Abstract
Introduction:
Although there have been important technological advances for the treatment of cardiac arrhythmias (e.g., catheter ablation technology), antiarrhythmic drugs (AADs) remain the cornerstone therapy for the majority of patients with arrhythmias. Most of the currently available AADs were coincidental findings and did not result from a systematic development process based on known arrhythmogenic mechanisms and specific targets. During the last 20 years, our understanding of cardiac electrophysiology and fundamental arrhythmia mechanisms has increased significantly, resulting in the identification of new potential targets for mechanism-based antiarrhythmic therapy.
Areas covered:
Here, we review the state-of-the-art in arrhythmogenic mechanisms and AAD therapy. Thereafter, we focus on a number of antiarrhythmic targets that have received significant attention recently: atrial-specific K+-channels, the late Na+-current, the cardiac ryanodine-receptor channel type-2, and the small-conductance Ca2+-activated K+-channel. We highlight for each of these targets available antiarrhythmic agents and the evidence for their antiarrhythmic effect in animal models and early clinical development.
Expert opinion:
Targeting AADs to specific subgroups of well-phenotyped patients is likely necessary to detect improved outcomes that may be obscured in the population at large. In addition, specific combinations of selective AADs may have synergistic effects and may enable a mechanism-based tailored antiarrhythmic therapy.
Keywords: antiarrhythmic drugs, atrial fibrillation, calcium handling, ion channels, sudden cardiac death
1. Introduction
Cardiovascular disease remains the most common cause of death and disability in the developed world [1]. A significant fraction of the cardiovascular disease-related deaths are a direct or indirect consequence of cardiac arrhythmias [2, 3]. For example, approximately 20% of all deaths occur suddenly, often due to ventricular tachyarrhythmias degenerating into ventricular fibrillation and sudden cardiac arrest [4]. Similarly, approximately half of the patients with chronic heart failure (HF) die from ventricular arrhythmias [5]. Atrial fibrillation (AF) is the most common clinically-relevant cardiac arrhythmia, affecting 30 million people worldwide. AF is also strongly associated with increased morbidity and mortality and is designated the underlying cause of death in more than 15% of deaths [6]. Despite significant technological advances, e.g., regarding catheter ablation therapy, antiarrhythmic drugs (AADs) remain the most commonly used treatment for cardiac arrhythmias. For example, the EURObservational Research Programme Atrial Fibrillation registry indicates that 36% of AF patients receive AADs for rhythm control therapy and more than 80% receive beta-blockers for rate control treatment [7]. Likewise, 15% of implantable cardiac defibrillator recipients receive adjunctive AAD therapy [8]. On average, these AADs reduce the arrhythmia burden in a wide range of patients [9, 10, 11]. However, currently available AADs have suboptimal efficacy and a relatively high risk of adverse effects, notably drug-induced proarrhythmia or organ toxicity [12, 13]. In addition, current AADs can strongly interact with numerous other drugs, e.g., by inhibiting P-glycoprotein-mediated drug transport, thereby further increasing the risk of adverse effects. Thus, there is a clear unmet clinical need for safer and more effective AADs.
During the last 25 years our understanding of the molecular and cellular basis of cardiac electrophysiology and the mechanisms underlying cardiac arrhythmias in diverse clinical conditions has increased substantially [14, 15, 16, 17]. This has resulted in the identification of a number of novel AAD targets and new compounds aiming to provide mechanism-based therapy. Although so far none of these attempts have been able to outperform existing AADs, a number of targets and drugs in preclinical development have shown promising results. Here, we first review the state-of-the-art in arrhythmogenic mechanisms and AAD therapy. Thereafter, we discuss important general considerations for the development of new AADs and apply these considerations to a number of recent compounds directed at a few promising targets.
2. Fundamental mechanisms of cardiac arrhythmias and antiarrhythmic therapy
2.1. Cardiac electrophysiology and arrhythmia mechanisms
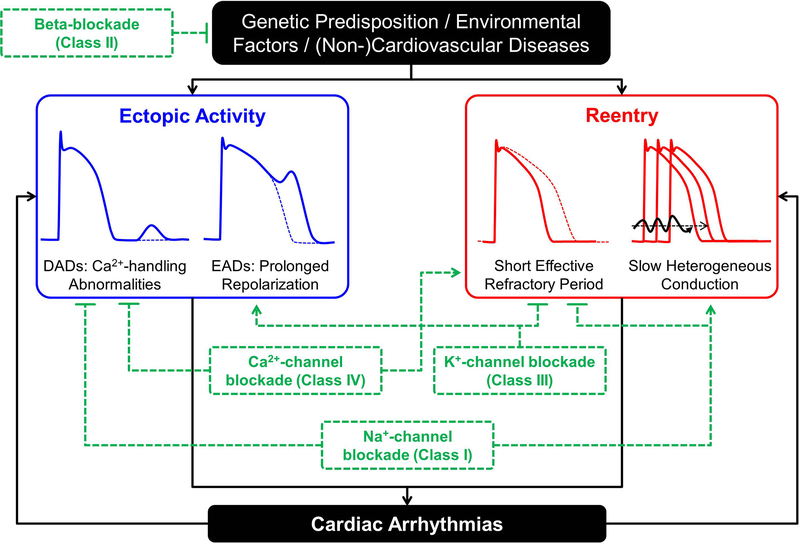
Cardiac arrhythmias require a vulnerable substrate and an acute initiating trigger that generally develop due to interactions between genetic predispositions, environmental factors, and cardiovascular or other diseases (Figure 1) [14, 15, 17]. In addition, if arrhythmias persist, for example in the case of AF or ventricular electrical storm, the arrhythmia itself can produce cardiac remodeling that further promotes arrhythmia maintenance and progression [14, 18]. Ectopic activity, the generation of electrical activity independent of the normal activation sequence initiated by the sinus node, can maintain an arrhythmia when occurring repetitively at high frequency, even in the absence of an arrhythmogenic sustrate. Furthermore, ectopic activity can produce unidirectional block of electrical activity, acting as the initiating trigger for reentry in the presence of a vulnerable substrate. Ectopic activity can result from abnormal automaticity, as well as early- or delayed afterdepolarizations (EADs and DADs, respectively). EADs are secondary depolarizations arising before full repolarization of the action potential (AP) that occur during excessive repolarization prolongation. DADs, on the other hand, result from spontaneous diastolic Ca2+-release events from the intracellular stores of the sarcoplasmic reticulum (SR) that produce a depolarizing transient-inward current after full repolarization of the AP [12, 19].
Figure 1. Fundamental arrhythmogenic mechanisms and antiarrhythmic therapies.
Ectopic activity, often resulting from delayed or early afterdepolarizations (DADs or EADs, respectively), and reentry, promoted by short and heterogeneous effective refractory periods, Ca2+-driven repolarization alternans and slow heterogeneous conduction, are the predominant arrhythmogenic mechanisms involved in the initiation and maintenance of cardiac arrhythmias. Both mechanisms are influenced by interactions between the genetic predisposition, environmental factors, and (non-) cardiovascular diseases. Almost all AADs exhibit both pro- and antiarrhythmic effects. For example, Class I AADs (blocking cardiac Na+ channels) decrease excitability, reducing the likelihood of ectopic activity and prolong effective refractory period, reducing reentry, but can promote reentry by slowing conduction velocity. Similarly, Class III AADs prolong effective refractory period, reducing reentry, but can promote EAD-mediated ectopic activity.
Reentry can occur around a fixed anatomical obstacle when the balance between conduction velocity (CV) and effective refractory period (ERP) allows tissue to become re-excitable before the reentrant impulse arrives [20]. As such, reentry is promoted by short ERP, spatially heterogeneous ERP and repolarization alternans, as well as slow, heterogeneous conduction. Reentry can also occur within a purely functional substrate. The stability of functional reentry depends on local repolarization and conduction properties and sink-to-source relationships between the wavefront’s excitatory current and the current employed to excite neighboring tissue [20], which can be modulated by different AADs.
Most AADs act at the level of the individual ion channel, altering total membrane current and/or intracellular ion fluxes, thereby affecting cardiac electrophysiological properties that modulate the risk of ectopic activity and reentry. In addition, many AADs indirectly regulate cardiac electrophysiology by blocking the regulation of the heart by the autonomic nervous system through inhibition of various G-protein-coupled receptor signaling pathways. As such, a detailed understanding of cardiac cellular electrophysiology is required to understand the properties and limitations of current and novel AADs. The main properties of cardiac cellular electrophysiology and intracellular Ca2+-handling, including differences between atrial and ventricular cardiomyocytes, have been discussed in detail elsewhere. Here, we provide a brief summary and we refer the interested reader to recent reviews on this topic [14, 17, 19, 21, 22, 23] for more information.
The AP upstroke is mediated by the Na+-current (INa). The subsequent repolarization profile controls the AP shape and is determined by the balance between multiple ion-currents, each with their specific kinetic characteristics and regulation. These currents include the depolarizing L-type Ca2+-current (ICa,L), responsible for initiating SR Ca2+-release through ryanodine-receptor channels type-2 (RyR2), producing the systolic Ca2+-transient and activating excitation-contraction coupling, as well as a wide range of repolarizing K+-currents [24]. These include the transient-outward K+ current (Ito), inward-rectifier K+-currents activated under basal conditions (IK1) or in response to vagal stimulation (acetylcholine-activated IK,ACh), and multiple delayed-rectifier K+-currents with distinct kinetics (e.g., slow IKs, rapid IKr and ultra-rapid IKur). Following SR Ca2+-release through RyR2 channels, Ca2+ homeostasis is maintained through re-uptake into the SR by the SR Ca2+-ATPase (SERCA2a) complex, as well as through Ca2+ extrusion via the Na+/Ca2+-exchanger type-1 (NCX1). Finally, Na+ and K+ homeostasis is maintained primarily through the Na+-K+-ATPase. Recent work has identified several additional ion-channels that modulate cardiac electrophysiology and arrhythmogenesis, including two-pore domain K+-channels [25, 26], small-conductance Ca2+-activated K+-channels (SK-channels) [27, 28], and transient-receptor potential (TRP)-channels [29], all of which represent novel potential therapeutic targets.
2.2. Antiarrhythmic therapies
Although attempts have been made to provide a more mechanistic classification of AADs (e.g., in the Sicilian Gambit [30]), AADs remain most commonly classified according to Vaughan Williams and Singh (Table 1) [9, 31]. This classification initially grouped AADs into those depressing cardiac excitability (Class I), those blocking β-adrenoceptors (Class II), and those prolonging repolarization (Class III). Later, it was identified that the mechanisms for Class I and Class III actions corresponded to Na+- and K+-channel inhibition, respectively. The discovery of the Ca2+-channel blocker verapamil in the 1970s resulted in the definition of Class IV AADs and other AADs with diverse mechanisms of action (e.g., adenosine, digitalis, ivabradine) were finally grouped together in Class V [31].
Table 1. Overview of commonly used antiarrhythmic drugs (AADs) and their electrophysiological targets.
Only targets with a half-maximal inhibitory concentration (IC50) of a similar order of magnitude as that of the primary target are listed [12].
| Class | AAD | Primary target | Other targets | Comments |
|---|---|---|---|---|
| IA | Quinidine | INa (intermediate kinetics) | IKr, Ito | |
| IB | Lidocaine | INa (fast kinetics) | ||
| IB | Mexiletine | INa (fast kinetics) | ||
| IB | Ranolazine | INa | IKr, RyR2, ICa,L | Preferentially inhibiting INaL |
| IC | Flecainide | INa (slow kinetics) | IKr, RyR2 | |
| IC | Propafenone | INa (slow kinetics) | IKr, RyR2, βAR | |
| II | Metoprolol | β1AR | ||
| II | Nebivolol | β1AR | RyR2, NOS | |
| II | Carvedilol | βAR | RyR2, αAR | |
| III | Amiodarone | IKr | IK,ACh, IK2P, ICa,L, INa, βAR, αAR, M2R | Exhibits effects of all four classes |
| III | Dronedarone | IKr | IK,ACh, IK2P, ICa,L, INa, βAR, αAR | Exhibits effects of all four classes |
| III | Dofetilide | IKr | ||
| III | Sotalol | IKr | βAR | |
| III | Vernakalant | IKr | INa, IKur, IK2P | |
| IV | Verapamil | ICa,L | IKr | |
| V | Ivabradine | If | ||
| V | Digitalis | INaK |
Inhibition of INa through Class I AADs decreases cardiomyocyte excitability and reduces the likelihood of ectopic activity (Figure 1). In addition, this process prolongs the ERP without changing AP duration (APD) through prolongation of the so-called post-repolarization refractoriness (i.e., the time between completion of an AP and the availability of sufficient Na+-channels to initiate the next AP), thereby reducing the likelihood of reentry. Finally, INa plays an important role in producing the depolarizing force for cell-to-cell communication. As such, its inhibition may slow CV, thereby promoting reentry. Class I AADs are further subdivided into Class IA-C, based on the affinity between different channel states and kinetics of inhibition (intermediate unbinding kinetics for Class IA, fast for Class IB, and slow for class IC), which, together with differential effects on other ion channels, determine the actual electrophysiological effects of each AAD. For example, the slow unbinding kinetics from the Na+ channel in combination with ischemia-induced Na+-channel inactivation likely explain why Class IA and IC AADs are contraindicated in patients with ischemic heart disease and myocardial infarction. In addition, heterogeneous prolongation of ventricular ERP due to inhibition of K+ channels may contribute to the proarrhythmic potential of Class IA and Class IC, but not Class IB AADs [32]. Conversely, Na+-channel inhibition may also destabilize reentrant circuits by altering the local sink-to-source relationship [15]. In addition to their therapeutic use in a variety of cardiovascular diseases, β-blockers have pronounced antiarrhythmic effects, for example by limiting the proarrhythmic effects of a sudden increase in sympathetic activation on cardiac electrophysiology and Ca2+-handling, resulting in their classification as Class II AADs. In addition, sympathetic stimulation is known to reduce the antiarrhythmic efficacy of Class I and Class III AADs. Thus, β-blockers help to preserve the antiarrhythmic effects of other AADs [33]. Inhibition of K+-currents, most notably IKr, which is inhibited by numerous antiarrhythmic and non-antiarrhythmic drugs and which is the primary target of Class III AADs, prolongs APD and ERP, thereby reducing the likelihood of reentry. However, excessive APD prolongation may promote EAD-mediated arrhythmogenesis (Figure 1). Accordingly, the use of Class III AADs is contraindicated in settings in which repolarization is prolonged. Finally, Class IV AADs inhibit ICa,L, lowering Ca2+ influx and potentially reducing intracellular Ca2+ overload, thereby decreasing the likelihood of spontaneous SR Ca2+-release events and DADs. In addition, Class IV AADs reduce conduction of electrical impulses through the atrioventricular node, where the AP upstroke is largely mediated by ICa,L. As such, Class IV AADs along with β-blockers (Class II AADs) are used to lower the ventricular response rate in patients with AF. However, ICa,L inhibition may also shorten APD, promoting reentry and can have pronounced negative inotropic effects. The use of Class IV AADs is therefore contraindicated in patients with impaired left ventricular function.
Taken together, as summarized in Figure 1, all AAD classes exhibit both pro- and antiarrhythmic properties, even when only their primary class effect is considered. However, it should be emphasized that almost all AADs affect multiple other targets in addition to the one of their primary site of action (Table 1), resulting in a complex electrophysiological profile that may be pro- or antiarrhythmic depending on the actual clinical condition.
3. General considerations for novel antiarrhythmic drugs
Most currently available AADs have been derived from naturally available compounds (e.g., quinidine adapted from a compound from the bark of the cinchona tree) or were originally developed for other purposes (e.g., amiodarone and sotalol were initially developed for the treatment of angina). The multiple electrophysiological effects of each of these compounds make mechanism-based therapy difficult, because it is unknown which targets contribute to the desired antiarrhythmic effect in a given patient and which may increase the risk of adverse effects. Recently, a more mechanistic approach to AAD development has been initiated. An important aspect that has received considerable attention is chamber specificity of AAD effects, particularly for rhythm control treatment of AF [12, 34, 35, 36]. Drugs that target atrial-specific ion currents, such as the ultra-rapid delayed-rectifier K+-current (IKur, discussed below), are expected to have fewer ventricular proarrhythmic side effects, allowing more effective treatment of AF. Other properties for novel AADs that should be considered include the disease-specific regulation of the target protein. For example, if an ion channel inhibited by a new AAD is upregulated under the clinical condition that is being addressed, then the effect of this AAD would be expected to be larger, whereas an AAD targeting a protein that is already downregulated is expected to be less effective. Similarly, drugs with forward use dependence (i.e., showing stronger effects at fast rates, typical for Class IC drugs such as flecainide) would be expected to have a better efficacy-to-safety ratio for the treatment of tachyarrhythmias (AF or ventricular tachycardia) than drugs with reverse use dependence (e.g., most Class III AADs) [37].
In addition to these AAD-related properties, a mechanism-based antiarrhythmic therapy requires careful phenotyping and stratification of patients based on known electrophysiological differences that may influence the efficacy or safety of antiarrhythmic therapy. For example, women have a shorter atrial ERP but longer ventricular APD and ERP, at least in part due to regulation of ion-channel expression and function by sex hormones [38]. As such, women have an increased risk for drug-induced ventricular arrhythmias. Similarly, other genetic or acquired differences in electrophysiological properties may favor specific proarrhythmic mechanisms in an individual patient. Ideally, such electrophysiological differences can be exploited in the future to improve the efficacy and safety of AAD therapy. For example, initial data have suggested that pharmacogenetic-guided selection of Class I vs. Class III drugs in patients with AF may improve the response to AAD therapy [39, 40]. This approach is currently being tested in a preliminary feasibility study (NCT02347111). Similarly, recent work has shown that a genetic risk score can be used as a predictor for drug-induced proarrhythmia in response to AAD therapy [41]. These approaches may facilitate a personalized AAD therapy with improved efficacy-to-safety ratio. However, such precision medicine critically depends on the availability of multiple AADs with distinct electrophysiological targets (Figure 2).
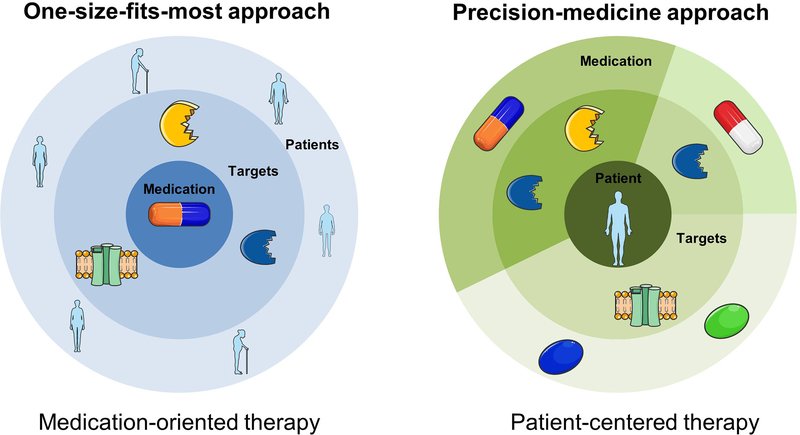
Figure 2. Paradigm shift in antiarrhythmic drug (AAD) therapy.
Most of the currently available AADs were coincidental findings, affect numerous electrophysiological targets and are applied to a heterogeneous group of patients in a ‘one-size-fits-most approach’ (left panel), resulting in limited efficacy and substantial adverse effects including proarrhythmia and extracardiac toxicity. Future ‘precision medicine’ approaches will likely provide a tailored, mechanism-based therapy by a deliberate combination of a number of selective AADs based on individual arrhythmia mechanisms (right panel).
4. Promising antiarrhythmic targets and agents
A large number of potential antiarrhythmic targets and agents have been discussed in a number of excellent recent review articles. For example, several reviews have focused on atrial-selective K+-channel blockers for AAD therapy in AF [34, 35, 36], AADs against atrial and ventricular arrhythmias targeting Ca2+-handling abnormalities [42, 43], or personalization of AAD therapy [40]. Here, we focus on several targets and the agents directed against these targets that have received considerable attention during the last few years.
4.1. The ultra-rapid delayed-rectifier K+-current (IKur) and acetylcholine-activated inward-rectifier K+-current (IK,ACh)
IKur is the prototypical atrial-selective target for Class III AADs. Inhibition of IKur would be expected to prolong atrial ERP, destabilizing reentrant arrhythmias, e.g., in the setting of AF, without potential proarrhythmic ventricular effects. In atrial cardiomyocytes or multicellular preparations from patients with persistent (“chronic”) AF, inhibition of IKur with agents such as MK-0448, XEN-D0101 / XEN-D0103, F373280, and BMS919373, consistently prolongs APD [34, 44]. However, in atrial cardiomyocytes from patients with sinus rhythm or paroxysmal AF, inhibition of IKur shortened APD at pacing rates corresponding to normal resting heart rates [34, 36, 44], thus potentially favoring the (re)initiation of AF episodes. In vivo, MK-0448 prolongs atrial ERP and terminates sustained AF in dogs, but has no effect on ERP in humans [45], possibly due to the aforementioned rate- and disease-dependent effects and administration to patient with atrial flutter instead of AF [36]. A number of other properties further complicate the therapeutic use of IKur blockers. For example, several loss-of-function mutations in Kv1.5, the pore-forming subunit of the IKur channel, have been identified in patients with familial AF [46] and inhibition of IKur in a computer model of the human atrial cardiomyocyte generated proarrhythmic EADs in the setting of simulated sympathetic stimulation [47], suggesting that excessive inhibition of IKur may have proarrhythmic effects. At high doses, compounds such as MK-0448 may also block other K+-currents (e.g., IKs), potentially leading to drug-induced ventricular proarrhythmia. Recently, even more selective IKur blockers have been proposed (e.g., MK-1832 [48]), but their antiarrhythmic effects in humans are unknown. Finally, some, but not all studies suggest that IKur is downregulated in patients with chronic AF [49], which would be expected to lower the efficacy of blocking this current. Phase-II clinical studies with different IKur blockers studying the antiarrhythmic efficacy are currently ongoing (e.g., NCT01831856 for F373280) or have been completed (e.g., NCT02156076 for BMS919373 or EUCTR2013–004456-38 for XEN-D0103), but to the best of our knowledge no positive results have been published for these trials.
Similar to IKur, IK,ACh is predominantly expressed in atrial cardiomyocytes and has received significant attention as atrial-selective therapeutic target [15, 34, 35, 36]. IK,ACh is activated by the neurotransmitter acetylcholine via muscarinic receptors. Although maximal muscarinic-receptor activated IK,ACh is decreased in patients with AF, the receptor-independent ‘constitutively active’ component (IK,AChc) is significantly increased and may contribute to reentry-promoting ERP shortening [50]. Many currently available AADs including flecainide and dronedarone also affect IK,ACh and a number of moderately selective IK,ACh blockers have been developed. Most of these compounds (e.g., NTC-801, AZD2927, A7071, XEN-R0706) have shown antiarrhythmic effects in animal models and some, but not all, prolong repolarization in human atrial tissue samples (reviewed in [34, 35, 36]), suggesting potential anti-AF effects in patients. Some of these compounds have also been evaluated in early clinical studies, but efficacy was limited and development of these compounds has been halted [34, 36].
4.2. The two-pore-domain K+-current (IK2P)
The family of two-pore-domain K+ channels (K2P) encompasses a wide-range of K+-channels that are regulated by numerous signals including temperature, pH and membrane stretch [22, 36]. Several members of this family, including the TASK-1 (K2P3.1) and TREK-1 (K2P2.1) channels are expressed in the heart, contributing to the background or steady-state K+-current. Inhibition of these channels would be expected to have a Class III effect, prolonging repolarization and destabilizing reentrant arrhythmias. In the human heart TASK-1 is only expressed in the atria, enabling atrial-selective antiarrhythmic therapy of AF, similar to IKur blockers. In fact, several IKur blockers appear to have a comparable affinity for TASK-1 as for IKur [51], suggesting that their antiarrhythmic effects may be partially mediated by TASK-1 blockade. Indeed, in contrast to IKur, the expression of TASK-1 is significantly upregulated in patients with chronic AF, which should increase the antiarrhythmic efficacy of TASK-1 inhibition [25]. On the other hand, the expression of TASK-1 is downregulated in patients with left ventricular dysfunction, suggesting that TASK-1 inhibition may be less effective under these conditions [26], although the consequences of TASK-1 inhibition will also depend on the remodeling of other ion channels that determine the atrial repolarization reserve. Several existing AADs also partially inhibit TREK-1 (e.g., vernakalant) or TASK-1 (e.g., amiodarone) at clinically relevant concentrations [52], further supporting the potential antiarrhythmic effect of K2P-channel inhibition. A moderately selective compound (A293) for TASK-1 channels is available for experimental studies and a more selective TASK-1 inhibitor (ML365) has recently been described [34, 53]. In isolated guinea pig hearts with augmented TASK-1 by changes in pH, both A293 and ML365 selectively prolonged atrial ERP, but only had limited antiarrhythmic effects in the setting of acute AF [54]. Studies in mice are further complicated by the fact that in contrast to humans TASK-1 is expressed in both atria and ventricles. In agreement, TASK-1 knockout mice have prolonged QTc interval, suggesting an increased risk of ventricular proarrhythmia upon TASK-1 inhibition in mice [36, 55]. Finally, because TASK-1 is expressed in numerous other cell types, TASK-1 inhibitors may also have unwanted extra-cardiac side effects [36]. These data further highlight the need for selecting the appropriate patient cohort for a given AAD. Gene therapy provides an alternative approach to locally modify K2P-channel expression. Recent work has shown that adenovirus-mediated overexpression of TREK-1 in the right atrium normalizes the prolonged atrial ERP in the setting of combined AF and HF and improved sinus rhythm maintenance [56]. Although the exact antiarrhythmic mechanisms require further study, these data support K2P channels as potentially effective antiarrhythmic targets.
4.3. The late Na+-current (INaL)
Class I AADs targeting the cardiac Na+-channel are among the oldest AADs. There is a wide range of Na+-channel blocking agents with specific affinities for different channel states (e.g., open-state versus inactivated-state block) and different kinetic properties (with off rates of channel dissociation ranging from milliseconds to several seconds). Both genetic (long-QT syndrome type-3) and acquired (e.g., hypoxia, ischemia) pathological conditions can disrupt normal Na+ gating, resulting in an increased late (also known as persistent or non-inactivating) component of the Na+-current (INaL). Increased INaL prolongs repolarization duration and promotes EAD-mediated arrhythmogenesis [31, 57]. In addition, increased INaL may alter intracellular Na+ homeostasis, subsequently causing proarrhythmic changes in intracellular Ca2+ due to effects on NCX1 [57]. As such, inhibition of INaL would be expected to be antiarrhythmic and agents preferentially targeting INaL have received significant attention recently [31].
Ranolazine was originally discovered for the therapy of angina and is a multi-channel blocker inhibiting primarily IKr and INaL at therapeutic concentrations, with an approximately 10-fold selectivity for INaL over peak INa [57]. A variety of experimental studies and case series in patients with increased INaL due to long-QT syndrome type-3 [58], recurrent ventricular arrhythmias refractory to classical AAD therapy [59], or frequent ventricular ectopic activity [60], support the therapeutic potential of ranolazine for ventricular arrhythmias in patients [61]. Similarly, a number of small-scale clinical studies have evaluated ranolazine for rhythm control of AF and recent meta-analyses [62, 63] support the antiarrhythmic effects of ranolazine in AF patients. Recently, ranolazine was also evaluated in a larger cohort of high-risk patients with implantable cardiac defibrillators (RAID trial), showing a non-significant 16% reduction in the primary endpoint of ventricular arrhythmia, and a significant 27% reduction in ventricular tachycardia requiring anti-tachypacing versus placebo [64]. Thus, although ranolazine is not officially approved as an AAD, it appears to have important antiarrhythmic properties. Based on these results, a number of AADs selectively targeting INaL have been developed. For example, the selective INaL inhibitor GS-458967 has more potent antiarrhythmic effects than ranolazine in a number of animal models of atrial and ventricular arrhythmias [65, 66]. However, the compound also produces use-dependent block of Na+ channels in the central and peripheral nervous system, which results in a very small therapeutic index [66]. GS-6615 (also known as eleclazine) is an alternative INaL blocker developed to overcome some of the limitations of GS-458967 [67]. In particular, eleclazine has a lower affinity for neuronal Nav1.1 channels, retains a strong selectivity for INaL and is able to suppress drug-induced Torsades des Pointes arrhythmias more potently than ranolazine [67]. In addition, eleclazine has strong antiarrhythmic properties against ischemia-induced ventricular arrhythmias in rabbits [67] and autonomically-induced AF [68] and VT [69] in pigs. These positive results in animal studies prompted a number of early clinical trials, investigating the safety and antiarrhythmic effect of eleclazine in patients with implantable cardioverter defibrillators (NCT02104583), in the setting of hypertrophic cardiomyopathy (Liberty-HCM; NCT02291237), and in patients with gain-of-function mutations in the Na+-channel leading to long-QT syndrome type-3 (NCT02300558). However, all three of these studies have recently been discontinued, likely due to limited efficacy or safety concerns [70]. Thus, the therapeutic value of selective INaL inhibition in patients remains uncertain and requires further investigation in subsequent prospective clinical studies.
4.4. The cardiac RyR2 and abnormal intracellular Ca2+ handling
Spontaneous SR Ca2+-release events and subsequent Ca2+-driven DADs potentially leading to triggered activity have been implicated in both atrial (e.g., paroxysmal and chronic AF [14]) and ventricular arrhythmias (e.g., in the setting of heart failure or with catecholaminergic polymorphic ventricular tachycardia [CPVT] due to mutations in RyR2 or the SR Ca2+ buffer calsequestrin [19]). As such, modification of RyR2 gating to reduce the number of large spontaneous SR Ca2+-releases continues to receive significant attention [71]. Several clinically available compounds are known to target RyR2. For example, dantrolene is a muscle relaxant that in addition to RyR1 in skeletal muscle cells also affects RyR2 in cardiomyocytes. Dantrolene has antiarrhythmic effects in different animal models [72, 73] and normalizes Ca2+-handling abnormalities in atrial cardiomyocytes from patients with AF and ventricular cardiomyocytes from patients with HF without affecting APD or contractility [74]. The exact molecular antiarrhythmic effect of dantrolene on RyR2 remains incompletely understood, but is not related to direct channel-blocking activity and requires presence of calmodulin, a Ca2+-binding protein and regulator of RyR2 [71, 72, 73]. A number of Na+-channel blockers including tetracaine, flecainide and ranolazine have also been shown to target RyR2 [42]. However, the exact state-dependent blocking mechanism critically determines the antiarrhythmic effect [75]. In particular, tetracaine blocks RyR2 in the closed state, elevating SR Ca2+ load and increasing the likelihood of potentially proarrhythmic Ca2+ waves [75]. However, tetracaine-derivatives (e.g., EL9) have been developed recently, which show antiarrhythmic effects in CPVT models without effects on heart rate, QRS duration or inotropy, although[76] the exact mechanisms of action of EL9 are still unknown [76]. In contrast to tetracaine, flecainide and ranolazine are open-state blockers that increase the number of microscopic Ca2+ sparks but reduce the number of proarrhythmic Ca2+ waves [42, 75]. However, the physiological relevance of flecainide-mediated RyR2 inhibition remains a topic of active debate and may be part of a complex triple mode of action also involving reduced excitability and changes in intracellular Na+ and Ca2+ levels [77, 78, 79, 80]. The relative contribution of each mode of action is likely distinct for different experimental and clinical conditions. The β-blockers carvedilol and nebivolol also reduce single RyR2 channel open time (despite increasing open frequency), suppress spontaneous Ca2+ waves and reduce arrhythmogenesis in CPVT animal models [81, 82]. Interestingly, a non-β-blocking nebivolol enantiomer, (l)-nebivolol, as well as non-β-blocking carvedilol derivatives (e.g., VK-II-86), also suppress spontaneous SR Ca2+-release events and CPVT. Taken together, these data support RyR2 modulation as a potential target for novel AADs [81, 82], although the exact molecular approach that is needed to stabilize RyR2 closure during diastole (including different forms of state-dependent pore block and correction of physiological regulation of channel stability) requires further delineation and validation. Alternatively, upstream therapy to reduce RyR2 dysfunction has also been proposed. For example, Ca2+/calmodulin-dependent kinase-II (CaMKII)-mediated hyperphosphorylation has been implicated in Ca2+-handling abnormalities in both AF and HF, contributing to acute arrhythmia initiation and long-term cardiac remodeling [83]. As such, CaMKII inhibition has been proposed as an alternative approach to prevent RyR2 dysfunction [42, 43], although the potential side effects of CaMKII inhibition outside the heart make the selective targeting of CaMKII within the cardiac RyR2 complex a big challenge.
Despite encouraging preclinical data there is a paucity of clinical data on specific RyR2-targeting compounds. Based on initial work with less selective compounds such as K201 / JTV519 [42, 75], the class of rycal compounds has been proposed as novel potential AADs targeting leaky RyR2 [84]. Some lead compounds from this class (e.g., ARM036, also known as aladorian) exhibit antiarrhythmic effects in an animal model of HF [84] and have been tested in phase IIa studies for future use in patients with HF and arrhythmias (e.g., ISRCTN14227980). Although aladorian itself has been discontinued for undisclosed reasons, it might provide a basis for the development of second generation rycal compounds. On the other hand, recent data also suggest that although RyR2 inhibition reduces spontaneous SR Ca2+-release events, it may promote proarrhythmic Ca2+-driven cardiac alternans [85]. Thus, the exact therapeutic value of RyR2 modulation is still unclear and a carefully balanced RyR2 modulation might be the best approach to achieve clinically relevant antiarrhythmic efficacy.
4.5. The small-conductance Ca2+-activated K+-current (ISK)
Functional cardiac SK channels were first demonstrated in 2003 [86]. Under physiological conditions, SK channels show predominant atrial expression and have been implicated in the regulation of atrial electrical activity in a variety of studies [27, 36]. SK channels couple to L-type Ca2+-channels and respond to RyR2-mediated SR Ca2+ release. Moreover, SK2-channel trafficking is Ca2+-dependent, such that a rise in Ca2+ during tachyarrhythmias is predicted to increase the membrane localization of SK2 channels [27, 42]. Accordingly, short-term atrial burst-pacing in dogs, which leads to cellular Ca2+ overload, accelerates SK2 membrane trafficking to the plasma membrane and causes proarrhythmic AP shortening in pulmonary veins [87]. Similarly, upregulation of SK-channel activity in failing rabbit hearts contributes to post-shock AP shortening and recurrent spontaneous ventricular fibrillation initiated by NCX1-mediated late phase-3 EADs [28]. On the other hand, the expression of SK channels is upregulated in end-stage HF, which may protect against excessive repolarization prolongation and stabilize the resting membrane potential. Genetic variants in SK channels have been associated with AF in genome-wide association studies, but the remodeling of SK channels in AF patients remains controversial, with both increased and decreased SK currents reported in patients with AF [36, 88, 89].
Inhibition of SK channels would be expected to prolong ERP, reducing the likelihood of reentry. However, inhibition of SK channels re-expressed in ventricular cardiomyocytes of the failing heart may further reduce repolarization reserve and could promote ventricular arrhythmias. In agreement, blockade of SK channels has been shown to be either antiarrhythmic or proarrhythmic in various animal models [27]. Apamin, the active neurotoxin in bee venom, is a highly-selective SK-channel blocker that is often used to identify the SK current experimentally. Because apamin cannot be applied to humans, other compounds with different mechanisms of SK-channel inhibition, including modulation of the Ca2+ sensor (e.g., NS8593) and direct channel pore block (e.g., UCL1684 and ICAGEN) [36] have been developed. Currently used AADs do not appear to target SK channels, with only dofetilide and propafenone showing some inhibition at suprapharmacological concentrations [90]. Interestingly, an SK-channel activator (NS309) has recently been described to attenuate Ca2+-dependent arrhythmia in hypertrophic rat hearts by regulating SK channels on the mitochondrial membrane, thereby reducing mitochondrial production of reactive oxygen species that promote RyR2 dysfunction [91]. By contrast, the SK-channel blocker UCL1684 promoted oxidative stress. Taken together, modulation of SK channels appears a promising antiarrhythmic strategy, but data about the antiarrhythmic effects in patients are lacking. Again, proper selection of the right cohort of patients will be critical given the potential proarrhythmic effects of SK-channel modulation. Importantly, SK-channel inhibition appears to be the only antiarrhythmic drug strategy that has not yet been disproven in the clinical setting and first-in-man studies employing selective SK-channel inhibitors in AF patients are expected in the near future.
4.6. Transient receptor potential (TRP) channels
The family of transient receptor potential channels includes dozens of channels divided over 7 subfamilies, including canonical (TRPC), vallinoid (TRPV), and melastatin-related (TRPM) variants. TRP channels respond to a wide range of signals including pressure, temperature, pain, etc. [92]. A number of TRP channels are expressed in the heart and have been implicated in the regulation of cellular Ca2+-influx, thereby modulating cardiac function and remodeling [92]. For example, TRPC3 and TRPM7 have been shown to regulate Ca2+-influx in atrial fibroblasts, promoting their transition to myofibroblasts and increasing reentry-promoting collagen synthesis [93]. In agreement, inhibition of TRPC3 with pyrazole-3 suppressed AF in an animal model [93]. Interestingly, several SK-channel blockers also act as blockers of TRPM7 [94]. Several TRP channels including TRPC1 [95] and TRPM4 [29] are also expressed in cardiomyocytes, thereby directly modulating cardiac electrophysiology and potentially contributing to arrhythmogenesis. Indeed, mutations in TRPM4 have been associated with cardiac conduction disease, Brugada syndrome and long-QT syndrome [96, 97]. Thus, modulation of TRP channels may have antiarrhythmic effects. Their involvement in a wide range of pathological conditions has produced significant interest in drugs targeting TRP channels [98]. However, given their diverse functions, selective targeting of cardiac TRP channels will likely be required to develop safe and effective AADs. Although significant progress is being made in the development of compounds targeting TRP channels, few selective compounds are available at present and their antiarrhythmic effects in patients are unknown [94, 98].
5. Conclusion
AADs still remain the cornerstone treatment for patients with heart rhythm disorders. All currently available AADs have multiple electrophysiological targets and diverse pro- and antiarrhythmic effects. Thus, there is a clear unmet clinical need for safer and more effective antiarrhythmic drugs. Recent research has identified a number of targets with promising properties (selective chamber occurrence, upregulation under disease conditions), including atrial-selective K+-currents (IKur, TASK-1), INaL, RyR2 and SK-channels. Several compounds modulating these targets have been developed and have shown promising antiarrhythmic effects in preclinical studies. However, so far none of these compounds have been successful in clinical trials, indicating that there is still an important translational gap in the development of modern AADs. A better preclinical target validation in humanized animal models in combination with verification of importance in human tissue specimen might help to improve the translational potential of novel antiarrhythmia targets. Future studies will also need to improve our understanding of the fundamental arrhythmia mechanisms in individual patients to identify the optimal target group for the next generation of antiarrhythmic agents. Finally, the goal will be to develop selective compounds for these targets devoid of toxic side effects, which would preclude clinical application independent of the antiarrhythmic efficacy, to improve the treatment options for patients with arrhythmias.
6. Expert Opinion
Recent advances in our understanding of cardiac electrophysiology have enabled new mechanism-based AAD therapies. Promising drugs in early clinical development attempt to exploit chamber-selectivity and, to a lesser extent, other electrophysiological properties to produce more effective and safer therapeutic strategies. Despite encouraging results in preclinical studies, the efficacy of these drugs in patients have so far been rather disappointing. Thus, there is indeed still a large translational gap between preclinical and clinical development of new AADs [15]. However, this might be expected given the complexity of AAD development, which requires a careful balance between antiarrhythmic effects and proarrhythmic potential that is applicable under a wide range of conditions that dynamically remodel cardiac electrophysiology. Indeed, even AF itself is known to produce extensive ion-channel remodeling that modulates the effect of AADs. Furthermore, the development of AADs is hindered by interspecies differences in electrophysiology, making it difficult to predict drug effects in humans prior to testing in patients. Indeed, modulation of the recently identified IK2P is theoretically interesting, but data in large animal models are scarce and in vivo data in patients are lacking. Similarly, SK channels are receiving significant attention as promising AAD targets in the setting of AF, but their antiarrhythmic potential in patients has not yet been demonstrated.
Besides addressing intrinsic limitations of the new AADs per se, the importance of selecting the proper patient population, particularly in the first clinical trials is essential for further AAD development. A more targeted use of AADs for specific subpopulations of patients (precision medicine) in the first clinical trials might be the best way to unmask potential antiarrhythmic effects, thereby enabling detection of improved outcomes that may be obscured in the bigger pool of less well-phenotyped patient populations. In addition, using specific combinations of selective AADs may have synergistic effects and enable a mechanism-based tailored antiarrhythmic therapy. Indeed, virtually all AADs used in clinical practice affect multiple ion channels. For example, amiodarone is the most effective AAD available to date and inhibits a wide range of ion channels, including Na+-channels, multiple K+-channels and L-type Ca2+-channels, as well as α- and β-adrenoceptors, thereby exhibiting effects of AAD classes I-IV (Table 1). However, amiodarone’s application is limited by its severe extra-cardiac toxicity and several derivatives (including dronedarone and budiodarone) have been developed [99]. These derivatives are also multi-channel blockers, albeit with distinct affinities for individual ion channels, and their moderate antiarrhythmic effects in some patient populations are associated with lower toxicity than amiodarone [100, 101]. The selective dopamine reuptake inhibitor vanoxerine, originally developed for the treatment of Parkinsons disease, has shown antiarrhythmic effects by blocking IKr, INa and ICa,L, but its development as an AAD was recently halted due to safety concerns in the RESTORE SR trial (NCT02454283) [102]. Similarly, ranolazine inhibits both INaL, IKr and RyR2, all of which may contribute to its antiarrhythmic effects. Recently, experimental data in human cardiomyocytes and the small-scale HARMONY trial have shown that a combination of ranolazine with low-dose dronedarone has more pronounced antiarrhythmic effects than each AAD alone [103, 104]. Thus, targeting a combination of ion channels likely has synergistic antiarrhythmic effects. However, it may be desirable to achieve multi-channel block through a specific combination of selective compounds instead of the ‘shotgun approach’ offered by amiodarone or dronedarone with or without additional AADs, whereby the exact antiarrhythmic mechanism remains unknown. For example, recent work has shown that additional inhibition of specific K+-currents (ISK, IKr or IKur) also potentiated the AF-selective antiarrhythmic effects of Na+-channel inhibition with specific state-dependent blockers [105, 106, 107], providing a proof-of-concept for mechanism-based AAD combinations. These drug combinations may include compounds directed at the targets discussed in this review. The mechanism-based AAD combination approach represents a paradigm-shift from an AAD-centered ‘one-size-fits-most approach’ to a patient-oriented ‘precision medicine’ (Figure 2). This approach will naturally raise new challenges, for example regarding pharmacokinetic drug-drug interactions affecting efficacy or safety that will have to be evaluated in both animal models and clinical studies, and will make clinical evaluation in appropriately powered trials more challenging. Nonetheless, advances in clinical trial design and improved understanding of antiarrhythmic therapies are, at least in theory, expected to enable mechanism-based tailored antiarrhythmic therapy resulting in improved therapeutic options for the millions of patients suffering from cardiac arrhythmias.
Article Highlights.
Antiarrhythmic drugs, commonly grouped in Vaughn Williams Class I-IV according to their primary electrophysiological target, remain the most common treatment for patients with heart rhythm disorders.
All antiarrhythmic drugs have multiple electrophysiological targets and diverse pro- and antiarrhythmic effects and there is a clear unmet clinical need for safer and more effective antiarrhythmic drugs.
Recent research has identified a number of targets with promising properties (e.g., selective chamber occurrence, upregulation under disease conditions), including atrial-specific K+-currents (IKur, TASK-1), INaL, RyR2 and SK channels.
Several compounds modulating these targets have been developed and have shown promising antiarrhythmic effects in preclinical studies. However, so far none of these compounds have been successful in clinical trials, although inhibition of TASK-1 and SK channels has not been tested yet.
Future studies will need to improve our understanding of the fundamental arrhythmia mechanisms in individual patients to identify the optimal target group for the next generation of antiarrhythmic agents.
Acknowledgments
Funding
The authors’ work is supported by the Netherlands Organization for Scientific Research (ZonMW Veni 91616057 to J. Heijman), the CardioVascular Onderzoek Nederland (CVON) and Netherlands Heart Foundation PREDICT project (Young Talent Program to J. Heijman), the National Institutes of Health (HL131517 to D. Dobrev), the DZHK (German Center for Cardiovascular Research, 81X2800108, 81X2800161, and 81X2800136 to D. Dobrev) and the German Research Foundation (DFG, Do 769/4–1 to D. Dobrev).
Footnotes
Declaration of Interest
D. Dobrev is on the Scientific Advisory Board of OMEICOS and received speaker’s fees from Boston Scientific, Daiichi Sankyo and Servier. His laboratory executed a research contract for Omeicos. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–867. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116:1887–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrhythmias Kjekshus J. and mortality in congestive heart failure. Am J Cardiol. 1990;65:42I–8I. [DOI] [PubMed] [Google Scholar]
- 6.Fedeli U, Ferroni E, Pengo V. Mortality associated to atrial fibrillation still on the rise: United States, 1999 to 2014. Int J Cardiol. 2016;222:788–9. [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Laroche C, Dan GA, et al. A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: baseline results of EURObservational Research Programme Atrial Fibrillation (EORP-AF) Pilot General Registry. Europace. 2014;16:308–19. [DOI] [PubMed] [Google Scholar]
- 8.Dev S, Peterson PN, Wang Y, et al. Prevalence, correlates, and temporal trends in antiarrhythmic drug use at discharge after implantable cardioverter defibrillator placement (from the National Cardiovascular Data Registry [NCDR]). Am J Cardiol. 2014;113:314–20. [DOI] [PubMed] [Google Scholar]
- 9.Schleifer JW, Sorajja D, Shen WK. Advances in the pharmacologic treatment of ventricular arrhythmias. Expert Opin Pharmacother. 2015;16:2637–51. [DOI] [PubMed] [Google Scholar]
- 10.Qin D, Leef G, Alam MB, et al. Comparative effectiveness of antiarrhythmic drugs for rhythm control of atrial fibrillation. J Cardiol. 2016;67:471–6. [DOI] [PubMed] [Google Scholar]
- 11.Santangeli P, Muser D, Maeda S, et al. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: A systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016;13:1552–9. [DOI] [PubMed] [Google Scholar]
- 12.Heijman J, Voigt N, Dobrev D. New directions in antiarrhythmic drug therapy for atrial fibrillation. Future Cardiol. 2013;9:71–88. [DOI] [PubMed] [Google Scholar]
- 13.Frommeyer G, Eckardt L. Drug-induced proarrhythmia: risk factors and electrophysiological mechanisms. Nat Rev Cardiol. 2016;13:36–47. [DOI] [PubMed] [Google Scholar]
- 14.Heijman J, Voigt N, Nattel S, et al. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–99. [DOI] [PubMed] [Google Scholar]
- 15.Heijman J, Algalarrondo V, Voigt N, et al. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis. Cardiovasc Res. 2016;109:467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu Z, Weiss JN. Mechanisms of ventricular arrhythmias: from molecular fluctuations to electrical turbulence. Annu Rev Physiol. 2015;77:29–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartos DC, Grandi E, Ripplinger CM. Ion Channels in the Heart. Compr Physiol. 2015;5:1423–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuji Y, Heijman J, Nattel S, et al. Electrical storm: recent pathophysiological insights and therapeutic consequences. Basic Res Cardiol. 2013;108:336. [DOI] [PubMed] [Google Scholar]
- 19.Wagner S, Maier LS, Bers DM. Role of sodium and calcium dysregulation in tachyarrhythmias in sudden cardiac death. Circ Res. 2015;116:1956–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comtois P, Kneller J, Nattel S. Of circles and spirals: bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace. 2005;7 Suppl 2:10–20. [DOI] [PubMed] [Google Scholar]
- 21.Abriel H, Rougier JS, Jalife J. Ion channel macromolecular complexes in cardiomyocytes: roles in sudden cardiac death. Circ Res. 2015;116:1971–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt N, Grunnet M, Olesen SP. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev. 2014;94:609–53. [DOI] [PubMed] [Google Scholar]
- 23.Landstrom AP, Dobrev D, Wehrens XH. Calcium signaling and cardiac arrhythmias. Circ Res. 2017;120:1969–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiamvimonvat N, Chen-Izu Y, Clancy CE, et al. Potassium currents in the heart: functional roles in repolarization, arrhythmia and therapeutics. J Physiol. 2017;595:2229–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt C, Wiedmann F, Voigt N, et al. Upregulation of K2P3.1 K+ Current Causes Action Potential Shortening in Patients With Chronic Atrial Fibrillation. Circulation. 2015;132:82–92. [DOI] [PubMed] [Google Scholar]
- 26.•.Schmidt C, Wiedmann F, Zhou XB, et al. Inverse remodelling of K2P3.1 K+ channel expression and action potential duration in left ventricular dysfunction and atrial fibrillation: implications for patient-specific antiarrhythmic drug therapy. Eur Heart J. 2017. Study highlighting the complexity of ion channel remodeling in patients with different comorbidities and the implications for personalized AAD therapy. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XD, Lieu DK, Chiamvimonvat N. Small-conductance Ca2+ -activated K+ channels and cardiac arrhythmias. Heart Rhythm. 2015;12:1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang PC, Chen PS. SK channels and ventricular arrhythmias in heart failure. Trends Cardiovasc Med. 2015;25:508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simard C, Hof T, Keddache Z, et al. The TRPM4 non-selective cation channel contributes to the mammalian atrial action potential. J Mol Cell Cardiol. 2013;59:11–9. [DOI] [PubMed] [Google Scholar]
- 30.Cardiology TTFotWGoAotESo. The ‘Sicilian Gambit’. A new approach to the classification of antiarrhythmic drugs based on their actions on arrhythmogenic mechanisms. Eur Heart J. 1991;12:1112–31. [PubMed] [Google Scholar]
- 31.Karagueuzian HS, Pezhouman A, Angelini M, et al. Enhanced Late Na and Ca Currents as Effective Antiarrhythmic Drug Targets. Front Pharmacol. 2017;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osadchii OE. Impact of Na+ channel blockers on transmural dispersion of refractoriness and arrhythmic susceptibility in guinea-pig left ventricle. Eur J Pharmacol. 2012;691:173–81. [DOI] [PubMed] [Google Scholar]
- 33.Sager PT. Modulation of antiarrhythmic drug effects by beta-adrenergic sympathetic stimulation. Am J Cardiol. 1998;82:20I–30I. [DOI] [PubMed] [Google Scholar]
- 34.El-Haou S, Ford JW, Milnes JT. Novel K+ Channel Targets in Atrial Fibrillation Drug Development--Where Are We? J Cardiovasc Pharmacol. 2015;66:412–31. [DOI] [PubMed] [Google Scholar]
- 35.Voigt N, Dobrev D. Atrial-Selective Potassium Channel Blockers. Card Electrophysiol Clin. 2016;8:411–21. [DOI] [PubMed] [Google Scholar]
- 36.Ravens U, Odening KE. Atrial fibrillation: Therapeutic potential of atrial K+ channel blockers. Pharmacol Ther. 2016. [DOI] [PubMed] [Google Scholar]
- 37.Weirich J, Antoni H. Rate-dependence of antiarrhythmic and proarrhythmic properties of class I and class III antiarrhythmic drugs. Basic Res Cardiol. 1998;93 Suppl 1:125–32. [DOI] [PubMed] [Google Scholar]
- 38.Gillis AM. Atrial Fibrillation and Ventricular Arrhythmias: Sex Differences in Electrophysiology, Epidemiology, Clinical Presentation, and Clinical Outcomes. Circulation. 2017;135:593–608. [DOI] [PubMed] [Google Scholar]
- 39.Parvez B, Vaglio J, Rowan S, et al. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol. 2012;60:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darbar D The Role of Pharmacogenetics in Atrial Fibrillation Therapeutics: Is Personalized Therapy in Sight? J Cardiovasc Pharmacol. 2016;67:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.•.Strauss DG, Vicente J, Johannesen L, et al. Common Genetic Variant Risk Score Is Associated With Drug-Induced QT Prolongation and Torsade de Pointes Risk: A Pilot Study. Circulation. 2017;135:1300–10. This study highlights an opportunity for recent genetic discoveries to improve individualized risk-benefit assessment for AAD therapies by predicting the risk of drug-induced arrhythmias. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heijman J, Voigt N, Ghezelbash S, et al. Calcium Handling Abnormalities as a Target for Atrial Fibrillation Therapeutics: How Close to Clinical Implementation? J Cardiovasc Pharmacol. 2015;66:515–22. [DOI] [PubMed] [Google Scholar]
- 43.Mustroph J, Neef S, Maier LS. CaMKII as a target for arrhythmia suppression. Pharmacol Ther. 2016. [DOI] [PubMed] [Google Scholar]
- 44.Ford J, Milnes J, El Haou S, et al. The positive frequency-dependent electrophysiological effects of the IKur inhibitor XEN-D0103 are desirable for the treatment of atrial fibrillation. Heart Rhythm. 2016;13:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavri BB, Greenberg HE, Kraft WK, et al. MK-0448, a specific Kv1.5 inhibitor: safety, pharmacokinetics, and pharmacodynamic electrophysiology in experimental animal models and humans. Circ Arrhythm Electrophysiol. 2012;5:1193–201. [DOI] [PubMed] [Google Scholar]
- 46.Olesen MS, Nielsen MW, Haunso S, et al. Atrial fibrillation: the role of common and rare genetic variants. Eur J Hum Genet. 2014;22:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grandi E, Pandit SV, Voigt N, et al. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res. 2011;109:1055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolkenberg SE, Nolt MB, Bilodeau MT, et al. Discovery of MK-1832, a Kv1.5 inhibitor with improved selectivity and pharmacokinetics. Bioorg Med Chem Lett. 2017;27:1062–9. [DOI] [PubMed] [Google Scholar]
- 49.Dobrev D, Ravens U. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Res Cardiol. 2003;98:137–48. [DOI] [PubMed] [Google Scholar]
- 50.Dobrev D, Friedrich A, Voigt N, et al. The G protein-gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–706. [DOI] [PubMed] [Google Scholar]
- 51.Kiper AK, Rinne S, Rolfes C, et al. Kv1.5 blockers preferentially inhibit TASK-1 channels: TASK-1 as a target against atrial fibrillation and obstructive sleep apnea? Pflugers Arch. 2015;467:1081–90. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt C, Wiedmann F, Schweizer PA, et al. Inhibition of cardiac two-pore-domain K+ (K2P) channels--an emerging antiarrhythmic concept. Eur J Pharmacol. 2014;738:250–5. [DOI] [PubMed] [Google Scholar]
- 53.Flaherty DP, Simpson DS, Miller M, et al. Potent and selective inhibitors of the TASK-1 potassium channel through chemical optimization of a bis-amide scaffold. Bioorg Med Chem Lett. 2014;24:3968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skarsfeldt MA, Jepps TA, Bomholtz SH, et al. pH-dependent inhibition of K2P3.1 prolongs atrial refractoriness in whole hearts. Pflugers Arch. 2016;468:643–54. [DOI] [PubMed] [Google Scholar]
- 55.Decher N, Wemhoner K, Rinne S, et al. Knock-out of the potassium channel TASK-1 leads to a prolonged QT interval and a disturbed QRS complex. Cell Physiol Biochem. 2011;28:77–86. [DOI] [PubMed] [Google Scholar]
- 56.Lugenbiel P, Wenz F, Syren P, et al. TREK-1 (K2P2.1) K+ channels are suppressed in patients with atrial fibrillation and heart failure and provide therapeutic targets for rhythm control. Basic Res Cardiol. 2017;112:8. [DOI] [PubMed] [Google Scholar]
- 57.Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac “late sodium current.”. Pharmacol Ther. 2008;119:326–39. [DOI] [PubMed] [Google Scholar]
- 58.Chorin E, Hu D, Antzelevitch C, et al. Ranolazine for Congenital Long-QT Syndrome Type III: Experimental and Long-Term Clinical Data. Circ Arrhythm Electrophysiol. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Curnis A, Salghetti F, Cerini M, et al. Ranolazine therapy in drug-refractory ventricular arrhythmias. J Cardiovasc Med (Hagerstown). 2017. [DOI] [PubMed] [Google Scholar]
- 60.Yeung E, Krantz MJ, Schuller JL, et al. Ranolazine for the suppression of ventricular arrhythmia: a case series. Ann Noninvasive Electrocardiol. 2014;19:345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pulford BR, Kluger J. Ranolazine Therapy in Cardiac Arrhythmias. Pacing Clin Electrophysiol. 2016;39:1006–15. [DOI] [PubMed] [Google Scholar]
- 62.Guerra F, Romandini A, Barbarossa A, et al. Ranolazine for rhythm control in atrial fibrillation: A systematic review and meta-analysis. Int J Cardiol. 2017;227:284–91. [DOI] [PubMed] [Google Scholar]
- 63.Gong M, Zhang Z, Fragakis N, et al. Role of ranolazine in the prevention and treatment of atrial fibrillation: A meta-analysis of randomized clinical trials. Heart Rhythm. 2017;14:3–11. [DOI] [PubMed] [Google Scholar]
- 64.Zareba W, Daubert JP, Beck CA, et al. Late-breaking clinical trials II: Ranolazine in high-risk icd patients (RAID) trial. Heart Rhythm. 2017;14:944. [Google Scholar]
- 65.Belardinelli L, Liu G, Smith-Maxwell C, et al. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J Pharmacol Exp Ther. 2013;344:23–32. [DOI] [PubMed] [Google Scholar]
- 66.Koltun DO, Parkhill EQ, Elzein E, et al. Discovery of triazolopyridine GS-458967, a late sodium current inhibitor (Late INai) of the cardiac NaV 1.5 channel with improved efficacy and potency relative to ranolazine. Bioorg Med Chem Lett. 2016;26:3202–6. [DOI] [PubMed] [Google Scholar]
- 67.Zablocki JA, Elzein E, Li X, et al. Discovery of Dihydrobenzoxazepinone (GS-6615) Late Sodium Current Inhibitor (Late INai), a Phase II Agent with Demonstrated Preclinical Anti-Ischemic and Antiarrhythmic Properties. J Med Chem. 2016. [DOI] [PubMed] [Google Scholar]
- 68.Fuller H, Justo F, Nearing BD, et al. Eleclazine, a new selective cardiac late sodium current inhibitor, confers concurrent protection against autonomically induced atrial premature beats, repolarization alternans and heterogeneity, and atrial fibrillation in an intact porcine model. Heart Rhythm. 2016;13:1679–86. [DOI] [PubMed] [Google Scholar]
- 69.Bacic D, Carneiro JS, Bento AA, et al. Eleclazine, an inhibitor of the cardiac late sodium current, is superior to flecainide in suppressing catecholamine-induced ventricular tachycardia and T-wave alternans in an intact porcine model. Heart Rhythm. 2017;14:448–54. [DOI] [PubMed] [Google Scholar]
- 70.Beat H. End of the Road for Eleclazine and Liberty HCM Study. 2016 [cited 2017 April 18, 2017]. Available from: https://hcmbeat.com/2016/12/27/end-of-the-road-for-eleclazine-and-liberty-hcm-study/.
- 71.Bers DM. Stabilizing ryanodine receptor gating quiets arrhythmogenic events in human heart failure and atrial fibrillation. Heart Rhythm. 2017;14:420–1. [DOI] [PubMed] [Google Scholar]
- 72.Walweel K, Oo YW, Laver DR. The emerging role of calmodulin regulation of RyR2 in controlling heart rhythm, the progression of heart failure and the antiarrhythmic action of dantrolene. Clin Exp Pharmacol Physiol. 2017;44:135–42. [DOI] [PubMed] [Google Scholar]
- 73.Uchinoumi H, Yang Y, Oda T, et al. CaMKII-dependent phosphorylation of RyR2 promotes targetable pathological RyR2 conformational shift. J Mol Cell Cardiol. 2016;98:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hartmann N, Pabel S, Herting J, et al. Antiarrhythmic effects of dantrolene in human diseased cardiomyocytes. Heart Rhythm. 2017;14:412–9. [DOI] [PubMed] [Google Scholar]
- 75.Zaza A, Rocchetti M. Calcium store stability as an antiarrhythmic endpoint. Curr Pharm Des. 2015;21:1053–61. [DOI] [PubMed] [Google Scholar]
- 76.Li N, Wang Q, Sibrian-Vazquez M, et al. Treatment of catecholaminergic polymorphic ventricular tachycardia in mice using novel RyR2-modifying drugs. Int J Cardiol. 2017;227:668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith GL, MacQuaide N. The direct actions of flecainide on the human cardiac ryanodine receptor: keeping open the debate on the mechanism of action of local anesthetics in CPVT. Circ Res. 2015;116:1284–6. [DOI] [PubMed] [Google Scholar]
- 78.Williams AJ, Bannister ML, Thomas NL, et al. Questioning flecainide’s mechanism of action in the treatment of catecholaminergic polymorphic ventricular tachycardia. J Physiol. 2016;594:6431–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang PC, Moreno JD, Jeng MT, et al. Reply from Yang Pei-Chi, Moreno Jonathan D., Jeng Mao-Tsuen, Wehrens Xander H. T., Noskov Sergei and Clancy Colleen E.. J Physiol. 2016;594:6433–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steele DS, Hwang HS, Knollmann BC. Triple mode of action of flecainide in catecholaminergic polymorphic ventricular tachycardia. Cardiovasc Res. 2013;98:326–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Q, Xiao J, Jiang D, et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan Z, Xiao Z, Wei J, et al. Nebivolol suppresses cardiac ryanodine receptor-mediated spontaneous Ca2+ release and catecholaminergic polymorphic ventricular tachycardia. Biochem J. 2016;473:4159–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dobrev D, Wehrens XH. Role of RyR2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ Res. 2014;114:1311–9; discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thireau J, Farah C, Bouly M, et al. Abstract 18592: Anti-arrhythmic Effect of a Novel Rycal, S44121 / Arm036, in a Post-myocardial Infarction Mouse Model of Heart Failure. Circulation. 2014;130:A18592. [Google Scholar]
- 85.Zhong X, Sun B, Vallmitjana A, et al. Suppression of ryanodine receptor function prolongs Ca2+ release refractoriness and promotes cardiac alternans in intact hearts. Biochem J. 2016;473:3951–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu Y, Tuteja D, Zhang Z, et al. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278:49085–94. [DOI] [PubMed] [Google Scholar]
- 87.Qi XY, Diness JG, Brundel BJ, et al. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation. 2014;129:430–40. [DOI] [PubMed] [Google Scholar]
- 88.Zhou XB, Sun Q, Voigt N, et al. Increased trafficking of small-conductance Ca2+-activated K plus channels to plasma membrane modulates action potential duration in human paroxysmal atrial fibrillation. Eur Heart J. 2014;35:183. [Google Scholar]
- 89.Skibsbye L, Poulet C, Diness JG, et al. Small-conductance calcium-activated potassium (SK) channels contribute to action potential repolarization in human atria. Cardiovasc Res. 2014;103:156–67. [DOI] [PubMed] [Google Scholar]
- 90.Simo-Vicens R, Sauter DRP, Grunnet M, et al. Effect of antiarrhythmic drugs on small conductance calcium - activated potassium channels. Eur J Pharmacol. 2017;803:118–23. [DOI] [PubMed] [Google Scholar]
- 91.••.Kim TY, Terentyeva R, Roder KH, et al. SK channel enhancers attenuate Ca2+-dependent arrhythmia in hypertrophic hearts by regulating mito-ROS-dependent oxidation and activity of RyR. Cardiovasc Res. 2017;113:343–53. Experimental study showing the antiarrhythmic effects of SK-channel enhancers through activation of mitochondrial SK-channels, thereby regulating oxidative stress and Ca2+-handling abnormalities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inoue R, Jensen LJ, Shi J, et al. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–31. [DOI] [PubMed] [Google Scholar]
- 93.Harada M, Luo X, Qi XY, et al. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation. 2012;126:2051–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chubanov V, Ferioli S, Gudermann T. Assessment of TRPM7 functions by drug-like small molecules. Cell Calcium. 2017. [DOI] [PubMed] [Google Scholar]
- 95.Zhang YH, Wu HJ, Che H, et al. Functional transient receptor potential canonical type 1 channels in human atrial myocytes. Pflugers Arch. 2013;465:1439–49. [DOI] [PubMed] [Google Scholar]
- 96.Guinamard R, Bouvagnet P, Hof T, et al. TRPM4 in cardiac electrical activity. Cardiovasc Res. 2015;108:21–30. [DOI] [PubMed] [Google Scholar]
- 97.Hof T, Liu H, Salle L, et al. TRPM4 non-selective cation channel variants in long QT syndrome. BMC Med Genet. 2017;18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev. 2014;66:676–814. [DOI] [PubMed] [Google Scholar]
- 99.Dobrev D, Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 2010;375:1212–23. [DOI] [PubMed] [Google Scholar]
- 100.Ezekowitz MD, Nagarakanti R, Lubinski A, et al. A randomized trial of budiodarone in paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2012;34:1–9. [DOI] [PubMed] [Google Scholar]
- 101.Heijman J, Heusch G, Dobrev D. Pleiotropic effects of antiarrhythmic agents: dronedarone in the treatment of atrial fibrillation. Clin Med Insights Cardiol. 2013;7:127–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Piccini JP, Pritchett EL, Davison BA, et al. Randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of a single oral dose of vanoxerine for the conversion of subjects with recent onset atrial fibrillation or flutter to normal sinus rhythm: RESTORE SR. Heart Rhythm. 2016;13:1777–83. [DOI] [PubMed] [Google Scholar]
- 103.•.Reiffel JA, Camm AJ, Belardinelli L, et al. The HARMONY Trial: Combined Ranolazine and Dronedarone in the Management of Paroxysmal Atrial Fibrillation: Mechanistic and Therapeutic Synergism. Circ Arrhythm Electrophysiol. 2015;8:1048–56. Clinical evidence for the synergystic effects of combined AAD therapy with ranolazine and dronedarone. [DOI] [PubMed] [Google Scholar]
- 104.Hartmann N, Mason FE, Braun I, et al. The combined effects of ranolazine and dronedarone on human atrial and ventricular electrophysiology. J Mol Cell Cardiol. 2016;94:95–106. [DOI] [PubMed] [Google Scholar]
- 105.••.Aguilar M, Xiong F, Qi XY, et al. Potassium Channel Blockade Enhances Atrial Fibrillation-Selective Antiarrhythmic Effects of Optimized State-Dependent Sodium Channel Blockade. Circulation. 2015;132:2203–11. A combined experimental and computational approach highlights how antiarrhythmic therapy can be optimized by modulation of a specific combination of ion channel targets. [DOI] [PubMed] [Google Scholar]
- 106.Burashnikov A, Belardinelli L, Antzelevitch C. Inhibition of IKr potentiates development of atrial-selective INa block leading to effective suppression of atrial fibrillation. Heart Rhythm. 2015;12:836–44. [DOI] [PubMed] [Google Scholar]
- 107.Kirchhoff JE, Diness JG, Sheykhzade M, et al. Synergistic antiarrhythmic effect of combining inhibition of Ca2+-activated K+ (SK) channels and voltage-gated Na+ channels in an isolated heart model of atrial fibrillation. Heart Rhythm. 2015;12:409–18. [DOI] [PubMed] [Google Scholar]