Abstract
Addition of new neurons and oligodendroglia in the postnatal/adult mammalian brain present distinct forms of grey and white matter plasticity. Substantial effort has been devoted to understanding the cellular and molecular mechanisms controlling postnatal neurogenesis and gliogenesis, revealing important parallels to principles governing the embryonic stages. While during CNS development, scripted temporal and spatial patterns of neural and glial progenitor proliferation/differentiation are necessary to create the nervous system architecture, it remains unclear what may be the driving forces that maintain and sustain postnatal neural stem cell (NSC) and oligodendrocyte precursor cell (OPC) production of new neurons and glia. In recent years, neuronal activity has been identified as an important modulator of these processes. Utilizing the distinct properties of neurotransmitter ionotropic and metabotropic channels to signal downstream cellular events, NSCs and OPCs share common features in their readout of neuronal activity patterns. Here we review the current evidence for neuronal activity-dependent control of NSC/OPC proliferation and differentiation in the postnatal brain, highlight some potential mechanisms used by the two progenitor populations, and discuss future studies that might advance these research areas further.
INTRODUCTION
Resident progenitor cells in the postnatal brain that give rise to new neurons and glia present exciting possibilities for tissue regeneration and remodeling. Under physiological conditions, it is now well accepted that postnatal neural stem cells (NSCs) and ongoing neurogenesis are retained in two distinct mammalian brain regions: the hippocampal dentate gyrus (DG) and the subependymal zone (SEZ) region of the lateral ventricles (LV) (Ihrie & Alvarez-Buylla. 2011, Kempermann et al. 2015). Another class of proliferating progenitors, the oligodendrocyte progenitor cell (OPC) are widely distributed in the central nervous system (CNS) (Pringle et al. 1992), with significantly higher cellular density within the white matter (Dawson et al. 2003). Functionally, 1) newly generated neurons in the adult hippocampus contribute importantly to learning and memory (Goncalves et al. 2016, Kropff et al. 2015, Sahay et al. 2011); 2) LV neurogenesis and gliogenesis play critical roles in olfactory-based social learning (Mak et al. 2007, Mak & Weiss. 2010, Sakamoto et al. 2014), as well as post-injury tissue remodeling (Benner et al. 2013, Faiz et al. 2015, Kuo et al. 2006); and 3) OPC activation/differentiation results in new myelination that is necessary for efficient neuronal activity propagation (Arancibia-Carcamo et al. 2017, Ford et al. 2015, Pajevic et al. 2014), and motor learning (McKenzie et al. 2014). These stem/progenitor cells found in the rodent brain are also present in the postnatal and adult human CNS (Bergmann et al. 2015, Sanai et al. 2011, Yeung et al. 2014). Thus, their study can help us better understand how control of progenitor cell proliferation/differentiation contributes to neural plasticity and pathologies.
Significant progress has been made in our understanding of the cellular and molecular processes regulating postnatal NSC and OPC development. In the hippocampal DG, GFAP+ astrocyte-like NSCs (also called type 1 cells) residing within the subgranular zone (SGZ) divide to give rise to transient proliferative progenitors (type 2 cells), which then produce DCX+ neuroblasts that mature into local glutamatergic DG granule neurons (Kempermann et al. 2015, Yu et al. 2014). Postnatal LV neurogenesis is initiated by cerebral spinal fluid (CSF)-contacting GFAP+ glia in the SEZ functioning as NSCs, producing Mash1+ transiently amplifying progenitors (also called C cells), which in turn differentiate into DCX+ neuroblasts (also called A cells) that migrate via the rostral migratory stream to become predominantly inhibitory interneurons in the olfactory bulb (Ihrie & Alvarez-Buylla. 2011, Lledo et al. 2008). OPCs are NG2+ proliferating progenitors that reside in wide-ranging CNS areas, with roughly 50% higher cellular density in the white vs. gray matter (Dawson et al. 2003). Outside of established neurogenic regions, OPCs form the major population of actively dividing cells in the adult brain, with white matter OPCs having faster cell cycle times (Simon et al. 2011). OPC proliferation results in self-renewal as well as new progeny giving rise to myelinating oligodendrocytes (Young et al. 2013).
In adult tissues, retaining a dedicated stem cell population requires extra energy and resources, and when they acquire oncogenic mutations over time these proliferative cells can become tumorigenic (Alcantara Llaguno et al. 2009), leading to harmful sequelae to the host tissue (Tomasetti & Vogelstein. 2015). In other organ systems, stem/progenitor cells play an important role during normal cellular replacement, maintaining tissue homeostasis. Unsurprisingly, much like other stem cells, conserved, cell-intrinsic molecular pathways control important steps in self-renewal and differentiation of postnatal NSCs and OPCs (Christian et al. 2014, Frisen. 2016, Kriegstein & Alvarez-Buylla. 2009, Lopez Juarez et al. 2016). Extracellular factors and cell-cell interactions within the local microenvironmental niche also play critical roles (Bjornsson et al. 2015, Miller & Gauthier-Fisher. 2009, Paez-Gonzalez et al. 2011, Zuchero & Barres. 2013). However, CNS cells generally have much lower turnover rates than those in tissues such as the skin or gut lining. The need for precise neurogenesis and gliogenesis during development is rather clear: the clockwork of neural progenitor proliferation and differentiation generates a full range of neurons/glia in the correct temporal and spatial patterns, enabling proper assembly of functional neural circuits (Gallo & Deneen. 2014, Kriegstein & Alvarez-Buylla. 2009, Mitew et al. 2014, Yu et al. 2009). What are the organizing principles and factors sustaining postnatal NSC/OPC proliferation and regeneration through mammalian evolution (Figure 1)? We are just beginning to decipher the biological underpinning of these processes, and neuronal activity has emerged as a common driver to stimulate postnatal NSC as well as OPC proliferation.
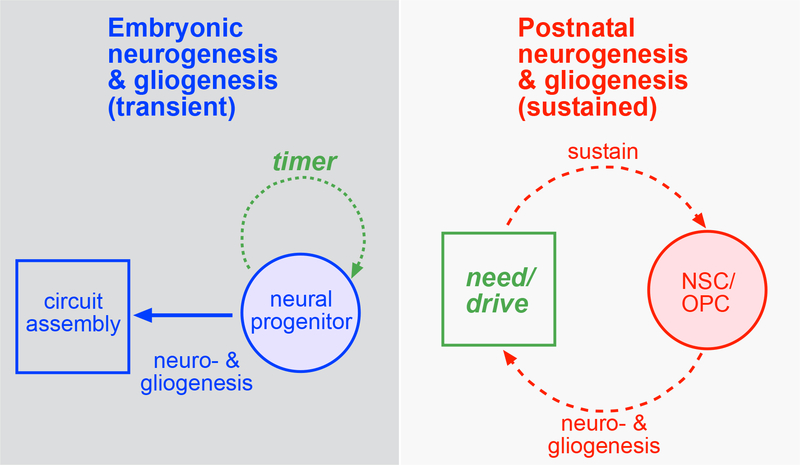
Figure 1.
Comparing rules governing embryonic vs. postnatal neurogenesis and gliogenesis.
Schematics representing progenitor proliferation concepts during embryonic development and in the postnatal CNS. Embryonic development and progenitor proliferation take place over defined/short time scale (left panel), where choreographed generation of neurons and glia in time-dependent fashion (timer, green dashed arrow) results in proper neural circuit assembly. In the postnatal/adult CNS, NSC and OPC sustain their proliferation capacity for much longer time scale (right panel), requiring a different conceptual framework for neurogenesis and gliogenesis. A cyclical stimulus (need/drive, green box) -based feedback promotes continued NSC/OPC proliferation and differentiation.
Low-level, sustained neuronal activity patterns that result in consistently low concentrations of neurotransmitter released are termed tonic firing patterns. On the other hand, more robust, synaptic, and temporally salient neuronal activities are referred to as phasic activity, occurring on much finer time-scales. While connected neurons in circuits have innate capacities to interpret and respond to distinct activity patterns from their synaptic partners, a key question has been whether NSCs and OPCs have similar abilities. Activity-dependent vesicular release from synaptic sites facilitate fast, high concentration neurotransmitter access to receptors, while bulk/volume neurotransmitter release that occurs at non-synaptic sites or from synaptic spillover nearby provide low concentrations to receptors via diffusion over larger areas. For neurotransmitters acetylcholine (ACh), γ-amino butyric acid (GABA), and glutamate, a common feature is their signaling through two receptor types on the target cells: 1) ionotropic receptors with fast kinetics (nicotinic, GABAA, AMPA/KA and NMDA receptors) (Fritschy & Panzanelli. 2014, Greger et al. 2017, Iacobucci & Popescu. 2017, Yakel. 2014), and 2) metabotropic GPCRs with slower kinetics (muscarinic receptor, GABAB, mGluRs) (Kruse et al. 2014, Niswender & Conn. 2010, Pin & Bettler. 2016). An important property of ionotropic receptors is their rapid desensitization following neurotransmitter-induced activation (Plested. 2016). This well-described channel property results in short bursts of neurotransmitter release having a qualitatively different effect on the target cell than having the neurotransmitter constantly present or absent. These principles of cellular signaling through two different receptors, plus temporal dynamics on receptor activation/desensitization, underlie ACh, GABA, and glutamate’s impacts on neural circuit activity. This context-dependent complexity in signaling capacity can provide a palette richness for NSCs and OPCs to functionally read-out subtle changes in local neurotransmitter availability as a function of neuronal activity patterns.
With recent advances in cutting-edge techniques applied to long standing questions about functional connections between neuronal activity and postnatal NSC/OPC proliferation, our goal in this review is to provide an up-to-date view in this field of research, and summarize general themes regulating NSC and OPC proliferation in the adult CNS, which may have been under appreciated previously. This review will focus particularly on the neurotransmitters acetylcholine, GABA, and glutamate; as vehicles used by progenitors to readout neuronal activity patterns, as well as local circuit properties allowing for higher-level brain inputs that connect to behavioral paradigms/disease states. Due to space constraints, we apologize in advance for areas which we were unfortunately unable to cover.
Comparing neuronal activity-dependent control of NSC vs. OPC proliferation
There are some important parallels and potential differences underlying neuronal activity-dependent control of postnatal/adult NSC and OPC proliferation. On the neurogenic side, there is a substantial body of literature supporting important roles for neurotransmitter signaling, mostly via experiments using systemic/pharmacological approaches (Berg et al. 2013, Young et al. 2011). Postnatal/adult NSCs often proliferate in close proximity to neurons firing action potentials, but until recent years, experimental approaches were unable to pinpoint neurotransmitter signaling in postnatal NSCs as: 1) a byproduct of communications between nearby neurons (i.e. synaptic spillover effect); 2) feedback control of progenitor progeny by non-vesicular release; and/or 3) direct communication via neuronal activity from discrete circuits. The findings that GABA released from newborn DCX+ neuroblasts can feedback-modulate NSC proliferation (Liu et al. 2005), as well as GABA spill-over from local parvalbumin+ (PV+) interneuron regulating DG NSC proliferation/differentiation have shown bulk release/non-synaptic mechanisms for neurotransmitter function in the postnatal/adult neurogenic niches (Song et al. 2012). In this fashion, they are growth factor or cytokine-like, controlling NSC properties in broad strokes that do not necessarily correspond to the precision of neuronal activity patterns. On the contrary, serotonergic neurons from the Raphe nuclei send their projections extensively in the brain, including the ventricular surfaces that contain the LV neurogenic niche (Mathew. 1999). Serotonin released from these axonal fibers on the ependymal surface may directly modulate NSC proliferation (Tong et al. 2014). And recently, proopiomelanocortin+ (POMC+) neurons from the hypothalamus were identified to project selectively to a regionally-specific population of postnatal LV NSCs (Paul et al. 2017). Pharmacogenetic manipulations of these POMC+ neurons showed that their activity can regulate the proliferation of NSC subtypes (Paul et al. 2017).
For postnatal oligodendrogenesis, seminal studies by Barres and Raff in 1993 showed that when postnatal retinal ganglion cell axons were either transected, or had their activity blocked by intravitreal injection of TTX, it lead to decreased OPC numbers in the optic nerve (Barres & Raff. 1993). This decrease in OPC numbers was rescued with application of PDGF, a potent mitogen for OPCs (Barres & Raff. 1993). Studies looking into potential cellular mechanisms regulating this process found that ATP, released from active axons, can increase intracellular Ca2+ in OPCs via P2X and P2Y receptors, mimicking the effects of neuronal firing (Matute. 2008, Stevens et al. 2002, Wigley et al. 2007). Following axonal activation, ATP is quickly degraded to ADP (which can bind P2Y receptors but not P2X receptors), AMP, and adenosine (specific for P1 type receptors) by membrane surface bound ectonucleotidases. Adenosine is then up taken by nucleoside transporters into neurons and glial cells (Noji et al. 2004), terminating the purinergic signaling process. Rather than acting as a mitogen, ATP release from active axons reduced PDGF-stimulated OPC proliferation. Similarly, adenosine was found to be a potent negative regulator of OPC proliferation. In the absence of PDGF however, P2Y receptor activation paradoxically led to increased OPC migration and a mild increase in proliferation (Agresti et al. 2005, Stevens et al. 2002). Subsequent to the 1993 landmark paper, in vivo pharmacological, genetic, and lesion studies provided confounding results looking into the roles of neuronal activity on OPC proliferation. When neuronal activity was blocked in adult demyelinating lesions with focal TTX delivery in vivo (Gautier et al. 2015), or when whiskers were removed at birth leading to reduced barrel cortical inputs (Hill et al. 2014, Mangin et al. 2012) , these approaches resulted in increased numbers of OPCs. Likewise, when neuronal activity of DRGs was increased in vitro, OPC proliferation was significantly reduced (Stevens et al. 2002). But consistent with the notion that neuronal activity is a positive signal, electrical stimulation of adult cortical motor neurons increased OPC proliferation along the corticospinal tract and subcortical white matter (Gibson et al. 2014, Li et al. 2010). OPCs receive synaptic inputs from axons in both gray (Bergles et al. 2000, Jabs et al. 2005, Lin & Bergles. 2004) and white matter (Káradóttir et al. 2005, Káradóttir et al. 2008, Kukley et al. 2007, Ziskin et al. 2007). These synaptic contacts appeared to occur predominantly on entirely unmyelinated axons or unmyelinated segments of the axon (Kukley et al. 2007, Tomassy et al. 2014, Ziskin et al. 2007). OPCs and their synaptic neurons showed synchronized spontaneous activity, indicating that OPCs have the capacity to sense the pattern of neural activity in circuits (Lin et al. 2005, Mangin et al. 2008, Muller et al. 2009). This notion is further supported by functional studies where environmental enrichment or motor training induced OPC cell cycle exit and differentiation (Simon et al. 2011), followed by increased numbers of BrdU+ or EdU+ OPCs (McKenzie et al. 2014, Okuda et al. 2009). The current view is that the increased OPC proliferation that occured during motor-learning is a response aiming to replensh the progenitor numbers to homeostatic levels after activity-induced differentiation (Hughes et al. 2013, Xiao et al. 2016).
Behavioral paradigms such as exposure to enriched environments can robustly promote adult hippocampal neurogenesis (Kempermann et al. 1997), strongly indicating further complexity in higher order neural circuitry control of NSC proliferation and differentiation. This functional influence parallels learning-induced OPC differentiation and oligodendrogenesis (Mount & Monje. 2017). It is interesting to note that while neurotransmitter modulation on postnatal/adult NSC proliferation has been acknowledged over the past decade, identifying neuronal activity as a direct control of this process is a relatively recent advance. In contrast, while there is strong evidence and appreciation that neuronal activity can regulate OPC proliferation, the widely accepted view was through an indirect effect via growth factors to active OPCs. Despite the fact that OPCs express plethora of neurotransmitter receptors (detailed in the following sections), the roles played by those neurotransmitters released following neuronal action potential in regulating OPC proliferation is just beginning to be understood.
Cholinergic regulation
The neurotransmitter ACh activates both nicotinic and muscarinic acetylcholine receptors (nAChR and mAChR, respectively) on target cells. nAChRs are pentameric, ionotropic channels consisting of several subunits: alpha, beta, gamma, delta, and epsilon. In the peripheral and central nervous system, nAChRs mediate fast cholinergic transmission, and the subunit compositions of the various nAChRs determine their ionic permeability (e.g. Na+, K+, Ca2+), ACh affinity, channel kinetics, and channel desensitization (Dani. 2001). mAChRs are metabotropic transmembrane proteins, coupled to G proteins, and activate downstream intracellular signaling pathways to provide sustained ACh-mediated responses (Caulfield. 1993, Dutar & Nicoll. 1988). Activity patterns of specific cholinergic neuronal populations can range from spontaneous, low frequency firing to those that fire irregularly or respond strongly to specific salient stimulation on much finer time-scales (Manns et al. 2000, Parikh & Sarter. 2008). The types of cholinergic neuron activity patterns will influence local ACh concentration and temporal profiles of downstream ACh signaling in the target cells, as well as the speed of ACh breakdown by acetylcholinesterases, which are particularly efficient in synaptic clefts (Unal et al. 2012) (Figure 2). One notable feature about nAChRs is their rapid desensitization following ACh-induced activation (Giniatullin et al. 2005, Quick & Lester. 2002) (Figure 2b). This well-described channel property results in short bursts of ACh release having a qualitatively different effect on the target cell than ACh constantly being present or absent (Figure 2c,d). These principles of ACh neurotransmitter signaling through two receptors, plus temporal dynamics on nAChR activation/desensitization, underlie the modulatory effects of ACh’s impact on neural circuit activity.
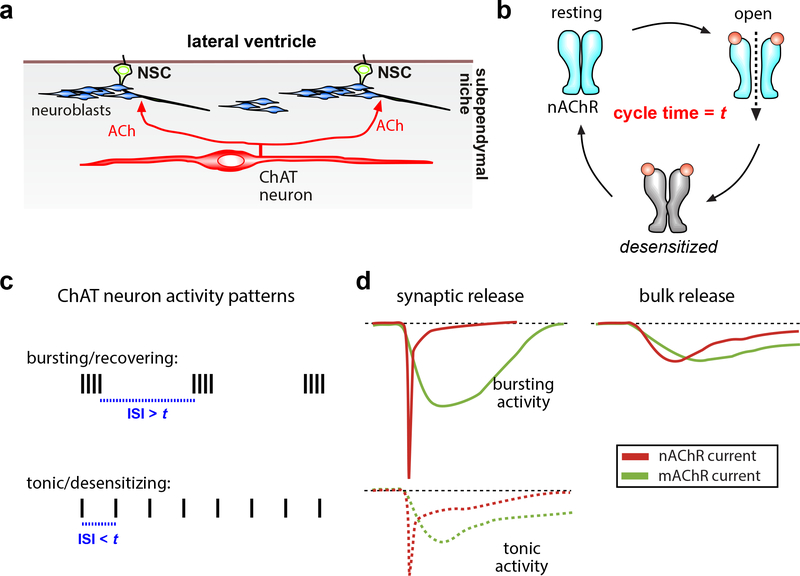
Figure 2.
Neurogenic niche ChAT neuron and ACh receptor dynamics conveying activity patterns.
(a) Schematic representation of ChAT neuron residing in the postnatal LV neurogenic niche, a source of ACh acting on NSCs to produce migrating neuroblasts. (b) nAChR in resting, open, and desensitized states (t = receptor subtype specific cycle time). (c) Patterns of ACh release will desensitize nAChR when neuronal inter-stimulus intervals (ISI) < t, but promote nAChR recovery for reactivation when ISI > t, giving rise to distinct nAChR activation dynamics in the target cells. (d) With a high density of local nAChR and mAChR receptors, synaptic ACh release results in rapid and fast-inactivating nicotinic currents, and slower, longer-lasting muscarinic currents. Upon release ACh is quickly degraded by extracellular acetylcholinesterase. Tonic release of ACh will result in nAChR desensitization. Bulk/volume ACh stimulates larger fields of receptors at low density, and may generate small and prolonged cholinergic currents.
In the nervous system, mAChRs and nAChRs are present on neurons at both synaptic and extra-synaptic sites (Huh & Fuhrer. 2002, Mrzljak et al. 1993, Vizi & Lendvai. 1999), as well as on glial cells (De Angelis et al. 2012, Loreti et al. 2006). While characterization of ACh receptor expression in neurogenic niches has not been extensive, LV NSCs have been reported to express α3- and α4- subunit containing nAChRs (Paez-Gonzalez et al. 2014), similar to those residing in the rostral migratory stream showing α3β4 nAChR activity (Sharma. 2013). In contrast to NSCs and DCX+ neuroblasts, LV niche Mash1+ transiently amplifying progenitors did not appear to express functional ACh receptors (Paez-Gonzalez et al. 2014). In the DG niche, IHC and functional analyses have revealed the presence of M1 and M4 subunit mAChRs (Kaneko et al. 2006), as well as α7 nAChR subunit expression in immature hippocampal neurons (John et al. 2015). BrdU-pulsed proliferating DG cells also express M1 and M4 subunit mAChRs (Mohapel et al. 2005). The availability of effective AChR agonists and antagonists have allowed pharmacological investigations into the roles for cholinergic signaling during neurogenic proliferation. mAChR agonists such as bethanechol, pilocarpine, and oxotremorine enhanced NSC proliferation when added to in vitro cultures (Calza et al. 2003), hippocampal slices (Itou et al. 2011), or in vivo (Ma et al. 2004, Veena et al. 2011), while muscarinic antagonists had the opposite effect (Kotani et al. 2006). M1/M4 mAChR agonists have been shown to control DG neurogenic proliferation and differentiation (Van Kampen & Eckman. 2010), and mAChR activation was similarly effective in enhancing cortical progenitor proliferation (Ma et al. 2000). In the DG niche, pharmacological activation of the α7-subunit containing nAChRs can increase cellular proliferation (Narla et al. 2013), and α4 nAChR agonists have also been shown to control DG neurogenic proliferation and differentiation (Takarada et al. 2012). β2-subunit nAChR mutant mice have reduced DG proliferation over the life of the animal (Harrist et al. 2004). In the LV niche, nicotinic stimulation appears to increase neurogenesis, as direct nicotine application in vivo increased Nestin+ cellular proliferation, resulting in subsequent BrdU-labeled NeuN+ granule neurons in the olfactory bulb (Mudo et al. 2007). Interestingly, high doses of nicotine delivered chronically in vivo have an opposite effect in decreasing DG neurogenesis (Abrous et al. 2002), potentially as a result of receptor desensitization. Together these pharmacological results showed that postnatal NSCs are sensitive to the timing and local concentrations of ACh released, and that cholinergic receptor subtypes may mediate differential effects on cellular proliferation.
To understand the specific cholinergic circuits involved in controlling NSC proliferation, experimental attempts targeting cholinergic neuron subpopulations have been utilized to determine the sources of ACh acting on NSCs. Genetically, expression of Cre recombinase from the choline acetyltransferase (ChAT, required for acetylcholine synthesis) gene regulatory element is an efficient method to target cholinergic neurons, although this approach does not distinguish neuronal subtypes. In fact, there are a few approaches that can distinguish distinct subpopulations of cholinergic neurons. While less elegant, one such approach is anatomic lesions, which do allow for some regional specificity to assess cholinergic influences on NSC proliferation/differentiation. Transection of the fimbria-fornix, which disrupts basal forebrain cholinergic projections to the hippocampus (Rosenberg et al. 1988), resulted in a concurrent decrease in DG BrdU incorporation (Fontana et al. 2006), consistent with decreased SGZ neurogenesis . N-methyl-d-aspartate (NMDA) injection to the medial septal cholinergic nuclei, creating a local excitotoxic lesion, also reduced SGZ neurogenesis (Van der Borght et al. 2005). Cholinergic neurons in the basal forebrain, medial septum, nucleus basalis of Meynert, and diagonal band of Broca specifically express the p75 neurotrophin receptor (p75NTR). This allowed for their specific cellular elimination by stereotaxic injection of 192-IgG-SAP (192-Saporin), a chemical conjugate of p75NTR mouse clonal antibody to the ribosome-inactivating protein saporin. 192-Saporin-mediated removal of medial septal cholinergic neurons resulted in decreased SGZ neurogenesis (Itou et al. 2011), as well as decreased cellular proliferation within the LV niche (Cooper-Kuhn et al. 2004).
To determine whether activity patterns from cholinergic neurons can differentially modulate NSC proliferation, another approach is to alter intrinsic excitability of these neurons. Ank3 is a large adapter protein localized to neuronal axon initial segment, important for clustering voltage-gated ion channels required to generate precise action potentials (Jenkins & Bennett. 2001). Conditional Ank3 deletion in ChAT+ neurons resulted in their inability to precisely initiate and scale action potentials to electrical stimulation, which resulted in marked reduction in DCX+ neuroblasts and NSC proliferation in the LV niche (Paez-Gonzalez et al. 2014). These results revealed that cholinergic circuit activity and precision are required to sustain the robustness of postnatal neurogenesis. Examining cholinergic sources along the LV wall, a ChAT+ neuronal population with distinct morphology and activity to those from the striatum were identified in the subependymal space (subep-ChAT neurons) (Paez-Gonzalez et al. 2014) (Figure 2a). In vivo optogenetic stimulation of subep-ChAT neurons significantly increased the numbers of Ki67+ proliferating cells and neurogenic progenitors in the LV niche, as well as frequency-dependent inward nAChR and mAChR currents in NSCs (Paez-Gonzalez et al. 2014). These findings revealed subep-ChAT neurons as integral components of the cholinergic circuit controlling postnatal LV neurogenesis.
Unlike neighboring striatal cholinergic neurons which are spontaneously active, the subep-ChAT neurons (normally active in vivo) did not exhibit basal-level spontaneous activity in acute brain slice preparation (Paez-Gonzalez et al. 2014). The sources for excitatory/inhibitory inputs onto subep-ChAT neurons are currently unclear, although CNS cholinergic neurons such as those found in the striatum, basal forebrain nuclei, hypothalamus, medial habenula, pontomesencephalic tegmentum, and medullary tegmentum, tend to have highly stereotyped patterns of afferent connectivity, serving as potential blueprints for subep-ChAT neuron connectivity. Conceptually, since CNS cholinergic neurons groups are broadly interconnected, it is an intriguing possibility that subep-ChAT neurons participate in and sample the cholinergic plexus to transform cascades of activity within the cholinergic system into functional neurogenesis. As the cholinergic circuit is complex, it remains likely that there are other cholinergic neurons whose activity contributes to postnatal LV neurogenesis control.
Characterization of AChR expression and function in OPCs has not been extensive, but transcriptional and IHC analysis indicate that OPCs express transcript for nAChR subunits: α3, α4, α5, α7, β2 and to a lesser extent β4 (Rogers et al. 2001, Zhang et al. 2014) and mAChRs subunits: M1, M2, M3 and M4 (Abiraman et al. 2015, Cohen & Almazan. 1994, Ragheb et al. 2001, Zhang et al. 2014), with most reports indicating that M3 is the most prominent subunit of mAChRs (Abiraman et al. 2015, De Angelis et al. 2012, Ragheb et al. 2001). Functional analysis of nAChRs, using whole-cell patch clamping and calcium imaging, have reported that hippocampal OPCs express α7-subunit containing nAChRs, but not α4β2 nAChR (Vélez-Fort et al. 2009), whereas white matter OPCs express α4β2 nAChR and not α7-subunit containing nAChRs (Rogers et al. 2001). Functional analyses using calcium imaging and real-time biochemical measurements of intracellular signaling, have revealed the presence of functional muscarinic receptors in OPCs (Cohen & Almazan. 1994, Kastritsis & McCarthy. 1993). Pharmacological investigations into the roles of ACh signaling have shown that cholinergic stimulation via nAChRs increases OPC differentiation with no effect on proliferation (Imamura et al. 2015). Whereas muscarinic stimulation, when applied in vitro, provides a potent proliferative signal to OPCs, by upregulating PDGFRα (Cohen & Almazan. 1994, De Angelis et al. 2012), but not in vivo (Abiraman et al. 2015). In recent years, a number of high-throughput screenings for novel myelin regenerative therapies have revealed muscarinic signaling as a strong negative regulator of OPC differentiation, particularly via M1 and M3 receptor activation, highlighting the importance of cholinergic signaling. A number of anti-muscarinic compounds, such as benztropine, clemastine and solifenacin, have been identified as potential therapeutic agents for myelin regenerative therapies for multiple sclerosis, and clinical trials for some of these compounds are ongoing (Abiraman et al. 2015, Deshmukh et al. 2013, Mei et al. 2014, Mei et al. 2016).
GABAergic regulation
GABA is a key excitatory neurotransmitter in the developing brain, as well as the main inhibitory neurotransmitter in the adult CNS, whose functional activity depends on intracellular chloride concentrations of the target cells, set by transporters that either accumulate intracellular chloride (NKCC1) or extrude chloride (KCC2) (Delpire. 2000) (Figure 3a). GABA is synthesized mainly from glutamate by glutamate decarboxylases (GAD65 and GAD67) and is degraded by GABA-transaminase. Extracellular GABA levels are tightly regulated by high-affinity sodium- and chloride-dependent GABA transporters (Borden. 1996), which efficiently remove GABA from synaptic clefts. GABA binds to its ionotropic receptors GABAA and GABAC, which are ligand-gated chloride channels, and its metabotropic receptor GABAB, to trigger downstream signaling events (Figure 3b–d). GABAA receptors are hetero-pentamers primarily composed of α1–6, β1–3, and γ1–3 subunits (other subunits include δ, ε, π, ρ, τ) (Fritschy & Panzanelli. 2014), and are the more frequently investigated GABA receptor in the context of postnatal NSC proliferation/differentiation. GABAB receptors are G-protein couple receptors linked to potassium channels, and thus far few studies have examined their potential roles in the context of postnatal NSC and OPC proliferation.
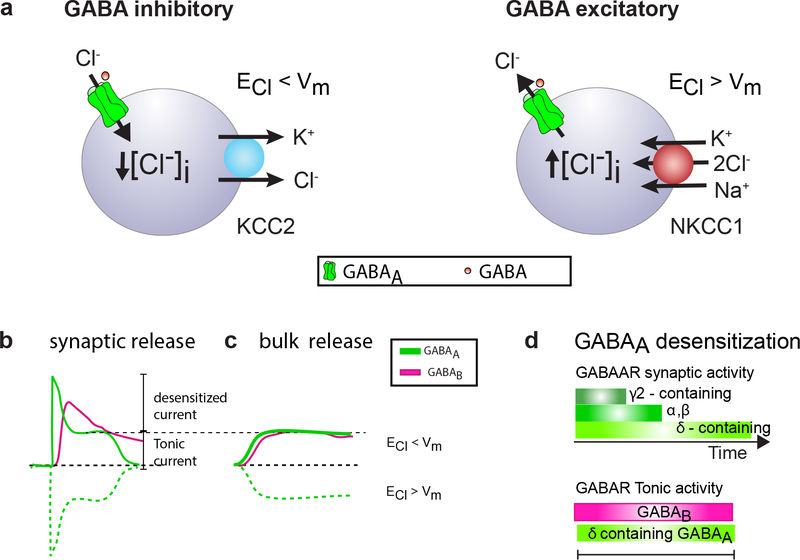
Figure 3.
GABAergic signalling in progenitor cells.
(a) The Na+- K+- 2Cl– cotransporter 1 (NKCC1) and K+- Cl– cotransporter 2 (KCC2) determine the intracellular Cl– concentration ([Cl−]i). It is the intracellular Cl− concentration that dictates whether GABA is inhibitory (low [Cl−]i – leading to Cl− influx, when GABAA receptors are activated), like it is in mature neurons, or excitatory (high [Cl−]i - leading to Cl− outflux, when GABAA receptors are activated) as for adult NPCs and OPCs. (b) Depending on the equilibrium potential of chloride (ECl−), whether it is above or below membrane potential, activation of GABAA receptors will either be a net outward current (hyperpolarizing; green line) or a net inward current (depolarizing; green dotted line). GABAAR currents, are fast and have multiple desensitization kinetics (depending on subunits), but most show incomplete desensitization (tonic current); activation of GABAB receptors (purple line) is slower compared to GABAAR and long lasting. (c) Volume release of GABA stimulates GABAA receptors that have incomplete desensitization and GABAB receptors, often for prolonged periods. (d) The encoding of GABA signaling is determined by the subunits expressed. In general GABA receptors containing γ subunits desensitize faster than α&β containing receptors, whereas δ containing receptors have the longest desensitization time constant. The GABAA receptors, that incompletely desensitize, such as δ containing GABAA receptors, along with GABAB receptors mediate tonic GABA stimulation.
GABA receptors are widely expressed in the CNS. Within the LV neurogenic niche, functional GABAA receptors have been identified in NSCs/SEZ astrocytes (Liu et al. 2005, Wang et al. 2003a), as well as migrating DCX+ neuroblasts (Nguyen et al. 2003). Upon release in the LV niche, GABA activates GABAA receptors present on GFAP+ cells and newborn neuroblasts, limiting their proliferation (Liu et al. 2005). In addition, GABA signaling can influence other phases of postnatal LV neurogenesis, including neuroblast migration and terminal differentiation in the olfactory bulb (Bolteus & Bordey. 2004, Gascon et al. 2006). Within the hippocampus, birth and development of newborn hippocampal granule neurons can be categorized into distinct steps: a) NSC quiescence vs. activation and fate determination; b) intermediate progenitor proliferation; c) migration and removal of excessive newborn neuroblasts; d) initiation of newborn neuron integration; and e) functional maturation and maintenance of adult-born granule neurons. GABA signaling through the GABAA receptor depolarizes NSCs and immature neurons in the DG, and promotes new neuron maturation (Tozuka et al. 2005), integration into local circuitry (Ge et al. 2006), and survival (Jagasia et al. 2009).
Within the LV niche, newborn DCX+ neuroblasts have been shown to synthesize and release GABA in a calcium-dependent, non-vesicular manner (De Marchis et al. 2004, Liu et al. 2005, Nguyen et al. 2003). The numerous neuroblasts releasing GABA has complicated experimental efforts looking into neuronal/vesicular sources of GABA in the LV niche. Striatal GABAergic neurons are in close proximity to the LV niche. Quinolinic acid, an excitatory amino acid that induces cell death, was used in chemical lesion studies to interrogate striatal GABAergic interneuron function. Following quinolinic acid injection into the striatum there was an increased cellular proliferation in the LV niche, and potential rerouting of newborn cells to the striatal-injured areas (Tattersfield et al. 2004), suggesting that striatal GABAergic neurons may contribute to LV neurogenesis control. In support, vesicular GABA transporter expression has been detected in the LV niche, and nitric oxide-containing GABAergic striatal neurons extend processes to the LV niche (Young et al. 2014). Future studies targeting specific circuit components, both locally and distally to the LV niche, will be needed to address whether neuronal activity through distinct populations of GABAergic neurons can control postnatal NSC proliferation and/or differentiation.
The hippocampal neural circuitry is complex and tightly integrated, thus anatomical/chemical lesions to target its GABAergic components specifically for studying neurogenic control have been technically challenging to perform. Within the DG niche, local GABAergic interneurons and their axons are situated in close proximity to NSCs and their progeny. The neurogliaform POMC+ GABAergic interneurons been shown to provide functionally important synaptic inputs onto newborn DG granule neurons (Markwardt et al. 2011). PV+ interneurons are another component from the local inhibitory circuit whose activity-dependent released of GABA can activate GABAA receptors on NSCs. Optogenetic control of DG PV+ neuron activity in vivo has been shown to regulate quiescence vs. activation of resident adult NSCs (Song et al. 2012). This process appeared to be indirect, through GABA spillover from nearby PV+ neuron synaptic activity (Song et al. 2012) (Figure 3c). In contrast, PV+ interneurons can provide direct synaptic contacts with newborn DG neuroblasts, which regulate their subsequent development into granule neurons (Song et al. 2013).
On a functional level, the hippocampal GABAergic interneurons provide tight local inhibitory control of the principal cells, regulating DG field potential oscillations (Engel et al. 2009). These hippocampal interneurons show diversity in physical location, morphology, synaptic targets, and protein marker expression (Freund & Buzsaki. 1996, Maccaferri & Lacaille. 2003). In a heightened state of activity, for example, PV+ interneurons’ increased firing can inhibit quiescent NSC proliferation and concurrently enhance the survival of NSC progeny (Song et al. 2012), modulating new neuron addition and future circuitry wiring within the DG. Identifying relevant functional inputs onto hippocampal GABAergic interneurons will be important next steps in deciphering neuronal activity-dependent control of adult hippocampal neurogenesis.
OPCs express the NKCC1 transporter (Figure 3a), therefore activation of ionotropic GABAA receptors expressed by OPCs will lead to membrane depolarization and intracellular calcium rise (Gilbert et al. 1984, Hoppe & Kettenmann. 1989, Kirchhoff & Kettenmann. 1992, Wang et al. 2003b). RNA transcripts for GABAA subunits α1–5, β1 – 3, and γ1−3 have been detected from isolated OPCs (Zhang et al. 2014). Similarly, transcriptional, IHC analysis and biochemical measurements of intracellular signaling have revealed the presence of functional GABAB receptors in OPCs, mainly expressing GABAB1 subunits (Luyt et al. 2007, Zhang et al. 2014). In the hippocampus, somatosensory cortex, and the cerebellar white matter OPCs have been shown to receive GABAergic synaptic inputs (Káradóttir et al. 2008, Lin & Bergles. 2004, Vélez-Fort et al. 2010). In the somatosensory cortex OPCs receive inputs from multiple interneurons, but on average only one input from each interneuron. After postnatal development, these GABAergic inputs change from being synaptic to being extra-synaptic (Balia et al. 2013, Vélez-Fort et al. 2010). Within the cerebellar white matter migratory neuroblasts release GABA, via vesicular release, and generate transient synapses onto white matter OPCs and via this mechanism negatively regulate OPC proliferation (Zonouzi et al. 2015). Although when post-synaptic GABAA are reduced in OPC, by conditionally knocking out the post-synaptic specific γ2 subunit in OPCs, no apparent effect on OPC proliferation or differentiation was detected in the somatosensory cortex (Balia et al. 2017), presumably as GABA acting on extra-synaptic receptors can still regulate proliferation. Overall, it seems that GABA acting on GABAA receptors is a negative regulator of OPC proliferation, whereas when acting on GABAB receptors it augments OPC migration and proliferation, in vitro. However, when GABA concentrations are raised, either in vivo or in organotypic brain slices, OPC proliferation is dramatically reduced, indicating that the proliferative brake delivered by activation of GABAA receptors prevails over the proliferative potentiation mediated by GABAB receptors.
Glutamatergic regulation
Near 90% of all projection neurons in the brain are glutamatergic (Schmidt & Pierce. 2010), and glutamate can be released from these neurons along their axons in both gray and white matter. Glutamate activates both ionotropic and metabotropic glutamate receptors on receiving cells. Ionotropic glutamate receptors are divided into AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), NMDA (N-methyl-D-aspartate) and kainate (KA) receptors, all of which are selective cation channels, made of four subunits, with approximately equal permeability to Na+ and K+, but a differing permeability to Ca2+ ions. AMPA and kainate receptors containing subunits that have undergone RNA Q to R editing, or AMPA receptors that contain the GluA2 subunit, are calcium impermeable. Whereas, all NMDA receptor subunits are permeable to Ca2+ ions, although the permeability differs between subunits (Dingledine et al. 1999). Glutamate signaling is terminated by a family of high-affinity glutamate-sodium co-transporters, these transporters have been shown to be capable of rapidly removing glutamate released at synapses (Attwell & Gibb. 2005). The encoding of glutamate signaling is determined by specific subunits that make up the channel. In general NMDA receptors have a much lower EC50 for glutamate than AMPA receptors, around 1μM for prolonged exposure to glutamate, versus tens of μM for AMPA receptors (Patneau & Mayer. 1990) and more than 100 fold longer deactivation time and channel desensitization compared to AMPA and KA receptors, and a much lower dissociation constant (Attwell & Gibb. 2005). NMDAR, in contrast to AMPAR and KAR, need glycine as a co-agonist alongside glutamate to become activated and, at the resting membrane potential the pore of the NMDA receptor is almost completely blocked by Mg2+, and only upon membrane depolarization is the Mg2+ block removed enabling the channel to conduct ions (Dingledine et al. 1999). Metabotropic glutamate receptors, like other G protein coupled receptors, activate various downstream enzyme systems including adenylate cyclase and phospholipase C (Conn & Pin. 1997). These receptors have lower affinity for glutamate than NMDAR but higher than AMPA receptors, but also have long activation desensitization kinetics, needing prolonged glutamate concentration for activation, as could occur with high neuronal firing for an extended period or phasic release of glutamate. Thus, each glutamate receptor encodes glutamatergic neuronal firing at different timescales (Figure 4). The unique features of the NMDA receptor distinguish them from other ionotropic neurotransmitters. At low frequency synaptic transmission, AMPA receptors will be activated, due to their fast on and off kinetics, whereas NMDA receptors due to their long activation time and voltage-dependence (depolarization needed to repel the Mg2+ from the pore) the receptor has not been fully activated by the time glutamate transporters have removed glutamate from the synapse (~1–3ms) (Figure 4a). However, if a second input occurs (release of glutamate) within the timeframe of glutamate unbinding from the NMDAR that is sufficiently large to depolarize the membrane (via activation on AMPA and KAR) to repel the Mg2+ ion, NMDAR then becomes fully activated. This feature of slow unbinding of glutamate (~100 ms), and slow activation rate, high glutamate affinity, and the magnesium block and membrane potential, allow the NMDAR to act as an input coincidence detector (Figure 4a). The dual input requirement, along with its unique kinetics allows NMDARs to integrate incoming activity. Thus, signals at fast rates are encoded by AMPA, followed by NMDA receptors that can keep information regarding glutamate release for up to 100ms, and mGluRs can mediate information in the presence of glutamate for up to 20s (Attwell & Gibb. 2005, Blanke & VanDongen. 2009) (Figure 4b,c). The different timescale these receptors operate on, and different features, will determine the outcome of how glutamatergic signaling regulates progenitor cell proliferation. While very little is understood about glutamatergic signaling during postnatal NSC proliferation, NMDA receptor activity has been shown to be a critical regulator of newborn neuron survival and integration in both the adult DG and OB (Lin et al. 2010, Platel et al. 2010, Tashiro et al. 2006).
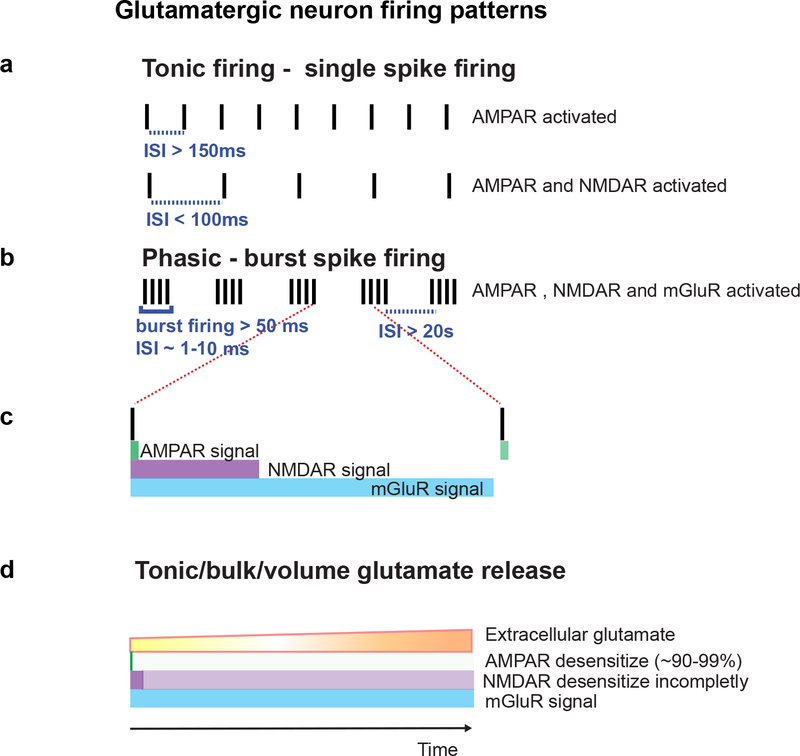
Figure 4.
Differential glutamate receptor activation dynamics encode neuronal activity patterns
Schematic diagram depicting the differential kinetics of glutamate receptors that dictates their decoding of neuronal firing patterns. (a) AMPAR mediate low firing rates activity pattern where ISI is longer than the dissociation of glutamate from NMDAR. At firing rates where ISI is shorter than the dissociation time of glutamate from NMDAR, the NMDARs will become active along with AMPARs and KARs. (b,c) At high neuronal activity, or during burst firing, a large amount of glutamate is released and for sufficiently long time to activate metabotropic receptors, which can stay active ~20s after the activity has ceased. (d) During bulk or tonic/volume release of glutamate, which occurs over a longer time scale than synaptic transmission, AMPA receptor quickly, ~ 3ms, and near completely desensitize (in general >99%, but depending on subunit expression some AMPAR desensitize less or <90%). Whereas NMDA receptors incompletely desensitize (after ~100ms) and thus remain tonically active, similarly mGluRs become active, few milliseconds after AMPAR and NMDAR activation and remain active.
OPC glutamate receptor expression has been relatively well characterized when compared to other neurotransmitter receptor expression analyzed in OPCs. Transcriptional, protein, IHC and functional analysis have identified that OPCs express ionotropic glutamate receptors (AMPA, KAR, and NMDAR) and mGluRs (please see (Spitzer et al. 2016) for more detailed information). Moreover, OPCs receive glutamatergic inputs from unmyelinated axons in both white and gray matter, thus are capable of sensing and decoding glutamatergic activity (Kukley et al. 2007, Tomassy et al. 2014, Ziskin et al. 2007). The availability of specific antagonists and agonist for different glutamate receptor subtypes have allowed pharmacological investigations into the roles for glutamate on OPCs proliferation. When specific agonists for AMPA/KA receptors were added to the culture medium of isolated OPCs or cultured brain slices (mimicking tonic stimulation) OPC proliferation was reduced, and increased when antagonists for AMPA/KA receptors were added to the culture medium (Fannon et al. 2015, Gallo et al. 1996, Yuan et al. 1998), indicating that some endogenous glutamate release in the culture was regulating basal OPC proliferation. Conversely, increasing glutamatergic activity in the adult mouse brain, either through optogenetic stimulation or electrical stimulation of glutamatergic cortical neurons (Gibson et al. 2014, Li et al. 2010), or through the acquisition of new motor skills (McKenzie et al. 2014) which arguably increases neuronal glutamate release, OPC proliferation was enhanced, not decreased (but see above section on neuronal activity where activity increasing OPCs proliferation could be due to a secondary homeostatic response). Similar to the in vitro studies, when glutamate activity was blocked in the regenerating white matter in vivo, or reduced by sensory deprivation leading to reduction in glutamatergic inputs to the mouse barrel cortex, OPC proliferation increased (Gautier et al. 2015, Hill et al. 2014, Mangin et al. 2012). The differences between these studies might be due to different secondary effects, such as homeostatic control of the OPC pool, stimulation of neighboring cells, or release of growth factors from stimulated axons, such as PDGF (Barres & Raff. 1993) or neuroligin-3 (Venkatesh et al. 2015). Genetic approaches can circumvent these secondary effects, and these studies have found that when deleting the AMPAR subunits GluR2, 3 & 4 (GluR1 is not expressed) in OPCs or reducing the vesicular release of glutamate from axons there was little to no effect on OPC proliferation (Etxeberria et al. 2016, Hines et al. 2015, Kougioumtzidou et al. 2017, Mensch et al. 2015). However, studies using pharmacological or genetic approaches to understand the function of the NMDAR, have synonymously reported that NMDA receptor signaling has no detectable effect on OPC proliferation (De Biase et al. 2011, Fannon et al. 2015, Guo et al. 2012, Li et al. 2013, Lundgaard et al. 2013), but NMDAR function have been implicated to have a role in activity dependent myelination (Li et al. 2013, Lundgaard et al. 2013), and myelin basic protein synthesis (Wake et al. 2011). The differential effects of AMPA, NMDA or glutamate signaling on OPCs either in vivo or in vitro could indicate the differences in signal encoding. Glutamatergic pre-synapses signal to OPCs via temporally fast kinetics, which were altered by most in vivo stimulation and genetic studies, but glutamate also signals by phasic tonic release, comparable to the prolonged applications that in vitro studies inevitably mimic. The recent advances in temporal and defined local control of neuronal activity will allow dissecting the differential role of glutamate signaling on OPC proliferation.
DISCUSSION
The preceding sections of this review focused on our current understanding of neuronal activity-dependent control of NSC and OPC proliferation in the postnatal/adult CNS. Conceptually, similar to a computer needing hardware upgrades to keep up with increasingly complex software, postnatal neurogenesis and gliogenesis may endow particular neural circuits with such capacity. Neuronal activity patterns directing NSCs and OPCs proliferation takes this idea one step further, and proposes that perhaps neural circuits may functionally instruct progenitors for their own neuron/oligodendrocyte additions over time, inducing a potentially powerful form of gray and white matter plasticity distinct from strengthening/weakening of existing neuronal connections. The parallels between NSC and OPC, where ACh increases but GABA decreases cellular proliferation, present several future experimental possibilities. Going forward, on the neurogenic side, an important task will be to clearly map neural circuits whose activity patterns can control NSC proliferation. The first challenge is to decipher neuronal projections contacting NSCs and/or their microenvironment that can modulate cellular proliferation and differentiation. Some advances have already been made in this important line of inquiry, but much more work remains to be done. Understanding how neuronal inputs can instruct NSC proliferation/differentiation involves more than just identifying anatomical projections, and will likely require further experimentation to decipher local modulators of neural transmitter signaling, as the microenvironmental niche plays critical roles. Further challenges are to understand how neural circuits identified in these contexts are linked to behavioral inputs that can alter their activity states, understand whether neural plasticity in these circuits can result in long lasting changes in NSC proliferation/differentiation, and how newborn neurons integrating into the olfactory bulb and hippocampus may influence the connectivity and output of these circuits. For both NSCs and OPCs, environmental enrichment and learning can stimulate progenitor proliferation as well as the subsequent addition of new neuron/oligodendrocyte progeny. It is also clear that neurotransmitters and growth factors act in concert to control NSC and OPC proliferation decisions. With multiple extracellular factors present, likely some concurrently, it is unclear how the receptor signaling pathways downstream of ligand binding converge, or interfere with one another, to execute progenitor fate decisions. Because neurodevelopmental programs demand a precise progenitor proliferation/differentiation timetable independent of neuronal activity inputs, it remains rather mysterious how the postnatal/adult NSCs and OPCs come to detect and be regulated by neuronal activity. Was the capacity always there during embryonic development; maybe it is acquired over time; or perhaps postnatal/adult progenitors represent distinct lineages? Future studies that address these issues will be highly informative for the field.
ACKNOWLEDGMENTS
We thank Brent Asrican, Patricia Paez-Gonzalez, Juan Song, and Kimberley Evans for helpful discussions and input. This work was supported by US National Institutes of Health grants R01MH105416 (C.T.K.), R01NS078192 (C.T.K.), R01NS096096 (C.T.K.), Lister Institute Research Prize (R.T.K.), and Allen Distinguished Investigator Program Award, through The Paul G. Allen Frontiers Group (R.T.K.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abiraman K, Pol SU, O’Bara MA, Chen G-D, Khaku ZM, et al. 2015. Anti-muscarinic adjunct therapy accelerates functional human oligodendrocyte repair. The Journal of neuroscience : the official journal of the Society for Neuroscience 35: 3676–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, et al. 2002. Nicotine self-administration impairs hippocampal plasticity. J. Neurosci 22: 3656–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, et al. 2005. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia 50: 132–44 [DOI] [PubMed] [Google Scholar]
- Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, et al. 2009. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15: 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia-Carcamo IL, Ford MC, Cossell L, Ishida K, Tohyama K, Attwell D. 2017. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. Elife 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Gibb A. 2005. Neuroenergetics and the kinetic design of excitatory synapses. Nature Reviews Neuroscience 6: 841–49 [DOI] [PubMed] [Google Scholar]
- Balia M, Benamer N, Angulo MC. 2017. A specific GABAergic synapse onto oligodendrocyte precursors does not regulate cortical oligodendrogenesis. Glia 89: 63. [DOI] [PubMed] [Google Scholar]
- Balia M, Vélez-Fort M, Passlick S, Schäfer C, Audinat E, et al. 2013. Postnatal down-regulation of the GABAA receptor γ2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic t… - PubMed - NCBI. Cerebral Cortex 25: 1114–23 [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. 1993. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361: 258–60 [DOI] [PubMed] [Google Scholar]
- Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, et al. 2013. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature 497: 369–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DA, Belnoue L, Song H, Simon A. 2013. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 140: 2548–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles D, Roberts J, Somogyi P, Jahr C. 2000. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405: 187–91 [DOI] [PubMed] [Google Scholar]
- Bergmann O, Spalding KL, Frisen J. 2015. Adult Neurogenesis in Humans. Cold Spring Harb Perspect Biol 7: a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson CS, Apostolopoulou M, Tian Y, Temple S. 2015. It takes a village: constructing the neurogenic niche. Dev. Cell 32: 435–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke ML, VanDongen AMJ. 2009. Activation Mechanisms of the NMDA Receptor In Biology of the NMDA Receptor, ed. Dongen AM Van. Boca Raton (FL) [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. 2004. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci 24: 7623–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden LA. 1996. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int 29: 335–56 [DOI] [PubMed] [Google Scholar]
- Calza L, Giuliani A, Fernandez M, Pirondi S, D’Intino G, et al. 2003. Neural stem cells and cholinergic neurons: regulation by immunolesion and treatment with mitogens, retinoic acid, and nerve growth factor. Proc. Natl. Acad. Sci. USA 100: 7325–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP. 1993. Muscarinic receptors--characterization, coupling and function. Pharmacol. Ther 58: 319–79 [DOI] [PubMed] [Google Scholar]
- Christian KM, Song H, Ming GL. 2014. Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci 37: 243–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RI, Almazan G. 1994. Rat Oligodendrocytes Express Muscarinic Receptors Coupled to Phosphoinositide Hydrolysis and Adenylyl Cyclase. European Journal of Neuroscience 6: 1213–24 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. 1997. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205–37 [DOI] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Winkler J, Kuhn HG. 2004. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J. Neurosci. Res 77: 155–65 [DOI] [PubMed] [Google Scholar]
- Dani JA. 2001. Overview of nicotinic receptors and their roles in the central nervous system. Biol. Psychiatry 49: 166–74 [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. 2003. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24: 476–88 [DOI] [PubMed] [Google Scholar]
- De Angelis F, Bernardo A, Magnaghi V, Minghetti L, Tata AM. 2012. Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev. Neurobiol 72: 713–28 [DOI] [PubMed] [Google Scholar]
- De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, et al. 2011. NMDA Receptor Signaling in Oligodendrocyte Progenitors Is Not Required for Oligodendrogenesis and Myelination. Journal of Neuroscience 31: 12650–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchis S, Temoney S, Erdelyi F, Bovetti S, Bovolin P, et al. 2004. GABAergic phenotypic differentiation of a subpopulation of subventricular derived migrating progenitors. Eur J Neurosci 20: 1307–17 [DOI] [PubMed] [Google Scholar]
- Delpire E 2000. Cation-Chloride Cotransporters in Neuronal Communication. News Physiol Sci 15: 309–12 [DOI] [PubMed] [Google Scholar]
- Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, et al. 2013. A regenerative approach to the treatment of multiple sclerosis. Nature 502: 327–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. 1999. The glutamate receptor ion channels. Pharmacol.Rev 51: 7–61 [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. 1988. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J. Neurosci 8: 4214–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J Jr., Bragin A, Staba R, Mody I. 2009. High-frequency oscillations: what is normal and what is not? Epilepsia 50: 598–604 [DOI] [PubMed] [Google Scholar]
- Etxeberria A, Hokanson KC, Dao DQ, Mayoral SR, Mei F, et al. 2016. Dynamic Modulation of Myelination in Response to Visual Stimuli Alters Optic Nerve Conduction Velocity. The Journal of Neuroscience 36: 6937–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiz M, Sachewsky N, Gascon S, Bang KW, Morshead CM, Nagy A. 2015. Adult Neural Stem Cells from the Subventricular Zone Give Rise to Reactive Astrocytes in the Cortex after Stroke. Cell Stem Cell 17: 624–34 [DOI] [PubMed] [Google Scholar]
- Fannon J, Tarmier W, Fulton D. 2015. Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia [DOI] [PubMed] [Google Scholar]
- Fontana X, Nacher J, Soriano E, del Rio JA. 2006. Cell proliferation in the adult hippocampal formation of rodents and its modulation by entorhinal and fimbria-fornix afferents. Cereb. Cortex 16: 301–12 [DOI] [PubMed] [Google Scholar]
- Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, et al. 2015. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat Commun 6: 8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. 1996. Interneurons of the hippocampus. Hippocampus 6: 347–470 [DOI] [PubMed] [Google Scholar]
- Frisen J 2016. Neurogenesis and Gliogenesis in Nervous System Plasticity and Repair. Annu Rev Cell Dev Biol 32: 127–41 [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P. 2014. GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur J Neurosci 39: 1845–65 [DOI] [PubMed] [Google Scholar]
- Gallo V, Deneen B. 2014. Glial development: the crossroads of regeneration and repair in the CNS. Neuron 83: 283–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. 1996. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. The Journal of Neuroscience 16: 2659–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon E, Dayer AG, Sauvain MO, Potter G, Jenny B, et al. 2006. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci 26: 12956–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier HOB, Evans KA, Volbracht K, James R, Sitnikov S, et al. 2015. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nature Communications 6: 8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. 2006. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439: 589–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, et al. 2014. Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science (New York, N.Y.) 344: 1252304–04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P, Kettenmann H, Schachner M. 1984. gamma-Aminobutyric acid directly depolarizes cultured oligodendrocytes. Journal of Neuroscience 4: 561–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. 2005. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 28: 371–8 [DOI] [PubMed] [Google Scholar]
- Goncalves JT, Schafer ST, Gage FH. 2016. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167: 897–914 [DOI] [PubMed] [Google Scholar]
- Greger IH, Watson JF, Cull-Candy SG. 2017. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 94: 713–30 [DOI] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ko EM, Delgado M, Horiuchi M, et al. 2012. Disruption of NMDA Receptors in Oligodendroglial Lineage Cells Does Not Alter Their Susceptibility to Experimental Autoimmune Encephalomyelitis or Their Normal Development. Journal of Neuroscience 32: 639–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrist A, Beech RD, King SL, Zanardi A, Cleary MA, et al. 2004. Alteration of hippocampal cell proliferation in mice lacking the beta 2 subunit of the neuronal nicotinic acetylcholine receptor. Synapse 54: 200–6 [DOI] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A. 2014. Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nature Neuroscience 17: 1518–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. 2015. Neuronal activity biases axon selection for myelination in vivo. Nature Neuroscience 18: 683–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe D, Kettenmann H. 1989. GABA triggers a Cl− efflux from cultured mouse oligodendrocytes. Neuroscience Letters 97: 334–39 [DOI] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. 2013. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nature Neuroscience 16: 668–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh KH, Fuhrer C. 2002. Clustering of nicotinic acetylcholine receptors: from the neuromuscular junction to interneuronal synapses. Mol. Neurobiol 25: 79–112 [DOI] [PubMed] [Google Scholar]
- Iacobucci GJ, Popescu GK. 2017. NMDA receptors: linking physiological output to biophysical operation. Nat Rev Neurosci 18: 236–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. 2011. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron 70: 674–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura O, Arai M, Dateki M, Ogata T, Uchida R, et al. 2015. Nicotinic acetylcholine receptors mediate donepezil-induced oligodendrocyte differentiation. Journal of Neurochemistry 135: 1086–98 [DOI] [PubMed] [Google Scholar]
- Itou Y, Nochi R, Kuribayashi H, Saito Y, Hisatsune T. 2011. Cholinergic activation of hippocampal neural stem cells in aged dentate gyrus. Hippocampus 21: 446–59 [DOI] [PubMed] [Google Scholar]
- Jabs R, Pivneva T, Huttmann K, Wyczynski A, Nolte C, et al. 2005. Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J.Cell Sci 118: 3791–803 [DOI] [PubMed] [Google Scholar]
- Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, et al. 2009. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci 29: 7966–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. 2001. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol 155: 739–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D, Shelukhina I, Yanagawa Y, Deuchars J, Henderson Z. 2015. Functional alpha7 nicotinic receptors are expressed on immature granule cells of the postnatal dentate gyrus. Brain Res. 1601: 15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Okano H, Sawamoto K. 2006. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells. 11: 1145–59 [DOI] [PubMed] [Google Scholar]
- Káradóttir R, Cavelier P, Bergersen LH, Attwell D. 2005. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438: 1162–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, Hamilton NB, Bakiri Y, Attwell D. 2008. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nature Neuroscience 11: 450–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastritsis CH, McCarthy KD. 1993. Oligodendroglial lineage cells express neuroligand receptors. Glia 8: 106–13 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386: 493–5 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH. 2015. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff F, Kettenmann H. 1992. GABA Triggers a [Ca2+]i Increase in Murine Precursor Cells of the Oligodendrocyte Lineage. Eur.J.Neurosci 4: 1049–58 [DOI] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. 2006. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience 142: 505–14 [DOI] [PubMed] [Google Scholar]
- Kougioumtzidou E, Shimizu T, Hamilton NB, Tohyama K, Sprengel R, et al. 2017. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife 6: e28080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. 2009. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32: 149–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropff E, Yang SM, Schinder AF. 2015. Dynamic role of adult-born dentate granule cells in memory processing. Curr Opin Neurobiol 35: 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Kobilka BK, Gautam D, Sexton PM, Christopoulos A, Wess J. 2014. Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov 13: 549–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. 2007. Vesicular glutamate release from axons in white matter. Nature neuroscience 10: 311–20 [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, et al. 2006. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell 127: 1253–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiao L, Liu X, Yang W, Shen W, et al. 2013. A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61: 732–49 [DOI] [PubMed] [Google Scholar]
- Li Q, Brus-Ramer M, Martin JH, McDonald JW. 2010. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neuroscience Letters 479: 128–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Sim S, Ainsworth A, Okada M, Kelsch W, Lois C. 2010. Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain circuits. Neuron 65: 32–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-c, Bergles DE. 2004. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nature Neuroscience 7: 24–32 [DOI] [PubMed] [Google Scholar]
- Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. 2005. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron 46: 773–85 [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. 2005. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci 8: 1179–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Merkle FT, Alvarez-Buylla A. 2008. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 31: 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Juarez A, He D, Richard Lu Q. 2016. Oligodendrocyte progenitor programming and reprogramming: Toward myelin regeneration. Brain Res 1638: 209–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti S, Vilaro MT, Visentin S, Rees H, Levey AI, Tata AM. 2006. Rat Schwann cells express M1-M4 muscarinic receptor subtypes. J. Neurosci. Res 84: 97–105 [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, et al. 2013. Neuregulin and BDNF Induce a Switch to NMDA Receptor-Dependent Myelination by Oligodendrocytes. PLoS Biology 11: e1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyt K, Slade TP, Dorward JJ, Durant CF, Wu Y, et al. 2007. Developing oligodendrocytes express functional GABAB receptors that stimulate cell proliferation and migration. Journal of Neurochemistry 100: 822–40 [DOI] [PubMed] [Google Scholar]
- Ma W, Li BS, Zhang L, Pant HC. 2004. Signaling cascades implicated in muscarinic regulation of proliferation of neural stem and progenitor cells. Drug News Perspect. 17: 258–66 [DOI] [PubMed] [Google Scholar]
- Ma W, Maric D, Li BS, Hu Q, Andreadis JD, et al. 2000. Acetylcholine stimulates cortical precursor cell proliferation in vitro via muscarinic receptor activation and MAP kinase phosphorylation. Eur. J. Neurosci 12: 1227–40 [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Lacaille JC. 2003. Interneuron Diversity series: Hippocampal interneuron classifications--making things as simple as possible, not simpler. Trends Neurosci 26: 564–71 [DOI] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, et al. 2007. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat. Neurosci 10: 1003–11 [DOI] [PubMed] [Google Scholar]
- Mak GK, Weiss S. 2010. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat. Neurosci 13: 753–8 [DOI] [PubMed] [Google Scholar]
- Mangin J-M, Kunze A, Chittajallu R, Gallo V. 2008. Satellite NG2 Progenitor Cells Share Common Glutamatergic Inputs with Associated Interneurons in the Mouse Dentate Gyrus. The Journal of Neuroscience 28: 7610–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin J-M, Li P, Scafidi J, Gallo V. 2012. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nature Neuroscience 15: 1192–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. 2000. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J. Neurosci 20: 1505–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt SJ, Dieni CV, Wadiche JI, Overstreet-Wadiche L. 2011. Ivy/neurogliaform interneurons coordinate activity in the neurogenic niche. Nat. Neurosci 14: 1407–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew TC. 1999. Association between supraependymal nerve fibres and the ependymal cilia of the mammalian brain. Anat Histol Embryol 28: 193–7 [DOI] [PubMed] [Google Scholar]
- Matute C 2008. P2X7 Receptors in Oligodendrocytes: A Novel Target for Neuroprotection. Molecular Neurobiology 38: 123–28 [DOI] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, Paes de Faria J, Emery B, et al. 2014. Motor skill learning requires active central myelination. Science (New York, N.Y.) 346: 318–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Fancy SPJ, Shen Y-AA, Niu J, Zhao C, et al. 2014. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nature Medicine 20: 954–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Lehmann-Horn K, Shen Y-AA, Rankin KA, Stebbins KJ, et al. 2016. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife 5: e18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, et al. 2015. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nature Neuroscience 18: 628–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FD, Gauthier-Fisher A. 2009. Home at last: neural stem cell niches defined. Cell Stem Cell 4: 507–10 [DOI] [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. 2014. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 276: 29–47 [DOI] [PubMed] [Google Scholar]
- Mohapel P, Leanza G, Kokaia M, Lindvall O. 2005. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol. Aging 26: 939–46 [DOI] [PubMed] [Google Scholar]
- Mount CW, Monje M. 2017. Wrapped to Adapt: Experience-Dependent Myelination. Neuron 95: 743–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Goldman-Rakic PS. 1993. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 90: 5194–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudo G, Belluardo N, Mauro A, Fuxe K. 2007. Acute intermittent nicotine treatment induces fibroblast growth factor-2 in the subventricular zone of the adult rat brain and enhances neuronal precursor cell proliferation. Neuroscience 145: 470–83 [DOI] [PubMed] [Google Scholar]
- Muller J, Reyes-Haro D, Pivneva T, Nolte C, Schaette R, et al. 2009. The principal neurons of the medial nucleus of the trapezoid body and NG2(+) glial cells receive coordinated excitatory synaptic input. J Gen Physiol 134: 115–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla S, Klejbor I, Birkaya B, Lee YW, Morys J, et al. 2013. alpha7 nicotinic receptor agonist reactivates neurogenesis in adult brain. Biochem. Pharmacol 86: 1099–104 [DOI] [PubMed] [Google Scholar]
- Nguyen L, Malgrange B, Breuskin I, Bettendorff L, Moonen G, et al. 2003. Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J Neurosci 23: 3278–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. 2010. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50: 295–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji T, Karasawa A, Kusaka H. 2004. Adenosine uptake inhibitors. European Journal of Pharmacology 495: 1–16 [DOI] [PubMed] [Google Scholar]
- Okuda H, Tatsumi K, Makinodan M, Yamauchi T, Kishimoto T, Wanaka A. 2009. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. Journal of Neuroscience Research 87: 3546–53 [DOI] [PubMed] [Google Scholar]
- Paez-Gonzalez P, Abdi K, Luciano D, Liu Y, Soriano-Navarro M, et al. 2011. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron 71: 61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Gonzalez P, Asrican B, Rodriguez E, Kuo CT. 2014. Identification of distinct ChAT(+) neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat. Neurosci 17: 934–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajevic S, Basser PJ, Fields RD. 2014. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276: 135–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. 2008. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann. N.Y. Acad. Sci 1129: 225–35 [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. 1990. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. Journal of Neuroscience 10: 2385–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Chaker Z, Doetsch F. 2017. Hypothalamic regulation of regionally distinct adult neural stem cells and neurogenesis. Science 356: 1383–86 [DOI] [PubMed] [Google Scholar]
- Pin JP, Bettler B. 2016. Organization and functions of mGlu and GABAB receptor complexes. Nature 540: 60–68 [DOI] [PubMed] [Google Scholar]
- Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. 2010. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron 65: 859–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plested AJ. 2016. Structural mechanisms of activation and desensitization in neurotransmitter-gated ion channels. Nat Struct Mol Biol 23: 494–502 [DOI] [PubMed] [Google Scholar]
- Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. 1992. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development 115: 535–51 [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA. 2002. Desensitization of neuronal nicotinic receptors. J. Neurobiol 53: 457–78 [DOI] [PubMed] [Google Scholar]
- Ragheb F, Molina-Holgado E, Cui QL, Khorchid A, Liu HN, et al. 2001. Pharmacological and functional characterization of muscarinic receptor subtypes in developing oligodendrocytes. J Neurochem 77: 1396–406 [DOI] [PubMed] [Google Scholar]
- Rogers SW, Gregori NZ, Carlson N, Gahring LC, Noble M. 2001. Neuronal nicotinic acetylcholine receptor expression by O2A/oligodendrocyte progenitor cells. Glia 33: 306–13 [PubMed] [Google Scholar]
- Rosenberg MB, Friedmann T, Robertson RC, Tuszynski M, Wolff JA, et al. 1988. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science 242: 1575–8 [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, et al. 2011. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472: 466–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Kageyama R, Imayoshi I. 2014. The functional significance of newly born neurons integrated into olfactory bulb circuits. Front Neurosci 8: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, et al. 2011. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478: 382–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. 2010. Cocaine-induced neuroadaptations in glutamate transmission. Annals of the New York Academy of Sciences 1187: 35–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G 2013. The dominant functional nicotinic receptor in progenitor cells in the rostral migratory stream is the alpha3beta4 subtype. J. Neurophysiol 109: 867–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Gotz M, Dimou L. 2011. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia 59: 869–81 [DOI] [PubMed] [Google Scholar]
- Song J, Sun J, Moss J, Wen Z, Sun GJ, et al. 2013. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat. Neurosci 16: 1728–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, et al. 2012. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489: 150–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer S, Volbracht K, Lundgaard I, Karadottir RT. 2016. Glutamate signalling: A multifaceted modulator of oligodendrocyte lineage cells in health and disease. Neuropharmacology 110: 574–85 [DOI] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. 2002. Adenosine: A Neuron-Glial Transmitter Promoting Myelination in the CNS in Response to Action Potentials. Neuron 36: 855–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarada T, Nakamichi N, Kitajima S, Fukumori R, Nakazato R, et al. 2012. Promoted neuronal differentiation after activation of alpha4/beta2 nicotinic acetylcholine receptors in undifferentiated neural progenitors. PLoS One 7: e46177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. 2006. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442: 929–33 [DOI] [PubMed] [Google Scholar]
- Tattersfield AS, Croon RJ, Liu YW, Kells AP, Faull RL, Connor B. 2004. Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington’s disease. Neuroscience 127: 319–32 [DOI] [PubMed] [Google Scholar]
- Tomasetti C, Vogelstein B. 2015. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347: 78–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen H-H, Kasthuri N, Hayworth KJ, et al. 2014. Distinct Profiles of Myelin Distribution Along Single Axons of Pyramidal Neurons in the Neocortex. Science 344: 319–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong CK, Chen J, Cebrian-Silla A, Mirzadeh Z, Obernier K, et al. 2014. Axonal control of the adult neural stem cell niche. Cell Stem Cell 14: 500–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. 2005. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47: 803–15 [DOI] [PubMed] [Google Scholar]
- Unal CT, Golowasch JP, Zaborszky L. 2012. Adult mouse basal forebrain harbors two distinct cholinergic populations defined by their electrophysiology. Front. Behav. Neurosci 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Borght K, Mulder J, Keijser JN, Eggen BJ, Luiten PG, Van der Zee EA. 2005. Input from the medial septum regulates adult hippocampal neurogenesis. Brain Res. Bull 67: 117–25 [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Eckman CB. 2010. Agonist-induced restoration of hippocampal neurogenesis and cognitive improvement in a model of cholinergic denervation. Neuropharm 58: 921–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veena J, Srikumar BN, Mahati K, Raju TR, Shankaranarayana Rao BS. 2011. Oxotremorine treatment restores hippocampal neurogenesis and ameliorates depression-like behaviour in chronically stressed rats. Psychopharm 217: 239–53 [DOI] [PubMed] [Google Scholar]
- Vélez-Fort M, Audinat E, Angulo MC. 2009. Functional α7-containing nicotinic receptors of NG2-expressing cells in the hippocampus. Glia 57: 1104–14 [DOI] [PubMed] [Google Scholar]
- Vélez-Fort M, Maldonado PP, Butt AM, Audinat E, Angulo MC. 2010. Postnatal Switch from Synaptic to Extrasynaptic Transmission between Interneurons and NG2 Cells. The Journal of Neuroscience 30: 6921–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, et al. 2015. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 161: 803–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi ES, Lendvai B. 1999. Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res. Rev 30: 219–35 [DOI] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. 2011. Control of Local Protein Synthesis and Initial Events in Myelination by Action Potentials. Science 333: 1647–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A. 2003a. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol 550: 785–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yan Y, Kintner DB, Lytle C, Sun D. 2003b. GABA-Mediated Trophic Effect on Oligodendrocytes Requires Na-K-2Cl Cotransport Activity. Journal of Neurophysiology 90: 1257–65 [DOI] [PubMed] [Google Scholar]
- Wigley R, Hamilton N, Nishiyama A, Kirchhoff F, Butt AM. 2007. Morphological and physiological interactions of NG2-glia with astrocytes and neurons. Journal of Anatomy 210: 661–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, et al. 2016. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nature Neuroscience 19: 1210–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel JL. 2014. Nicotinic ACh receptors in the hippocampal circuit; functional expression and role in synaptic plasticity. J Physiol 592: 4147–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, et al. 2014. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159: 766–74 [DOI] [PubMed] [Google Scholar]
- Young Kaylene M, Psachoulia K, Tripathi Richa B, Dunn S-J, Cossell L, et al. 2013. Oligodendrocyte Dynamics in the Healthy Adult CNS: Evidence for Myelin Remodeling. Neuron 77: 873–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SZ, Lafourcade CA, Platel JC, Lin TV, Bordey A. 2014. GABAergic striatal neurons project dendrites and axons into the postnatal subventricular zone leading to calcium activity. Front Cell Neurosci 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SZ, Taylor MM, Bordey A. 2011. Neurotransmitters couple brain activity to subventricular zone neurogenesis. Eur. J. Neurosci 33: 1123–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DX, Marchetto MC, Gage FH. 2014. How to make a hippocampal dentate gyrus granule neuron. Development 141: 2366–75 [DOI] [PubMed] [Google Scholar]
- Yu YC, Bultje RS, Wang X, Shi SH. 2009. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 458: 501–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. 1998. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development 125: 2901–14 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, et al. 2014. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. The Journal of Neuroscience 34: 11929–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. 2007. Vesicular release of glutamate from unmyelinated axons in white matter. Nature Neuroscience 10: 321–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonouzi M, Scafidi J, Li P, McEllin B, Edwards J, et al. 2015. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nature Neuroscience 18: 674–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Barres BA. 2013. Intrinsic and extrinsic control of oligodendrocyte development. Curr Opin Neurobiol 23: 914–20 [DOI] [PMC free article] [PubMed] [Google Scholar]