Abstract
X-linked hypophosphatemia (XLH) is associated with a pervasive, severe degenerative osteoarthritis. We conducted a retrospective chart review/patient survey using the Knee or Hip Osteoarthritis Outcome Score Physical Function Short Form. Fourteen total knee arthroplasties and 7 total hip arthroplasties among 11 patients were included. The mean KOOS-PS score was 31.4 ± 9.7 with a mean follow up of 6.9 years. Mean HOOS-PS score was 14.8 ± 12.9 at a mean follow up of 7.6 years. One knee failed due to aseptic loosening and one hip was revised due to polyethylene wear. In conclusion, total joint arthroplasty is beneficial in XLH.
Keywords: X-linked hypophosphatemia, Osteomalacia, Phosphate-wasting disorders, Knee or hip osteoarthritis outcome score physical function short form, Knee arthroplasty, Hip arthroplasty
1. Introduction
X-linked hypophosphatemia (XLH) is the most common form of heritable rickets with an incidence of approximately 1 in 20,000.1 XLH arises from an inactivating mutation in the phosphate-regulating gene with homology to endopeptidases located on the X chromosome (PHEX) gene.2The loss of PHEX activity results in an excess of fibroblast growth factor 23 (FGF23), a phosphatonin that causes renal phosphate wasting and low levels of 1,25(OH)2 vitamin D3.3 These effects combine to result in rickets and osteomalacia in children and osteomalacia in adults. Patients present in childhood with short stature, bowing of the legs and medial tibial rotational torsion.4
In XLH, joint degeneration occurs prematurely, is more pervasive, and involves multiple joints.5, 6, 7 Therapeutic interventions to minimize the impact on joints in the phosphate-wasting environment are limited by a lack of understanding in the etiology of the degenerative arthritis of XLH. Studies conducted in a murine model of XLH, the Hyp mouse, revealed significant changes to the normal bi-zonal architecture of articular cartilage, lacking a mineralized zone of articular chondrocytes while assuming a homogeneous single zone of chondrocytes in an unmineralized matrix.5 However, treatment of Hyp mice with oral phosphorus and calcitriol restores the normal architecture of the cartilage suggesting that preservation of the articular joint with ongoing therapy may positively impact the progression of the disorder. Nonetheless, there is currently no consensus on the treatment of adult patients with XLH.5,8,9 The recent introduction of a monoclonal antibody targeting the phosphatonin FGF23 may likewise impact the progression of the disorder.10, 11, 12
However, there is little research aimed at finding a better way to manage the arthritis associated with XLH. One study by Larson et al. of eight patients with XLH showed that joint replacement is an effective treatment for XLH associated arthritis.13 An extensive literature search revealed no further research on this topic. In this study, we aimed to further validate the findings by Larson et al.
2. Materials and methods
We conducted a retrospective chart review and a patient survey. Institutional review board approval was obtained. Participants were identified and recruited from the XLH Network, which is a patient advocacy organization. Patients were surveyed using the Knee or Hip Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS or HOOS-PS). The KOOS-PS and HOOS-PS were developed using the Rasch method and have been validated to assess physical function.14,15 The KOOS-PS is seven items and the HOOS-PS is a five-item questionnaire. Raw scores were collected and converted using the published conversion chart. Scores range from 0 (no difficulty) to 100 (extreme difficulty). In addition, we reviewed operative notes, clinic notes, and imaging studies.
Inclusion criteria were age 18 and over, a diagnosis of XLH, and either a total hip or knee replacement with a minimum follow up time of two years post arthroplasty. Exclusion criteria were age under 18, no diagnosis of XLH, a joint replacement other than the hip or knee, and a lack of two year follow up time.
A linear mixed model was used to test if either cement or medication use before or after surgery were predictive of better functioning after surgery, as measured by the KOOS-PS and HOOS-PS. For cement, models were run separately for hip and knee replacements. For medical treatment before or after surgery, models were run jointly for hips and knees. All models included fixed effects for follow-up in months since surgery as well as either medication or cement use, depending on the variable being tested. A random effect included the intercept that varied by patient to account for the correlation of scores given by the same patient. Statistical significance was set at an alpha level of .05 and analyses were conducted in SPSS v24. All means are reported ± standard deviation.
3. Results
3.1. Knee arthroplasty
A total of 14 TKAs were reported among nine patients (Table 1). The mean age at time of surgery was 50 ± 9 years old and mean BMI was 29.5 ± 5.9. Eight patients were female and one patient was male. The mean KOOS-PS score reported was 31.4 ± 9.7 with a mean follow up time of 6.86 ± 3.5. Implant survivorship was 93%.
Table 1.
| Patient | Laterality | Age at Time of Surgery | BMI | KOOS-PS Score | Follow-up Time (yrs) | Tibial Component | Femoral Component | Cement | Tournequet Time (mins) | Medical Treatment | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Right | 59.5 | 25.0 | 35.3 | 2.9 | N/A | PS* | Yes | N/A | No | Patellar tendon avulsion |
| 2 | Left | 48.4 | 34.0 | 33.6 | 2.6 | FB* | Constrained | Yes | 70 | Yes | Compartment syndrome |
| 3 | Left | 51.3 | 28.0 | 24.9 | 7.0 | MB* | PS* | Yes | 100 | No | None |
| 3 | Right | 50.2 | 28.0 | 24.9 | 8.2 | MB* | PS* | Yes | 120 | No | Revision 6 yrs post primary TKA for aseptic loosening |
| 4 | Left | 53.9 | 34.4 | 24.9 | 4.0 | FB* | PS* | Yes | 63 | Yes | None |
| 4 | Right | 56.2 | 34.4 | 10.5 | 3.2 | FB* | PS* | No | 37 | Yes | None |
| 5 | Left | 52.2 | 39.1 | 42 | 6.3 | FB* | PS* | Yes | N/A | No | None |
| 5 | Right | 52.7 | 39.1 | 40.3 | 5.8 | FB* | N/A | Yes | N/A | No | None |
| 6 | Left | 52.3 | 23.9 | 40.3 | 7.9 | MB* | PS* | Yes | 84 | No | None |
| 6 | Right | 52.3 | 23.9 | 42 | 7.9 | MB* | PS* | Yes | 90 | No | None |
| 7 | Left | 31.0 | 24.0 | 27.5 | 13.3 | FB* | PS* | Femur only | 88 | No | None |
| 7 | Right | 30.1 | 24.0 | 24.9 | 14.3 | FB* | PS* | Femur only | 112 | No | None |
| 8 | Left | 58.7 | 33.0 | 24.9 | 5.2 | FB* | PS* | Yes | 48 | Yes | None |
| 9 | Left | 45.6 | 22.7 | 44 | 7.3 | FB* | CR* | Yes | N/A | No | None |
*FB: Fixed-Bearing MB: Mobile-Bearing PS: Posterior Stabilized CR: Cruciate Retaining.
Ten tourniquet times were listed in the operative reports, with a mean of 81.2 ± 26.8 min. One custom guide was used and one semi-constrained LCCK implant was used. Four tibial components were mobile bearing, nine were fixed bearing, and one operative report did not indicate the components used. Eleven femoral components were posterior stabilized, one was cruciate retaining, one was a constrained condylar knee implant, and one was not indicated in the operative report. Eleven implants were cemented, while two were a hybrid construct with only the femur cemented, and one was cementless (Fig. 1). A significant difference was noted by cement status (p = 0.002). The estimated mean KOOS-PS was lowest for the cementless knee (19.2 ± 4.0), next lowest for partially cemented knees (26.0 ± 10.4) and highest for the fully cemented knees (33.8 ± 3.2).
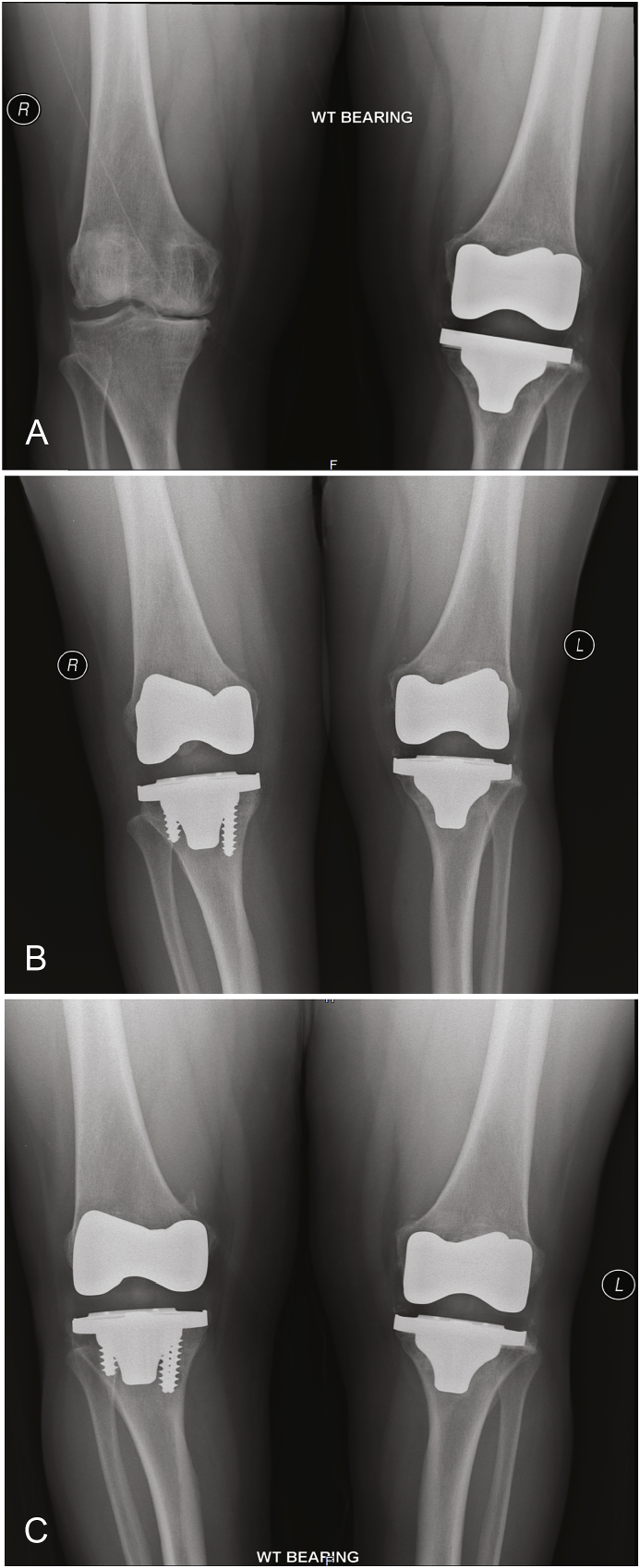
Fig. 1.
This is a 55 year old female (Table 1, patient 4) who presented with bilateral knee pain. (A) is an anteroposterior (AP) film of her bilateral knees 2 years post-operatively from her left TKA, which was a cemented posterior stabilized implant. (B) is an AP film one year post-operatively from her right TKA, which was a cementless posterior stabilized implant, and three yrs. post-operatively from her left TKA. (C) is an AP film three years post-operatively from her left TKA and five years post-operatively from her right TKA, showing no evidence of loosening or implant migration.
3.2. Complications
One patient with a cemented implant required revision 6 years post-operatively due to tibial component loosening (Fig. 2). One patient suffered a patellar tendon avulsion intra-operatively treated with a patellar tendon repair. One patient developed compartment syndrome with unknown etiology two days post operatively requiring emergent fasciotomy.
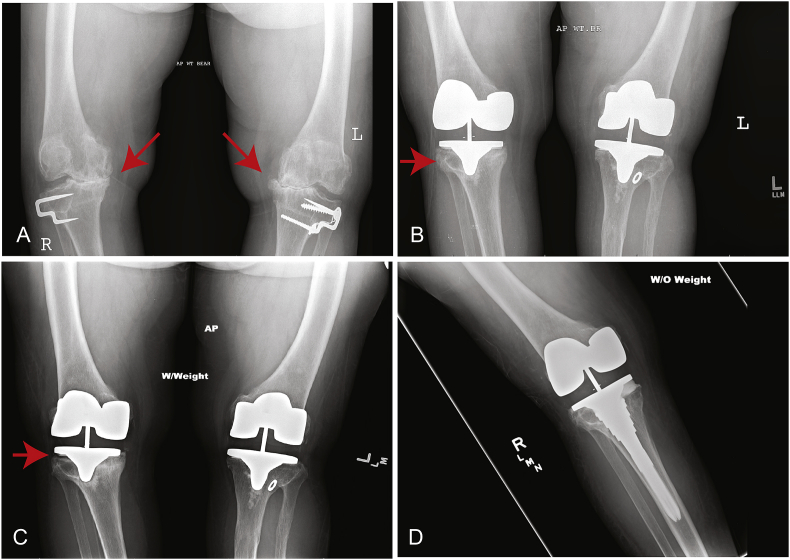
Fig. 2.
This is a 50 year old female (Table 1, Patient 3) who presented with severe medial compartment osteoarthritis bilaterally (A). (B) She underwent cemented bilateral TKA, and this is an anteroposterior (AP) film one year post-operatively from her right TKA and two years post-operatively from her left TKA. The tibial component on the right side is malaligned with respect to the tibial canal (arrow). (C) This is an AP film six years post-operatively from her right TKA. She was not experiencing any pain, but there is clear evidence of tibial component loosening (arrow) and migration. (D) This is an AP film after revision right TKA.
3.3. Hip arthroplasty
A total of seven THAs were reported among five patients (Table 2). The mean age at time of surgery was 50 ± 10 years old and mean BMI was 31.4 ± 5.0. One patient was male and four were female. The mean HOOS-PS score reported was 14.8 ± 12.9 with a mean follow up time of 7.6 years ±5.7 post-arthroplasty. Implant survivorship was 86%.
Table 2.
| Patient | Laterality | Age at Surgery | BMI | HOOS-PS | Follow-up time (yrs) | Bearing | Femoral Head Size | Cemented Femoral Component | Medical Treament | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | Left | 53.2 | 29.2 | 4.6 | 6.9 | COC* | 32 | No | Yes | No |
| 10 | Right | 51.6 | 29.2 | 4.6 | 8.5 | COC* | 32 | No | Yes | No |
| 11 | Right | 41.6 | 33.3 | 12.7 | 7.9 | C/PE* | 36 | No | No | Periprosthetic Fracture |
| 5 | Left | 55.9 | 39.1 | 37.7 | 2.5 | M/HCLPE* | 36 | No | No | No |
| 8 | Left | 59.8 | 33.0 | 4.6 | 4.2 | M/HCLPE* | 32 | No | No | No |
| 8 | Right | 60.4 | 33.0 | 12.7 | 3.5 | M/HCLPE* | 32 | No | No | No |
| 9 | Right | 33.6 | 22.7 | 26.9 | 19.5 | N/A | N/A | Yes | No | Revision 18.9 years after primary THA |
*COC: Ceramic on Ceramic C/PE: Ceramic on Polyethylene M/HCLPE: Metal on Highly Cross-Linked Polyethylene.
One revision stem was used, while the rest of the femoral components were primary. Two were ceramic on ceramic bearings, one was ceramic on polyethylene, three were metal on highly cross-linked polyethylene, and one did not indicate the bearing in the operative report. Four femoral heads were 32 mm, two were 36 mm, and one did not indicate the size. One femoral component was cemented, while the rest were press fit (Fig. 3). There was no significant difference in HOOS-PS scores between cemented and cementless prostheses (p = 0.165).
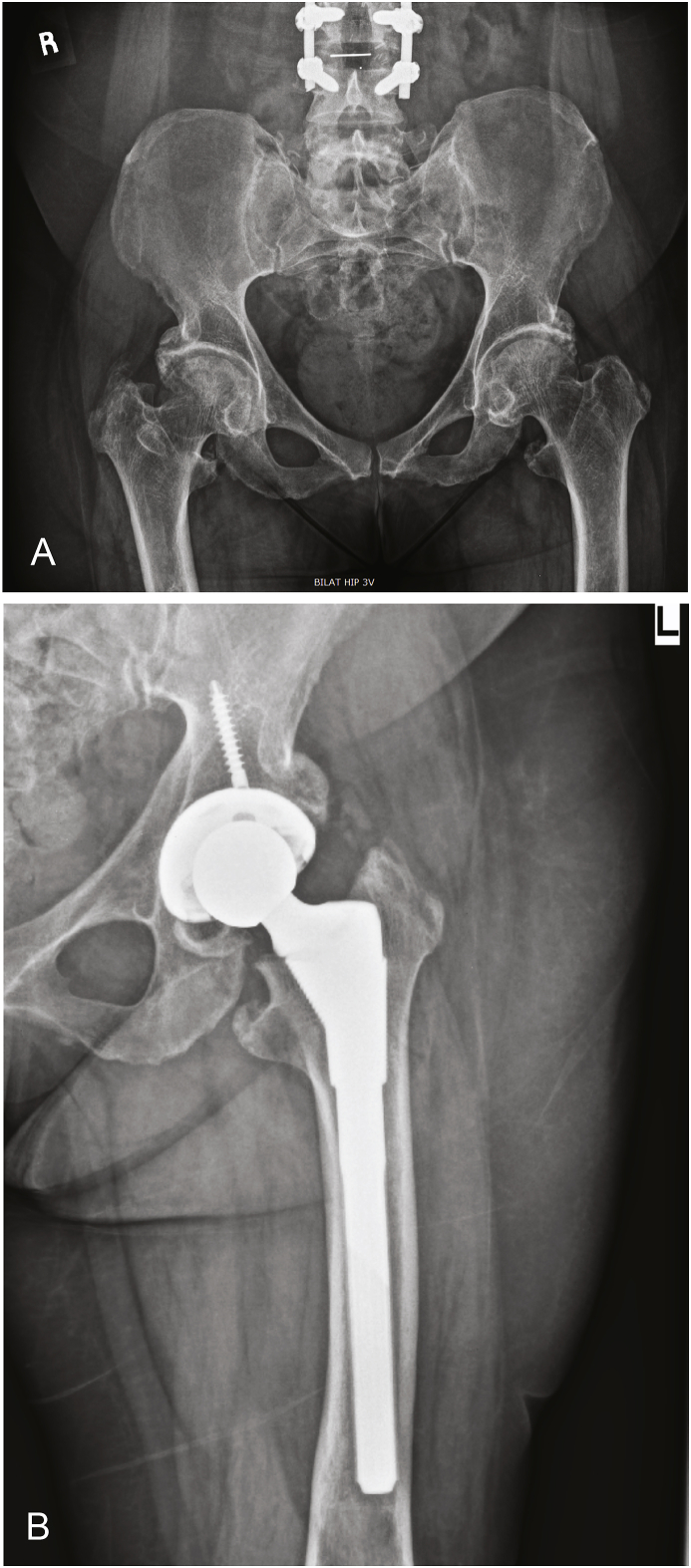
Fig. 3.
This is a 59 year old female (Table 2, Patient 8) presenting with bilateral hip pain who underwent bilateral cementless THA. (A) shows a pre-operative AP pelvis showing decreased bone quality and bilateral osteoarthritis (B) shows a post-operative AP X-Ray of the left hip with stable implant fixation and no evidence of complication.
3.4. Complications
One patient required revision 18.9 years post primary THA due to polyethylene wear. The femoral component was cemented and was not revised.
One patient suffered a periprosthetic fracture post operatively (Fig. 4). This patient had a cementless implant with a ceramic on polyethylene bearing. This patient's HOOS-PS score was 12.7 post-operatively, even considering her periprosthetic fracture, with a follow up time of 7.9 years.
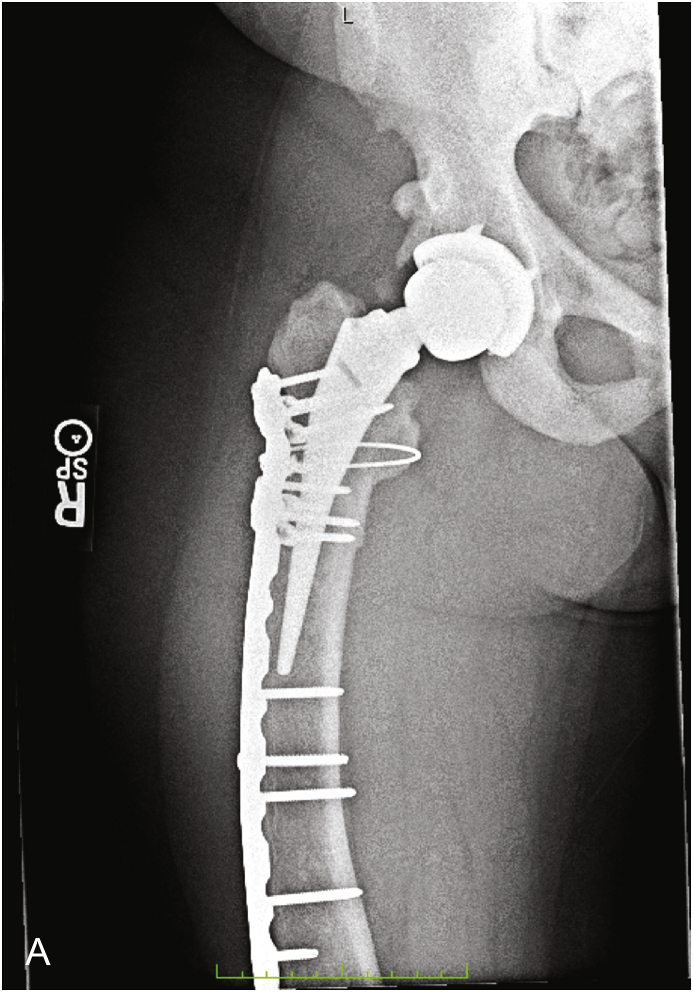
Fig. 4.
This is a 41 year old female (Table 2, Patient 11) who initially underwent cementless total hip arthroplasty and subsequently suffered a periprosthetic fracture. This is an anteroposterior film of her right hip post open reduction internal fixation of the fracture.
3.5. Medical treatment
Three patients (four knees) who underwent TKA and one patient (two hips) who underwent THA received medical treatment before or after surgery for XLH. Four patients received the standard phosphate and calcitriol treatment while one received teriparatide. We did not find a significant difference in KOOS-PS (treatment M = 23.5; no treatment M = 34.6; p = 0.083) or HOOS-PS (treatment M = 4.6; no treatment M = 18.9; p = 0.440) scores between patients who received medical treatment and those who did not, although our small sample size makes it difficult to draw conclusions from this data.
4. Discussion
This series is the second and largest study of total joint arthroplasty in patients with XLH. At midterm follow-up, we found that TKA and THA resulted in KOOS-PS and HOOS-PS scores that are similar to outcome scores in the unaffected population.16, 17, 18 One knee failed six years post-operatively due to aseptic loosening, and one hip was revised at 18.9 years due to polyethylene wear.
In this small series, we found a significant difference between patients who received cementless versus cemented implants. However, due to our sample size of one patient with a cementless knee, we are unable to draw any conclusions from this data. Patients with XLH have poor bone quality, which historically has been an indication for using cemented prostheses.19,20 However, patients often present at a younger age than the unaffected population and therefore may be candidates for cementless implants.20
Bone quality in XLH has been compared to that of renal osteodystrophy, a similar metabolic bone disease.13,21 Several studies have recommended cemented implants in renal osteodystrophy due to the potential for poor ingrowth,22,23 while others have shown no difference between cemented verses cementless implants.24,25 A recent study recommended the use of cemented implants in THA in patients with metabolic bone disease.26 However, this study did not specifically mention XLH in the discussion, although there was a discussion of osteomalacia. The results of the present study support the theory that cementless implants may be as effective as cemented implants in patients with metabolic bone disease. However, we only studied patients with XLH and had a small number of patients.
There has been one previous study by Larson et al. of THA and TKA in the XLH population.13 This study had 8 TKAs and 6 THAs from 8 total patients at a single institution. They found that total joint arthroplasty was an effective treatment for rickets-associated arthritis. Our small series reinforces Larson et al.‘s conclusion that TKA and THA are effective treatments for XLH associated arthritis.
In terms of medical treatment, it is recommended that patients be treated before and after surgery to reduce the risk of implant loosening. Patients should be referred to an endocrinologist before surgery, as the recommendation is to start medical treatment three-six months prior to joint replacement.4 We did not find that patients who did receive medical treatment either before or after surgery had improved outcomes as opposed to those who did not, although we had a small sample size making it difficult to interpret our results. In addition, the recently approved anti-FGF23 monoclonal antibody, burosumab, may improve bone ingrowth potential in the setting of familial phosphate-wasting disorders.10, 11, 12
Our study is limited by the small size of our sample due to the rarity of XLH. Additionally, any conclusions drawn from the data must be taken with caution because of the small sample size and the fact that different surgeons from different centers performed the surgeries. Surgical technique and expertise almost certainly differed between patients, which must be taken into account when interpreting these results.
5. Conclusion
TKA and THA are effective in the XLH population. Surgeons who attempt total joint replacement in this population should be prepared for a technically difficult surgery. Any conclusions drawn from this study should be taken with caution due to our small sample size and tailored to the patient. Although our findings support the use of total joint arthroplasty in the treatment of degenerative arthritis in individuals with XLH, more research is needed in this field to further determine which types of implants work best in the XLH population. Additionally, more research is needed to determine if cementless implants are a reasonable option for young patients with metabolic bone disease.
Funding
This work was supported, in part, by a grant from Ultragenyx Pharmaceutical, Novato, CA.
Acknowledgements
The authors wish to acknowledge the individuals with XLH who participated in this study; we are grateful for their continued commitment to better understanding the adult disorder. We also thank the readers at Ultragenxy Pharmaceutical for their helpful discussion.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2018.12.007.
Contributor Information
Emily S. Mills, Email: Emily.mills@quinnipiac.edu.
Louis Iorio, Email: louis.iorio@quinnipiac.edu.
Richard S. Feinn, Email: richard.feinn@quinnipiac.edu.
Kevin M. Duignan, Email: kevin.duignan@quinnipiac.edu.
Carolyn M. Macica, Email: carolyn.macica@quinnipiac.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Endo I., Fukumoto S., Ozono K. Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: prevalence, biochemical data and treatment. Endocr J. 2015;62(9):811. doi: 10.1507/endocrj.EJ15-0275. [DOI] [PubMed] [Google Scholar]
- 2.Du L., Desbarats M., Viel J., Glorieux F.H., Cawthorn C., Ecarot B. cDNA cloning of the murine Pex gene implicated in X-linked hypophosphatemia and evidence for expression in bone. Genomics. 1996;36(1):22. doi: 10.1006/geno.1996.0421. [DOI] [PubMed] [Google Scholar]
- 3.Prie D., Urena Torres P., Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009;75(9):882. doi: 10.1038/ki.2008.643. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter T.O., Imel E.A., Holm I.A., Jan de Beur S.M., Insogna K.L. A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26(7):1381. doi: 10.1002/jbmr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang G., Vanhouten J., Macica C.M. An atypical degenerative osteoarthropathy in Hyp mice is characterized by a loss in the mineralized zone of articular cartilage. Calcif Tissue Int. 2011;89(2):151. doi: 10.1007/s00223-011-9502-4. [DOI] [PubMed] [Google Scholar]
- 6.Hardy D.C., Murphy W.A., Siegel B.A., Reid I.R., Whyte M.P. X-linked hypophosphatemia in adults: prevalence of skeletal radiographic and scintigraphic features. Radiology. 1989;171(2):403. doi: 10.1148/radiology.171.2.2539609. [DOI] [PubMed] [Google Scholar]
- 7.Reid I.R., Hardy D.C., Murphy W.A., Teitelbaum S.L., Bergfeld M.A., Whyte M.P. X-linked hypophosphatemia: a clinical, biochemical, and histopathologic assessment of morbidity in adults. Medicine. 1989;68(6):336. [PubMed] [Google Scholar]
- 8.Liang G., Katz L.D., Insogna K.L., Carpenter T.O., Macica C.M. Survey of the enthesopathy of X-linked hypophosphatemia and its characterization in Hyp mice. Calcif Tissue Int. 2009;85(3):235. doi: 10.1007/s00223-009-9270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter T.O., Keller M., Schwartz D. 24,25 Dihydroxyvitamin D supplementation corrects hyperparathyroidism and improves skeletal abnormalities in X-linked hypophosphatemic rickets--a clinical research center study. J Clin Endocrinol Metabol. 1996;81(6):2381. doi: 10.1210/jcem.81.6.8964881. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X., Imel E.A., Ruppe M.D. Pharmacokinetics and pharmacodynamics of a human monoclonal anti-FGF23 antibody (KRN23) in the first multiple ascending-dose trial treating adults with X-linked hypophosphatemia. J Clin Pharmacol. 2016;56(2):176. doi: 10.1002/jcph.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Peyret T., Gosselin N.H., Marier J.F., Imel E.A., Carpenter T.O. Population pharmacokinetic and pharmacodynamic analyses from a 4-month intradose escalation and its subsequent 12-month dose titration studies for a human monoclonal anti-FGF23 antibody (KRN23) in adults with X-linked hypophosphatemia. J Clin Pharmacol. 2016;56(4):429. doi: 10.1002/jcph.611. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter T.O., Imel E.A., Ruppe M.D. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest. 2014;124(4):1587. doi: 10.1172/JCI72829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson A.N., Trousdale R.T., Pagnano M.W., Hanssen A.D., Lewallen D.G., Sanchez-Sotelo J. Hip and knee arthroplasty in hypophosphatemic rickets. J Arthroplasty. 2010;25(7):1099. doi: 10.1016/j.arth.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Perruccio A.V., Stefan Lohmander L., Canizares M. The development of a short measure of physical function for knee OA KOOS-Physical Function Shortform (KOOS-PS) - an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16(5):542. doi: 10.1016/j.joca.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Davis A.M., Perruccio A.V., Canizares M. The development of a short measure of physical function for hip OA HOOS-Physical Function Shortform (HOOS-PS): an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16(5):551. doi: 10.1016/j.joca.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 16.van de Groes S.A., Koeter S., de Waal Malefijt M., Verdonschot N. Effect of medial-lateral malpositioning of the femoral component in total knee arthroplasty on anterior knee pain at greater than 8 years of follow-up. Knee. 2014;21(6):1258. doi: 10.1016/j.knee.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Aveline C., Roux A.L., Hetet H.L. Pain and recovery after total knee arthroplasty: a 12-month follow-up after a prospective randomized study evaluating Nefopam and Ketamine for early rehabilitation. Clin J Pain. 2014;30(9):749. doi: 10.1097/AJP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 18.Rosenlund S., Broeng L., Holsgaard-Larsen A., Jensen C., Overgaard S. Patient-reported outcome after total hip arthroplasty: comparison between lateral and posterior approach. Acta Orthop. 2017;88(3):239. doi: 10.1080/17453674.2017.1291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tejwani N.C., Schachter A.K., Immerman I., Achan P. Renal osteodystrophy. J Am Acad Orthop Surg. 2006;14(5):303. doi: 10.5435/00124635-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Dalury D.F. Cementless total knee arthroplasty: current concepts review. Bone Joint Lett J. 2016;98-B(7):867. doi: 10.1302/0301-620X.98B7.37367. [DOI] [PubMed] [Google Scholar]
- 21.Kocaoglu M., Bilen F.E., Sen C., Eralp L., Balci H.I. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J Bone Joint Surg Br. 2011;93(1):52. doi: 10.1302/0301-620X.93B1.24788. [DOI] [PubMed] [Google Scholar]
- 22.Cheng E.Y., Klibanoff J.E., Robinson H.J., Bradford D.S. Total hip arthroplasty with cement after renal transplantation. Long-term results. J Bone Joint Surg Am. 1995;77(10):1535. doi: 10.2106/00004623-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Bradford D.S., Janes P.C., Simmons R.S., Najarian J.S. Total hip arthroplasty in renal transplant recipients. Clin Orthop Relat Res. 1983;107 [PubMed] [Google Scholar]
- 24.Toomey H.E., Toomey S.D. Hip arthroplasty in chronic dialysis patients. J Arthroplasty. 1998;13(6):647. doi: 10.1016/s0883-5403(98)80008-9. [DOI] [PubMed] [Google Scholar]
- 25.Murzic W.J., McCollum D.E. Hip arthroplasty for osteonecrosis after renal transplantation. Clin Orthop Relat Res. 1994;299:212. [PubMed] [Google Scholar]
- 26.Moya-Angeler J., Lane J.M., Rodriguez J.A. Metabolic bone diseases and total hip arthroplasty: preventing complications. J Am Acad Orthop Surg. 2017;25(11):725. doi: 10.5435/JAAOS-D-16-00067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.