Wistar rats exposed for 3 months to a 20% ethanol intermittent-access voluntary drinking paradigm displayed a reduction in epididymal fat, blood glucose and non-HDL and total cholesterol. These effects were accompanied by decreased expression of Hmgcr, Srebp-2, Cox-2 and RelA, indicating downregulation of genes involved in cholesterol synthesis and inflammation.
Abstract
Aims
Epidemiological studies and experimental data from rodent models have reported a non-linear relationship between consumption of alcohol and cardiovascular disease (CVD) risk that suggests that light-to-moderate drinking as opposed to excessive consumption may provide some cardiovascular benefits. The present study examined potential mechanisms by which moderate alcohol consumption may provide a protective effect against CVD.
Short summary
Wistar rats exposed for 3 months to a 20% ethanol intermittent-access voluntary drinking paradigm displayed a reduction in epididymal fat, blood glucose and non-HDL and total cholesterol. These effects were accompanied by decreased expression of Hmgcr, Srebp-2, Cox-2 and RelA, indicating downregulation of genes involved in cholesterol synthesis and inflammation.
Methods
Twenty-four male Wistar rats voluntarily consumed a 20% v/v ethanol solution on alternate days for 13 weeks (ethanol-treated) or were given access to water alone (non-ethanol-exposed control).
Results
There was no difference in body weight gain between the two groups, however, epididymal fat weight was lower in ethanol-fed rats (P = 0.030). Blood glucose, total cholesterol, non-high-density lipoprotein (HDL) and oxidized low-density lipoprotein (LDL) levels were lower in the ethanol group compared to controls (P < 0.05). There was a significant reduction in the expression of hydroxymethylglutaryl-coenzyme A reductase and sterol regulatory element-binding protein-2 in ethanol-treated rats (P < 0.05), suggesting that ethanol may have lowered cholesterol levels via downregulation of genes involved in cholesterol synthesis. Paraoxonase-1, which is associated with inhibition of LDL cholesterol oxidation, was upregulated in the ethanol group (P = 0.029). Ethanol-treated rats exhibited significantly lower levels of high-mobility box group protein 1 (P ≤ 0.05). Cyclooxygenase-2 and RelA gene expression were significantly lower in ethanol-treated rats (P < 0.05), indicating possible anti-inflammatory effects.
Conclusions
These findings suggest that moderate ethanol consumption may potentially contribute to improved cardiovascular outcomes by reducing body fat, improving blood cholesterol and blood glucose, and modulation of gene expression involved in inflammation and/or cholesterol synthesis.
INTRODUCTION
Cardiovascular diseases (CVDs), namely, coronary artery disease and ischemic stroke, hypertension and atrial fibrillation have close associations with chronic high alcohol consumption. An increasing body of evidence continues to validate a J- or U-shaped correlation between alcohol consumption and CVD risk factors (Carnevale and Nocella, 2012; Bergmann et al., 2013; Chiva-Blanch et al., 2013; Wakabayashi, 2013a; Klein et al., 2014; Matsumoto et al., 2014; O’Keefe et al., 2014). This J- or U-shaped correlation indicates potential cardioprotective effects of light-to-moderate alcohol consumption, while high levels of consumption confer increased risk (Krenz and Korthuis, 2012). Improvements in lipid profile have been proposed as a mechanism behind the protective effects of light-to-moderate alcohol consumption, however, meta-analyses examining the effects of alcohol on lipid profile have reported inconsistent results pertaining to changes in triglycerides (TG), low-density lipoprotein (LDL) cholesterol and total cholesterol (TC) (Brinton, 2012; Klop et al., 2013). In addition to the discrepancies found, mechanisms behind these changes in lipid profile are not well established.
CVDs are promoted by chronic inflammation and oxidative stress (Chiva-Blanch et al., 2013). Chronic inflammation has been linked to excessive alcohol intake by inducing oxidative stress (Grasselli et al., 2014). Effects of light-to-moderate ethanol consumption on inflammation and antioxidant status have yielded conflicting results (Estruch et al., 2011; Chiva-Blanch et al., 2013). Chiva-Blanch et al. (2013) reported inconsistent effects of moderate alcohol consumption on inflammation depending on beverage type and the inflammatory markers measured. Wine consumption has been shown to decrease oxidative stress by improving antioxidant capacity and/or promoting favorable changes in antioxidant enzyme activity (Fernandez-Pachon et al., 2009; Estruch et al., 2011). Moderate beer consumption has been researched on a smaller scale and has demonstrated similar results to wine in regard to oxidative stress (van der Gaag et al., 2000; Chiva-Blanch et al., 2013).
Existing studies analyzing the effects of ethanol on CVD risk factors have produced inconsistent findings and the mechanisms behind potential protective effects of moderate ethanol consumption on CVD risk factors are not well determined. The purpose of the present study was to further elucidate the mechanisms of moderate ethanol consumption on risk factors for CVD utilizing an outbred Wistar rat model and controlled experimental design. It was hypothesized that a pattern of moderate, alternating day ethanol consumption would improve lipid profile via changes in hepatic fat metabolism gene expression, decrease inflammation by the downregulation of inflammatory genes and decrease global oxidative stress by improving antioxidant defenses.
MATERIALS AND METHODS
Animals and diets
This study involved a randomized controlled design conducted over a 13-week period. Twenty-four 15-week-old male outbred Wistar rats (490–512 g; Harlan, Placentia, CA) were individually housed in tub cages in a vivarium at San Diego State University that maintained a 12:12-h light/dark cycle and an ambient temperature of ~23°C. The animals were randomly assigned to a control group (water and ad libitum diet; n = 12) and a treatment group (water and ad libitum diet and a 20% v/v ethanol solution on alternate days for 13 weeks; n = 12, see the following). The ad libitum diet consisted of regular chow (LabDiet, St. Louis, MO). All study procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at San Diego State University and were in accordance with National Institutes of Health guidelines.
Ethanol exposure
Rats were exposed for 13 weeks to either a 20% ethanol intermittent-access drinking paradigm (n = 12) or were given access water alone as their sole source of fluid (n = 12). This alcohol exposure paradigm has previously been shown to result in blood alcohol concentrations (BACs) averaging ~30–50 mg/dl in outbred Wistar rats when measured 30–120 min into a standard drinking session (Simms et al., 2008; Cippitelli et al., 2012). The first seven daily sessions served to acclimate the animals to the testing procedures and all rats received free access to food and water only. Following the acclimation period, ethanol-exposed rats began 22-h intake sessions involving voluntary access to a 20% (v/v) ethanol solution vs water, alternating with 22-h abstinence periods involving voluntary access to water only (45 ethanol drinking sessions total over 13 weeks). The position of ethanol and water bottles was rotated each ethanol session to control for position preferences. The control group was given voluntary access to water only during the entire duration of the chronic exposure period. All fluids were weighed to the nearest gram and replaced daily, and body weights were measured every 48 h.
Sample collection
One day following their final experimental session, rats were euthanized by CO2 gas overdose and blood samples were collected. Serum samples were collected after centrifugation at 1200× g for 15 min at 4°C. Tissues including the liver and epididymal fat were harvested and stored with serum samples at −80° C until time of analysis.
Serum lipids and blood glucose
Serum lipid profile measurements were determined using serum TC, TG and high-density lipoprotein (HDL) cholesterol assay kits (Stanbio, Boerne, TX). Non-HDL cholesterol was calculated using the following formula: non-HDL cholesterol = TC – HDL – (TG/5). Blood glucose levels were measured using the Glucose LiquiColor assay kit (Stanbio).
Serum oxidized LDL
Oxidized LDL (oxLDL) was measured using an oxLDL sandwich ELISA kit from MyBioSource (San Diego, CA). Serum samples were incubated with anti-rat oxLDL antibody, then biotinylated detection antibody. Avidin–horseradish peroxidase conjugate was added to produce a blue color in direct proportion to initial oxLDL concentration. Following the addition of a stop solution, absorbance was read at 450 nm.
Serum apolipoprotein A1
Apolipoprotein A1 (ApoA1) was measured in serum using a sandwich ELISA assay kit (MyBioSource.com, San Diego, CA). Serum samples were incubated with biotinylated ApoA1 antibody followed by horseradish peroxidase–avidin, then substrate solution was added to produce a blue color in direct proportion to initial ApoA1 concentration. Stop solution was added and the absorbance was read at 450 nm.
Liver cholesterol concentration
Cholesterol concentrations were measured in liver tissue using a cholesterol quantitation kit (Sigma, St. Louis, MO). Liver tissue (100 mg) was extracted using chloroform:isoporpanol:IGEPAL CA-630 in a microhomogenizer, then the samples were centrifuged and air-dried to remove insoluble material and organic solvents. Dried lipids were dissolved in the provided buffer solution, after which the samples were combined with cholesterol probe, cholesterol enzyme mix and cholesterol esterase in microplate wells. Following incubation for 60 min at 37°C, the absorbance was read at 570 nm.
Serum HMGB1 and TLR4 activities
High-mobility box group 1 protein (HMGB1) and Toll-like receptor 4 (TLR4) were measured using sandwich ELISA kits from MyBioSource (San Diego, CA). Serum samples were incubated with anti-rat HMGB1 antibody and anti-rat TLR4 antibody, respectively. The samples were subsequently incubated with biotinylated anti-rat antibody, followed by avidin–horseradish peroxidase conjugate. Substrate solution was added to produce a blue color in direct proportion to initial HMGB1 or TLR4 concentration, and absorbance was read at 450 nm.
Hepatic gene expression
The TRIzol method was employed to extract total ribonucleic acid (RNA) from the liver. Reverse transcription was performed using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) with oligo (dT)12-18 primers. The mRNA levels of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Hmgcr, Rn00565598_m1), fatty acid synthase (Fas, Rn00569117_m1), sterol regulatory element-binding protein-1 (Srebp-1, Rn01495769_m1), sterol regulatory element-binding protein-2 (Srebp-2, Rn01306296_m1), paraoxonase-1 (Pon-1, Rn01455909_m1), cyclooxygenase-2 (Cox-2, Rn00568225_m1), NF-kappa-B p65 subunit (RelA, Rn01502266_m1) and aldehyde dehydrogenase-2 (Aldh2, Rn00583474_m1) were assessed by quantitative Real Time-PCR (ViiA7 system, Applied Biosystems, Foster City, CA) using TaqMan Universal PCR probes. Calculations of target gene relative quantitation values were performed using the ΔΔCT numerical quantity and normalized to r18S expression. Each set of PCR contained a minus RT as a negative control.
Serum antioxidant enzyme assays
Serum antioxidant enzyme activity levels of superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), glutathione peroxidase (GPx) and glutathione reductase (GR) were determined using the respective Cayman assay kit protocol (Cayman Chemical Co., Ann Arbor, MI). Each assay was performed by manual instructions and read by spectrophotometer. Briefly, xanthine oxidase and hypoxanthine were used in serum samples to determine SOD activity. CAT activity was initiated upon the addition of hydrogen peroxide (H2O2) and measured as the reaction between the enzyme itself and methanol. GST activity was measured by the rate of conjugation of 1-chloro-2,4-dinitrobenzene with reduced glutathione (GSSG). Absorbance was measured every minute for 12 consecutive minutes. Both GPx and GR use the oxidation of NADPH to NADP+, which was accompanied by a decrease in absorbance, to determine enzyme activity. This process indirectly determines GPx activity while directly measuring GR activity because GPx requires hydroperoxide to produce GSSG. GR, then, oxidizes GSSG back to glutathione (GSH) and NADP+. Absorbances were read at 450 nm (SOD), 540 nm (CAT) 340 nm (GPx, GR and GST).
Serum thiobarbituric acid reactive substances
Thiobarbituric acid reactive substances (TBARS) assay kit (Cayman Chemical Co.) was used to measure oxidative stress through lipid peroxidation. Sodium dodecyl sulfate solution and color reagent were mixed with serum samples and allowed to boil for 1 h. Reactions were then terminated by immediate relocation of boiling samples onto ice. Absorbance was read at 535 nm after stabilization at room temperature for 30 min using a spectrophotometer.
Serum total antioxidant capacity
Total antioxidant levels were measured in serum using an Antioxidant Assay Kit (Sigma, St. Louis, MO). Standards and samples were prepared following the manufacturer’s protocol instructions. Trolox was used as a standard and oxidation of 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid was determined spectrophotometrically at 405 nm.
Data analysis
Data were analyzed using SPSS Version 21.0 (IBM, Armonk, New York) and Statistica Version 13 (StatSoft, Inc). Ethanol intake in ethanol-exposed rats was calculated for each session during the 13-week chronic exposure phase to determine levels and patterns of ethanol consumption across time. Individual session data were averaged in three-session blocks prior to analysis (15 blocks total). Ethanol intake (g/kg) and ethanol and water intake (ml/kg) for ethanol-exposed subjects were analyzed using repeated measures analysis of variance (ANOVA) with block and solution as within-subject factors, followed by Newman-Keuls test where appropriate (α = 0.05). Student’s t-test was employed to evaluate the effects of ethanol treatment on body weight, weight gain, epididymal fat content, lipid profile, hepatic gene expression, antioxidant enzyme activity, TBARS and total antioxidant capacity (TAC). Data are presented as mean ± standard error (SE) for the respective measurements. The correlations between variables were tested using Spearman rho. Statistical significance was set at P < 0.05.
RESULTS
Ethanol intake
The intermittent-access 20% ethanol drinking paradigm resulted in increased g/kg intake of ethanol across the 13-week exposure phase (P < 0.001). Mean ethanol intake increased from 1.33 ± 0.38 g/kg/22 h during the first three sessions to 4.88 ± 0.62 g/kg/22 h by the end of the chronic exposure phase, with mean overall alcohol consumption of 3.58 ± 0.60 g/kg/22 h. Analysis of ml/kg ethanol and water intake in ethanol-exposed subjects revealed a similar increase in ethanol intake, while water intake declined (main effect of solution: P < 0.01; main effect of block: P < 0.001; solution × block interaction: P < 0.001).
Body weight gain and epididymal fat
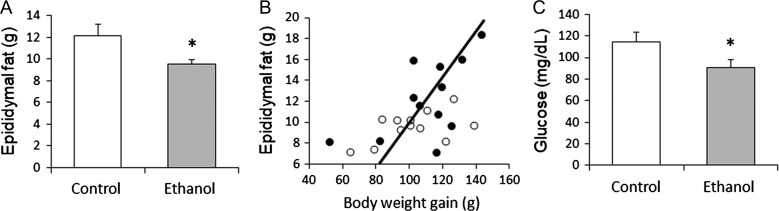
There were no significant differences found in initial body weight (490 ± 8.83 g vs 516 ± 12.9 g), final body weight (592 ± 14.2 g vs 726 ± 16.4 g) or body weight gain (102 ± 6.06 g vs 110 ± 6.89 g) between the ethanol treatment group and the control group. Epididymal fat weight at the end of the experiment was significantly lower in ethanol-fed rats (P = 0.030; Fig. 1A). There was a significant positive correlation between epididymal fat weight and weight gain (r = 0.585, P = 0.005) (Fig. 1B).
Fig. 1.
(A) Epididymal fat weight. Epididymal fat weight was significantly lower in ethanol-fed rats (P = 0.03). (B) Correlation of body weight gain to epididymal fat. Weight gain was positively correlated with epididymal fat (r = 0.585, P = 0.005). Closed circle: control group, open circle: ethanol group. (C) Blood glucose. Blood glucose was significantly lower in rats consuming ethanol compared to control rats (P = 0.03). *P < 0.05 considered significant.
Blood glucose, lipid profile, oxLDL and ApoA1
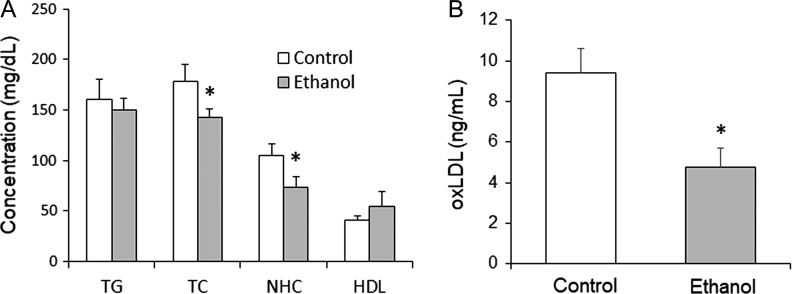
Ethanol-treated rats displayed significantly lower blood glucose levels than non-ethanol-exposed control rats (P = 0.030; Fig. 1C). Consumption of ethanol on alternating days also resulted in significant reductions in serum total cholesterol (P = 0.036) and serum non-HDL cholesterol (P = 0.031) levels. Differences in TG and HDL cholesterol were not found to be significant (Fig. 2A). The ethanol group had significantly lower serum levels of oxLDL than controls (P = 0.006) (Fig. 2B). Serum levels of ApoA1 were not significantly different between the ethanol and control groups (340.9 ± 24.7 μg/mL vs 335.9 ± 33.2 μg/mL).
Fig. 2.
(A) Serum lipid profile. Serum lipid profile of rats after 13 weeks of moderate ethanol consumption every other day. Moderate ethanol consumption produced a significant reduction in TC (P = 0.036) and NHC (non-HDL cholesterol) (P = 0.031). HDL cholesterol and TG were not significantly different. (B) oxLDL. Ethanol consumption lowered oxLDL level (P = 0.006). *P ≤ 0.05 considered significant.
Liver cholesterol concentration
Liver cholesterol concentration was significantly lower in ethanol-treated rats (18.1 ± 2.13 μg/mg) than in control animals (23.6 ± 1.36 μg/mg) (P = 0.039).
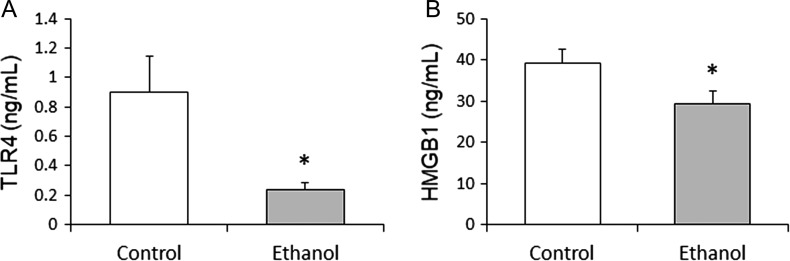
Serum HMGB1 and TLR4
Compared with the control group, the ethanol group had significantly lower serum levels of TLR4 (P = 0.019) and HMGB1 (P = 0.050) (Fig. 3).
Fig. 3.
(A) TLR4 and (B) HMGB1. Moderate ethanol consumption significantly lowered TLR4 (P = 0.019) and HMGB1 (P = 0.050) levels. *P ≤ 0.05 considered significant.
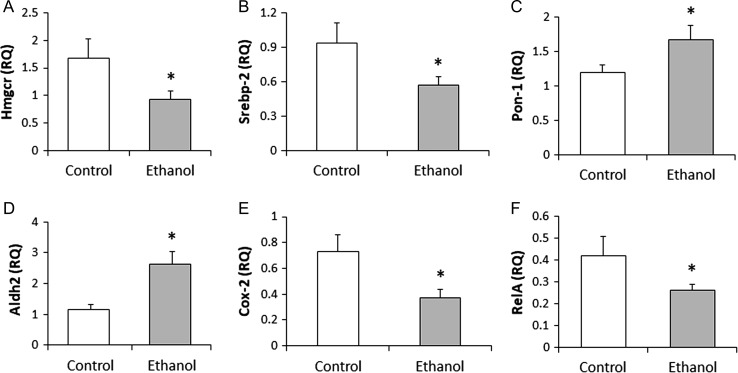
Hepatic gene expression
Significant decreases in Hmgcr (P = 0.034) and Srebp-2 (P = 0.026) gene expression and an increase in Pon-1 (P = 0.029) expression were observed in the ethanol treatment group compared to the control group (Fig. 4A, B and C). Ethanol treatment also significantly increased Aldh2 expression (P = 0.002) (Fig. 4D). Cox-2 (P = 0.016) and RelA (P = 0.047) genes both displayed a decrease in expression in ethanol-treated rats relative to controls (Fig. 4E and F). There were no significant differences between the groups for Fas (ethanol group: 0.28 ± 0.08 RQ vs control: 0.38 ± 0.08 RQ) and Srebp-1 (ethanol group: 0.40 ± 0.10 RQ vs control: 0.48 ± 0.10 RQ) gene expression.
Fig. 4.
Gene expression related to lipid metabolism and inflammation. The ethanol group showed significantly decreased hepatic expression of Hmgcr (P = 0.034), Srebp2 (P = 0.026), Cox-2 (P = 0.016) and RelA (P = 0.047) as well as significantly increased expression of and Pon-1 (P = 0.029) and Aldh2 (P = 0.002). *P ≤ 0.05 considered significant. Aldh2: aldehyde dehydrogenase-2; Cox2: cyclooxygenase-2; Hmgcr: 3-hydroxy-3-methylglutaryl-CoA reductase; Pon-1: paraoxonase-1; RelA: NF-kappa-B p65 subunit; RQ: relative quantification; Srebp2: sterol regulatory element-binding protein 2.
Oxidative stress and antioxidant capacity and antioxidant enzymes
Oxidative stress, as measured by TBARS, significantly decreased in ethanol-fed rats (P = 0.028; Table 1). TAC measurements were not significant. Activities of five antioxidant enzymes were measured (SOD, CAT, GST, GPx and GR) and only CAT (P = 0.047) showed significantly enhanced activity with ethanol treatment (Table 1).
Table 1.
TBARS, TAC and antioxidant enzyme activities
| Control | Ethanol | |
|---|---|---|
| TBARS, μmol/L | 3.35 ± 0.40a | 2.31 ± 0.12b |
| TAC, mmol/L | 0.26 ± 0.07 | 0.47 ± 0.20 |
| SOD, U/mL | 25.3 ± 4.45 | 23.6 ± 3.16 |
| CAT, nmol/min/mL | 240 ± 30.9a | 339 ± 34.9b |
| GST, nmol/min/mL | 7.22 ± 0.86 | 7.12 ± 0.43 |
| GPx, nmol/min/mL | 25.9 ± 4.05 | 37.8 ± 4.51 |
| GR, nmol/min/mL | 32.8 ± 5.87 | 48.2 ± 18.69 |
Data are presented as means ± SE. Data were tested using repeated measures ANOVA followed by SNK test for between-group comparisons. Data within rows with varying superscript letters are statistically different (P < 0.05). N = 24, 12 animals per group. CAT: catalase; GST: glutathione S-transferase; GPx: glutathione peroxidase; GR: glutathione reductase; SOD: superoxide dismutase; TAC: total antioxidant capacity; TBARS: thiobarbituric acid reactive substances.
Correlations
Blood glucose levels showed a positive correlation with weight gain (coefficient = 0.432; P = 0.035), epididymal fat amount (coefficient = 0.360; P = 0.042) and TC (coefficient = 0.419; P = 0.018; Table 2). Although not significant, there was a positive trend between blood glucose and TG (P = 0.072). Greater body weight gain and epididymal fat amount were inversely correlated with Pon-1 (coefficient = −0.433 and −0.533, respectively) and Aldh2 (coefficient = −0.449 and −0.404, respectively; P < 0.05; Table 2). Pon-1 level was inversely correlated with oxLDL (coefficient = −0.511, P = 0.030) and TBARS (coefficient = -0.532, P = 0.016). Serum LDL was positively correlated with TLR4 level (coefficient = 0.569, P = 0.005).
Table 2.
Correlations between glucose and gene expression of Pon-1 and Aldh2 with weight gain, body fat, TC and TG
| Glucose | Pon-1 | Aldh2 | |
|---|---|---|---|
| Weight gain | 0.432 (P = 0.035) | −0.433 (P = 0.028) | −0.449 (P = 0.041) |
| Epididymal fat | 0.360 (P = 0.042) | −0.533 (P = 0.011) | −0.404 (P = 0.035) |
| TC | 0.419 (P = 0.018) | −0.017 (P = 0.946) | −0.337 (P = 0.146) |
| TG | 0.360 (P = 0.072) | 0.121 (P = 0.611) | 0.109 (P = 0.638) |
Values are provided as coefficients (P-value). Aldh2, aldehyde dehydrogenase-2; Pon-1, paraoxonase-1; TC, total cholesterol; TG, triglyceride.
DISCUSSION
Ethanol consumption has shown a relationship to the development of CVD, with light-to-moderate drinking linked to improved cardiovascular outcomes, while excessive consumption is associated with increased risk (Krenz and Korthuis, 2012). Many CVD events, which can lead to death, are related to an inflammatory disorder of the arteries, initiated by risk factors such as hypercholesterolemia, inflammation and oxidative stress (Ramji and Davies, 2015; Soeki and Sata, 2016). Serum LDL cholesterol levels continue to be one of the most important CVD risk factors (Klop et al., 2013). In this study, Wistar rats consuming ethanol in a voluntary drinking paradigm, previously shown to result in moderate blood alcohol levels in this strain (~30–50 mg/dl; Simms et al., 2008; Cippitelli et al., 2012) exhibited decreased non-HDL, TC and liver cholesterol levels. These cholesterol-lowering effects of moderate alcohol consumption are consistent with many studies, including meta-analysis (Mathews et al., 2015; Vu et al., 2016; Wakabayashi, 2013a, b, 2016), although a few meta-analyses have found no relationship (Brien et al., 2011; Matsumoto et al., 2014). The disparate reported effects of moderate alcohol consumption on LDL cholesterol in the clinical literature may be due to several factors, including differing populations (genetics), genders and alcohol beverage type. Although ethanol consumption typically raises HDL cholesterol levels in humans, HDL cholesterol was not significantly different between the groups in this study. There was, however, a trend toward higher HDL in the ethanol group. As ethanol increases HDL cholesterol in a dose-dependent fashion (Volcik et al., 2008), a larger ethanol dose could have produced significant results for HDL. Pon-1 is HDL-associated protein and was significantly higher in the ethanol group, which may have contributed to the numerically higher HDL in ethanol-fed animals.
The reduction in serum cholesterol and hepatic cholesterol observed in this study may be mechanistically related to a reduction in Hmgcr and Srebp-2 gene expression. HMG-CoA reductase (Hmgcr) is a key rate-limiting enzyme in cholesterol synthesis (Yin et al., 2009; Klein et al., 2014), while Srebp-2 is the primary transcription factor that regulates the expression of Hmgcr (Sakakura et al., 2001; Yin et al., 2009; Wang et al., 2010). The current findings concur with a prior study demonstrating that moderate ethanol consumption decreases hepatic Hmgcr gene expression in alcohol-preferring rats consuming ethanol daily (Klein et al., 2014). In contrast, chronic excessive alcohol consumption increases Srebp-2 and Hmgcr expression (Yin et al., 2009; Wang et al., 2010), as well as increases hepatic Srebp-2 activation and amount (Wang et al., 2010). Taken together, these data support the present results of reduction in Hmgcr and Srebp-2 expression as a possible mechanism leading to proposed cardiovascular benefits of moderate ethanol consumption. However, the present data differ from the findings of Yin et al. (2009) demonstrating that low-dose (3.6% total kcal) ethanol feeding in mice significantly increased the expression of Srebp-2 and Hmgcr after 4 weeks. The variation in results may be attributed to the duration or schedule of ethanol exposure (alternating day exposure for 13 weeks in the present paradigm) and/or important alcohol-dietary interactions, as ethanol in the Yin et al.’s study was administered in a high-fat Lieber–DeCarli diet (vs regular chow diet in the current study), commonly used to induce alcohol liver steatosis.
CVD is a complex disease involving many immune and inflammatory mechanisms. The increase in proinflammatory cytokine production, resulting from a number of risk factors, activates NF-κB in the cytoplasm, which releases the RelA subunit. The RelA subunit then travels into the nucleus and activates the transcription of inflammatory genes like Cox-2 (Tsatsanis et al., 2006). The present study observed significant decreases in RelA and Cox-2 expression in ethanol-consuming rats, indicating that moderate ethanol intake may produce anti-inflammatory effects as a potential mechanism underlying its cardioprotective benefits. Previous studies have shown that moderate ethanol consumption reduces proinflammatory cytokine production including TNF-α (Mandrekar et al., 2006; Joosten et al., 2012) and IL-1β (Mandrekar et al., 2006; Joosten et al., 2012; Zhou et al., 2016). Moderate vodka consumption downregulated Nf-κb in human leukocytes when compared to placebo (orange juice), indicating that preventing the activation of NF-κB may lead to the inhibition of proinflammatory cytokines (Mandrekar et al., 2006; Joosten et al., 2012). In addition to reduced expression of Nf-κb, our study showed significant reductions in serum TLR4 and HMGB1 following moderate ethanol consumption. HMGB1 activates TLR4, which in turn increases production of inflammatory cytokines through the NF-κB and MAPK pathways (Re and Strominger, 2001; Leclerc et al., 2013). Therefore, moderate levels of ethanol may reduce production of proinflammatory cytokines by downregulating TLR4 and HMGB1 expression, which in turn reduce expression of Nf-κb. The current findings along with prior data indicate that moderate ethanol consumption exhibits a positive influence on some of the risk factors contributing to the onset of the inflammatory process initiating CVD (Wakabayashi, 2016). By contrast, chronic excessive alcohol consumption has been shown to increase the aforementioned proinflammatory cytokines (Carnevale and Nocella, 2012; Wakabayashi, 2016; Zhou et al., 2016). The relationship between alcohol intake and inflammatory markers, including C reactive protein (CRP) and interleukin 6 (IL-6), follows a similar pattern: lowered by moderate doses of alcohol, but increased by high doses (McCarty, 1999; Imhof et al., 2001). As immune cells generally contain a higher concentration of TLR4, a future study should determine TLR4 concentration in these cells.
Although excessive alcohol consumption increases oxidative stress, low levels of ethanol may have antioxidant effects due to NADH produced when ethanol is metabolized via alcohol dehydrogenase and aldehyde dehydrogenase (Vasdev et al., 2006). Consistent with an antioxidant effect, ethanol has been shown to reduce the formation of lipid peroxides and to decrease urinary 8-hydroxydeoxyguanosine, a measure of oxidative stress (Bonnefont-Rousselot et al., 2001; Yoshida et al., 2001). The present study demonstrated significant increases in Aldh2 expression and CAT activity, as well as a decrease in TBARS, as a result of moderate ethanol feeding. Other studies have confirmed that ethanol consumption increases CAT activity in myocardial tissue (Piano and Phillips, 2014) and red blood cells of alcoholic patients (Grasselli et al., 2014), suggesting that the increase in CAT activity is an adaptive/protective response to ethanol consumption. A few studies have indicated that Aldh2 functions to protect against oxidative stress in human mitochondria (Ohta et al., 2004) and in diabetic rat pulmonary tissue upon low-dose ethanol treatment (Hu et al., 2014). Increased Aldh2 expression and CAT activity may contribute to the lower level of oxidative stress seen in ethanol-consuming animals in the present study.
In addition to the contribution of Aldh2 gene expression and CAT activity as potential mechanisms behind the decrease seen in TBARS, the changes observed in Pon-1 gene expression may contribute to the reduction as well. Pon-1 is a multifunctional antioxidant enzyme that protects against lipid peroxidation (Rao et al., 2003). The present study shows an inverse correlation between Pon-1 and TBARS. Pon-1 functions to inhibit LDL oxidation, destroys oxLDL cholesterol to inactive products (disposal of oxLDL cholesterol), attenuates macrophage cholesterol biosynthesis, stimulates macrophage efflux and detoxifies homocysteine, with highest activity in the liver and blood (Lakshman et al., 2010; Klein et al., 2014). In the present study, oxLDL was significantly lower in ethanol-treated rats compared with control animals and Pon-1 expression was inversely correlated with oxLDL. The demonstrated increase in hepatic Pon-1 expression would facilitate a decrease in the level of oxLDL cholesterol, potentially contributing to the decrease seen in TBARS. These findings concur with other reports of an upregulation of Pon-1 expression with moderate alcohol consumption (Rao et al., 2003; Lakshman et al., 2010). Evidence for a decrease in expression with excessive alcohol consumption (Rao et al., 2003) suggests that the increase in Pon-1 expression may be a cardioprotective mechanism specific to moderate levels of alcohol consumption.
In both humans and rats, obesity is associated with elevated blood glucose levels and insulin resistance. The lowering of blood glucose and reduction in epididymal fat weight observed in the ethanol group suggest a possible ethanol action on insulin response. Aldh2 has been suggested to contribute to amelioration of alcohol-induced myocardial insulin resistance and endoplasmic reticulum stress (Li et al., 2009). Consistent with the results of the present study, moderate alcohol intake has previously demonstrated favorable effects on glucose metabolism, and increased insulin sensitivity may play an important role in this effect (Davies et al., 2002; Sierksma et al., 2004; Bonnet et al., 2012). Sierksma et al. (2004) demonstrated that the improved insulin sensitivity may be mediated through alcohol-induced increases in adiponectin which modulates glucose uptake and fatty acid oxidation. Long-term intake of low-concentration (0.1%) ethanol has rather been shown to downregulate adiponectin levels and PPARγ through increased activation of p38 MAPK pathways (Tian et al., 2014). These effects implicate the potential importance of alcohol-induced alterations in adiponectin and PPARγ and p38 MARK pathways in underlying mechanisms of moderate ethanol consumption, however, further research in this area is required.
CONCLUSION
The present results demonstrate that moderate levels of alcohol consumption are associated with a reduction of several CVD risk factors, including a more favorable lipid profile via downregulation of genes involved in cholesterol synthesis, decrease in inflammation by downregulation of Cox-2 and Rela expression and decreased oxidative stress by an increase in Pon-1 and Aldh2 expression and CAT activity. These data may contribute to a better understanding of the underlying mechanisms mediating protective effects of moderate alcohol consumption against CVD.
A limitation of this study is that food intake was not measured. It is possible that alcohol displaced some food calories and that reduced caloric intake is at least partially responsible for the reduction in non-HDL cholesterol. To address this limitation, food intake should be assessed in a future study. In addition, a measurement of protein levels of Hmgcr, Srebp-2 and Pon-1 will further confirm the effects of ethanol on mRNA expression. The rats in this study were fed a standard chow diet; however, a high-fat diet increases dyslipidemia and other CVD risk factors and exacerbates the liver injury that occurs with excessive ethanol intake (Tsukamoto, et al., 1986). For these reasons, it would be of interest to determine whether ethanol shows protective effects under conditions of a high-fat diet. Future studies that assess CVD development through plaque formation as a function of moderate ethanol intake, as well as interactive effects of different levels of alcohol consumption with other dietary components, will also further illuminate the significant impact of alcohol on cardiovascular outcomes.
FUNDING
This work was supported by the National Institutes of Health [R21 AA023291] and San Diego State University [NUTR302L].
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- Bergmann MM, Rehm J, Klipstein-Grobusch K, et al. (2013) The association of pattern of lifetime alcohol use and cause of death in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Int J Epidemiol 42:1772–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefont-Rousselot D, Rouscilles A, Bizard C, et al. (2001) Antioxidant effect of ethanol toward in vitro peroxidation of human low-density lipoproteins initiated by oxygen free radicals. Radiat Res 155:279–87. [DOI] [PubMed] [Google Scholar]
- Bonnet F, Disse E, Laville M, et al. (2012) Moderate alcohol consumption is associated with improved insulin sensitivity, reduced basal insulin secretion rate and lower fasting glucagon concentration in healthy women. Diabetologia 55:3228–37. [DOI] [PubMed] [Google Scholar]
- Brien SE, Ronksley PE, Turner BJ, et al. (2011) Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton EA. (2012) Effects of ethanol intake on lipoproteins. Curr Atheroscler Rep 14:108–14. [DOI] [PubMed] [Google Scholar]
- Carnevale R, Nocella C (2012) Alcohol and cardiovascular disease: still unresolved underlying mechanisms. Vasc Pharmacol 57:69–71. [DOI] [PubMed] [Google Scholar]
- Chiva-Blanch G, Arranz S, Lamuela-Raventos RM, et al. (2013) Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: evidences from human studies. Alcohol Alcohol 48:270–77. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Singley E, et al. (2012) Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacol Biochem Behav 100:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ, Baer DJ, Judd JT, et al. (2002) Effects of moderate alcohol on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA 287:2559–62. [DOI] [PubMed] [Google Scholar]
- Estruch R, Sacanella E, Mota F, et al. (2011) Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: a randomised cross-over trial. Nutr Metab Cardiovasc 21:46–53. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pachon MS, Berna G, Otaolaurruchi E, et al. (2009) Changes in antioxidant endogenous enzymes (activity and gene expression levels) after repeated red wine intake. J Agric Food Chem 57:6578–83. [DOI] [PubMed] [Google Scholar]
- Grasselli E, Compalati AD, Voci A, et al. (2014) Altered oxidative stress/antioxidant status in blood of alcoholic subjects is associated with alcoholic liver disease. Drug Alcohol Depend 143:112–19. [DOI] [PubMed] [Google Scholar]
- Hu JF, Zhang GJ, Wang L, et al. (2014) Ethanol at low concentration attenuates diabetes induced lung injury in rats model. J Diabetes Res 2014:107152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof A, Froehlich M, Brenner H, et al. (2001) Effect of alcohol consumption on systemic markers of inflammation. The Lancet 357:763–7. 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- Joosten MM, van Erk MJ, Pellis L, et al. (2012) Moderate alcohol consumption alters both leucocyte gene expression profiles and circulating proteins related to immune response and lipid metabolism in men. Br J Nutr 108:620–27. [DOI] [PubMed] [Google Scholar]
- Klein JD, Sherrill JB, Morello GM, et al. (2014) A snapshot of the hepatic transcriptome: ad libitum alcohol intake suppresses expression of cholesterol synthesis genes in alcohol-preferring (P) rats. PLoS One 9:e110501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klop B, do Rego AT, Cabezas MC (2013) Alcohol and plasma triglycerides. Curr Opin Lipidol 24:321–26. [DOI] [PubMed] [Google Scholar]
- Krenz M, Korthuis RJ (2012) Moderate ethanol ingestion and cardiovascular protection: from epidemiologic associations to cellular mechanisms. J Mol Cell Cardiol 52:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman R, Garige M, Gong M, et al. (2010) Is alcohol beneficial or harmful for cardioprotection? Genes Nutr 5:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc P, Wähämaa H, Idborg H, et al. (2013) IL-1β/HMGB1 complexes promote the PGE2 biosynthesis pathway in synovial fibroblasts. Scand J Immunol 77:350–60. 10.1111/sji.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Gilbert SA, Li Q, et al. (2009) Aldehyde dehydrogenase-2 (ALDH2) ameliorates chronic alcohol ingestion-induced myocardial insulin resistance and endoplasmic reticulum stress. J Mol Cell Cardiol 47:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, White B, et al. (2006) Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin Exp Res 30:135–39. [DOI] [PubMed] [Google Scholar]
- Mathews MJ, Liebenberg L, Mathews EH (2015) The mechanism by which moderate alcohol consumption influences coronary heart disease. Nutr J 14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto C, Miedema MD, Ofman P, et al. (2014) An expanding knowledge of the mechanisms and effects of alcohol consumption on cardiovascular disease. J Cardiopulm Rehabil Prev 34:159–71. [DOI] [PubMed] [Google Scholar]
- McCarty MF. (1999) Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Med Hypotheses 52:465–77. 10.1054/mehy.1997.0684. [DOI] [PubMed] [Google Scholar]
- Ohta S, Ohsawa I, Kamino K, et al. (2004) Mitochondrial ALDH2 deficiency as an oxidative stress. Ann N Y Acad Sci 1011:36–44. [DOI] [PubMed] [Google Scholar]
- O’Keefe JH, Bhatti SK, Bajwa A, et al. (2014) Alcohol and cardiovascular health: the dose makes the poison… or the remedy. Mayo Clin Proc 89:382–93. [DOI] [PubMed] [Google Scholar]
- Piano MR, Phillips SA (2014) Alcoholic cardiomyopathy: pathophysiologic insights. Cardiovasc Toxicol 14:291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramji DP, Davies TS (2015) Cytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev 26:673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MN, Marmillot P, Gong M, et al. (2003) Light, but not heavy alcohol rinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism 52:1287–94. [DOI] [PubMed] [Google Scholar]
- Re F, Strominger JL (2001) Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem 276:37692–9. 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- Sakakura Y, Shimano H, Sone H, et al. (2001) Sterol regulatory element-binding proteins induce an entire pathway of cholesterol synthesis. Biochem Biophys Res Commun 286:176–83. [DOI] [PubMed] [Google Scholar]
- Sierksma A, Patel H, Ouchi N, et al. (2004) Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-α, and insulin sensitivity. Diabetes Care 27:184–9. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, et al. (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeki T, Sata M (2016) Inflammatory biomarkers and atherosclerosis. Int Heart J 57:134–39. [DOI] [PubMed] [Google Scholar]
- Tian C, Jin X, Ye X, et al. (2014) Long term intake of 0.1% ethanol decreases serum adiponectin by suppressing PPARγ expression via p38 MAPK pathway. Food Chem Toxicol 65:329–34. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Venihaki M, et al. (2006) Signaling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol 38:1654–61. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Towner SJ, Clofalo LM, et al. (1986) Ethanol-induced liver fibrosis in rats fed high fat diet. Hepatology 6:814–22. 10.1002/hep.1840060503. [DOI] [PubMed] [Google Scholar]
- van der Gaag MS, van den Berg R, van den Berg H, et al. (2000) Moderate consumption of beer, red wine and spirits has counteracting effects on plasma antioxidants in middle-aged men. Eur J Clin Nutr 54:586–91. [DOI] [PubMed] [Google Scholar]
- Vasdev S, Gill V, Singal PK (2006) Beneficial effect of low ethanol intake on the cardiovascular system: possible biochemical mechanisms. Vasc Health Risk Manag 2:263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volcik KA, Ballantyne CM, Fuchs FD, et al. (2008) Relationship of alcohol consumption and type of alcoholic beverage consumed with plasma lipid levels: differences between Whites and African Americans of the ARIC study. Ann Epidemiol 18:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu KN, Ballantyne CM, Hoogeveen RC, et al. (2016) Causal role of alcohol consumption in an improved lipid profile: the atherosclerosis risk in communities (ARIC) study. PLoS One 11:e0148765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi I. (2013. a) Relationship between alcohol intake and lipid accumulation product in middle-aged men. Alcohol Alcohol 48:535–42. [DOI] [PubMed] [Google Scholar]
- Wakabayashi I. (2013. b) Relationships between alcohol intake and atherogenic indices in women. J Clin Lipidol 7:454–62. [DOI] [PubMed] [Google Scholar]
- Wakabayashi I. (2016) A U-shaped relationship between alcohol consumption and cardiometabolic index in middle-aged men. Lipids Health Dis 15:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZG, Yao T, Song ZY (2010) Chronic alcohol consumption disrupted cholesterol homeostasis in rats: down-regulation of low-density lipoprotein receptor and enhancement of cholesterol biosynthesis pathway in the liver. Alcohol Clin Exp Res 34:471–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HQ, Je YT, Kim M, et al. (2009) Analysis of hepatic gene expression during fatty liver change due to chronic ethanol administration in mice. Toxicol Appl Pharmacol 235:312–20. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Shioji I, Kishida A, et al. (2001) Moderate alcohol consumption reduces urinary 8-hydroxydeoxyguanosine by inducing of uric acid. Ind Health 39:322–9. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zheng J, Li S, et al. (2016) Alcoholic beverage consumption and chronic diseases. Int J Environ Res Public Health 13:E522. [DOI] [PMC free article] [PubMed] [Google Scholar]