Abstract
Background:
The Advisory Committee on Immunization Practices (ACIP) has routinely recommended zoster vaccine live (ZVL) for adults ≥60 since 2008; only 33% of eligible adults received it by 2016. A recombinant zoster vaccine (RZV) was licensed in 2017 and ACIP recommended in January 2018. Our objectives were to assess among primary care physicians 1) practices and attitudes regarding ZVL and 2) awareness of RZV.
Methods:
We administered an Internet and mail survey from July to September 2016 to national networks of 953 primary care physicians.
Results:
Response rate was 65% (603/923). Ninety-three % of physicians recommended ZVL to adults ≥60, but fewer recommended it to adults ≥60 with a prior history of zoster (88%), adults >85 (62%) and adults ≥60 on low-dose methotrexate (42%). Several physicians recommended ZVL in ways that are not recommended by ACIP including to adults 50–59 (50%), adults ≥60 with HIV (33%), and adults ≥60 on high dose prednisone (≥20mg/day) (27%). Nineteen percent of physicians stocked and administered ZVL and did not refer patients elsewhere for vaccination, 37% did not stock and only referred patients to receive it, and 44% both stocked/administered and referred elsewhere. Twenty-three % (n=115) of physicians who had ever administered ZVL in the office (n=490) had stopped, citing primarily financial issues (90%). Only 5% were ‘very aware’ of RZV.
Conclusions:
Physicians report not recommending ZVL to certain ACIP-recommended groups, but report recommending it to some groups for which the vaccine should be avoided. Implementation of recommendations for RZV will need to consider financial barriers and the complex patchwork of office-based and pharmacy delivery ZVL has encountered.
Introduction
A third of U.S. adults are afflicted with herpes zoster (HZ) during their lifetime and the incidence increases with age.1 Antiviral agents may reduce the duration and severity of the HZ rash,2 but available treatments for the associated pain and its most common complication, postherpetic neuralgia (PHN), are often ineffective and wrought with side effects, particularly in the elderly population most affected.3 Zoster vaccine live (ZVL or Zostavax®), licensed by the FDA in 2006, was the first vaccine for the prevention of HZ. ZVL decreases the risk of HZ by 51% and the risk of PHN by 67% in adults ≥60 years in the first four years following vaccine receipt.4 Since 2008, the Advisory Committee on Immunization Practices (ACIP) has recommended this vaccine for adults in this age group.1 In March of 2011, the U.S. Food and Drug Administration approved the use of ZVL in adults aged 50–59 years. The ACIP did not recommend the vaccine for individuals 50–59 years, initially due to shortages of the vaccine and subsequently because of concerns regarding the cost effectiveness and duration of protection in this age group.5
Ten years post-licensure experience in the U.S. and internationally with ZVL have accumulated and no significant safety concerns have been documented in numerous studies.6–13 However, other issues regarding vaccine storage, efficacy, duration of protection and reimbursement have arisen. Unlike most adult vaccines, ZVL is a live virus vaccine requiring freezer storage and cautious use, or in some instances, contraindication for use, among immunocompromised patients. The efficacy of ZVL declines dramatically with advancing age at receipt, from over 60% in adults 60–69 years to 38% in adults ≥70 years and only 18% in adults ≥80.4 Data now show this vaccine has a limited duration of protection with one study documenting 4% efficacy against HZ incidence eight years after vaccination14 and another study demonstrating no protection against HZ or PHN ten years after vaccination15 calling for inquiries into possible revaccination strategies.16,17 Finally, this is the most expensive vaccine routinely recommended for adults and one of the first vaccines to be covered under Medicare Part D.18 Medicare Part D drug plans are designed to provide pharmacy rather than medical benefits and therefore most physicians have no direct way to bill and be reimbursed by these plans.
A recombinant zoster vaccine (RZV or Shingrix®) showed between 96.6% and 97.9% in all age groups studied: 50–59, 60–69 and ≥ 70 years.19 In another study, RZV demonstrated a vaccine efficacy of 89.8% in adults ≥ 70 years.20 In pooled analysis from two studies, vaccine efficacy against PHN was 88.8%.20 RZV has not only been shown to prevent HZ, but also reduce the severity of HZ in the rare individuals who develop HZ after being vaccinated.21 In addition to its greater short-term efficacy, RZV is refrigerator stable and is not contraindicated in immunocompromised individuals. However, RZV had more side effects than ZVL compared to placebo, mostly pain and redness at the injection site, as well as myalgias and fatigue. Also, RZV requires a two-dose administration two months apart and lacks the post-marketing safety data that ZVL has accumulated. In October 2017, the FDA licensed RZV22 and then ACIP recommended it be used for the prevention of HZ in immunocompetent persons ≥50 years.23 ACIP also recommended RZV for anyone who had previously received ZVL and preferentially recommended RZV over ZVL.23
Prior work has shown that vaccine pre-licensure surveys are acceptable predictors of physician behaviors regarding vaccines post-licensure24 and therefore our objectives for this study were to assess among primary care physicians shortly before licensure of RZV: 1) practices and 2) attitudes regarding ZVL and 3) awareness and likelihood of recommending and (4) anticipated barriers to administering RZV.
Methods
Study Setting
From July 2016 to September 2016, we administered a survey to a national network of physicians who had agreed to participate in surveys about vaccine policy issues and who spent at least half their time practicing in primary care. The human subjects review board at the University of Colorado Denver approved this study as exempt research not requiring written informed consent.
Study Population
The Vaccine Policy Collaborative Initiative,25 a survey mechanism to assess physician attitudes about vaccine issues, in collaboration with the Centers for Disease Control and Prevention (CDC), conducted the survey. We developed a network of primary care physicians by recruiting general internists (GIM) and family physicians (FP) from the memberships of the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP). Seventy-seven percent of family physicians are members of the AAFP and it cannot be estimated how many general internists are members of the ACP.26,27We performed quota sampling28 to ensure that networks of physicians were similar to the ACP and AAFP memberships with respect to region, urban versus rural location, and practice setting. We previously demonstrated that survey responses from network physicians were similar compared to those of physicians randomly sampled from American Medical Association physician databases with respect to reported demographic characteristics, practice attributes, and attitudes about vaccination issues.28
Survey Design
We used 4-point Likert scales for assessing frequency of a particular practice (‘Often’ to ‘Never)’, attitudes about ZVL (‘Strongly agree’ to ‘Strongly disagree’), and anticipated barriers to RZV (‘Major barrier’ to ‘Not a barrier’). For assessing physician recommendations for ZVL in various patient populations, response options included a 4-point Likert scale from ‘Strongly recommend’ to ‘Recommend against,’ and included two additional response options of ‘Would have to look this up,’ and ‘Don’t see these types of patients.’ We provided respondents with information about RZV, including efficacy and safety data and the need for two doses, and asked about prior awareness of this vaccine. When we asked about the likelihood of recommending the new RZV if it is approved, recommended, and covered by insurance in the same way as ZVL, response options included a 4-point Likert scale from ‘Very likely’ to ‘Very unlikely,’ as well as a ‘Don’t Know’ category. A national advisory panel of GIM (n=9) and FP (n=6) pre-tested the survey, which we modified based on their feedback. We pilot-tested the survey among 33 GIM and 17 FP nationally and further modified the survey instrument based on their feedback.
Survey Administration
Based on physician preference, we sent the survey over the Internet29 or through U.S. mail. We sent the Internet group an initial e-mail with up to 8 e-mail reminders, and we sent the mail group an initial mailing and up to 2 additional reminders. Non-respondents in the Internet group were also sent up to 2 mail surveys in case of problems with e-mail correspondence. We patterned the mail protocol on Dillman’s Tailored Design Method.30
Statistical analysis
We pooled Internet and mail surveys for analyses because other studies have found that physician attitudes are similar when obtained by either method.30–32 We compared respondents with non-respondents on all available characteristics using t-tests, Pearson’s chi-squared tests and Mantel-Haenszel chi-squared tests; characteristics of non-respondents were obtained from the recruitment survey for the sentinel networks. We compared GIM and FP responses using Mantel-Haenszel and Pearson’s chi-squared tests. We used chi-squared and Wilcoxon tests to evaluate associations with recommending ZVL inconsistently with ACIP recommendations. Most results were similar for GIM and FP physicians, and are therefore presented together with any differences highlighted in the text. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina).
Results
Survey response and characteristics of respondents
The response rate was 65% (603/923). Characteristics of respondents and non-respondents and further characteristics of respondents’ practices and patient populations are shown in Table 1. The majority of respondents were in private practice and at least 50% were male. GIM respondents were evenly distributed across U.S. regions and the largest proportion practiced in the urban setting. A smaller proportion of FP respondents were from the Northeast region and the greatest proportion of FP respondents practiced in the suburban setting.
Table 1:
Demographic and Practice Characteristics of Respondents in a Study of Physicians’ Perspective on Zoster Vaccine Live and Recombinant Zoster Vaccine, United States, 2016
| Characteristic | Respondents (n=603) |
Non- Respondents (n=320) |
|---|---|---|
| Age in years, mean (SD)* | 54.4 (8.4) | 56.2 (8.7) |
| Male, %* | 53 | 65 |
| Region, % | ||
| Midwest | 25 | 25 |
| Northeast | 20 | 17 |
| South | 32 | 41 |
| West | 24 | 18 |
| Location of Practice, % | ||
| Urban | 49 | 42 |
| Suburban | 46 | 53 |
| Rural | 5 | 5 |
| Setting, %* | ||
| Private practice | 71 | 79 |
| Hospital/clinic | 22 | 15 |
| HMO | 6 | 6 |
| # of providers in your practice, % | ||
| 1 | 12 | 18 |
| 2–4 | 27 | 25 |
| 5–10 | 32 | 31 |
| >10 | 29 | 25 |
| Proportion of patients ≥ 65,% | ||
| <10% | 4 | N/A |
| 10–24% | 18 | N/A |
| 25–49% | 38 | N/A |
| ≥50% | 39 | N/A |
| Proportion of patients with Medicare Part D, % | N/A | |
| <10% | 9 | N/A |
| 10–24% | 30 | N/A |
| 25–49% | 30 | N/A |
| ≥50% | 17 | N/A |
| Don’t know | 13 | N/A |
GIM= General Internists FP= Family Physicians
p<0.05 for comparison of GIM and FP with FP respondents being more likely to be female and more likely to be from the west region than GIM respondents; GIM respondents more likely to be from a hospital/clinic setting than FP respondents.
Practices Regarding Zoster Vaccine Live
Physician recommendations for ZVL in various patient groups are presented in Figure 1. In terms of groups with an ACIP recommendation, only 62% and 42% of physicians recommended ZVL for adults > 85 and adults on low dose methotrexate (<0.4mg/kg), respectively. In terms of groups without an ACIP recommendation, 50% of physicians recommended ZVL to adults 50–59 years and 33% recommended ZVL to adults ≥60 years with HIV and a CD4 count ≥200. Family physicians, physicians from the South and physicians in smaller practices (mean / median 13 / 5 vs. 23 / 6 providers in the practice) were more likely to recommend ZVL to immunocompetent patients 50–59 years (p<0.05). Family physicians were also more likely to recommend ZVL to patients ≥60 on immune mediator or modulator therapy or chemotherapy (p=<0.05). Forty-nine percent of physicians reported that a quarter or more of their patients who have insurance coverage for ZVL decline the vaccine despite it being recommended. Nineteen percent of physicians stocked and administered ZVL and did not refer patients elsewhere for vaccination, 37% did not stock and only referred patients to receive it, and 44% both stocked/administered and referred elsewhere. Of those who referred at least some patients elsewhere for vaccination (n=464), referral locations “often” or “sometimes” used included pharmacies (94%), public health departments (26%), and clinics (5%). Twenty-two percent ‘often’ or ‘sometimes’ referred patients to a pharmacy to purchase the vaccine and instructed the patients to return to the practice to have it administered.
Figure 1.

Physician Strength of Recommendation for Zoster Vaccine Live for the Following Types of Patients, United States, 2016 (n=590)
GIM= General Internists FP= Family Physicians
† 5% don’t see these types of patients (n=31)
‡ 19% don’t see these types of patients (n=114)
* p<0.05 for differences between GIM and FP (Fisher’s Exact chi-squared test or chi-squared test, as appropriate) with GIM being more likely to recommend against herpes zoster vaccine to ≥ 60 years-old on low dose methotrexate (28% vs. 12%), on high dose prednisone (58% vs. 34%), on a recombinant human immune mediator or immune modulator (62% vs. 33%), or on chemotherapy (66% vs. 40%) and FP being more likely to recommend herpes zoster vaccine to immunocompetent adults 50-59 years old (56% vs. 45%). Some percentages do not add up to 100% due to rounding.
Sixty-four percent of physicians currently administered ZVL in the office to at least some patients; 20% had stopped administering and 16% had never administered it in the office. The most common reasons physicians had stopped administering ZVL (n=115) included cost and reimbursement issues (90%), difficulties maintaining freezer storage (35%), and insufficient patient demand (19%).
Attitudes and Experience Regarding Performance of Zoster Vaccine Live
Attitudes and experience with ZVL were generally favorable, with most physicians agreeing that the vaccine had decreased the burden of illness from HZ and PHN (Figure 2). However, many physicians agreed (39% ‘Strongly,’ 45% ‘Somewhat’) that they had seen people who developed HZ despite getting the vaccine. GIM had seen more serious reactions from ZVL than FP (17.5% vs. 9.6%, p=<0.05).
Figure 2.
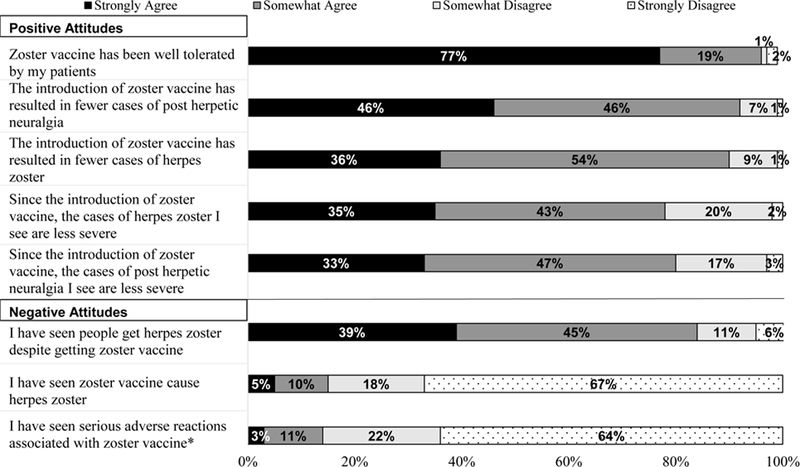
Physician Attitudes & Experiences with Zoster Vaccine Live, United States, 2016 (n=590)
GIM = General Internists FP = Family Physicians
*p<0.05 for comparison between GIM and FP; GIM were more likely to report having seen a serious adverse reaction with zoster vaccine (18% vs. 10% ‘Strongly agree’ or ‘Somewhat agree’).
Some percentages do not add up to 100% due to rounding.
Awareness of and Likelihood of Recommending Recombinant Zoster Vaccine
Only 5% and 21% of respondents were ‘Very’ or ‘Somewhat’ aware of RZV, respectively. FP were more likely than GIM to be ‘Not at all aware,’ (84% vs. 66%, p=<0.0001). Figure 3 demonstrates physician likelihood of recommending RZV to various immunocompetent patients after reading the informational statements about the vaccine provided in the survey.
Figure 3:
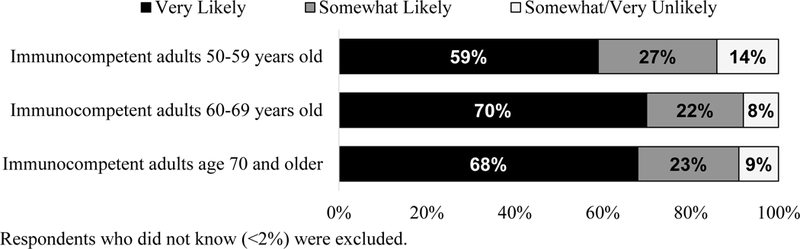
Physician Likelihood of Recommending Recombinant Zoster Vaccine to Various Patient Groups, United States, 2016.
Respondents who did not know (<2%) were excluded.
Anticipated Barriers to Recombinant Zoster Vaccine
Figure 4 shows physician anticipated barriers to RZV. The most commonly reported barrier was that if the vaccine is not fully covered by insurance, patients would be unwilling to pay out of pocket for it (40% ‘Major,’ 37% ‘Moderate’ barrier). Eighty percent of respondents estimated their patients would pay no more than $74 out of pocket. FP was more likely than GIM to report if the vaccine is not covered by insurance, patients would be unwilling to pay out of pocket for it (81.3 vs. 72.8%) and that the two-dose regimen would be barriers (53.5 vs. 42.6%).
Figure 4.
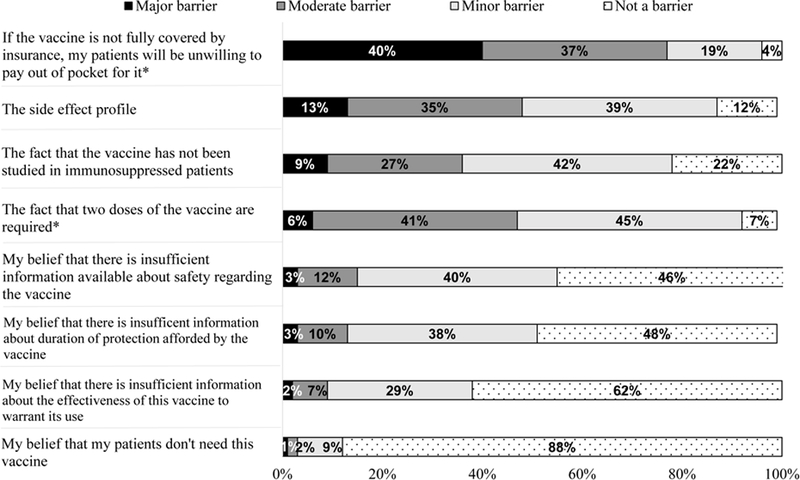
Physician Anticipated Barriers to the Recombinant Zoster Vaccine, United States, 2016 (n=590)
GIM = General Internists FP = Family Physicians
*p<0.05 for comparison between GIM and FP; FP were more likely to say if the vaccine is not fully covered by insurance, patients will be unwilling to pay out of pocket for it (81% vs. 73% ‘Major/’Moderate barrier’) and that two doses of the vaccine are required is a barrier (54% vs. 43% ‘Major/Moderate barrier‘).
Some percentages do not add up to 100% due to rounding.
Discussion
Approximately a decade after the ZVL recommendation, almost all physicians reported they are recommending it for adults ≥60 years and generally reported a favorable experience with the vaccine being well tolerated and its use resulting in fewer and less severe cases of HZ and PHN. However, the majority of physicians had seen patients develop HZ despite having received ZVL, possibly reflecting ZVL’s baseline efficacy and waning protection over time. Physicians described a complex patchwork of office-based and pharmacy delivery of the vaccine and reported recommending ZVL to some patients not recommended to receive it by ACIP.
The fact that physicians are not recommending ZVL to certain vaccine eligible groups (e.g. individuals >85 years old and ≥60 on low dose methotrexate), but recommending it to some groups for which this live virus vaccine is contraindicated (e.g. individual ≥ 60 on high dose prednisone or an immune mediator) speaks to problems in interpretation and implementation that can arise even with a relatively simple age-based indication for a vaccine. A notable minority of physicians (21%) recommended against ZVL in patients on low dose methotrexate even though the recommendations state this is not a contraindication.; 31% reported they would have to look up whether such a patient should receive ZVL.1 Physicians’ reservations regarding using ZVL in patients on low dose methotrexate likely stem from safety concerns, however, patients needing low dose methotrexate for an underlying condition are at higher risk for HZ33 and use of ZVL in patients on low dose methotrexate has been shown to be safe.34 Similarly, eleven percent of physicians recommended against patients older than eighty-five receiving ZVL even though the recommendation states to vaccinate adults sixty and older without making any caveats for the oldest old, individuals over 85.35 Unvaccinated individuals who live to 85 years have a 50% risk of HZ.2
Clearly there is a lot of interest among physicians to vaccinate adults against HZ who previously did not or continue to not have an ACIP recommendation. ZVL is FDA approved for adults 50–59 years, but not recommended due to waning of protection and cost-effectiveness.36 Physicians also reported recommending ZVL to patients with HIV with a CD4 count ≥200 even though ACIP makes no recommendation for ZVL to this patient population.37 Some have argued that ZVL should be administered to patients with HIV with a CD4 count ≥200, the same CD4 threshold used for other live-attenuated viral vaccines.38 Also, many physicians are recommending ZVL to patients on immunosuppressive therapy that is considered a contraindication to ZVL.1
Unsurprisingly most physicians in this study were not aware of RZV as the study was conducted approximately a year before RZV was FDA approved. Now that RZV has been preferentially recommended, it is important to keep in mind similarities between ZVL and RZV that might affect RZV’s delivery. ZVL is currently not well utilized, with only 33% of the recommended population receiving the vaccine.39 Previous literature has highlighted the importance of financial barriers to ZVL delivery both in terms of cost of the vaccine and Medicare Part D coverage of the vaccine for Medicare beneficiaries.18 Physicians believe patients often decline ZVL for financial reasons, even when they have coverage.40 Many Part D plans categorize ZVL as an expensive Tier 3 or 4 drug meaning higher co-pays for patients41 despite the Centers for Medicare & Medicaid Service’s encouragement that Part D plans place vaccines into a Tier 6 category where there is no copay for patients.42 Like ZVL, RZV will be covered by Medicare Part D. RZV is estimated to cost $280, $140 per dose23, so will be more expensive than ZVL which costs approximately $213.43 We do not know how much the $67 price difference will affect interest in the vaccine, but our physician respondents anticipated that patient out-of-pocket expense for the RZV could be problematic. Higher vaccine prices might also affect how many physician practices choose to stock RZV; a sizable portion of respondents (20%) had stopped administering ZVL in the office mostly due to cost and reimbursement issues. Medicare Part D coverage is likely at the root of our finding of the varying practices physicians employ to deliver ZVL, with most physicians reporting they do a combination of in-office administration (likely for the privately insured non-Medicare population) and referring to pharmacies (likely for the Medicare Part D population) because Medicare Part D is a pharmacy benefit that is more easily billed by pharmacists. Although fewer physicians reported referring patients to the pharmacy to purchase ZVL and return to the clinic for administration (i.e. ‘brown-bagging’) than a previous study (36%)18, a considerable minority (22%) are still employing this practice which may have a large impact on the cold chain and how well ZVL performs. RZV is refrigerator stable and would presumably be less affected by such a practice. Since RZV is recommended beginning at age 50, more adults may have the opportunity to be vaccinated prior to becoming Medicare-eligible.
Several physicians, particularly family physicians, reported recommending ZVL to immunosuppressed populations (Figure 1). The current RZV recommendation does not address this physician inclination to vaccinate these immunosuppressed populations as it is only recommended for immunocompetent individuals. However, RZV is not contraindicated in the immunosuppressed, which may influence physicians’ decisions regarding using the vaccine off-label and off-recommendation in this high-risk group. RZV has been shown to be immunogenic and to have a clinically acceptable safety profile in HIV-infected adults,44 however, the two trials considered in RZV’s FDA approval and ACIP recommendation, ZOE-5010 and ZOE-70,20 did not include immunosuppressed patients. Physicians reported the fact that RZV had not been studied in immunocompromised populations could be a barrier to RZV’s use.
Barriers unique to RZV identified by physician respondents included its greater reactogenicity than ZVL and the two-dose regimen. For ZVL, in the Shingles Prevention Study subgroup analysis of adverse events, local reactions, mostly manifesting as erythema, were more common in the intervention than placebo group (48.3% vs. 16%), but there was no difference between intervention and placebo in terms of systemic reactions.4 For RZV, similarly, but to a greater degree, local reactions, primarily pain at the injection site, were more common in the intervention group than the placebo group in ZOE-50 (81.5% vs. 11.9%) and in ZOE-70 (74.1 vs. 9.9%). By contrast, systemic reactions (myalgias in ZOE-50 and fatigue in ZOE-70) were also more common in the intervention groups than the placebo groups in these two studies (66.1% vs. 29.5% for ZOE-50 and 53% vs. 25% for ZOE-70).19,20 In post-hoc analysis of phase 3 clinical trials less than 1% of ZVL recipients reported a grade 3 reaction, a reaction related to vaccination that was severe enough to prevent normal activities.45 In comparison, in pooled data from ZOE-50 and ZOE-70, 16.5% of RZV recipients reported grade 3 reactions.46
The two-dose regimen adds a layer of complexity to delivery and suggests the need for implementation of a vaccine reminder/recall system which currently healthcare practices rarely use.47 Retail pharmacies, as a primary provider of Medicare Part D vaccines, will also need to consider using these systems. Even with a recall system in place, the anticipated second co-pay or side effects experienced may cause individuals to be less likely to return for the second dose. Although not a particular concern for our physician respondents, less than four years of safety and efficacy data is available for RZV.
Our study has strengths and limitations. Results were generated from primary care physicians from across the nation and we achieved an excellent response rate for a physician survey. One needs to be cautious using these survey results to draw any comparative conclusion regarding the two vaccines since physicians were responding based on actual experience with ZVL and only hypothetically regarding RZV. Although our sample was designed to be representative of ACP and AAFP memberships, the attitudes, experiences, and practices of sentinel physicians may not be fully generalizable. Non-respondents may have held different views than respondents. The survey relied on self-report of practice rather than observation of practice.
Physicians report a favorable experience with the ZVL product, but difficulties with administration due to financial concerns. Physicians are interested in recommending RZV, likely due to its superior efficacy and possibly the broader application to a wider age range of patients. It will be important to monitor physician attitudes and practices related to zoster vaccination as RZV becomes widely available in order to determine how the unique characteristics of this new vaccine affect its implementation.
Footnotes
Conflict of Interest: None of the authors has a conflict of interest.
Bibliography
- 1.Harpaz R, Ortega-Sanchez IR, Seward JF, Advisory Committee on Immunization Practices Centers for Disease Control and Prevention. Prevention of Herpes Zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008;57(RR-5):1–30; quiz CE32–34. [PubMed] [Google Scholar]
- 2.Cohen JI. Clinical practice: Herpes zoster. N Engl J Med. 2013;369(3):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–1533. [DOI] [PubMed] [Google Scholar]
- 4.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep 2011;60(44):1528. [PubMed] [Google Scholar]
- 6.Baxter R, Tran TN, Hansen J, et al. Safety of Zostavax--a cohort study in a managed care organization. Vaccine. 2012;30(47):6636–6641. [DOI] [PubMed] [Google Scholar]
- 7.Mills R, Tyring SK, Levin MJ, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects with a history of herpes zoster. Vaccine. 2010;28(25):4204–4209. [DOI] [PubMed] [Google Scholar]
- 8.Morrison VA, Oxman MN, Levin MJ, et al. Safety of zoster vaccine in elderly adults following documented herpes zoster. J Infect Dis. 2013;208(4):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray AV, Reisinger KS, Kerzner B, et al. Safety and tolerability of zoster vaccine in adults >/=60 years old. Human vaccines. 2011;7(11):1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmader KE, Levin MJ, Gnann JW Jr., et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54(7):922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simberkoff MS, Arbeit RD, Johnson GR, et al. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med. 2010;152(9):545–554. [DOI] [PubMed] [Google Scholar]
- 12.Tseng HF, Liu A, Sy L, et al. Safety of zoster vaccine in adults from a large managed-care cohort: a Vaccine Safety Datalink study. J Intern Med. 2012;271(5):510–520. [DOI] [PubMed] [Google Scholar]
- 13.Willis ED, Woodward M, Brown E, et al. Herpes zoster vaccine live: A 10year review of post-marketing safety experience. Vaccine. 2017;35(52):7231–7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng HF, Harpaz R, Luo Y, et al. Declining Effectiveness of Herpes Zoster Vaccine in Adults Aged >/=60 Years. J Infect Dis. 2016;213(12):1872–1875. [DOI] [PubMed] [Google Scholar]
- 15.Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60(6):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le P, Rothberg MB. Determining the Optimal Vaccination Schedule for Herpes Zoster: a Cost-Effectiveness Analysis. J Gen Intern Med. 2017;32(2):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oxman MN, Harbecke R, Koelle DM. Clinical Usage of the Candidate Adjuvanted HZ/su Zoster Vaccine: re-vaccination of recipients of live attenuated zoster vaccine and co-administration with a seasonal influenza vaccine. J Infect Dis. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurley LP, Lindley MC, Harpaz R, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med. 2010;152(9):555–560. [DOI] [PubMed] [Google Scholar]
- 19.Lal H, Cunningham AL, Heineman TC. Adjuvanted Herpes Zoster Subunit Vaccine in older adults. N Engl J Med. 2015;373(16):1576–1577. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med. 2016;375(11):1019–1032. [DOI] [PubMed] [Google Scholar]
- 21.Curran D, Oostvogels L, Heineman T, et al. Quality of Life impact of a Recombinant Zoster Vaccine in adults >/=50 Years of Age. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruber FM. BLA Approval 2017; https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM581750.pdf. Accessed 11/6/2017.
- 23.Kaplan SCDC Panel Recommends a New Shingles Vaccine 2017; https://www.nytimes.com/2017/10/25/health/cdc-shingles-vaccine.html. Accessed 11/6/17.
- 24.Seewald L, Hurley L, Crane LA, et al. Things are not as bad as they seem: physicians’ ability to predict their clinical practice when a new vaccine becomes available. Healthcare policy = Politiques de sante. 2013;8(4):71–85. [PMC free article] [PubMed] [Google Scholar]
- 25.University of Colorado. Vaccine policy collaborative initiative 2017; http://www.ucdenver.edu/academics/colleges/medicalschool/programs/ACCORDS/childrensoutcomesreserach/VaccinePolicyCollaborativeInitiative/Pages/default.aspx. Accessed October 9, 2017.
- 26.Weissman A. question. In: Hurley L, ed 2018.
- 27.Schoof B. question - AAFP Member Data. In: Hurley L, ed 2018.
- 28.Crane LA, Daley MF, Barrow J, et al. Sentinel physician networks as a technique for rapid immunization policy surveys. EvalHealth Prof. 2008;31(1):43–64. [DOI] [PubMed] [Google Scholar]
- 29.Vovici Feedback [computer program]. Melville, NY: Verint Systems Inc; 2015. [Google Scholar]
- 30.Dillman DA, Smyth J, Christian LM. Internet, Mail and Mixed-Mode Surveys: The Tailored Desgin Method, 3rd Edition Vol 3rd. New York, NY: John Wiley Co.; 2009. [Google Scholar]
- 31.Atkeson LR, Adams AN, Bryant LA, Zilberman L, Saunder KL. Considering Mixed Mode Surveys for Questions in Political Behavior: Using the Internet and Mail to Get Quality Data at Reasonable Costs. Political Behavior. 2011;33(1):161–178. [Google Scholar]
- 32.McMahon SR, Iwamoto M, Massoudi MS, et al. Comparison of e-mail, fax, and postal surveys of pediatricians. Pediatrics. 2003;111(4 Pt 1):e299–e303. [DOI] [PubMed] [Google Scholar]
- 33.Smitten AL, Choi HK, Hochberg MC, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57(8):1431–1438. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matters TD. The Demographics of Aging. http://transgenerational.org/aging/demographics.htm. Accessed 11/6/2017.
- 36.Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR, Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729–731. [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Adult Immunization Schedule by Medical and Other Indications, Recommended Immunization Schedule for Adults Aged 19 Years or Older by Medical Conditions and Other Indications, United States, 2017. 2017; https://www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.html. Accessed September 20, 2017.
- 38.Shafran SD. Live attenuated herpes zoster vaccine for HIV-infected adults. HIV Med. 2016;17(4):305–310. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Vaccination Coverage Among Adults in the United States, National Health Interview Survey, 2016. 2018; https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/NHIS-2016.html. Accessed April 25, 2018.
- 40.Hurley LP, Lindley MC, Allison MA, et al. Primary care physicians’ perspective on financial issues and adult immunization in the Era of the Affordable Care Act. Vaccine. 2017;35(4):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carr T Why Does My Shingles Vaccine Cost So Much? Medicare may not provide good coverage for this vital protection. 2017; https://www.consumerreports.org/health/why-the-shingles-vaccine-cost-so-much/. Accessed September 29, 2017.
- 42.U.S. Government Accountability Office. Medicare: many factors, including administrative challenges, affect access to Part D vaccinations. 2011; http://www.gao.gov/products/GAO-12-61. Accessed February 24, 2016.
- 43.Centers for Disease Control and Prevention. Vaccines for Children Program (VFC) 2017; https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html. Accessed November 6, 2017.
- 44.Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211(8):1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popmihajlov Z, Pang L, Brown E, Su S-C, Kaplan S, Willis E. Low Rates of Severe Injection-Site and Systemic Adverse Events Within 7 Days Postvaccination With ZOSTAVAX™, a Post-Hoc Analysis of Two Pivotal Phase 3 Trials. 26th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2016; https://www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=48271. [Google Scholar]
- 46.Centers for Disease Control and Prevention. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE): Recombinant Zoster Vaccine (RZV) and Herpes Zoster Live-Attenuated Vaccine (ZVL). 2018; https://www.cdc.gov/vaccines/acip/recs/grade/herpes-zoster.html. Accessed April 23, 2018.
- 47.Pereira JA, Quach S, Heidebrecht CL, et al. Barriers to the use of reminder/recall interventions for immunizations: a systematic review. BMC Med Inform Decis Mak 2012;12(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]


