Abstract
Background and Aims
The presence, location and morphology of silica bodies are informative anatomical characters in angiosperms, mainly in Poales. In Podostemaceae, a strictly aquatic family, these structures are mentioned frequently, but there is limited insight into their location and morphological features. In the present study we focused on describing and analysing the morphological diversity of silica bodies in leaves of neotropical Podostemaceae at the intra- and interspecific levels to determine their taxonomic and phylogenetic relevance.
Methods
We studied 103 specimens distributed across 40 species. Silica body morphological traits were analysed under light and scanning electron microscopy. Additionally, data from three species of Hypericaceae (sister group) were retrieved from the literature. A phylogenetic framework based on four molecular markers was built in order to reconstruct ancestral character states related to silica bodies in neotropical Podostemaceae.
Key Results
Silica bodies were detected in epidermal, subepidermal and perivascular cells, presenting different shapes and surface morphology. Presence and location were used for primary differentiation while surface morphology and lumen (presence and shape) were used for finer distinctions. Intraspecific comparisons among samples showed that the length and width of these structures were highly variable. Maximum parsimony and maximum likelihood analyses for ancestral character reconstruction were congruent. Three out of five characters showed a statistically strong phylogenetic signal.
Conclusions
Silica bodies were reported for the first time for 19 taxa, and their morphological diversity is greater than reported in previous studies. Their presence can be considered an apomorphy in Podostemaceae. Although some significant differences were detected in length and width, qualitative characters are more informative at both specific and generic ranks.
Keywords: Rheophyte, Malpighiales, phytoliths, silicon, ancestral character state reconstruction
INTRODUCTION
Many angiosperms retain inorganic compounds, such as calcium oxalate crystals and silica bodies (Sangster and Hodson, 1986; Horner and Wagner, 1995). Silicon (Si) is the second most abundant element in soil after oxygen (Sposito, 2008). It is commonly assimilated by plant roots as monosilicic acid (Si(OH)4) dissolved in water (Hart, 2015) and deposited as intracellular and/or extracellular silica bodies (SiO2.nH2O; also known as biosilica or phytoliths) in vegetative and/or reproductive structures. These bodies vary in size, shape and surface morphology (e.g. Gu et al., 2013), depending on in which type of cell and/or tissue they accumulate. The physiological benefits of silicon in plants include promoting resistance to pathogens and insects, resisting drought conditions and improving essential nutrient intake (Guntzer et al., 2012, and references therein). Hart (2015) reports that silica bodies can be preserved in the environment for tens of millions of years at temperatures up to 1000 °C, and have been helpful tools in archaeological studies (e.g. Evett and Bartolome, 2013). The taxonomic relevance of the presence, location and form of silica bodies in angiosperms, and their use as informative characters at several taxonomic ranks, is highlighted by many studies (e.g. Brown, 1984; Prychid et al., 2003a; da Costa et al., 2011). The majority of these studies have focused on monocot families, such as Cyperaceae and Poaceae (e.g. Figueiredo et al., 1971; Lu and Liu, 2003).
The eudicotyledonous Podostemaceae Rich. ex Kunth is the largest family of strictly aquatic angiosperms, encompassing 50 genera and 280 species (Bove, 2018). It is a member of the clusioid clade (Malpighiales), which includes five families with the following phylogenetic topology: ((Podostemaceae + Hypericaceae) Calophyllaceae) + (Clusiaceae + Bonnetiaceae) (Ruhfel et al., 2011). The family is broadly distributed across tropical regions, with few species in temperate areas (e.g. Podostemum ceratophyllum Michx.). In the neotropics, it ranges from central Mexico to northern Paraguay and Argentina and is represented by 20 genera and about 150 species (Bove and Philbrick, 2010). Morphological and molecular data support recognition of three subfamilies: Tristichoideae, Weddellinoideae and Podostemoideae (e.g. Engler, 1930; Koi et al., 2012).
Podostemaceae grow tightly adhered to the surface of submerged solid substrates, mostly rocky, in swift currents of river rapids and waterfalls (rheophytes). As the water level drops, plants flower and set fruit (Cook and Rutishauser, 2007). Many species possess a thalloid photosynthetic body due to the dorsiventral flattening of roots, shoots or a combination of both (Rutishauser, 1997). Leaves may be one cell layer thick and scale-like to compound and up to 2 m long. Some of the anatomical peculiarities reported include: a lack of aerenchyma, which is a marked disparity when compared with other hydrophytes (Arber, 1920; Lopes, 2012); silica bodies in peripheral tissues (Metcalfe and Chalk, 1950); laticiferous tubes found in some neotropical taxa (Schnell, 1967; Rutishauser and Grubert, 1994); and dimorphic chloroplasts, recently reported in Cladopus japonicus Imamura, an Asiatic species of Podostemoideae (Fujinami et al., 2011).
The first author to detect silica bodies in Podostemaceae was Tulasne (1852: 6), observing them in stem epidermal cells of a species of Podostemum, even though he did not know its composition ‘… cellulae superficiales materie solida hyalina dilute brunnea aqua calda non soluta nec iode ullo modo colorata … qua materia non disrupta facile …’ (... peripheral cells with solid hyaline structures, brown when diluted in hot water, not soluble in iodine or even by coloration techniques … difficult to break …). This material was identified as silica by Cario (1881) and Warming (1881). Since then, silica bodies have been reported in epidermal and/or subepidermal cells of vegetative (Ancíbor, 1990; Ameka et al., 2002; Rutishauser et al., 2005; Koi and Kato, 2007) and reproductive organs (Sá-Haiad et al., 2010) of several neotropical and palaeotropical taxa. However, Jäger-Zürn (2011) pointed out that silica bodies have been only incidentally recorded in Podostemaceae.
Little has been proposed regarding the use of silica bodies in terms of taxonomic and phylogenetic perspectives in Podostemaceae (Table 1). da Costa et al. (2011) concluded that shape and surface morphology of silica bodies are valuable taxonomic characters at the subfamily level in Podostemaceae, and emphasized the need for further investigations to evaluate their utility at lower hierarchical ranks. Lopes (2012) analysed the vegetative anatomy of eight neotropical species (five of them in Podostemum) from three genera, and concluded that these species can be distinguished by the shape of their silica bodies.
Table 1.
Studies that report silica bodies characters in neotropical species of Podostemaceae
| Author (year) | Taxa | Description type |
|---|---|---|
| Cario (1881) | Tristicha trifaria (Bory ex Willd.) Spreng. | Location, shape and ornamentation under LM |
| Warming (1881) |
Podostemum ovatum C.T. Philbrick & Novelo (as Mniopsis glazioviana) Podostemum weddellianum (Tul.) C.T. Philbrick & Novelo (as Mniopsis weddelliana) Podostemum ceratophyllum Michx. |
Location, shape and ornamentation under LM |
| Warming (1888) |
Mourera aspera (Bong.) Tul. Podostemum muelleri Warm. |
Location, shape and ornamentation under LM |
| Wächter (1897) | Weddellina squamulosa Tul. | Location under LM |
| Warming (1899) |
Podostemum distichum (Cham.) Wedd. (as P. glaziovianum) Podostemum scaturiginum (Mart.) C.T. Philbrick & Novelo (as Mniopsis scaturiginum) |
Location, shape and ornamentation under LM |
| Schnell (1967) |
Apinagia flexuosa (Tul.) P. Royen Apinagia richardiana (Tul.) P. Royen Marathrum capillaceum (Pulle) P. Royen Mourera fluviatilis Aubl. |
Location under LM |
| Tur (1987) |
Podostemum muelleri (as P. uruguayense) Tristicha trifaria |
Location under LM |
| Ancíbor (1990) |
Podostemum muelleri (as P. galvone) Podostemum rutifolium Warm. Podostemum muelleri (as P. uruguayense) Podostemum weddellianum (Tul.) C.T. Philbrick & Novelo |
Location and shape under LM and TEM |
| Rutishauser et al. (2005) | Diamantina lombardii Novelo, C.T. Philbrick & Irgang | Location under LM |
| Sá-Haiad et al. (2010) | Podostemum weddellianum | Location under LM |
| da Costa et al. (2011) |
Diamantina lombardii
Tristicha trifaria Weddellina squamulosa |
Location, shape and ornamentation under LM and SEM, with taxonomic and phylogenetic implications |
| Lopes (2012) |
Apinagia riedelii (Bong.) Tul. (as A. yguazuensis) Podostemum comatum Hicken Podostemum distichum Podostemum irgangii C.T. Philbrick & Novelo Podostemum muelleri Podostemum rutifolium Tristicha trifaria |
Location, shape and ornamentation under LM and SEM, with taxonomic implications |
LM, light microscopy; SEM, scanning electron microscopy; TEM, transmission electron microscopy.
Phylogenetic analyses have indicated that neotropical Podostemoideae are non-monophyletic (Kita and Kato, 2001; Ruhfel et al., 2011, 2016). Further studies by Koi et al. (2012) proposed a division of New World Podostemoideae into four clades: Diamantina ((Mourera-Apinagia clade) (Podostemum (Ceratolacis + Old world clade))). Investigations on the phylogenetic relationships of neotropical Podostemaceae based on molecular data (ITS, rbcL and trnL) revealed that Apinagia and Marathrum are not monophyletic (Tippery et al., 2011). Additionally, an unexpected clade encompassing species of five morphologically diverse genera (Apinagia, Jenmaniella, Lophogyne, Marathrum and Monsostylis) was resolved (clade J in their fig. 3), strongly supported but with uncertain morphological integrity, i.e. morphological synapomorphies that define the clade remain unknown. These results point to an incongruence between molecular and morphological datasets and emphasize the need to identify additional morphological characters. Variation in silica bodies, in terms of both their presence and their morphology, may help to address this problem.
Our aim is to investigate the presence, location and morphology of silica bodies in leaves of neotropical Podostemaceae. Our goals are to (1) analyse the inter- and intraspecific variation in silica body form and evaluate their utility as taxonomic characters at the clade, generic and specific levels; and (2) evaluate the distribution of silica body characters across the phylogeny of neotropical Podostemaceae.
MATERIALS AND METHODS
Taxon sampling and anatomical analysis
Podostemaceae taxon sampling was based on the terminal branches of the phylogenetic studies conducted by Ruhfel et al. (2011) and Tippery et al. (2011). We analysed 103 specimens representing 40 species and 18 genera, spread across the three subfamilies and the main clades of neotropical Podostemoideae (Table 2). To evaluate the stability of silica body features at the intraspecific level, we investigated at least three specimens of each species, preferably from different localities, when possible (Supplementary Data Appendix S1). The material was obtained from herbarium sheets and spirit collections housed in the herbaria of Museu Nacional (R) and Western Connecticut State University (WCSU); herbarium acronyms are according to Thiers (2017).
Table 2.
Species of Podostemaceae included in the present study, organized by subfamily and genus; the number in parentheses represents the total species in each genus
| Genera | Studied species |
|---|---|
| Tristichoideae | |
| Tristicha Thouars (1) | T. trifaria (Bory ex Willd.) Spreng. |
| Weddellinoideae | |
| Weddellina Tul. (1) | W. squamulosa Tul. |
| Podostemoideae | |
| Apinagia Tul. (32) | A. fimbrifolia P. Royen |
| A. fluitans P. Royen | |
| A. longifolia (Tul.) P. Royen | |
| A. richardiana (Tul.) P. Royen | |
| A. riedelii Tul. | |
| A. staheliana (Went.) P. Royen | |
| Autana C.T. Philbrick (1) | A. andersonii C.T. Philbrick |
| Castelnavia Tul. & Wedd. (6) | C. fluitans Tul. & Wedd. |
| C. monandra Tul. & Wedd. | |
| C. multipartita Tul. & Wedd. | |
| C. noveloi C.T. Philbrick & C.P. Bove | |
| C. pendulosa (C.T. Philbrick & C.P. Bove) C.T. Philbrick & C.P. Bove | |
| C. princeps Tul. & Wedd. | |
| Ceratolacis (Tul.) Wedd. (2) | C. pedunculatum C.T. Philbrick, Novelo & Irgang |
| Cipoia C.T. Philbrick, Novelo & Irgang (2) | C. inserta C.T. Philbrick, Novelo & Irgang |
| C. ramosa C.P. Bove, C.T. Philbrick & Novelo | |
| Diamantina Novelo, C.T. Philbrick & Irgang (1) | D. lombardii Novelo, C.T. Philbrick & Irgang |
| Jenmaniella Engl. (6) | J. ceratophylla Engl. |
| J. fimbriata P. Royen | |
| Lophogyne Tul. (1) | L. lacunosa (Gardn.) C.P. Bove & C.T. Philbrick |
| Marathrum Humb. & Bonpl. (15) | M. aeruginosum P. Royen |
| M. foeniculaceum Humb. & Bonpl. | |
| M. plumosum (Novelo & C.T. Philbrick) C.T. Philbrick & C.P. Bove | |
| M. tenue Liebm. | |
| M. utile Tul. | |
| Monostylis Tul. (1) | M. capillacea Tul. |
| Mourera Aubl. (8) | M. aspera (Bong.) Tul. |
| M. elegans (Tul.) Baillon | |
| M. fluviatilis Aubl. | |
| M. weddelliana Tul. | |
| Noveloa C.T. Philbrick (2) | N. coulteriana (Tul.) C.T. Philbrick |
| Oserya Tul. & Wedd. (5) | O. perpusilla (Went.) P. Royen |
| Podostemum Michx. (11) | P. ceratophyllum Michx. |
| P. scaturiginum (Mart.) C.T. Philbrick & Novelo | |
| P. weddellianum (Tul.) C.T. Philbrick & Novelo | |
| Rhyncholacis Tul. (24) | R. applanata K.I. Goebel |
| R. penicillata Matthiesen | |
| Wettsteiniola Suess. (3) | Wettsteiniola sp. |
This study focused on leaves. Stems and roots were not included because of their limited availability on many specimens. To detect and study the morphology of silica bodies, fragments of apical, medial and basal portions of fully developed leaves were stored in 30 % hydrogen peroxide and glacial acetic acid solution (1: 1 v/v; Franklin, 1945) for 24 h. Subsequently, the samples were soaked in heated clove oil and phenol crystals (adapted from Johansen, 1940). To study the anatomical location of these structures in leaf blades, leaf samples were fixed in FAA70 (Johansen, 1940), preserved in 70 % ethanol, dehydrated with a crescent alcohol concentration series, and embedded in (2 hydroxyethyl)-methacrylate (HistoResin Embedding Kit; Leica, Heidelberg, Germany). Cross and longitudinal (5 µm) sections were obtained on a KD-3358 rotatory microtome (Zhejiang Jinhua Kedi Instrumental Equipment Co., China) using a steel knife. Sections were stained in aqueous 0.05 % Toluidine Blue O (O’Brien et al., 1964) for 30 s, mounted in Canada balsam and examined with light microscopy. The terminology used to describe shape and surface morphology followed Madella et al. (2005), with adaptations when necessary. Images were captured using an Olympus BX-51 microscope with the image-capture system Q-Color5 and Image-Pro Express software. For scanning electron microscopy analysis, leaf fragments were acetolysed according to da Costa et al. (2016). The acetolysed samples were placed on aluminium stubs, sputter-coated with gold–palladium and examined using a JEOL JSM-5800 microscope operating at 10 kV.
Statistical analysis
For each specimen that presented silica bodies we determined the following statistics for both length and width: range (minimum and maximum), arithmetic mean and coefficient of variation. Because some of the obtained data were not normally distributed (Shapiro–Wilk test, P < 0.05), the Kruskal–Wallis test was implemented to assess intraspecific differences in length and width followed by a Bonferroni post-hoc test (P < 0.01).
Phylogenetic framework and ancestral states reconstructions
To understand the evolution of the characters under a phylogenetic perspective, we inferred a phylogenetic tree for 38 species of Podostemaceae and three of Hypericaceae (outgroup) using four molecular markers (matK, rbcL, trnL and ITS). All sequences were obtained from GenBank (Appendix S2). Cipoia inserta and Wettsteiniola sp. were not used because their sequences were unavailable in the GenBank database. DNA sequences were initially aligned with MUSCLE (Edgar, 2004) and then verified manually in Mesquite ver. 3.31 (Maddison and Maddison, 2017). Maximum likelihood (ML) analyses were conducted using RaxML ver. 8.1.21 (Stamatakis, 2014) implemented in raxmlGUI ver. 1.5 (Silvestro and Michalak, 2012) under the GTRGAMMA model of nucleotide substitution. Tree searches were carried out using ML + ‘rapid bootstrap’ option with 1000 replicates. FigTree ver. 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree) was used to visualize and annotate trees.
A matrix containing the anatomically discrete data obtained was designed following the recommendations of Sereno (2007). Information about silica bodies for three Hypericaceae species (outgroup), each one representing a subfamily, was inferred from the following literature: Baas (1970) for Cratoxylum arborescens (Vahl.) Blume; Łotocka and Osińska (2010) for Hypericum perforatum L.; and Almeida-Cortez and Melo-de-Pinna (2006) for Vismia guianensis (Aubl.) Choisy.
The topology derived from combined molecular data under ML analysis was used to reconstruct ancestral character states in Mesquite ver. 3.31 (Maddison and Maddison, 2017) using maximum parsimony (MP) and likelihood (ML) approaches. For MP reconstructions, the number of steps was calculated considering unordered character states with equiprobable transitions. ML reconstructions were additionally implemented because of two advantages. First, they estimate the uncertainty of ancestral character reconstruction, and secondly they help to quantify the inferred ambiguities. The probability model implemented was Markov chain (Mk) with one parameter.
To estimate the presence of phylogenetic signal (i.e. if there is a tendency for related species to resemble each other), we implemented the null hypothesis test (Maddison and Slatkin, 1991). This procedure involves randomization of phylogenetic trees or character states and a posteriori comparison of the number of parsimony steps obtained. In this study we randomized character states (5000 replications) for each character, preserving the topology of the original tree. Distributions of the numbers of character steps obtained by randomization were compared with the number of steps of the original reconstruction at a 0.01 confidence level (one-tailed test).
RESULTS
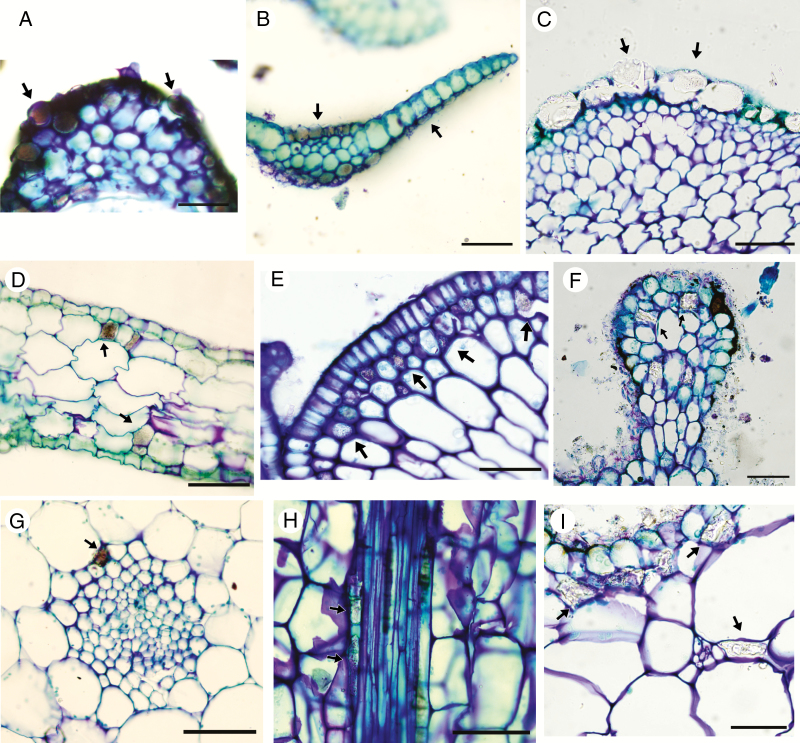
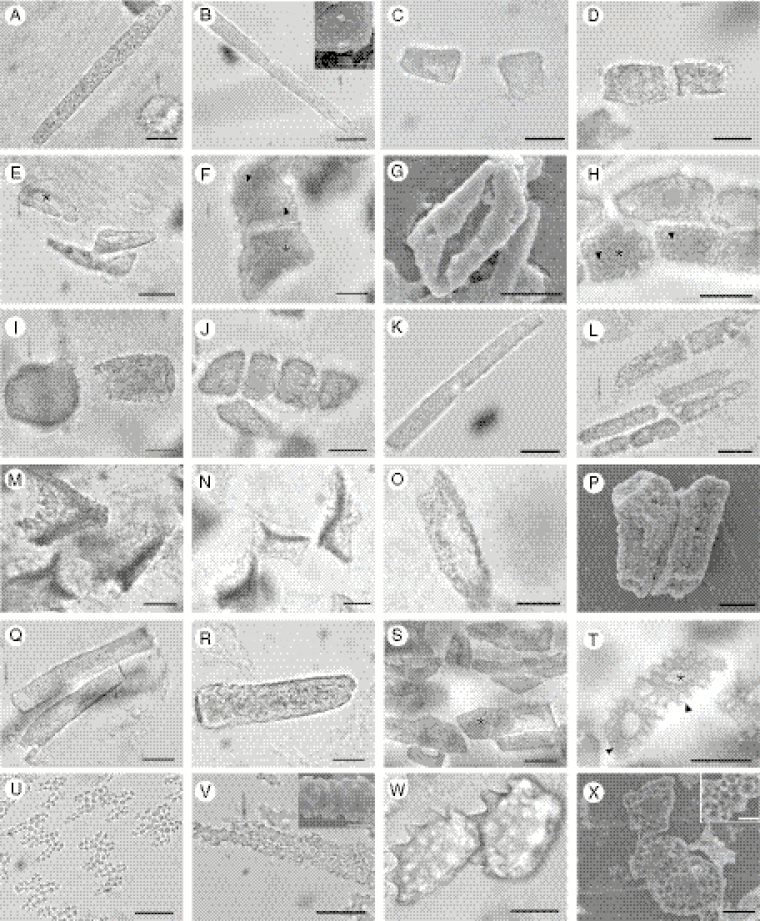
Silica bodies were found in leaves of all three subfamilies of Podostemaceae, and in 24 of 40 species examined, encompassing 11 of 18 genera. These structures were intracellular in all analysed species, i.e. filling the entire intracellular space. The presence or absence of silica bodies was a stable trait in all species and in almost all genera, except Apinagia and Marathrum; the polyphyletic nature of these two genera will be discussed below. Silica bodies were found at three anatomical locations in the leaves: epidermis (Fig. 1A–C), subepidermis (Fig. 1D–F) and perivascular cells (Fig. 1G–I), filling the intracellular space. When present they occur in all epidermal cells (in a continuous manner) and sparsely in subepidermal and perivascular cells. We identified six shapes: rectangular (Fig. 2A–B, K–L, O–R, V), short-rectangular (Fig. 2C–D, I–J), polyhedral (Fig. 2E–H, S–T), irregular (Fig. 2M–N), lobate (Fig. 2U) and oblong (Fig. 2W–X). Six types of silica body surface structure were recognized: granulate (Fig. 2A–D, I–L, Q–R), smooth (Fig. 2E, S), perforate (Fig. 2F–H), verrucose (Fig. 2M–P), crenate (Fig. 2U–V) and undulate (Fig. 2T, W–X). Additionally, we found a central lumen presenting a circular (Diamantina; Fig. 2H), elongate (Ceratolacis and Podostemum; Fig. 2E, S–T) and irregular (Cipoia; Fig. 2F) shape. With the exception of Tristicha trifaria, epidermal silica bodies are orientated such that their ornamented side faces the outer periclinal wall, i.e. a dorsiventral arrangement.
Fig. 1.
Location of silica bodies (arrows) on leaves of studied species under light microscopy. (A–C) Epidermal silica bodies in transverse sections. (A) Cipoia inserta. (B) Tristicha trifaria. (C) Weddellina squamulosa. (D–F) Subepidermal silica bodies. (D) Transverse section of Apinagia longifolia. (E) Transverse section of Marathrum plumosum. (F) Longitudinal section of an outgrowth of Mourera aspera. (G–I) Perivascular silica bodies. (G) Transverse section of Apinagia richardiana. (H) Longitudinal section of Apinagia staheliana. (I) Transverse section of Mourera aspera. Scale bars = 50 µm; except (D) = 100 µm.
Fig. 2.
Shape and surface morphology of silica bodies in leaves of studied taxa under light and scanning electron microscopy (SEM). (A–B) Rectangular and (C–D) short-rectangular with granulate surface in Apinagia. (A) A. fluitans; (B) A. riedelii (upper inset shows surface details under SEM); (C) A. richardiana; (D) A. longifolia. (E) Polyhedral with smooth surface and elongate lumen in Ceratolacis pedunculatum. (F–G) Polyhedral and rectangular with perforated surface in Cipoia ramosa and C. inserta, respectively; arrowheads (F) indicate perforations (note irregular lumen). (H) Rectangular with perforated surface (arrowheads) and circular lumen (*) in Diamantina lombardii. (I–J) Short-rectangular and (K–L) rectangular with granulate surface in Marathrum. (I, K) M. foeniculaceum; (J, L) M. plumosum. (M–N) Irregular and (O, P) rectangular with verrucose surface in Mourera. (M, O) M. aspera; (N, P) M. fluviatilis. (Q) Rectangular with granulate surface in Noveloa coulteriana. (R) Rectangular with densely granulate surface in Oserya perpusilla. (S) Polyhedral and rectangular with smooth surface and elongate lumen in Podostemum ceratophyllum. (T) Polyhedral with undulate surface and elongate lumen in Podostemum scaturiginum. (U) Rectangular and (V) lobate with crenate surface in Tristicha trifaria. (W–X) Oblong with undulate surface in Weddellina squamulosa. Scale bars = 20 µm, except in the B, V and X insets (10 µm).
Arithmetical means of silica body length varied from 15.4 µm (Tristicha trifaria) to 178.8 µm (Noveloa coulteriana) and width from 6.1 µm (Apinagia staheliana) to 26.4 µm (Cipoia ramosa). In the intraspecific comparison (Table S1) the coefficient of variation showed high variability among samples. Length and width differences were significantly different among at least two of three sampled specimens of all species, except Apinagia richardiana, Ceratolacis pedunculatum and Mourera fluviatilis. Interestingly, the three specimens of Noveloa coulteriana analysed had statistically different mean lengths but similar mean widths. Because quantitative data were not stable at the intraspecific level for most species, no further analyses were conducted.
Below we present general descriptions for each genus that possesses silica bodies, a taxonomic key to genera and the major state transitions on the phylogeny (Figs 4–6).
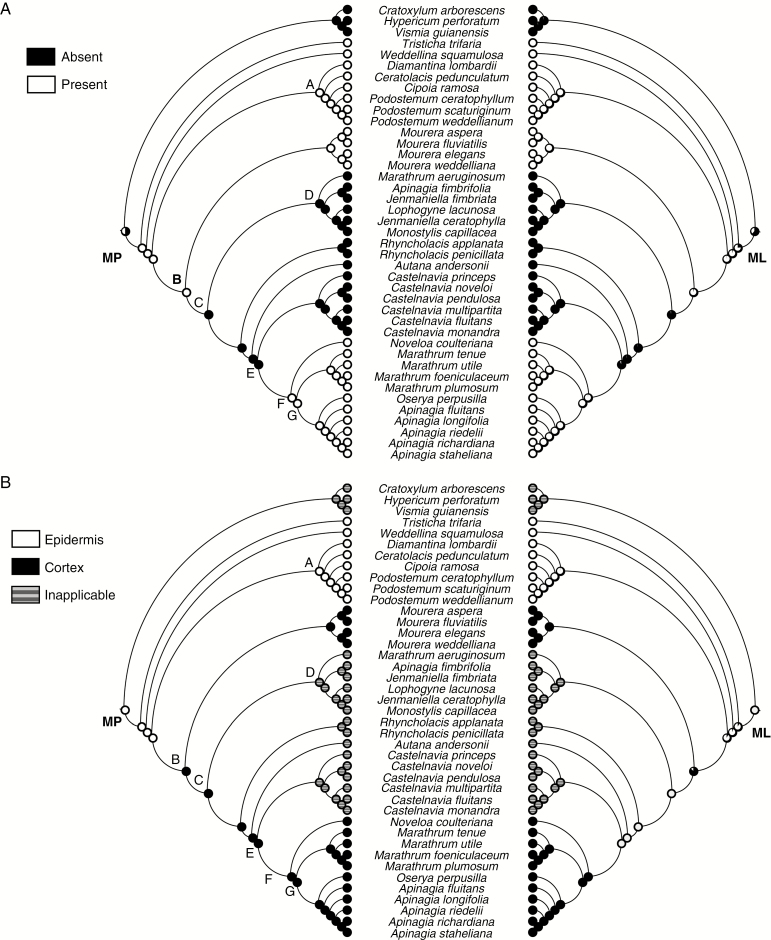
Fig. 4.
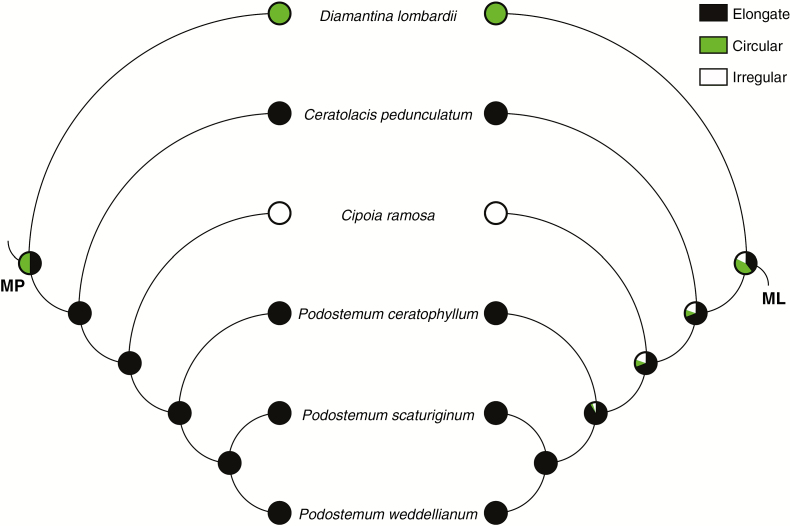
Ancestral character state reconstructions of silica body presence/absence (A) and location (B) in neotropical Podostemaceae from maximum parsimony (left) and maximum likelihood (right) analyses. Pie charts at nodes represent parsimony and likelihood proportions, respectively.
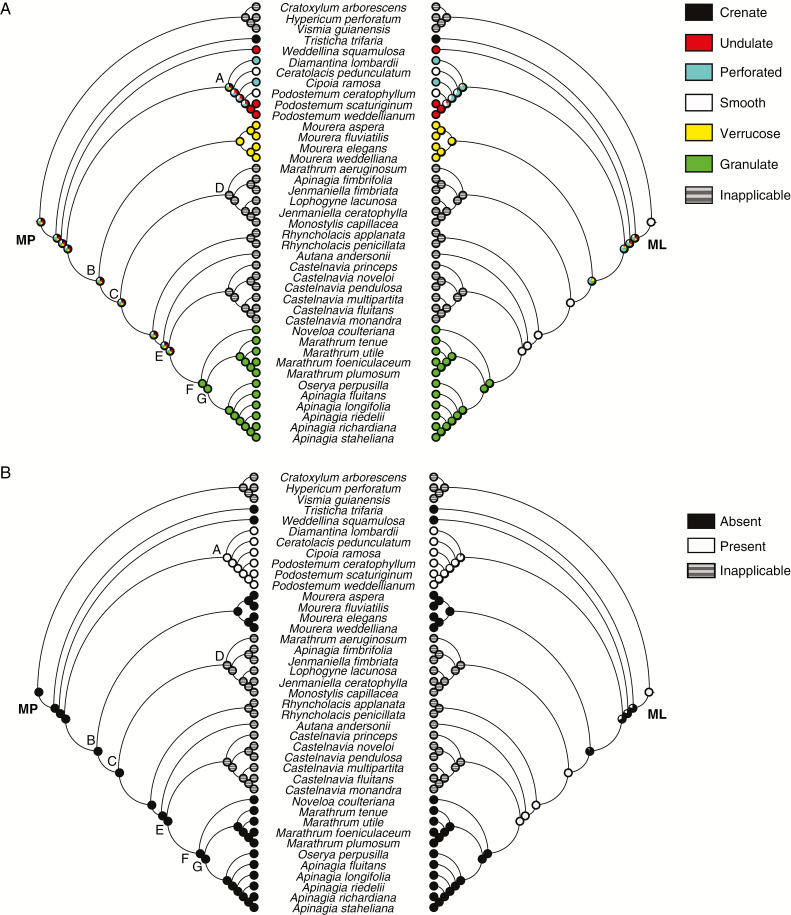
Fig. 5.
Ancestral character state reconstructions of silica body morphology (A) and the presence of a lumen (B) in neotropical Podostemaceae from maximum parsimony (left) and maximum likelihood (right) analyses. Pie charts at nodes represent parsimony and likelihood proportions, respectively.
Fig. 6.
Ancestral character state reconstruction of silica body lumen morphology in neotropical Podostemaceae from maximum parsimony (left) and maximum likelihood (right) analyses. Pie charts above and below branches represent parsimony and likelihood proportions, respectively.
Generic descriptions
Apinagia (except A. fimbrifolia).
Silica bodies occurred in apical, medial and basal regions of leaves, located in adaxial and abaxial subepidermal and/or perivascular cells (Fig. 1D, G–H). They presented rectangular and short-rectangular shape (Fig. 2A–D), granulate surface and a lumen was absent. Average length ranged from 26.5 (A. longifolia) to 102.4 µm (A. fluitans) and width from 7.3 to 17.9 µm (A. longifolia).
Ceratolacis.
Silica bodies occurred at the basal leaf (rare or absent at medial and apical portions), located in adaxial and abaxial epidermal cells. They presented rectangular and polyhedral shapes, a smooth surface and an elongated lumen (Fig. 2E). Average length ranged from 53.4 to 62.4 µm and width from 11.1 to 12.6 µm.
Cipoia.
Silica bodies occurred in apical, medial and basal regions of leaves, located in adaxial and abaxial epidermal cells. They presented rectangular and polyhedral shapes, a perforated surface and irregular shaped lumens (Fig. 2F–G). Average length ranged from 35.7 (C. ramosa) to 56.2 µm (C. inserta) and width from 14.2 (C. inserta) to 26.4 µm (C. ramosa).
Diamantina.
Silica bodies occurred in apical, medial and basal regions of leaves, located in adaxial and abaxial epidermal cells (Fig. 1A). They presented rectangular and polyhedral shapes, a perforated surface and circular lumens (Fig. 2H). Average length ranged from 17.6 to 30.1 µm and width from 9.7 to 15 µm.
Marathrum (except M. aeruginosum).
Silica bodies occurred in apical, medial and basal regions of leaves, located in adaxial and abaxial subepidermal and perivascular cells (Fig. 1E). They presented short-rectangular and rectangular shapes (Fig. 2I–L), a granulate surface and a lumen was absent. Average length ranged from 26.6 (M. plumosum) to 80.4 µm (M. foeniculaceum) and width from 10.7 to 23.8 µm (M. tenue). Marathrum aeruginosum was the only analysed species of this genus that did not possess silica bodies.
Mourera.
Silica bodies occurred in apical, medial and basal regions of leaves, and were located in perivascular and/or adaxial and abaxial subepidermal cells (Fig. 1F, I). They presented irregular and rectangular shapes (Fig. 2M–P), a verrucose surface and a lumen was absent. Average length ranged from 38.6 (M. fluviatilis) to 72.5 µm (M. elegans) and width from 15.7 (M. elegans) to 30.9 µm (M. aspera).
Noveloa.
Silica bodies occurred in apical, medial and basal regions of leaves, and were located in adaxial and abaxial subepidermal and perivascular cells. They presented rectangular shapes, a granulate surface (Fig. 2Q) and a lumen was absent. Average length ranged from 95.9 to 178.8 µm and width from 20.9 to 25.2 µm.
Oserya.
Silica bodies occurred in all leaf portions, located in adaxial and abaxial subepidermal cells. They presented a rectangular shape (Fig. 2R), granulate surface and absent lumen. Average length ranged from 39.8 to 87.8 µm and width from 14.2 to 17 µm.
Podostemum.
Silica bodies occurred in apical, medial and basal regions of leaves, located in adaxial and abaxial epidermal cells. They presented rectangular and polyhedral shapes, a smooth (Fig. 2S) or undulate (Fig. 2T) surface and an elongated lumen. Average length ranged from 33.7 (P. scaturiginum) to 70.1 µm (P. ceratophyllum) and width from 15.6 (P. ceratophyllum) to 20.3 µm (P. scaturiginum).
Tristicha.
Silica bodies occurred in apical, medial and basal regions of leaves, located in adaxial and abaxial epidermal cells (Fig. 1B). They presented a lobate (‘H’) shape in epidermal cells in the midvein region (Fig. 2U), and rectangular shape in the remaining epidermal cells (Fig. 2V). Their surface was crenate and a lumen was absent. Average length ranged from 15.4 to 19.6 µm and width from 8.5 to 11 µm.
Weddellina.
Silica bodies occurred in apical, medial and basal regions of the scale-like leaves, located in abaxial epidermal cells (Fig. 1C). They presented an oblong shape, had an undulate surface (Fig. 2W–X) and a lumen was absent. Average length ranged from 33.3 to 48.1 µm and width from 21 to 33.7 µm.
Silica bodies were not found in the leaves of species in the following genera: Autana, Castelnavia, Jenmaniella, Lophogyne, Monostylis, Rhyncholacis and Wettsteiniola. Apinagia fimbrifolia and Marathrum aeruginosum also lacked silica bodies.
Genus-level identification
The dichotomous key to neotropical Podostemaceae genera (except Macarenia P. Royen) presented below is based on the presence/absence of silica bodies, and their location and morphology. Quantitative data were not used.
1a. Silica bodies absent …….…… Autana, Apinagia fimbrifolia, Castelnavia, Jenmaniella, Lophogyne, Marathrum aeruginosum, Monostylis, Rhyncholacis and Wettsteiniola
1b. Silica bodies present 2
2a. Silica bodies restricted to epidermis 3
2b. Silica bodies restricted to cortical cells 7
3a. Lumen absent 4
3b. Lumen present 5
4a. Surface crenate Tristicha
4b. Surface undulate Weddellina
5a. Surface perforate 6
5b. Surface smooth or undulate; lumen elongate Ceratolacis and Podostemum
6a. Lumen circular Diamantina
6b. Lumen irregular Cipoia
7a. Rectangular and irregular or only irregular shape Mourera
7b. Only rectangular shape 8
8a. Silica bodies restricted to subepidermal cells Oserya
8b. Silica bodies occurring in subepidermal and perivascular cells or restricted to the latter Apinagia (except A. fimbrifolia), Marathrum (except M. aeruginosum) and Noveloa
Ancestral state reconstruction and phylogenetic signal
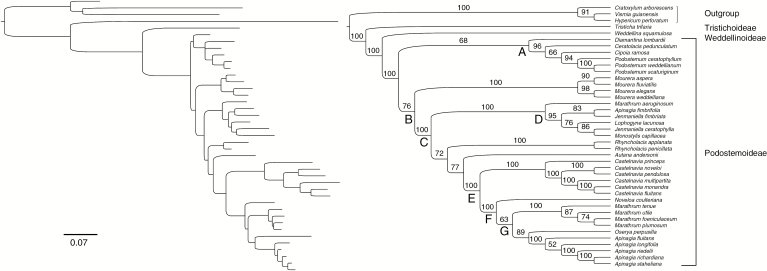
The matrix of molecular data resulted in 4545 nucleotide bases for the 41 taxa. The ML tree obtained (Fig. 3) shows that Podostemaceae, its subfamilies and most genera are strongly supported as monophyletic. Podostemoideae includes two main lineages, clades A and B, that are moderately supported (~70 % boostrap support). Moreover, their respective subclades are moderately to strongly supported. The genera Apinagia, Marathrum and Jenmaniella were recovered as non-monophyletic.
Fig. 3.
Maximum likelihood phylogram (left) and cladogram (right) based on combined molecular data (ITS, matK, rbcL and trnL) of neotropical Podostemaceae. Scale bar represents the substitutions per site. Nodal values above branches indicate ML bootstrap percentages.
Characters and their respective states are listed in Table 3, while the data matrix is provided in Table 4. Because silica body shape was revealed as polymorphic (i.e. two or more character states for each terminal in some conditions), we did not use it in ancestral reconstructions.
Table 3.
List of characters of silica bodies on the leaves of neotropical Podostemaceae and their respective states
| Character | States |
|---|---|
| 1. Silica bodies | 0 = absent, 1 = present |
| 2. Silica bodies, location | 0 = epidermis, 1 = cortex |
| 3. Silica bodies, surface morphology | 0 = smooth, 1 = perforate, 2 = granulate, 3 = verrucose, 4 = undulate, 5 = crenate |
| 4. Silica bodies, lumen | 0 = absent, 1 = present |
| 5. Silica bodies, lumen, shape | 0 = irregular, 1 = circular, 2 = elongate |
Table 4.
Data matrix scored for species in this study; numbers refer to characters and their states expressed in Table 3 (dashes represent inapplicable data)
| Taxon | Character | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Podostemaceae | |||||
| Apinagia fimbrifolia | 0 | – | – | – | – |
| Apinagia fluitans | 1 | 1 | 2 | 0 | – |
| Apinagia longifolia | 1 | 1 | 2 | 0 | – |
| Apinagia richardiana | 1 | 1 | 2 | 0 | – |
| Apinagia riedelii | 1 | 1 | 2 | 0 | – |
| Apinagia staheliana | 1 | 1 | 2 | 0 | – |
| Autana andersonii | 0 | – | – | – | – |
| Castelnavia fluitans | 0 | – | – | – | – |
| Castelnavia monandra | 0 | – | – | – | – |
| Castelnavia multipartita | 0 | – | – | – | – |
| Castelnavia noveloi | 0 | – | – | – | – |
| Castelnavia pendulosa | 0 | – | – | – | – |
| Castelnavia princeps | 0 | – | – | – | – |
| Ceratolacis pedunculatum | 1 | 0 | 0 | 1 | 2 |
| Cipoia inserta | 1 | 0 | 1 | 1 | 0 |
| Cipoia ramosa | 1 | 0 | 1 | 1 | 0 |
| Diamantina lombardii | 1 | 0 | 1 | 1 | 1 |
| Jenmaniella ceratophylla | 0 | – | – | – | – |
| Jenmaniella fimbriata | 0 | – | – | – | – |
| Lophogyne lacunosa | 0 | – | – | – | – |
| Marathrum aeroginosum | 0 | – | – | – | – |
| Marathrum foeniculaceum | 1 | 1 | 2 | 0 | – |
| Marathrum plumosum | 1 | 1 | 2 | 0 | – |
| Marathrum tenue | 1 | 1 | 2 | 0 | – |
| Marathrum utile | 1 | 1 | 2 | 0 | – |
| Monostylis capillacea | 0 | – | – | – | – |
| Mourera aspera | 1 | 1 | 3 | 0 | – |
| Mourera elegans | 1 | 1 | 3 | 0 | – |
| Mourera fluviatilis | 1 | 1 | 3 | 0 | – |
| Mourera weddelliana | 1 | 1 | 3 | 0 | – |
| Noveloa coulteriana | 1 | 1 | 2 | 0 | – |
| Oserya perpusilla | 1 | 1 | 2 | 0 | – |
| Podostemum ceratophyllum | 1 | 0 | 0 | 1 | 2 |
| Podostemum scaturiginum | 1 | 0 | 4 | 1 | 2 |
| Podostemum weddellianum | 1 | 0 | 4 | 1 | 2 |
| Rhyncholacis applanata | 0 | – | – | – | – |
| Rhyncholacis penicillata | 0 | – | – | – | – |
| Tristicha trifaria | 1 | 0 | 5 | 0 | – |
| Weddellina squamulosa | 1 | 0 | 4 | 0 | – |
| Wettsteiniola sp. | 0 | – | – | – | – |
| Hypericaceae | |||||
| Cratoxylum arborescens | 0 | – | – | – | – |
| Hypericum perforatum | 0 | – | – | – | – |
| Vismia guianensis | 0 | – | – | – | – |
Silica bodies were found in subfamilies Tristichoideae, Weddellinoideae, and clades A and F, and the genus Mourera in Podostemoideae. These structures are absent in Hypericaceae (outgroup), in clade D, Autana, Castelnavia and Rhyncholacis (Podostemoideae). MP and ML analyses show congruency for all ancestral states and unambiguously reconstructions for most ancestral states (Fig. 4A); ambiguities were found only in the common ancestors of Podostemaceae + Hypericaceae (silica bodies reconstructed as absent, 60.4 % likelihood) and (Podostemoideae + Weddellinoideae) Tristichoideae (silica bodies reconstructed as present, with 76.5 % likelihood). Silica bodies were present in the common ancestor of Podostemoideae + Weddellinoideae (96.6 % likelihood). Because these structures were present in the common ancestor to all Podostemaceae, one loss event probably occurred in the ancestor of clade C; this event resulted in the absence of silica bodies in some subclades of this clade. Both analyses indicate that these structures appeared again in clade F (99 % likelihood). The absence of silica bodies in Podostemoideae is an apomorphy while the presence is the plesiomorphic state. Such an interpretation is explained by three steps; the test for phylogenetic signal indicated that this number is statistically smaller than expected if they had evolved randomly (mean 13.5; median 14; P < 0.01).
Epidermal silica bodies occur in all subfamilies of Podostemaceae, whereas cortical silica bodies is a condition restricted to Podostemoideae (Mourera and clade F). MP and ML analyses show congruency for all ancestral states, which were reconstructed unambiguously (Fig. 4B). Both analyses indicate the presence of epidermal silica bodies (91.4 % likelihood) in the common ancestor of Tristichoideae (Podostemoideae + Weddellinoideae). A transition to silica bodies in cortical cells occurred in the ancestor to clade B (99.9 % likelihood). Silica bodies in cortical cells is an apomorphy, while epidermal silica bodies is plesiomorphic in Podostemaceae. Change in the location of silica bodies is explained through one step; this number is statistically smaller than expected if this character had evolved randomly (mean 7.3; median 7; P < 0.01).
In relation to the surface of silica bodies, our results indicate that each morphological type was restricted to least inclusive clades (e.g. verrucose surface in the Mourera clade). Consequently, MP and ML analyses were ambiguously reconstructed for the majority of clades (Fig. 5A); the exceptions were the most recent ancestors of Mourera and clade F. In Podostemoideae, clade A presented greater diversity of surface types than clade B. MP and ML analyses indicate that the most probable character states in the common ancestors to Mourera and clade F were verrucose (99.2 % likelihood) and granulate (98.5 % likelihood), respectively; these character states can be interpreted as synapomorphies. The evolution of silica body surface requires at least seven steps to be explained; this number is statistically smaller than expected if this character had evolved randomly (mean 12.8; median 13; P < 0.01). Silica bodies with verrucose or granulate surfaces can be interpreted as apomorphic states in Podostemaceae.
The absence of a lumen is hypothesized as the character state of the ancestor to all Podostemaceae by MP analysis (Fig. 5B). Both analyses (MP and ML) indicate that this feature appeared in the ancestor of clade A (90.8 % likelihood). One step is necessary to explain the evolution of this character; this number is statistically smaller than expected if this character had evolved randomly (mean 6; median 6; P < 0.01). Considering that a lumen arose in clade A, MP and ML analyses ambiguously reconstructed the character ‘lumen shape’ to its common ancestor (Fig. 6; circular shape with 42.8 % likelihood). MP analysis indicates the presence of an elongate lumen in the common ancestor of Ceratolacis (Cipoia + Podostemum). There are two steps required to recover the evolutionary history of this character; this number is not statistically smaller than expected if this character had evolved randomly (mean 2; median 2; P = 1). Silica bodies with a lumen can be interpreted as an apomorphy in Podostemaceae, although further studies are needed to test this hypothesis. In addition, their irregular shape can be considered an apomorphy in clade A and their irregular shape a synapomorphy to Cipoia ramosa.
DISCUSSION
Our phylogenetic analyses corroborate previous studies (Ruhfel et al., 2011, 2016; Tippery et al., 2011; Koi et al., 2012). However, Diamantina lombardii was placed in clade A with moderate bootstrap support. This topology diverges from that proposed in preceding analyses which have placed this species as sister to the remaining Podostemoideae (Ruhfel et al., 2011) or as sister to the strictly neotropical clade (represented by our clade B), both of which are poorly supported. Further analyses including additional molecular markers are required for a more consistent phylogenetic framework.
Presence–absence of silica bodies
Silica bodies have been reported for some families of Malpighiales, which are not closely related to Podostemaceae, such as Euphorbiaceae (Berry and Wiedenhoeft, 2004), Erythroxylaceae (Rury, 1981) and Chrysobalanaceae (Welle, 1976). Our MP and ML analyses were unable to resolve whether these structures were present in the common ancestor of Podostemaceae + Hypericaceae. Inasmuch as anatomical analyses in Calophyllaceae (sister group of Podostemaceae + Hypericaceae) did not report the presence of silica bodies (e.g. Caddah et al., 2012), their appearance in Podostemaceae can be interpreted as an evolutionary novelty, and their absence in some taxa as an apomorphic character state (i.e. a reversion, when Malpighiales is taken into account). According to Sangster and Hodson (1986), Podostemaceae is the only submerged vascular aquatic plant family in which silicification is reported. Hydrostachyaceae (Cornales), another group of submerged freshwater angiosperms with a rheophytic habit, presents clustered crystals of calcium but no silica bodies (Solereder, 1908). The presence or absence of these structures is also reported in palaeotropical species of Tristichoideae and Podostemoideae (Rutishauser and Huber, 1991; Mathew et al., 2001; Ameka et al., 2002, 2003). The transformation series presence–absence–presence of silica bodies, as observed here, was also reported for Commelinales by Prychid et al. (2003b).
Our results confirm the presence of silica bodies in five species that have already been reported in the literature: Apinagia richardiana (Schnell, 1967), Diamantina lombardii (Rutishauser et al., 2005; da Costa et al., 2011), Mourera aspera (Warming, 1888), Tristicha trifaria (Cario, 1881; Costa et al., 2011) and Weddellina squamulosa (Wächter, 1897; da Costa et al., 2011). We added new data for 19 species, in addition to the 16 species analysed that do not possess silica bodies. Lopes (2012) mentioned the presence of these structures in five Podostemum species (P. distichum, P. irgangii, P. muelleri and P. rutifolium); herein we extend the report to another three species, which means all species examined thus far in this genus have them, giving robustness to the morphological significance of this character state (discussed below in Morphology of silica bodies section). Although Warming (1888) detected silica bodies in outgrowths on the adaxial leaf surface of Mourera aspera, subsequent analyses by Steude (1935) and Lopes (2012) did not mention these structures. Herein, we report their presence in this species as well as in another three species of Mourera. Jäger-Zürn (2011) mentioned that vegetative organs of the genera Apinagia, Marathrum and Mourera are devoid of silica bodies. However, we noted the presence of these structures in all analysed species of these genera, except Apinagia fimbrifolia and Marathrum aeruginosum. The presence/absence of these structures in Apinagia and Marathrum reported here needs to be taken in context. Both genera have been reported to be polyphyletic by Tippery et al. (2011). It is noteworthy that our analyses corroborate Tippery et al. (2011) and place the analysed species of these genera embedded in two different strongly supported clades: clade D, grouping A. fimbrifolia, M. aeruginosum and species of different genera that are devoid of silica bodies; and clade G, grouping Apinagia sensu stricto (s.s.) and Marathrum s.s. that possess silica bodies. An analogous condition was found in the palaeotropical genus Polypleurum (Tul.) Warm. (Jäger-Zürn, 2011); only P. wallichii (R. Br. ex Griff.) Warm. (as P. minus (Wedd)), out of four species analysed, presented silica bodies.
The presence/absence of silica bodies is a stable attribute at several hierarchical levels (clades, genera and species) in which monophyly is supported by morphological (e.g. Castelnavia; Philbrick et al., 2009) and molecular (Tippery et al., 2011) data. The absence of silica bodies in leaves is a homoplasious apomorphy to clade D. This clade was first identified by Tippery et al. (2011) in their phylogenetic analyses of neotropical taxa (their clade J). The lack of silica bodies is the first non-molecular synapomorphy proposed for this clade. The absence of silica bodies in Wettsteiniola sp., the only unsampled genus in the phylogenetic framework presented here, in addition to the prostate stems and flowers arising in fascicles from fused leaf bases, could indicate affinities between it and Rhyncholacis. The above-mentioned observations indicate that silica body presence/absence is a conservative character at the genus level in neotropical taxa.
Metcalfe and Chalk (1950) hypothesized that the main role of silica bodies in Podostemaceae was mechanical strength, due their accumulation in peripheral tissue, forming a ‘carapace’. Although we are not able to fully corroborate such a proposition, we have observed that leaves which possess silica bodies in the epidermis (e.g. Cipoia) are stiffer and darker than those which possess silica bodies in subepidermal or perivascular cells (e.g. Apinagia and Mourera). Interestingly, species in which silica bodies are present or absent can occur sympatrically, sometimes on the same rock (e.g. Monostylis capillacea and Tristicha trifaria; Bove & Philbrick collections 1852 and 1853, respectively). This evidence indicates that the presence of these structures is not related to environmental factors as suggested by Jäger-Zürn (2011), thus enhancing their taxonomic and phylogenetic utility.
Location of silica bodies
Silica bodies were found in epidermal, subepidermal and perivascular cells. Solereder (1908) and Cook and Rutishauser (2007) pointed out that they are only detected in the peripheral tissues (i.e. epidermal and subepidermal layers) of roots, stems and leaves, forming a carapace. According to Solereder (1908), these structures are found in a continuous arrangement in the epidermis and discontinuous in subepidermis and perivascular cells; we observed this pattern in all analysed species. The first report of the occurrence of silica bodies in cortical cells in Podostemaceae was done by Schnell (1967) for Apinagia flexuosa (Tul.) P. Royen (a species not included in this study) and A. richardiana. We report this same condition in another 14 species, making this the first report of this condition in Marathrum, Mourera, Noveloa and Oserya.
Our results corroborate the location of silica bodies reported in the literature for the following species: Apinagia richardiana (Schnell, 1967), Diamantina lombardii (Rutishauser et al., 2005; da Costa et al., 2011), Podostemum ceratophyllum (Warming, 1881), P. scaturiginum (Warming, 1899), Tristicha trifaria (Cario, 1881; da Costa et al., 2011) and Weddellina squamulosa (Wächter, 1897; Koi and Kato, 2007; da Costa et al., 2011). Mello et al. (2011), in their taxonomic work, treated the outgrowths on the adaxial leaf surface of Mourera aspera as papillae and mentioned that these structures are composed of a set of siliceous cells. In our studies, we found silica bodies in the outgrowths and in subepidermal cells of Mourera aspera and M. fluviatilis. Lopes (2012) mentioned the presence of epidermal silica bodies in five species of Podostemum; herein we report this same condition in another three species. The information summarized here indicates that the location of these structures is a conservative character at the genus level. Nonetheless, we recognize the need for further analysis, mainly in species-rich genera such as Apinagia and Marathrum, in order to corroborate this hypothesis.
It is of interest that epidermal silica bodies in Podostemoideae are restricted to clade A. Given that the neotropical species embedded in this group are related to Old World taxa of Podostemoideae (Koi et al., 2012; Ruhfel et al., 2016), additional analyses of palaeotropical species are needed to verify possible variation in the location of silica bodies in palaeotropical species in this clade.
Morphology of silica bodies
Schnell (1967) and Dickison (2000) pointed out that silica bodies commonly assume the shape of the cells in which they occur, as these structures occupy the whole intracellular space (Solereder, 1908). Our results show congruency with this information, as elongate shape was detected in silica bodies that occur in perivascular cells, which are longer than epidermal or subepidermal cells (e.g. Apinagia longifolia). We presume that the great variety of silica body shapes observed in epidermal cells (lobate, oblong and rectangular) is a result of the presence of cells with different architectures in this tissue.
Comparing our results with those described by da Costa et al. (2011), who analysed silica bodies from vegetative shoots, we detected some differences in terms of shape: rectangular and oblong (vs. rectangular and polyhedral, here described) for Diamantina lombardii; rectangular (vs. rectangular and lobate, here described) for Tristicha trifaria; and triangular, elipsoid, ovate or oblong (vs. oblong, here described) for Weddellina squamulosa. These differences with da Costa et al. (2011) are a consequence of reinterpretation in D. lombardii and W. squamulosa, and the inclusion of silica bodies from the stems in T. trifaria, which probably have different shaped cells from those of leaves. The shape of the silica bodies in the leaves of Podostemum scaturiginum illustrated by Warming (1899) is in agreement with our description. Ameka et al. (2002), who analysed only two species (one voucher each) of Saxicolella Engl., asserted that there was little chance that silica bodies are taxonomically valuable characters due their homogeneous morphology in most Podostemaceae. However, our results refute this assertion, because we detected five distinct shapes and six types of surface morphology, in addition to the presence and shape of lumens (see below) in some taxa.
Solereder (1908) mentioned that silica bodies in Podostemaceae can possess smooth, crenate, undulate or perforate surfaces. We also found granulate (Apinagia, Marathrum and Oserya) and verrucose (Mourera) surfaces. Lopes (2012) mentioned the presence of ‘punctate’ ornamentation in Podostemum distichum and P. irgangii, which are similar to those reported here for P. scaturiginum and P. weddellianum. Some of the surface types depicted here were also found in the stems of palaeotropical taxa: for example, the undulate surface in Polypleurum wallichii (as P. minus (Wedd.) Nagendran, Arekal & Subramanyam) reported by Jäger-Zürn (2011) and perforated surface in two species of Saxicolella (Ameka et al., 2002). It is noteworthy that in Podostemoideae, undulate and perforate surfaces are restricted to clade A. Considering taxa of this clade (with the exception of Diamantina lombardii) have been placed in the ‘primarily Old World clade’ (Ruhfel et al., 2011: 316), further analyses including palaeotropical taxa are needed to describe the diversity of silica body surfaces in this clade. A crenate surface, similar to that of Tristicha trifaria, has been reported in several palaeoecological analyses of sediments and attributed to a Podostemaceae-type (Kennett et al., 2010; Erra, 2010; Garnier et al., 2013; Yost et al., 2018). Warming (1899) postulated that the surface diversity is a result of the influence of cellular organelles (e.g. nucleus, chloroplast and starch grains) on the silica body. Although we are not aware of any study which could point in this direction, we hypothesized that some of these organelles may be present during the silica body ontogenetic series. Based on this hypothesis, the great surface diversity presented by epidermal silica bodies (crenate, undulate, perforate and smooth) could indicate the presence of distinct ontogenetic series in epidermal silica bodies. It is important to note that clade A presented the greatest diversity in surface of silica bodies. The ambiguity retrieved in ancestral state reconstruction of silica body morphology can be attributed mainly to the sub-sampling of the aforementioned clade, once palaeotropical taxa were not included our analysis.
Ameka et al. (2002) were the first to observe a lumen in silica bodies of Podostemaceae (depicted in their fig. 25) In our previous analyses of Diamantina lombardii (da Costa et al., 2011), we incorrectly interpreted the lumen as a perforation; here it is reinterpreted as a lumen because it is a structure that is centrally located and much larger than perforations. Considering that the presence of a lumen was restricted to clade A in which neotropical taxa are closely related to Old World taxa (Ruhfel et al., 2011, 2016), further analyses in these latter species are needed to corroborate the presence of the lumen as a synapomorphy to this clade. In Podostemoideae, dorsiventral silica bodies were restricted to clade A. It is noteworthy that this trait has been reported from the stems of Saxicolella amicorum and S. submersa (Ameka et al., 2002) and on leaves of Diamantina lombardii (da Costa et al., 2011).
Based on the results, there were no clear interspecific differences in terms of size of silica bodies. Our results contrast with those of Krishnan et al. (2000) for 80 species of Poaceae, in which the authors provided an identification key based on shape and size (length and width). The factors that influence the size of silica bodies in neotropical Podostemaceae remain to be determined.
CONCLUSION
Our investigation shows the diversity of silica body attributes in neotropical Podostemaceae and their taxonomic and phylogenetic significance. The absence of silica bodies in most families of the clusioid clade could indicate that the appearance of these structures in Podostemaceae is a novelty that first arose in the ancestor of this family (synapomophy). The quantitative attributes evaluated were highly variable in intraspecific comparisons and of little taxonomic or phylogenetic utility. In contrast, qualitative characters (presence, location, surface type and lumen characteristics) are conservative at the specific and, in the same instances, the generic rank. Among the detected attributes, their location, the presence of a lumen and surface type are relevant as taxonomic and phylogenetic characters for the delimitation of clades, genera and/or species. Additionally, the positive correlation between phylogenetic support and character state transitions reinforce the phylogenetic relevance of silica bodies.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Appendix S1: Voucher specimens used in anatomical analysis. Appendix S2: GenBank accession numbers for sequences used in this study. Table S1: Range, arithmetic mean and coefficient of variation of analysed specimens.
ACKNOWLEDGEMENTS
We are grateful to Dr Vânia Gonçalves-Esteves for the use of the Palynology lab (Museu Nacional-UFRJ), under her responsibility, for extraction of silica bodies, the staff of R and WCSU for providing access to specimen materials, Amanda Veiga for operating the SEM at Departamento de Invertebrados (Museu Nacional-UFRJ) and Dr Brad Ruhfel for his comments on an early draft of the manuscript. We also acknowledge two anonymous reviewers for their valuable comments. The work was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) through a masters degree fellowship to F.G.C.M.C., by Conselho Nacional de Desenvolvimento Científico e Technológico (CNPq) grants PROTAX 562251/2010–3, REFLORA 563534/2010–9 and Productivity Grant (307870/2014–6) to C.P.B. The study was also supported by a National Science Foundation Grant (DEB-0444589) and Connecticut State University-AAUP research grants to C.T.P.
LITERATURE CITED
- Almeida-Cortez JS, Melo-de-Pinna GFA. 2006. Morphology and anatomy of a leaf mine in Vismia guianensis (Aubl.) Choisy (Clusiaceae) in a fragment of Brazilian Atlantic forest. Brazilian Journal of Biology 66: 759–763. [DOI] [PubMed] [Google Scholar]
- Ameka GK, Pfeifer E, Rutishauser R. 2002. Developmental morphology of Saxicolella amicorum and S. submersa (Podostemaceae: Podostemoideae) from Ghana. Botanical Journal of the Linnean Society 139: 255–273. [Google Scholar]
- Ameka GK, Clerk GC, Pfeifer E, Rutishauser R. 2003. Developmental morphology of Ledermaniella bowlingii (Podostemaceae) from Ghana. Plant Systematics and Evolution 237: 165–183. [Google Scholar]
- Ancíbor E. 1990. Anatomia de las espécies argentinas de Podostemum Michaux, (Podostemaceae). Parodiana 6: 31–47. [Google Scholar]
- Arber A. 1920. Water plants: a study of aquatic angiosperms. Cambridge: Cambridge University Press. [Google Scholar]
- Baas P. 1970. Anatomical contributions to plant taxonomy. I. Floral and vegetative anatomy of Eliaea from Madagascar and Cratoxylum from Indo-Malesia (Guttiferae). Blumea 18: 369–391. [Google Scholar]
- Berry PE, Wiedenhoeft AC. 2004. Micrandra inundata (Euphorbiaceae), a new species with unusual wood anatomy from black-water river banks in southern Venezuela. Systematic Botany 29: 125–133. [Google Scholar]
- Bove CP. 2018. Check-list da família Podostemaceae do Estado do Mato Grosso do Sul, Brasil. Iheringia, ser. bot. 72: 328–334. [Google Scholar]
- Bove CP, Philbrick CT. 2010. Neotropical Podostemaceae. In: Milliken W, Klitgård B, Baracat A, eds. Neotropikey - Interactive key and information resources for flowering plants of the Neotropics. Kew Royal Botanical Gardens; Available at: http://www.kew.org/science/tropamerica/neotropikey/families/Podostemaceae.htm (accessed 1 September 2017). [Google Scholar]
- Brown DA. 1984. Prospects and limits of a phytolith key for grasses in the central United States. Journal of Archaeological Science 11: 345–368. [Google Scholar]
- Cario R. 1881. Anatomische Untersuchungen von Tristicha hypnoides Spreng. Botanische Zeitung 39: 25–33. [Google Scholar]
- Caddah MK, Mayer JLS, Bittrich V, Amaral MCE. 2012. Species limits in the Kielmeyera coriaceae complex (Calophyllaceae) – a multidisciplinary approach. Botanical Journal of the Linnean Society 168: 101–115. [Google Scholar]
- Cook CDK, Rutishauser R. 2007. Podostemaceae. In: Kubitzki K, ed. The families and genera of vascular plants, Vol. 9 Berlin: Springer, 304–344. [Google Scholar]
- da Costa FGCM, Bove CP, Arruda RCO, Philbrick CT. 2011. Silica bodies and their systematic implications at the subfamily level in Podostemaceae. Rodriguésia 62: 937–942. [Google Scholar]
- da Costa FGCM, Souza PC, Klein DE, Bove CP. 2016. Application of acetolysis in phytoliths extraction. Review of Palaeobotany and Palynology 228: 93–97. [Google Scholar]
- Dickison WC. 2000. Integrative plant anatomy. San Diego: Academic Press. [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. 1930. Reihe Podostemales. In: Engler A, Prantl K, eds. Die natürlichen Pflanzenfamilien, 2nd ed, 18a. Leipzig: Engelmann, 1–68. [Google Scholar]
- Erra G. 2010. Asignación sistemática y paleocomunidades inferidas a partir del estudio fitolítico de sedimentos cuaternarios de Entre Ríos, Argentina. Boletín de la Sociedad Argentina Botánica 45: 309–319. [Google Scholar]
- Evett RR, Bartolome JW. 2013. Phytolith evidence for the extent and nature of prehistoric Californian grasslands. Holocene 23: 1644–1649. [Google Scholar]
- Figueiredo RCL, Handro W, Ferri MG. 1971. Corpos silicosos em gramíneas dos cerrados V. In: Ferri MG, ed. III Simpósio sobre o cerrado. São Paulo: Edgard Blücher, 215–231. [Google Scholar]
- Franklin GL. 1945. Preparation of thin sections of synthetic resins and wood-resin composites, and a new macerating method for wood. Nature 155: 51. [Google Scholar]
- Fujinami R, Yoshihama I, Imaichi R. 2011. Dimorphic chloroplasts in the epidermis of Podostemoideae, a subfamily of the unique aquatic angiosperm family Podostemaceae. Journal of Plant Research 124: 601–605. [DOI] [PubMed] [Google Scholar]
- Garnier A, Neumann K, Eichhorn B, Lespez L. 2013. Phytolith taphonomy in the middle-to late-Holocene fluvial sediments of Ounjougou (Mali, West Africa). Holocene 23: 416–431. [Google Scholar]
- Gu Y, Zhao Z, Pearsall DM. 2013. Phytolith morphology research on wild and domesticated rice species in East Asia. Quaternary International 287: 141–148. [Google Scholar]
- Guntzer F, Keller C, Catherine K, Meunier JD. 2012. Benefits of plant silicon for crops: a review. Agronomy for Sustainable Development 32: 201–213. [Google Scholar]
- Hart TC. 2015. Phytoliths: the storytelling stones inside plants. American Scientist 103: 136–143. [Google Scholar]
- Horner HT, Wagner BL. 1995. Calcium oxalate formation in higher plants. In: Khan SR, ed. Calcium oxalate in biological systems. Boca Raton: CRC Press, 53–72. [Google Scholar]
- Jäger-Zürn I. 2011. Neglected features of probable taxonomic value in Podostemaceae: the case of Polypleurum. Flora 206: 38–46. [Google Scholar]
- Johansen DA. 1940. Plant microtechnique. New York: McGraw Hill. [Google Scholar]
- Kennett DJ, Piperno DR, Jones JG, et al. 2010. Pre-pottery farmers on the Pacific coast of southern Mexico. Journal of Archaeological Science 37: 3401–3411. [Google Scholar]
- Kita Y, Kato M. 2001. Infrafamilial phylogeny of the aquatic angiosperm Podostemaceae inferred from the nucleotide sequences of matK gene. Plant Biology 3: 156–163. [Google Scholar]
- Koi S, Kato M. 2007. Developmental morphology of the shoot in Weddellina squamulosa and implications for shoot evolution in the Podostemaceae. Annals of Botany 99: 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koi S, Kita Y, Hirayama Y, Rutishauser R, Huber KA, Kato M. 2012. Molecular phylogenetic analysis of Podostemaceae: implications for taxonomy of major groups. Botanical Journal of the Linnean Society 169: 461–492. [Google Scholar]
- Krishnan S, Samson NP, Ravichandran P, Narasimhan D, Dayanandan P. 2000. Phytoliths of Indian grasses and their potential use in identification. Botanical Journal of the Linnean Society 132: 241–252. [Google Scholar]
- Lopes EFM. 2012. Anatomia dos órgãos vegetativos do gênero Podostemum Michx sensu stricto e grupos externos (Podostemoideae, Podostemaceae). MSc dissertation, Universidade Federal do Paraná, Brazil. [Google Scholar]
- Łotocka B, Osińska E. 2010. Shoot anatomy and secretory structures in Hypericum species (Hypericaceae). Botanical Journal of the Linnean Society 163: 70–86. [Google Scholar]
- Lu H, Liu K. 2003. Phytoliths of common grasses in the coastal environment of southeastern USA. Estuarine Coastal and Shelf Science 58: 587–600. [Google Scholar]
- Madella M, Alexandre A, Ball T. 2005. International code for phytolith nomenclature 1.0. Annals of Botany 96: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. 2017. Mesquite: a modular system for evolutionary analysis. Version 3.2. Available at: http://mesquiteproject.org.
- Maddison WP, Slatkin M. 1991. Null models for the number of evolutionary steps in a character on a phylogenetic tree. Evolution 45: 1184–1197. [DOI] [PubMed] [Google Scholar]
- Mathew CJ, Jäger-Zürn I, Nileena CB. 2001. Dalzellia gracilis: a new species of Podostemaceae (Tristichoideae) from Kerala, India. International Journal of Plant Sciences 162: 899–909. [Google Scholar]
- Mello AS, Tavares AS, Trevisan R. 2011. Podostemaceae in southern Brazil. Rodriguésia 62: 867–885. [Google Scholar]
- Metcalfe CR, Chalk L. 1950. Anatomy of the dicotyledons: leaves, stem, and wood in relation to taxonomy, with notes on economic uses. Oxford: Clarendon Press. [Google Scholar]
- O’Brien TP, Feder N, Mccully ME. 1964. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59: 367–373. [Google Scholar]
- Philbrick CT, Bove CP, Edson TC. 2009. Monograph of Castelnavia (Podostemaceae). Systematic Botany 34: 715–729. [Google Scholar]
- Prychid CJ, Rudall PJ, Gregory M. 2003a Systematics and biology of silica bodies in monocotyledons. The Botanical Review 69: 377–440. [Google Scholar]
- Prychid CJ, Furness CA, Rudall PJ. 2003b Systematic significance of cell inclusions in Haemodoraceae and allied families: silica bodies and tapetal raphides. Annals of Botany 92: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhfel BR, Bittrich V, Bove CP, et al. 2011. Phylogeny of the clusoid clade (Malpighiales): evidence from the plastid and mitochondrial genomes. American Journal Botany 98: 306–325. [DOI] [PubMed] [Google Scholar]
- Ruhfel BR, Bove CP, Philbrick CT, Davis CC. 2016. Dispersal largely explains the Gondwanan distribution of the ancient tropical clusioid plant clade. American Journal of Botany 103: 1117–1128. [DOI] [PubMed] [Google Scholar]
- Rury PM. 1981. Systematic anatomy of Erythroxylum P. Browne: practical and evolutionary implications for the cultivated cocas. Journal of Ethnopharmacology 3: 229–263. [DOI] [PubMed] [Google Scholar]
- Rutishauser R. 1997. Structural and developmental diversity in Podostemaceae (river-weeds). Aquatic Botany 57: 29–70. [Google Scholar]
- Rutishauser R, Grubert M. 1994. The architecture of Mourera fluviatilis (Podostemaceae): mature structures and leaf development. Botanica Helvetica 104: 179–194. [Google Scholar]
- Rutishauser R, Huber KA. 1991. The developmental morphology of Indotristicha ramosissima (Podostemaceae, Tristichoideae). Plant Systematics and Evolution 178: 195–223. [Google Scholar]
- Rutishauser R, Pfeifer E, Novelo RA, Philbrick CT. 2005. Diamantina lombardii – an odd Brazilian member of the Podostemaceae. Flora 200: 245–255. [Google Scholar]
- Sá-Haiad B, Torres CA, Abreu VHR, et al. 2010. Floral structure and palynology of Podostemum weddellianum (Podostemaceae: Malpighiales). Plant Systematics and Evolution 290: 141–149. [Google Scholar]
- Sangster AG, Hodson MJ. 1986. Silica in higher plants. In: Evered D, O’Connor M, eds. Silicon biochemistry, Edition 121 New York: John Wiley & Sons, 90–111. [DOI] [PubMed] [Google Scholar]
- Schnell R. 1967. Études sur l’anatomie et la morphologie des Podostemacees. Candollea 22: 157–225. [Google Scholar]
- Sereno PC. 2007. Logical basis for morphological characters in phylogenetics. Cladistics 23: 565–587. [DOI] [PubMed] [Google Scholar]
- Silvestro D, Michalak I. 2012. raxmlGUI: A graphical front-end for RAxML. Organisms Diversity and Evolution 12: 335–337. [Google Scholar]
- Solereder H. 1908. Systematic anatomy of the dicotyledons: a handbook for laboratories of pure and applied botany, Vol. II Oxford: Clarendon Press. [Google Scholar]
- Sposito G. 2008. The chemistry of soils. New York: Oxford University Press. [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steude H. 1935. Beiträge zur Morphologie und Anatomie von Mourera aspera. Beihefte zum botanischen Centralblatt 53: 627–650. [Google Scholar]
- Thiers B. 2017. Index Herbariorum: a global directory of public herbaria and associated staff. [continuosly updated.] New York Botanical Garden’s Virtual Herbarium; Available at: http://sweetgum.nybg.org/ih/. [Google Scholar]
- Tippery NP, Philbrick CT, Bove CP, Les DH. 2011. Systematics and phylogeny of neotropical riverweeds (Podostemaceae: Podostemoideae). Systematic Botany 36: 105–118. [Google Scholar]
- Tulasne LR. 1852. Podostemacearum monographia. Archive du Muséum d’Histoire Naturelle 6: 1–208. [Google Scholar]
- Tur NM. 1987. Podostemaceae. In: Troncoso NS, Bacigalupo NM, eds. Flora Ilustrada de Entre Ríos (Argentina). Collección Científica del Instituto Nacional de Tecnología Agropecuária 6: 43–54. [Google Scholar]
- Wächter W. 1897. Beiträge zur Kenntniss einiger Wasserpflanzen. Flora oder Allgemeine botanische Zeitung 83: 367–397. [Google Scholar]
- Warming E. 1881. Familien Podostemaceae I. Det Kongelige Danske Videnskabernes Selskabs Skrifter, Naturvidenskabelig og Mathematisk Afdeling 2: 1–34. [Google Scholar]
- Warming E. 1888. Familien Podostemaceae III. Det Kongelige Danske Videnskabernes Selskabs Skrifter, Naturvidenskabelig og Mathematisk Afdeling 4: 443–514. [Google Scholar]
- Warming E. 1899. Familien Podostemaceae V. Det Kongelige Danske Videnskabernes Selskabs Skrifter, Naturvidenskabelig og Mathematisk Afdeling 9: 105–154. [Google Scholar]
- Welle BJH. 1976. On the occurrence of silica grains in the secondary xylem of the Chrysobalanaceae. IAWA Bulletin 2: 19–29. [Google Scholar]
- Yost CL, Jackson LJ, Stone JR, Cohen AS. 2018. Subdecadal phytolith and charcoal records from Lake Malawi, East Africa imply minimal effects on human evolution from the∼74 ka Toba supereruption. Journal of Human Evolution 116: 75–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.