Abstract
Lessons Learned.
Pazopanib shows a modest efficacy in metastatic alveolar soft part sarcoma.
Clinical outcomes were comparable to those in previous studies using antiangiogenic drugs.
Further prospective studies evaluating the benefit of pazopanib in alveolar soft part sarcoma with a larger sample are warranted to validate results.
Background.
Alveolar soft part sarcoma (ASPS) is a rare mesenchymal malignant tumor characterized by an unbalanced translocation, t(X;17)(p11.2;q25), which leads to the fusion of ASPSCR1 to the TFE3 transcription factor. Because this results in the upregulation of angiogenesis‐related transcripts, antiangiogenic drugs have been used in ASPS patients.
Methods.
This open‐label, single‐arm, multicenter, investigator‐initiated phase II trial was designed to evaluate efficacy and safety of pazopanib 800 mg once daily in patients with metastatic ASPS. The primary endpoint was investigator‐assessed overall response rate (ORR), and secondary endpoints were toxicity, progression‐free survival (PFS), and overall survival (OS). 68Ga‐RGD (Arg‐Gly‐Asp) positron emission tomography (PET) scan and gene expression profiling using NanoString platform were performed for biomarker analysis.
Results.
Six patients with histologically confirmed metastatic ASPS were enrolled between December 2013 and November 2014. Among six patients, one achieved a partial response (PR) (ORR 16.7%) and five patients showed stable disease (SD). With a median follow‐up of 33 months (range 18.7–39.3 months), median PFS was 5.5 months (95% confidence interval [CI] 3.4–7.6 months), and median OS was not reached. There were no severe toxicities except one patient with grade 3 diarrhea.
Conclusion.
Pazopanib showed modest antitumor activity with manageable toxicities for patients with metastatic ASPS.
Abstract
经验教训
• 帕唑帕尼治疗转移性腺泡状软组织肉瘤的疗效一般。
• 临床结果与既往使用抗血管生成药物的研究结果相差无几。
• 未来需要较大样本的前瞻性研究评估帕唑帕尼在腺泡状软组织肉瘤中疗效,以验证结果。
摘要
背景。腺泡状软组织肉瘤 (ASPS) 是一种罕见的间充质恶性肿瘤,其特点是易位不平衡,t(X;17)(p11.2;q25),进而造成 ASPSCR1 到 TFE3 转录因子的融合。由于造成血管生成相关转录的上调,抗血管生成药物已被用于 ASPS 患者。
方法。这项开放标签、单臂、多中心且由研究员发起的 II 期试验旨在评估转移性 ASPS 患者帕唑帕尼800 mg每天服用一次的疗效和安全性。主要疗效指标是研究员评估的总缓解率 (ORR),次要疗效指标是毒性、无进展生存期 (PFS) 和总生存期 (OS)。利用 NanoString 平台对68Ga‐RGD (Arg ‐ Gly ‐ Asp) 正电子发射断层扫描 (PET) 和基因表达谱进行了生物标志物分析。
结果。2013 年 12 月至 2014 年 11 月间,共有 6 名经组织学证实的转移性 ASPS 患者参与研究。这 6 名患者中,1 人部分缓解 (PR) (ORR 16.7%), 另 5 人病情稳定 (SD)。平均随访时间为 33 个月(范围是 18.7‐39.3 个月),中位 PFS 为 5.5 个月 [95% 置信区间 (CI)3.4‐7.6 个月],未达到中位 OS。未发现严重毒性,只有一位患者出现 3 级腹泻。
结论。帕唑帕尼对转移性 ASPS 患者表现出一般的抗肿瘤活性和可控制的毒性。
Discussion
ASPS is a rare histological subtype of soft‐tissue sarcomas (STS). It shows a poor prognosis in the metastatic setting, and the standard chemotherapy regimens do not improve treatment outcomes. Several antiangiogenic drugs have been studied in metastatic ASPS patients.
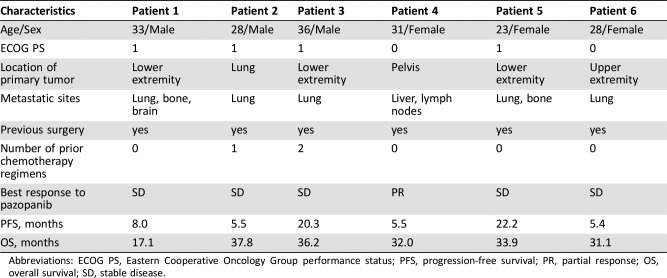
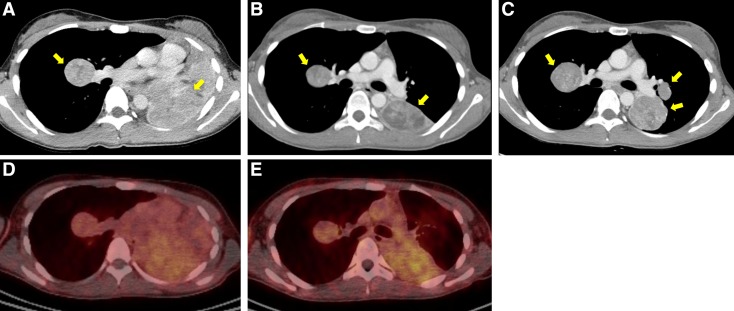
We performed an open‐label, single‐arm, phase II study to evaluate the efficacy and safety of pazopanib in patients with metastatic ASPS. We included patients who met the following key eligibility criteria: age ≥18 years; histologically confirmed diagnosis of metastatic or unresectable ASPS confirmed by positive immunostaining for TFE3; treatment‐naïve or received prior chemotherapy except vascular endothelial growth factor (VEGF) inhibitors. Six patients were enrolled into this trial. The median number of cycles administered was 7.5 (range 6–21), with a median follow‐up duration of 33 months (range 18.7–39.3 months). The baseline characteristics are presented in Table 1. One patient (16.7%) achieved PR after two cycles of pazopanib treatment. The remaining five (83.3%) showed SD during the treatment (tumor reduction, mean ± standard deviation, 9.4 ± 13.1%). The median PFS was 5.5 months (95% CI 3.4–7.6 months), and the 6‐month PFS rate was 50%. Among four patients with SD, there was one patient (patient 3) who showed disease stabilization over the long period of time. She was 23 years of age and diagnosed with ASPS with metastases to the lung and bone. As shown in Figure 1, computed tomography (CT) scan during pazopanib treatment showed clinical improvement, and the value of maximal standardized uptake value measured on 68Ga‐RGD PET/CT was also slightly decreased. The period of disease stabilization lasted 22 months. Treatment was discontinued for all six patients due to disease progression, and two patients died of disease progression.
Table 1. Patients' characteristics and treatment outcomes.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; PFS, progression‐free survival; PR, partial response; OS, overall survival; SD, stable disease.
Figure 1.
Computed tomography (CT) scan and 68Ga‐RGD positron emission tomography‐CT scan of patient 3. CT scan before treatment (A), after 2 months of pazopanib treatment (B), and at disease progression (C). 68Ga‐RGD PET/CT before treatment (D) and after 14 days of pazopanib treatment (E).
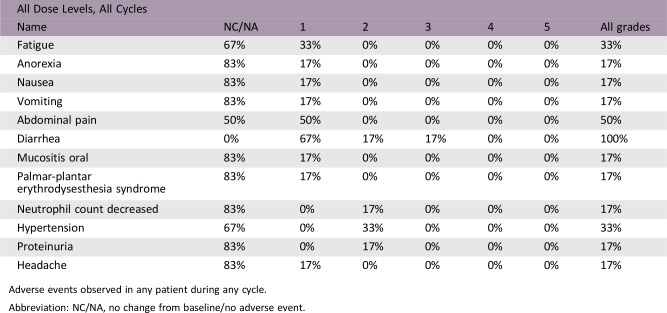
The most common treatment‐related toxicities were diarrhea (100%) and abdominal pain (50%; Adverse events table). There were no severe toxicities except one patient with grade 3 diarrhea.
To our knowledge, clinical outcomes in our study were comparable to those in previous studies in metastatic ASPS using antiangiogenic TKIs. However, our study was closed early because of the low accrual rate that was attributable to the rarity of the subtype (<1% of STS). In addition, a wait‐and‐see policy was adopted in ASPS patients who showed stable or slow‐growing metastases. Further large‐scale, prospective studies evaluating the efficacy of pazopanib in ASPS are warranted.
Trial Information
- Disease
Sarcomas – Adult
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
No designated number of regimens
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Overall response rate
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Progression‐free survival at 6 months
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Toxicity
- Additional Details of Endpoints or Study Design
Simon's two‐stage minimax design was used to evaluate a null hypothesis of an ORR <15% versus an alternative hypothesis of ≥40% ORR, with a significance level of 0.05 and a power of 0.80. The target accrual was nine patients in the first stage. If ≥2 responders were observed, then an additional 10 patients would be enrolled in the second stage.
- Investigator's Analysis
Active but results overtaken by other developments
Drug Information
- Drug 1
- Generic/Working Name
Pazopanib
- Trade Name
Votrient
- Company Name
Novartis
- Drug Type
Small molecule
- Drug Class
VEGF receptor
- Dose
800 mg per flat dose
- Route
p.o.
- Schedule of Administration
800 mg once daily administered continuouslyin 4‐week interval per cycle
Patient Characteristics
- Number of Patients, Male
3
- Number of Patients, Female
3
- Stage
Metastatic
- Age
Median (range): 29.5 (23–36)
- Number of Prior Systemic Therapies
Median (range): 0 (0–2)
- Performance Status: ECOG
-
0 — 2
1 — 4
2 — 0
3 — 0
Unknown —
- Other
Previous surgery: 6
- Cancer Types or Histologic Subtypes
Alveolar soft part sarcoma: 6
Primary Assessment Method
- Number of Patients Screened
7
- Number of Patients Enrolled
6
- Number of Patients Evaluable for Toxicity
6
- Number of Patients Evaluated for Efficacy
6
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 1 (16.7%)
- Response Assessment SD
n = 5 (83.3%)
- Response Assessment PD
n = 0 (0%)
- (Median) Duration Assessments PFS
5.5 months, CI: 3.4–7.6
- Outcome Notes
Median OS was not reached.
Adverse Events
Adverse events observed in any patient during any cycle.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated Reason
Did not fully accrue
- Investigator's Assessment
Active but results overtaken by other developments
Alveolar soft part sarcoma (ASPS) is a very rare and distinct histologic soft‐tissue sarcoma (STS), mainly arising in adolescents and young adults [1]. Despite its relatively slow progression, ASPS exhibits a very high propensity for metastases to other organs, typically the lung and the brain [2]. Conventional cytotoxic chemotherapy has not proved effective for the treatment of ASPS. Instead, surgical therapy is still the mainstay of treatment and ensures chance for long‐term survival [3], [4]. Microarray gene expression analysis and in vitro preclinical studies reported the markedly elevated expression of several genes involved in angiogenesis, including vascular endothelial growth factor (VEGF), ANGPTL2, HIF‐1α, MDK, c‐MET, and TIMP‐2, in ASPS tumors [5], [6]. The lack of therapeutic alternatives in metastatic ASPS, its highly vascular property, and the abnormal expression of genes related to angiogenesis prompted us to test the possible therapeutic activity of antiangiogenic drugs.
Pazopanib, a small molecule tyrosine kinase inhibitor, exhibits selective activity against VEGF receptors [7]. In a recent phase III study in metastatic STS (PALETTE), the median progression‐free survival (PFS) in patients receiving pazopanib was improved to 4.6 months compared with 1.6 months in patients receiving placebo [8]. However, the number of ASPS patients included in this trial was too small to evaluate its efficacy in this tumor type. Therefore, we designed this phase II study to assess the clinical efficacy and safety of pazopanib in patients with metastatic ASPS.
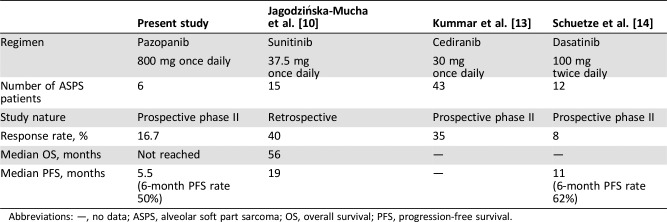
In the present study, we observed modest clinical benefit with pazopanib—with one (16.7%) and five patients (83%) with metastatic ASPS having partial response and stable disease (SD), respectively. The tumors of patients with SD remained stable for at least 4 months. Among four patients with SD, there was one patient who experienced disease stabilization for 22 months. The efficacy achieved in our study was comparable to that reported in previous phase II and III studies with pazopanib in patients with metastatic ASPS [8], [9]. Other drugs targeting the angiogenic pathways have also been investigated in ASPS. These include sunitinib, cediranib, dasatinib, and bevacizumab. The clinical benefit of sunitinib has been encouraging, based on a series of retrospective studies with median PFS ranging from 17 to 41 months, and the median OS ranging from 19 to 56 months [10], [11], [12]. Cediranib has also demonstrated encouraging efficacy in a phase II trial with an objective response rate of 35% and a disease control rate of 84% at 24 weeks [13]. Also, >10% of the ASPS patients treated with dasatinib showed disease stabilization for >1 year [14]. Finally, Azizi et al. reported that bevacizumab, a monoclonal antibody blocking VEGF‐α, induced tumor regression in a patient with metastatic ASPS [15]. To date, sunitinib seems to provide the most promising results for the treatment of metastatic ASPS compared with other antiangiogenic drugs (Table 2). However, it needs to be further validated by a prospective study on a larger scale before being recommended as the standard treatment for metastatic ASPS.
Table 2. Comparison of published studies of tyrosine kinase inhibitors for advanced ASPS.
Abbreviations: —, no data; ASPS, alveolar soft part sarcoma; OS, overall survival; PFS, progression‐free survival.
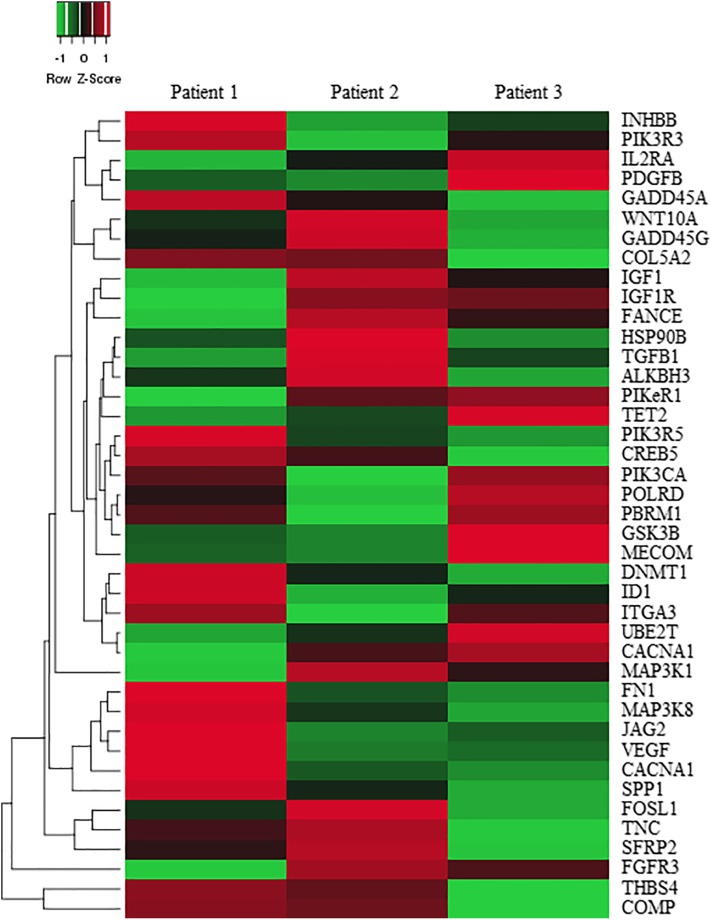
It is of note that stabilization of the disease was the most frequent response observed between 53% and 83% for patients treated with TKIs, including those receiving pazopanib in our study [10], [11], [13]. This is not surprising in that antiangiogenic TKIs target the angiogenesis signaling pathways on the endothelial cells rather than tumor itself. In this aspect, one could speculate that TKIs might have some limited benefit in their clinical efficacy due to their indirect mode of action. However, Stacchiotti et al. demonstrated the direct antitumor effect of sunitinib in short‐term ASPS cultures [11]. Pazopanib also has antitumor activity in tumor xenografts derived from non‐ASPS tumor cells [16]. Therefore, it is likely that the spectrum for activity of these multitargeted TKIs might be so broad as to inhibit both angiogenesis and tumor cell growth. Although the action mechanism of pazopanib is mediated via VEGF pathway, the underlying biological processes need to be addressed. In an effort to identify these key mediators, we analyzed the transcriptome of ASPS following the treatment with pazopanib. We analyzed the pretreatment and postprogression paired samples from three patients using the NanoString gene expression array. Using a p value <.05, we selected the top 41 differentially expressed genes (DEGs) between pretreatment and postprogression samples. DEGs with the greatest differential expression were the components of signaling pathways such as mitogen‐activated protein kinase, phosphoinositide 3‐kinase, and wingless‐type MMTV integration site family (Fig. 2). We also identified 10 up‐ or down‐regulated DEGs related to angiogenesis. Our result indicates that pazopanib might modulate multiple signaling pathways in a simultaneous manner.
Figure 2.
Heatmap illustrating the differential expression of 41 genes.
In conclusion, this study demonstrates that pazopanib has modest efficacy with tolerable toxicity in metastatic ASPS. However, our study had several limitations due to the rarity of the disease inherent to ASPS. First, this was a small population size of six patients due to an early closure. Second, our result on the efficacy of pazopanib should be interpreted with caution given that the spontaneous stabilization could occur in ASPS due to its indolent biology. Therefore, a larger, future study will be necessary to accrue a significant number of patients to validate the clinical benefit of pazopanib in patients with metastatic ASPS.
Figures and Tables
Acknowledgments
This study was supported by GlaxoSmithKline and Novartis Pharmaceuticals Corporation. Pazopanib is an asset of Novartis AG as of March 2, 2015. Medical writing assistance was provided by Seonah Ha, Ph.D.
Footnotes
ClinicalTrials.gov Identifier: NCT02113826
Sponsor(s): Tae Min Kim
Principal Investigator: Tae Min Kim
IRB Approved: Yes
Disclosures
Dong‐Wan Kim: Pfizer, Merck Sharp & Dohme (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Zarrin‐Khameh N, Kaye KS. Alveolar soft part sarcoma. Arch Pathol Lab Med 2007;131:488–491. [DOI] [PubMed] [Google Scholar]
- 2.Jaber OI, Kirby PA. Alveolar soft part sarcoma. Arch Pathol Lab Med 2015;139:1459–1462. [DOI] [PubMed] [Google Scholar]
- 3.Pappo AS, Parham DM, Cain A et al. Alveolar soft part sarcoma in children and adolescents: Clinical features and outcome of 11 patients. Med Pediatr Oncol 1996;26:81–84. [DOI] [PubMed] [Google Scholar]
- 4.van Ruth S, van Coevorden F, Peterse JL et al. Alveolar soft part sarcoma. A report of 15 cases. Eur J Cancer 2002;38:1324–1328. [DOI] [PubMed] [Google Scholar]
- 5.Kenney S, Vistica DT, Stockwin LH et al. ASPS‐1, a novel cell line manifesting key features of alveolar soft part sarcoma. J Pediatr Hematol Oncol 2011;33:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockwin LH, Vistica DT, Kenney S et al. Gene expression profiling of alveolar soft‐part sarcoma (ASPS). BMC Cancer 2009;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: Clinical development of a potent anti‐angiogenic drug. Crit Rev Oncol Hematol 2011;77:163–171. [DOI] [PubMed] [Google Scholar]
- 8.van der Graaf WT, Blay JY, Chawla SP et al. Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): A randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 2012;379:1879–1886. [DOI] [PubMed] [Google Scholar]
- 9.Sleijfer S, Ray‐Coquard I, Papai Z et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European Organisation for Research and Treatment of Cancer‐soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 2009;27:3126–3132. [DOI] [PubMed] [Google Scholar]
- 10.Jagodzinska‐Mucha P, Switaj T, Kozak K et al. Long‐term results of therapy with sunitinib in metastatic alveolar soft part sarcoma. Tumori 2017;103:231–235. [DOI] [PubMed] [Google Scholar]
- 11.Stacchiotti S, Negri T, Zaffaroni N et al. Sunitinib in advanced alveolar soft part sarcoma: Evidence of a direct antitumor effect. Ann Oncol 2011;22:1682–1690. [DOI] [PubMed] [Google Scholar]
- 12.Li T, Wang L, Wang H et al. A retrospective analysis of 14 consecutive Chinese patients with unresectable or metastatic alveolar soft part sarcoma treated with sunitinib. Invest New Drugs 2016;34:701–706. [DOI] [PubMed] [Google Scholar]
- 13.Kummar S, Allen D, Monks A et al. Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol 2013;31:2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuetze SM, Bolejack V, Choy E et al. Phase 2 study of dasatinib in patients with alveolar soft part sarcoma, chondrosarcoma, chordoma, epithelioid sarcoma, or solitary fibrous tumor. Cancer 2017;123:90–97. [DOI] [PubMed] [Google Scholar]
- 15.Azizi AA, Haberler C, Czech T et al. Vascular‐endothelial‐growth‐factor (VEGF) expression and possible response to angiogenesis inhibitor bevacizumab in metastatic alveolar soft part sarcoma. Lancet Oncol 2006;7:521–523. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Knick VB, Rudolph SK et al. Pharmacokinetic‐pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther 2007;6:2012–2021. [DOI] [PubMed] [Google Scholar]