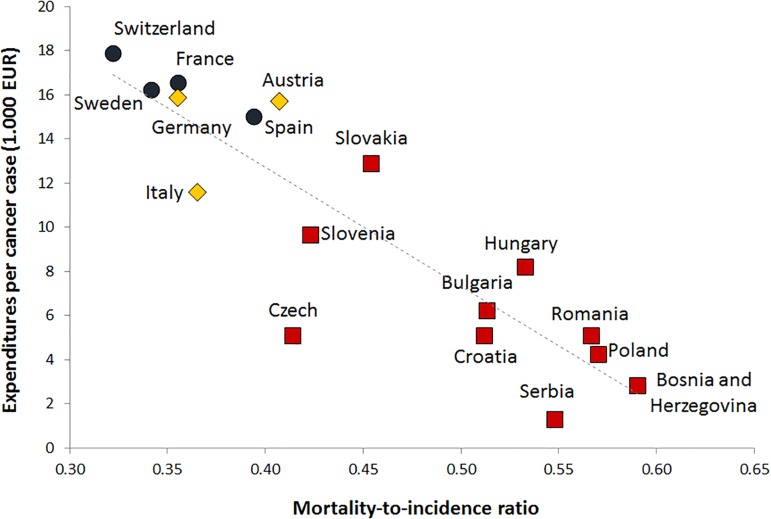
Annual expenditures on all oncology drugs per new cancer case was significantly negatively correlated with the M/I ratio for all cancer types taken together (Spearman's ρ = −0.90, p < .001; Figure 1). The higher the annual expenditure on oncology drugs was, the better and lower the M/I ratio. Within both studied populations, the correlation was markedly lower: ρ = −0.75, p = .052 in WE, and ρ = −0.67, p = .033 in CEE.
Figure 1.
Correlation of annual expenditures per new cancer case for ATC L01 class drugs during 2015, and mortality‐to‐incidence ratio: All cancers; red squares represent Central and Eastern Europe countries, yellow rhombuses represent neighboring Western Europe (WE) countries, and blue circles represent other WE countries [17], [20].
Discussion
There are large differences in outcomes of cancer care between EU countries, especially when WE and CEE are compared [22]. Improvement in these discrepancies should be attempted via prompt action using all known anticancer strategies [7]. One of the most important measures is better access to new, innovative, often expensive but effective oncology drugs. Our study showed that absolute expenditures on oncology drugs per capita and per cancer case are markedly lower in CEE compared with WE, although the percentage of GDP spent on oncology drugs is similar. The M/I ratio correlates strongly with the expenditure on oncology drugs. The lower incidence rates and higher mortality rates in CEE compared with WE [18] results in higher total numbers of patients actively treated with curative and palliative intent (prevalence of treated patients) in WE than in CEE [23]. Thus, the CEE/WE gap in investment in oncology drugs that we observed is relatively smaller. Despite the potentially smaller prevalence of patients on therapy, higher mortality and less favorable M/I ratio suggests the need for increased investment in CEE in relation to WE. However, the percentage of GDP spent on oncology drugs is equal in CEE and in WE. The consequence of this equal spending relative to GDP is lower absolute investment in CEE because the drug prices are not lower in CEE proportionally to the difference in GDP per capita between CEE and WE. Finally, because of the aging population with associated increase of cancer incidence, the financial burden of cancer will grow in CEE relative to WE.
We have found only a small positive correlation between the percentage of GDP spent on oncology drugs in 2013 and relative change in expenditures between 2013 and 2015. In other words, countries with lower expenditure in 2013 have not been trying to invest more. This conclusion may be confounded with different factors. If countries managed to negotiate lower drugs prices, the increasing drug use will not be obvious from the relative change in total expenditure. If the use of generic or biosimilar drugs had been increased, the explained effect might be similar. On the other side, the number of patients has most likely been increasing because of population growth and aging, earlier diagnosis with consequent increase in cancer incidence, new registered indications, and more intense anticancer treatments including more treatment lines [10], [24]. This finding is consistent with the finding that the relative change of share of oncology drugs in total sales of all prescription drugs has been lower than the relative change of absolute expenditure in all monitored countries except Serbia. This may be particularly worrying in CEE countries where unfavorable mortality trends have been observed and where the M/I ratio is markedly above WE countries. An analysis of cost and burden of cancer in the European Union 1995–2014 showed that expenditures on cancer as a share of total health care expenditures are unexpectedly low considering increasing cancer incidence, and the fact that cancer is the leading cause of mortality in the working population [12].
Based on our data, the availability of oncology drugs seems to be quite high, both in CEE and even more in WE countries. However, the criterion for availability of oncology drugs that we used was imprecise. The fact that the drug has any sales does not indicate the proportion of population in need that was actually treated with the drug. A recent survey performed by the European Society for Medical Oncology highlighted important discrepancies in reimbursement, access, and availability of cancer medicines across Europe [25]. Actual availability of novel drugs in populations depends on reimbursement policies and was reported as much lower. Ades et al. estimated the sizes of populations in need by multiplying country population size, incidence of breast cancer, percentage of human epidermal growth receptor 2 (HER2) positive, number of new such cases, proportion of patients treated in the adjuvant or metastatic setting, and duration of adjuvant and metastatic setting treatment [26]. They found that none of the Eastern European countries achieved the total coverage of the population in need before 2006, when trastuzumab was approved only in the metastatic setting [26]. After 2006, when trastuzumab was approved in the HER2 positive adjuvant setting, only the Czech Republic and Slovenia achieved total coverage if the incidence of HER2 positive tumor was estimated to 15%. In the 20% HER2‐positive scenario, no CEE country achieved total coverage.
Our analyses point toward the same direction. Availabilities of oncology drugs are different between WE and CEE, and this may affect the differences in cancer M/I ratio in these countries. High costs and economic burden of cancer therapeutics may affect patient access [15]. Because of the difference in investment in oncology drugs per cancer case shown in our study, this effect is higher in CEE than in WE. Health care payers with limited budgets make their decisions on the continuum between two extremes. One extreme option is investing in novel, more clinically beneficial and more expensive therapies. An inevitable consequence of this approach is limiting the treatment access to a relatively smaller number of patients. Another extreme option is investing more in the standard, older, generic, and less costly treatments addressed to the larger number of patients. In other words, the dilemma is whether to treat selected, limited numbers of patients with the best available treatments or to provide all patients with a less‐than‐state‐of‐the‐art treatment. However, expenditures on cancer drugs are part of the total health care expenditure, and the problem of oncology drug costs should be considered in the context of other health services. We have shown that costs of oncology drugs in CEE and WE constitute 16% and 19% of the costs of all prescription drugs, respectively. At the same time, cancer has become the leading cause of death in the EU, accounting for about 25% of all mortality [27]. A significant proportion of cancer survival may be attributed to new treatments [13], [14]. Furthermore, cancer prevalence is increasing at a higher pace than share of expenditures on cancer care in total health care expenditures [28], [29]. Finally, to correctly understand the expenditure on oncology drugs, it is important to express this figure as the proportion of overall costs of cancer care. Expenditure on oncology drugs in the EU in 2009 was 27% of all direct cancer care costs [11]. When costs of informal care (unpaid care provided by relatives or friends of patients) are added, the share of expenditures on oncology drugs drops to 18%. Finally, when costs of productivity loss due to morbidity and mortality are added, the correctly calculated share of oncology drugs costs within the total cost of cancer is 11% [11]. It is somewhat higher in CEE than in WE. This should partially be attributed to several factors: the lower costs of cancer‐related inpatient days, other formal treatment options, lower costs of informal care, lower costs of productivity losses, and to the interaction of differences in incidence of particular cancer types and differences in drugs and the total costs of particular cancer treatments and consequences. For example, CEE countries have higher incidences of lung cancer [1], [17], which has the highest productivity losses attributable to mortality [11].
Limitations of the Study
First, because of higher incidence and lower mortality rates, the prevalence of cancer is higher in WE than in CEE countries [23]. Throughout the study, we calculated expenditures per number of new cancer cases (incidence) or per capita, instead of per number of cancer patients actively treated with curative and palliative intent (prevalence of treated patients) when the financial costs are highest [30]. We did so because the published and available prevalence figures have lower reliability. We tried to control for the effects of this source of bias by calculating expenditures per new cancer case instead of per capita as was done in several other studies [7], [8]. However, this correction was limited because the prevalence of cases actively treated with ATC L01 class drugs may have a different correlation with the cancer incidence in WE and CEE countries. This may be caused by the differences in use and efficacy of other treatment options, differences in early diagnosis and the consequent average cancer stage at diagnosis, and differences in the adherence to clinical guidelines and the accuracy of incidence data. Second, we have not controlled for the effects of parallel trade. CEE countries have significantly more parallel export, and WE countries, particularly Germany, have more parallel import. Parallel trade is mediated by price differences and basically caused by international exchange rates and differences in patient demand and national income [31]. It affects countries in different ways and to different extents. Therefore, our results are the best‐case scenario for the expenditures in CEE. Actual expenditures are most likely even lower. Third, this was a cross‐sectional study, so we could not establish the temporal order of expenditures and M/I ratio. For this reason, we could not pose any causal claims. Fourth, incidence and mortality data have different reliability in different countries [6]. These differences may be associated with our key independent variable: expenditure on oncology drugs. Indeed, it was shown that accuracy of cancer registries for the total affected population is associated with the covered population's socioeconomic status [32]. Furthermore, it is possible that this effect varies between countries. This potential source of bias is common to the majority of analyses done on data based on cancer registries. This makes our analysis comparable but not necessarily valid. Fifth, we based our analysis on the overall expenditure on oncology drugs and ignored the differences in country‐specific prices and/or discounts that may confound the relationship between novel drug access and use and coverage of the population in need, with the M/I ratio. The likely effect of this potential source of bias is against our null hypothesis of no association between expenditures on oncology drugs and M/I ratio. We assumed such an effect because it is likely that drug prices are lower in lower‐ and middle‐income countries [33], [34], and it is well documented that they have less favorable M/I ratios. Moreover, the correlation between wealth and M/I ratio is higher in CEE than in WE countries [7]. Sixth, a similar source of bias lies in the fact that we calculated the total expenditure by summing up the expenditures for particular molecules, ignoring the possibility that less‐costly generic drugs or biosimilars are more often used in CEE than in WE, and consequently that the gap in drug use is smaller than what was indicated by the overall expenditure. Seventh, our key outcome was M/I ratio. Although M/I is a good approximation of the 5‐year relative survival for many tumor sites, its validity varies to some extent between different tumor sites and countries [19]. Eighth, total expenditure on the oncology drugs studied may also be a surrogate marker for expenditure in total health care for cancer patients. For example, less investment in modern radiotherapy equipment or scarcer availability of appropriate multidisciplinary management, both expensive commodities in their own right, may also contribute to the observed differences in M/I ratios. Ninth, we did the analysis on the national, not the individual, patient level and so risked the ecological inference fallacy. Therefore, our study findings should be interpreted on the national rather than individual patient level. Tenth, a large number of uncontrolled, unobserved, or even unknown possible confounders of our findings may exist, and the results should be interpreted cautiously with no direct causal inferences at all. Eleventh, we calculated the M/I ratio from the age‐standardized mortality and incidence rates regardless of cancer severity. However, what may be caused by the weaknesses of the secondary prevention and, consequently, more advanced stages at the detection of the same cancer may differ between different countries. The data on the cancer severity were not available to us.
Conclusion
Expenditures on oncology drugs per capita and per cancer case are markedly lower in CEE than in WE, although the spent percentage of GDP is roughly similar. The M/I ratio, which is significantly worse in CEE, is correlated with the expenditures on oncology drugs. Consequently, more investment in oncology drugs most likely will result in better M/I ratios in CEE countries. Policy makers should also be aware that expenditure on oncology drugs makes up only about 11% of total cancer costs, and that novel treatments increase survival and lower the costs associated with morbidity and mortality.
Acknowledgments
This work was supported by an unrestricted grant from Roche.
Author Contributions
Conception/design: Eduard Vrdoljak, Gyorgy Bodoky, Jacek Jassem, Razvan Popescu, Robert Pirker, Tanja Čufer, Semir Bešlija, Alexandru Eniu, Vladimir Todorović, Katerina Kopečková, Galia Kurteva, Zorica Tomašević, Agim Sallaku, Snezhana Smichkoska, Žarko Bajić, Branimir Sikic.
Provision of study material or patients: Eduard Vrdoljak, Gyorgy Bodoky, Jacek Jassem, Razvan Popescu, Robert Pirker, Tanja Čufer, Semir Bešlija, Alexandru Eniu, Vladimir Todorović, Katerina Kopečková, Galia Kurteva, Zorica Tomašević, Agim Sallaku, Snezhana Smichkoska, Žarko Bajić, Branimir Sikic.
Collection and/or assembly of data: Eduard Vrdoljak, Gyorgy Bodoky, Jacek Jassem, Razvan Popescu, Robert Pirker, Tanja Čufer, Semir Bešlija, Alexandru Eniu, Vladimir Todorović, Katerina Kopečková, Galia Kurteva, Zorica Tomašević, Agim Sallaku, Snezhana Smichkoska, Žarko Bajić, Branimir Sikic.
Data analysis and interpretation: Eduard Vrdoljak, Gyorgy Bodoky, Jacek Jassem, Razvan Popescu, Robert Pirker, Tanja Čufer, Semir Bešlija, Alexandru Eniu, Vladimir Todorović, Katerina Kopečková, Galia Kurteva, Zorica Tomašević, Agim Sallaku, Snezhana Smichkoska, Žarko Bajić, Branimir Sikic.
Manuscript writing: Eduard Vrdoljak, Gyorgy Bodoky, Jacek Jassem, Razvan Popescu, Robert Pirker, Tanja Čufer, Semir Bešlija, Alexandru Eniu, Vladimir Todorović, Katerina Kopečková, Galia Kurteva, Zorica Tomašević, Agim Sallaku, Snezhana Smichkoska, Žarko Bajić, Branimir Sikic.
Final approval of manuscript: Eduard Vrdoljak, Gyorgy Bodoky, Jacek Jassem, Razvan Popescu, Robert Pirker, Tanja Čufer, Semir Bešlija, Alexandru Eniu, Vladimir Todorović, Katerina Kopečková, Galia Kurteva, Zorica Tomašević, Agim Sallaku, Snezhana Smichkoska, Žarko Bajić, Branimir Sikic.
Disclosures
Eduard Vrdoljak: Amgen, Bristol‐Myers Squibb, Merck, Merck Sharp Dohme, Novartis, Roche, Sanofi (C/A); Gyorgy Bodoky: Amgen, Bristol‐Myers Squibb, Janssen, Merck, Novartis, Merrimack, Pfizer, Roche, Servier (SAB); Razvan Popescu: Amgen, Bayer, Bristol‐Myers Squibb, Eli Lilly, Merck, Merck Sharp & Dohme, Novartis, Roche, Sanofi (C/A), Bayer, Bristol‐Myers Squibb, Merck, Merck Sharp & Dohme, Novartis, Roche, Sanofi (SAB). Jacek Jassem: Roche, AstraZeneca, Amgen, Boehringer Ingelheim, Merck (C/A); Robert Pirker: AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Shartp & Dohme, Syntia (C/A); Alexandru Eniu: Roche, AstraZeneca (R/F); Vladimir Todorović: Roche, Sanofi, Pfizer, Inmed (H); Branimir I. Šikić: Threshold (C/A), Genentech/Roche, Novartis, Sanofi, CellDex, Gilead (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
References
- 1.Bosetti C, Bertuccio P, Malvezzi M et al. Cancer mortality in Europe, 2005‐2009, and an overview of trends since 1980. Ann Oncol 2013;24:2657–2671. [DOI] [PubMed] [Google Scholar]
- 2.Znaor A, van den Hurk C, Primic‐Zakelj M et al. Cancer incidence and mortality patterns in South Eastern Europe in the last decade: Gaps persist compared with the rest of Europe. Eur J Cancer 2013;49:1683–1691. [DOI] [PubMed] [Google Scholar]
- 3.La Vecchia C, Rota M, Malvezzi M et al. Potential for improvement in cancer management: reducing mortality in the European Union. The Oncologist 2015;20:495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malvezzi M, Bertuccio P, Levi F et al. European cancer mortality predictions for the year 2014. Ann Oncol 2014;25:1650–1656. [DOI] [PubMed] [Google Scholar]
- 5.Munro AJ. Comparative cancer survival in European countries. Br Med Bull 2014;110:5–22. [DOI] [PubMed] [Google Scholar]
- 6.Vrdoljak E, Torday L, Sella A et al. Insights into cancer surveillance in Central and Eastern Europe, Israel and Turkey. Eur J Cancer Care (Engl) 2015;24:99–110. [DOI] [PubMed] [Google Scholar]
- 7.Vrdoljak E, Bodoky G, Jassem J et al. Cancer control in Central and Eastern Europe: Current situation and recommendations for improvement. The Oncologist 2016;21:1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ades F, Senterre C, de Azambuja E et al. Discrepancies in cancer incidence and mortality and its relationship to health expenditure in the 27 European Union member states. Ann Oncol 2013;24:2897–2902. [DOI] [PubMed] [Google Scholar]
- 9.Grau C, Defourny N, Malicki J et al. Radiotherapy equipment and departments in the European countries: Final results from the ESTRO‐HERO survey. Radiother Oncol 2014;112:155–164. [DOI] [PubMed] [Google Scholar]
- 10.Lawler M, Le Chevalier T, Murphy MJ Jr et al. A catalyst for change: The European Cancer Patient's Bill of Rights. The Oncologist 2014;19:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luengo‐Fernandez R, Leal J, Gray A et al. Economic burden of cancer across the European Union: A population‐based cost analysis. Lancet Oncol 2013;14:1165–1174. [DOI] [PubMed] [Google Scholar]
- 12.Jönsson B, Hofmarcher T, Lindgren P et al. The cost and burden of cancer in the European Union 1995‐2014. Eur J Cancer 2016;66:162–170. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenberg FR. The impact of pharmaceutical innovation on premature mortality, cancer mortality, and hospitalization in Slovenia, 1997‐2010. Appl Health Econ Health Policy 2015;13:207–222. [DOI] [PubMed] [Google Scholar]
- 14.Sun E, Lakdawalla C, Reyes D et al. The determinants of recent gains in cancer survival: An analysis of the Surveillance, Epidemiology, and End Results (SEER) database. J Clin Oncol 2008;26 (suppl 15):6616a. [Google Scholar]
- 15.Albaba H, Lim C, Leighl NB. Economic considerations in the use of novel targeted therapies for lung cancer: Review of current literature. Pharmacoeconomics 2017;35:1195–1209. [DOI] [PubMed] [Google Scholar]
- 16.Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–1403. [DOI] [PubMed] [Google Scholar]
- 17.International Agency for Research on Cancer , World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available at http://globocan.iarc.fr/Pages/age-specific_table_sel.aspx. Accessed May 4, 2017.
- 18.Parkin D, Bray F. Evaluation of data quality in the cancer registry: Principles and methods Part II. Completeness. Eur J Cancer 2009;45:756–764. [DOI] [PubMed] [Google Scholar]
- 19.Asadzadeh Vostakolaei F, Karim‐Kos HE, Janssen‐Heijnen ML et al. The validity of the mortality to incidence ratio as a proxy for site‐specific cancer survival. Eur J Public Health 2011;21:573–577. [DOI] [PubMed] [Google Scholar]
- 20.QuintilesIMS. 2016. (IQVIA since November 2017) https://www.iqvia.com/. Accessed August 18, 2018.
- 21.EUROSTAT. GDP per capita, consumption per capita and price level indices. 2017. Available at http://ec.europa.eu/eurostat/statistics‐explained/index.php/GDP_per_capita,_consumption_per_capita_and_price_level_indices. Accessed August 18, 2018.
- 22.Vrdoljak E, Wojtukiewicz MZ, Pienkowski T et al. Cancer epidemiology in Central and South Eastern European countries. Croat Med J 2011;52:478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray F, Ren JS, Masuyer E et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013;132:1133–1145. [DOI] [PubMed] [Google Scholar]
- 24.EUROSTAT. Population structure and ageing. 2017. Available at http://ec.europa.eu/eurostat/statistics‐explained/index.php/Population_structure_and_ageing#Past_and_future_population_ageing_trends_in_the_EU. Accessed September 12, 2017.
- 25.Cherny NI, Sullivan R, Torode J et al. ESMO International Consortium Study on the availability, out‐of‐pocket costs and accessibility of anti‐neoplastic medicines in countries outside of Europe. Ann Oncol 2017;28:2633–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ades F, Senterre C, Zardavas D et al. Are life‐saving anticancer drugs reaching all patients? Patterns and discrepancies of trastuzumab use in the European Union and the USA. PLoS One 2017;12:e0172351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EUROSTAT. Cancer statistics. 2017. Available at http://ec.europa.eu/eurostat/statistics‐explained/index.php/Cancer_statistics. Accessed September 15, 2017.
- 28.Fitch K, Pelizzari P, Pyenson B. Cost drivers of cancer care: A retrospective analysis of Medicare and commercially insured population claim data 2004‐2014. Available at http://www.milliman.com/insight/2016/Cost‐drivers‐of‐cancer‐care‐A‐retrospective‐analysis‐of‐Medicare‐and‐commercially‐insured‐population‐claim‐data‐2004‐2014/. Accessed August 18, 2018.
- 29.Turck R. Oncology drug costs‐The imaginary crisis? Ann Oncol 2017;28:427–431. [DOI] [PubMed] [Google Scholar]
- 30.Soerjomataram I, Lortet‐Tieulent J, Ferlay J et al. Estimating and validating disability‐adjusted life years at the global level: A methodological framework for cancer. BMC Med Res Methodol 2012;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyle MK, Allsbrook JS, Schulman KA. Does reimportation reduce price differences for prescription drugs? Lessons from the European Union. Health Serv Res 2008;43:1308–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Angelis R, Sant M, Coleman MP et al. Cancer survival in Europe 1999‐2007 by country and age: Results of EUROCARE‐‐5‐A population‐based study. Lancet Oncol 2014;15:23–34. [DOI] [PubMed] [Google Scholar]
- 33.Vogler S, Vitry A, Babar ZU. Cancer drugs in 16 European countries, Australia, and New Zealand: A cross‐country price comparison study. Lancet Oncol 2016;17:39–47. [DOI] [PubMed] [Google Scholar]
- 34.Hill A, Redd C, Gotham D et al. Estimated generic prices of cancer medicines deemed cost‐ineffective in England: A cost estimation analysis. BMJ Open 2017;7:e011965. [DOI] [PMC free article] [PubMed] [Google Scholar]