Abstract
Background
Pin tract infection and loosening are major complications and challenges in the treatment of fractures by external fixation. To address this issue, we developed titanium pins coated with a fibroblast growth factor 2 (FGF-2)–apatite composite layer. The purpose of this initial clinical trial is to clarify the safety and feasibility of using these pins for the external fixation of distal radius fractures.
Methods
Unstable, displaced fractures of the distal radius that were medically suitable for external fixation were treated using external fixation pins coated and uncoated with an FGF-2–apatite composite layer. The coated pin group (n = 5) comprised 5 women (average age, 70.4 ± 5.9 years), whereas the uncoated pin group (n = 10) comprised 8 women and 2 men (average age, 64.4 ± 11.7 years). The average duration of external fixation was 40.8 ± 1.3 and 41.6 ± 2.1 days for the coated and uncoated pin groups, respectively.
Results
All patients achieved fracture union. One patient in the uncoated group had severe pin tract infection on the day of pin extraction. No pin loosening or difficulty in pin removal was observed in either group. Bacterial growth was present in 5% and 25% of the pin sites in the coated and uncoated groups, respectively (p = 0.059). No adverse events such as tumor formation were observed for more than 2 years after surgery in the coated pin group.
Conclusions
This study clarified the safety and feasibility of using pins coated with an FGF-2–apatite composite layer for the external fixation of distal radius fractures.
Keywords: Fibroblast growth factor 2 (FGF-2), Apatite, Coating, External fixation, Distal radius fractures, Initial clinical trial, Safety
1. Introduction
Pin tract infection and loosening present a challenge to the treatment of fractures by external fixation. Mild pin tract infections need improved wound care and treatment with antibiotics, whereas in severe cases, the affected pins are removed or changed.1 When pin loosening is associated with infections, the external fixator treatment is discontinued.
Previous studies on reducing pin tract complications include those on improving surgical procedures1 or pin tract care,2 loading bactericides such as silver and chlorhexidine onto pins,3 and coating pins with hydroxyapatite (HA).4 Although HA is a biocompatible and an osteoconductive material, a plasma-sprayed thick and porous HA coating is more susceptible to infection in animal studies than a thin HA coating or noncoating when they are contaminated with bacteria or implanted percutaneously.5
Coatings of fibroblast growth factor 2 (FGF-2)–apatite composite have led to increased bone-screw interface strength and reduced infection rates in screw tracts in animal studies. An FGF-2–apatite composite layer was formed on the surface of titanium implants using the coprecipitation method.6 FGF-2 promotes bone formation at optimal doses7 and accelerates angiogenesis and wound healing.8 In rats, an FGF-2–apatite composite layer with the optimal FGF-2 dose was demonstrated to enhance bone formation compared with that with an apatite layer with no FGF-2.9 In the rabbit tibial percutaneous implantation model, the FGF-2–apatite composite layer on the titanium screw increased bone-screw interface strength by 1.3 times and decreased pin tract infection rates by 0.47 times compared with those without the composite layer.6 The improved infection resistance is attributed to improved wound healing and formation of highly vascularized Sharpey's fiber-like tissue at the interface between the pin and soft tissue.10 These effects of FGF-2–apatite composite layers may occur in humans as well.
We conducted an initial clinical trial using the external fixation treatment with titanium pins coated with the FGF-2–apatite composite layer compared with uncoated pins to clarify the safety and feasibility of using these pins for the external fixation of distal radius fractures.
2. Materials and methods
2.1. Ethics approval and consent to participate
This study was conducted in compliance with the Declaration of Helsinki. The authors conducted the study according to the conditions approved by the ethics committees of University of Tsukuba Hospital and Mito Kyodo Hospital (H23-37 and H25-124) and National Institute of Advanced Industrial Science and Technology (I 2013-031, 031A, I 2014-031, 031ABC). Patients were informed about the study before participation and provided verbal and written consent to participate in the study.
2.2. Preparation and characterization of titanium pins coated with an FGF-2–apatite composite layer
Self-drilling Schanz screws (Seldrill™; DePuy Synthes, Zuchwil, Switzerland) were aseptically coated with the FGF-2–apatite composite layer by immersing the pins in a supersaturated calcium phosphate solution containing FGF-2 (4.0 μg/mL) at 37 °C for 48 h. The coating procedure was performed under the air cleanliness condition class 5 (ISO 14644-1), using a clean bench in the clean room (class 6) of the Cell Processing Factory at University of Tsukuba Hospital. The coating procedure is similar to, but slightly modified from, that of a previous study.9 The supersaturated calcium phosphate solution was prepared by mixing clinically available infusions, injection fluids, and pharmaceuticals, as follows: Meylon® injection 7% (NaHCO3; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan), water for injection (FUSO Pharmaceutical Industries, Ltd., Osaka, Japan), Klinisalz® (H2PO4−; KYOWA CritiCare Co., Ltd., Tokyo, Japan), dibasic potassium phosphate injection 20mEq kit (Terumo Co., Tokyo, Japan), Ringer's solution, calcium chloride corrective injection, normal saline (Otsuka Pharmaceutical Co., Ltd.), and Fiblast® spray (FGF-2; Kaken Pharmaceutical Co., Ltd., Tokyo, Japan).
Six pins per patient were coated with the FGF-2–apatite composite layer, with four pins being for implantation, one for a spare, and one for quality examination. In the quality examination, deposited calcium (Ca) and phosphorus (P) were measured using an inductively coupled plasma atomic emission spectrometer (SPS7800; Seiko Instruments, Inc., Tokyo, Japan) after dissolving the FGF-2–apatite composite layer with a citric acid solution. The amount of FGF-2 in the solution was determined by the Bradford method using a protein quantitation kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). The mitogenic activity due to the FGF-2 in the solution was examined by fibroblastic NIH3T3 cell proliferation and compared with that of a solution prepared by dissolving apatite layers without FGF-2. The FGF-2 mitogenic activity was expressed as the relative cell growth rate for the former solution compared with the latter. After coating, the supersaturated calcium phosphate solutions used were subjected to bacteriological examination, endotoxin tests (turbidimetric endotoxin detection system; Wako, Inc. Tokyo, Japan), and β-d-glucan tests.
2.3. Clinical study
The study was a prospective open-label controlled feasibility study. We enrolled 15 patients (aged ≥20 to ≤85 years) with unstable and displaced fractures of the distal radius that were medically suitable to using external fixation (FGF-2–apatite-coated pin group, n = 5; uncoated pin group, n = 10). Patients who had a skin disease, who had a severe systemic disease (heart, lung, liver, or kidney disease etc.), who had a malignant tumor within 5 years before the fracture, who were pregnant or were suspected to be pregnant, who lacked voluntary capacity to give verbal and written consent, or who were determined by the doctor as inappropriate for the surgery were excluded. The fractures were treated with external fixation using half-pins coated or uncoated with an FGF-2–apatite composite layer following fragment fixation with Kirschner wire pinning or a volar locking plate.
2.4. Treatment
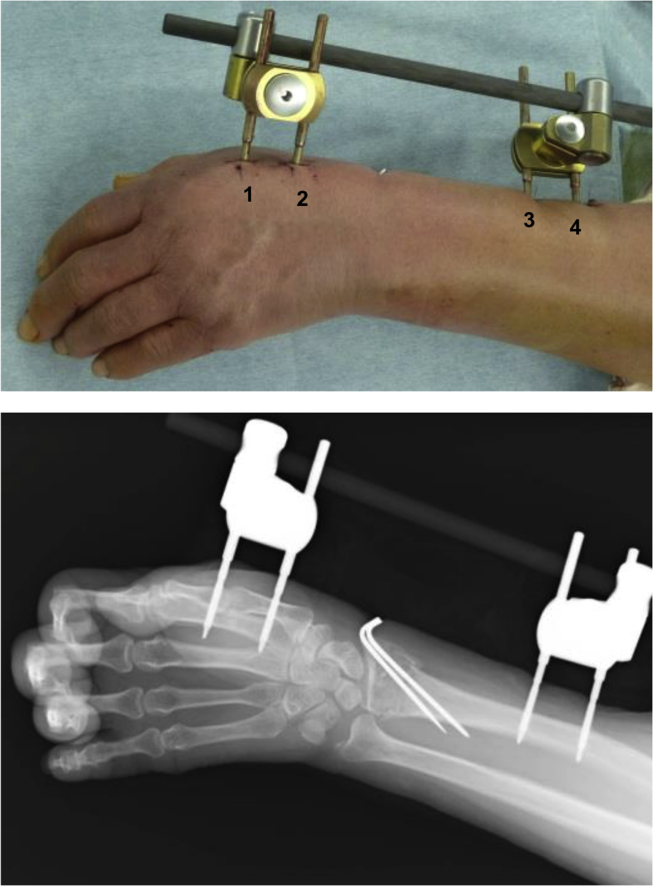
All surgical procedures were performed by one surgeon at the University of Tsukuba Hospital between February 2013 and January 2015 for the procedures using coated pins and by two surgeons at the University of Tsukuba Hospital or Mito Kyodo Hospital between January 2015 and August 2017 for those using uncoated pins. All operations were done with external fixation using the External Distal Radius Fixator (bridging type and unilateral type fixator; DePuy Synthes) following the original operation manual for standardizing pin insertion techniques. Two pins were inserted into the radial shaft, and two other pins were inserted into the second metacarpal (Fig. 1).
Fig. 1.
External fixation using pins coated with a FGF-2−apatite composite layer. Pins inserted at the distal and proximal sites of the second metacarpal and at the distal and proximal sites of the radial diaphysis were designated as pins 1, 2, 3, and 4, respectively.
All the patients were intravenously administered with 1 g prophylactic cephalosporin immediately preoperatively and 1 g every 8 h for 24 h postoperatively. The external fixators and pins were removed 6 weeks after surgery unless any adverse events (major infection, need for reoperation, or complication related to the coated pins) occurred.
2.5. Patient follow-up
The patients in both groups were medically assessed each week for 6 weeks, and the patients in the coated group were also assessed 2 years after pin insertion. The pin tracts were examined, cleaned with saline solution, and dressed with new gauze once a week by the surgeons who had performed the fracture surgery until pin removal at 6 weeks. The patients did not perform any daily pin tract care at home.
2.6. Evaluation measures
The intraoperative evaluation measure was insertion peak torque. The insertion peak torque was measured when the pins were inserted to the desired depth in the bone, using a digital torque wrench (type HTG2-5G; Imada, Toyohashi, Aichi, Japan).
Postoperative evaluation measures examined on and before pin extraction included pin tract rarefaction, pin tract fracture, neurovascular and tendon injury, necessity of additional operation, pin tract infection, pain levels, and extraction peak torques. Most of the measures were assessed at weekly visits. Pin tract rarefaction was assessed according to the Aro classification system using standard radiographs in the anteroposterior, lateral, and oblique projections taken immediately after surgery and at weekly visits.11 Group 1 was considered stable (no rarefaction); group 2 was unstable (rarefaction only at the entry cortex); group 3 showed loosening (rarefaction at both cortices).11 The rarefaction, which was examined independently by three physicians in charge of evaluation who were blinded to the identity of the patients and pin site, was defined as a radiolucent line with a width of more than 1 mm around a pin at the entry or exit cortex, or both. The Aro classification was determined based on the agreement of the judgments of two of the three physicians.
Pin tract fracture was assessed by the radiographs immediately after pin insertion. Neurovascular and tendon injury was assessed by physical examination. The necessity for additional operation was assessed by the surgeons. Pin tract infection was assessed at weekly visits according to the Checketts-Otterburn classification system (grades 1–6),12 with the addition of grade 0 to represent “no pin tract infection,” characterized by neither discharge nor redness around the pins. The distinction between grade 0 and grade 1 was made based on the 1-week cumulative discharge from the pin tracts, recorded as stains on the gauze on the pin tracts. Each pin site and gauze were photographed for assessment by three independent physicians in charge of evaluation who were blinded to the identity of the patients, pin site, and visit time point. The classification grade was based on the agreement of judgments of two of the physicians.
On pin extraction, extraction peak torque measurements and bacteriological examinations were performed as well as assessment of fracture and difficulty in extraction. An extraction torque value at or less than the specific value was regarded as “failure” of the treatment. Bacteriologic data were obtained with Gram stain and aerobic and anaerobic culture of swab samples taken from each pin tract.
Postoperative evaluation 2 years after surgery for the coated pin group was performed by standard radiographs, magnetic resonance imaging (MRI), and blood analysis to examine tissue abnormalities and general health at the previous pin tracts. MRI evaluation was performed in a blinded manner by a radiologist with no knowledge on this study.
2.7. Statistical analysis
The insertion and extraction torques, Ca per P (Ca/P) molar ratios, relative growth rates of NIH3T3 cells were statistically analyzed using the Student's t-test. Pin tract infection grade and bacterial examination results were statistically analyzed using the chi-square test. Patients' characteristics were statistically analyzed using the Mann-Whitney U test. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Titanium pins coated with FGF-2–apatite composite layers
The characteristics of titanium pins coated with an FGF-2–apatite composite layer are summarized in Table 1. The Ca/P molar ratio corresponds to that of stoichiometric HA (1.67), without a significant difference (p = 0.074). The layers retained their FGF-2 mitogenic activity. All the supersaturated calcium phosphate solutions remained aseptic during the coating process, as revealed by the bacteriologic and endotoxin tests of the solution.
Table 1.
Characteristics of the titanium pins coated with an FGF-2-apatite composite layer.
| Size | φ4.0/3.0 mm, Length 80/20 mm |
| Coated length | 57.2 ± 0.8 mm from tip |
| Ca (μg/pin) | 170.9 ± 47.7 |
| P (μg/pin) | 76.8 ± 20.7 |
| Apatite equivalent (μg/pin) | 428 ± 119 |
| Ca/P molar ratio | 1.71 ± 0.05 |
| FGF-2 (μg/pin) | 0.78 ± 0.29 (Bradford method) |
| FGF-2 activity | 1.31 ± 0.21 (p = 0.015 relative to 1.0) |
| Bacteria test | Negative |
3.2. Patients’ characteristics
The patients’ characteristics are shown in Table 2. There were no significant differences in age, bone mineral density, and young adult mean between the two groups.
Table 2.
Patients' characteristics.
| Patient | Age (yrs) |
Sex | AO classif. |
Combined surgery |
Fract. side |
BMD (g/cm2) |
YAM (%) |
|---|---|---|---|---|---|---|---|
| F1 | 62 | f | A3 | Pinning | left | 0.61 | 77 |
| F2 | 67 | f | C2 | Pinning | left | 0.71 | 90 |
| F3 | 77 | f | A3 | Pinning | right | 0.60 | 76 |
| F4 | 72 | f | A3 | Pinning | right | 0.50 | 64 |
| F5 | 74 | f | C2 | Pinning | left | 0.54 | 69 |
| Ave. |
70.4 ± 5.9 |
0.59 ± 0.1 |
75 ± 10 |
||||
| C1 | 58 | f | C2 | Pinning | left | 0.56 | 70 |
| C2 | 67 | f | A3 | Pinning | left | 0.58 | 73 |
| C3 | 52 | m | C2 | VLP | left | 1.01 | 108 |
| C4 | 74 | f | C2 | VLP | right | 0.56 | 86 |
| C5 | 64 | f | C2 | VLP | left | 0.77 | 82 |
| C6 | 43 | m | C3 | VLP | right | 0.94 | 102 |
| C7 | 67 | f | C3 | Pinning | left | 0.58 | 73 |
| C8 | 75 | f | C3 | VLP | right | 0.92 | 99 |
| C9 | 61 | f | C2 | Pinning | left | 0.60 | 76 |
| C10 | 83 | f | A3 | Pinning | right | 0.57 | 61 |
| Ave. | 64.4 ± 11.7 | 0.71 ± 0.2 | 83 ± 16 | ||||
BMD, bone mineral density; YAM, young adult mean; VLP, volar locking plate.
None of the patients had diabetes or used immunosuppressive drugs. In the uncoated pin group, one patient, who was injured in a high-energy trauma, sustained an open fracture (patient C6, Gustilo classification type 1).13
3.3. Outcome and safety
All patients achieved fracture union. The duration of external fixation was 40.8 ± 1.3 (range, 39–42) days for the coated pin group and 41.6 ± 2.1 (range, 39–45) days for the uncoated group. None of the patients exhibited pin tract fracture, neurovascular or tendon injury, need for an additional operation, or other complications. The MRIs for the coated pin group showed no abnormal findings in four of five patients; one did not undergo MRI due to personal reasons.
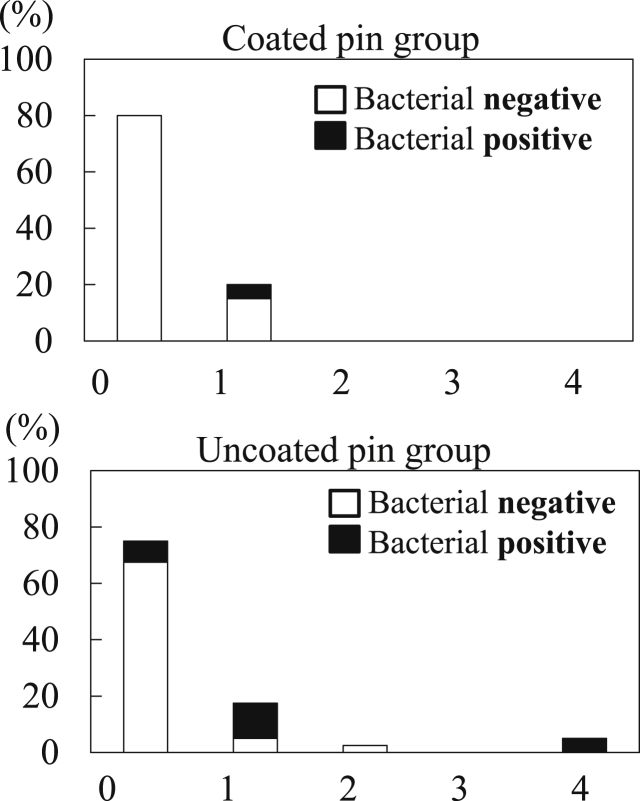
The bacteriological examination of pin sites on pin extraction showed one positive culture in the coated group (pin 4 of F5) and 10 positive cultures in the uncoated group (pins 3 and 4 of C4, pins 1–4 of C5, pins 2–4 of C6, and pin 3 of C9). The difference in the rate of positive cultures (1/20, 5%, vs. 10/40, 25%) was nearly significant (p = 0.059) between the two groups. The cultured bacteria were Staphylococcus epidermidis (F5, C5, and C6), Staphylococcus aureus (C4), and Gram-negative bacilli (C9).
The pin tract infection grades at 6 weeks after surgery are shown in Fig. 2. In the coated pin group, 16 (80%) of all pin tracts were grade 0. No major pin tract infections (grade 3 or higher) were observed during the entire treatment duration. Two pin tracts (pin 4 of F1 and pin 4 of F3) were grade 2 at 5 weeks after surgery and were treated with oral antibiotics (cefaclor, 750 mg/day) for 1 week. In the uncoated pin group, 30 (75%) of all pin tracts were grade 0. One case developed a severe infection. Case C4 was infected at 4 weeks after surgery and developed a grade 4 infection at 6 weeks despite administration of oral antibiotics for 2 weeks. Overall, there was no significant difference in pin tract infection grade between the two groups.
Fig. 2.
Pin tract infection grades in the modified Checketts-Otterburn classification system at 6 weeks after surgery.
Radiographically, all the pin tracts in both groups were Aro group 1, which meant absence of rarefactions.
No difficulties or fractures were encountered while inserting or removing the coated pins. The insertion and extraction peak torques exhibited ordinary values that ranged between 122 and 363 N mm and between 88 and 367 N mm, respectively (Table 3). There were no significant differences between the coated and uncoated pin groups as a whole and at each individual pin position for both insertion and extraction peak torques.
Table 3.
Insertion peak torque, extraction peak torque and fixation index defined as Extraction Peak Torque/Insertion Peak Torque.
| Group | Position | Peak Torque |
Fixation Index | |
|---|---|---|---|---|
| Insertion (Nmm) |
Extraction (Nmm) |
|||
| Coated | 1 | 363 ± 124 | 281 ± 110 | 0.79 ± 0.28 |
| Uncoated | 1 | 402 ± 88 | 319 ± 188 | 0.96 ± 0.19 |
| Coated | 2 | 122 ± 41 | 88 ± 39 | 0.86 ± 0.57 |
| Uncoated | 2 | 84 ± 53 | 111 ± 78 | 1.33 ± 1.27 |
| Coated | 3 | 326 ± 91 | 281 ± 81 | 0.90 ± 0.27 |
| Uncoated | 3 | 488 ± 254 | 443 ± 263 | 0.99 ± 0.40 |
| Coated | 4 | 358 ± 149 | 367 ± 102 | 1.24 ± 0.66 |
| Uncoated | 4 | 577 ± 528 | 479 ± 333 | 0.96 ± 0.32 |
Fixation index: extraction peak torque/insertion peak torque.
4. Discussion
The present study was the first clinical trial of external fixation titanium pins coated with an FGF-2–apatite composite layer, with 2 years of follow-up after pin removal. The study clarified the overall safety of using these pins for external fixation of distal radius fractures. Bone healing was accomplished in all five cases. There were no major complications, such as pin tract fracture, neurovascular or tendon injury, or other complications, leading to readmission for intravenous medication or further surgery. Additionally, there were no severe pin tract infections or clinical pin loosening, and no abnormal findings, such as hyperplasia or tumor, were observed at the pin tracts at the 2-year follow-up. The safety of the FGF-2–apatite composite layers results from their low FGF-2 dose. Previously, a dose of 800 μg of FGF-2 released from hydrogel was found to be safe in high tibial osteotomies in humans.14 This dose is more than 100 times higher than that of the FGF-2–apatite composite layers.
The titanium pins coated with an FGF-2–apatite composite layer showed a tendency of improved resistance to pin tract infection over uncoated titanium pins, as was demonstrated by the lower but not statistically significant (p = 0.0591) rate of positive bacterial culture on pin extraction. The Checketts-Otterburn classification was comparable between the coated and uncoated pin groups, although FGF-2 increases the volume of wound exudate and granulation tissues in wound healing.8 FGF-2 promotes angiogenesis and wound healing.8 Thus, FGF-2 can contribute to the inhibition of pin tract infection. Previous animal studies demonstrated higher resistance to pin tract infection of pins coated with an FGF-2–apatite composite layer over uncoated pins, which was associated with highly vascularized tissue at the interface between the pin and soft tissue.6,10
No patient had pin loosening radiographically or clinically in either group. All the pins were able to be removed without complications due to their moderate levels of extraction peak torques. An excessive level of extraction peak torque leads to difficulty in pin removal, which is sometimes associated with pins coated with plasma-sprayed HA.15 The thin and less porous nature of the FGF-2–apatite composite layers compared with the plasma-sprayed HA layers contributes to the moderate level of extraction peak torques. The adhesion strength of layers to pins could also be different between these two classes of layers.
The limitation of the present study is the small number of enrolled patients. Further studies are required, including randomized comparative and/or dose escalation studies.
We hypothesize that the titanium pins coated with FGF-2–apatite composite layers can be used clinically in the external fixation treatment for distal radius fractures. In conclusion, the present study demonstrated the safety of using titanium pins coated with an FGF-2–apatite composite layer for external fixation of distal radius fractures, without obvious adverse events due to the composite layer and no abnormal findings at the pin tracts even 2 years after surgery. Neither pin loosening nor severe pin tract infections were observed.
Funding
This study was supported by the grant from the Initiative for Accelerating Regulatory Science in Innovative Drug, Medical Device, and Regenerative Medicine from the Ministry of Health, Labour and Welfare, Japan. The funding source has no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Declaration of conflicting interests
The authors declare that they have no conflicts of interest.
Acknowledgment
We gratefully acknowledge the work of the past and present members of our institute who cooperated in conducting this study, namely Chizuko Fujisawa, Yuichi Hasegawa, Masataka Sakane, and Naoyuki Ochiai.
Contributor Information
Atsuo Ito, Email: atsuo-ito@aist.go.jp.
Yuki Hara, Email: yukihara@md.tsukuba.ac.jp.
References
- 1.Ferreira N., Marais L.C. Prevention and management of external fixator pin track sepsis. Strat Traum Limb Recon. 2012;7:67–72. doi: 10.1007/s11751-012-0139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.W-Dahl A., Toksvig-Larsen S., Lindstrand A. No difference between daily and weekly pin site care: a randomized study of 50 patients with external fixation. Acta Orthop Scand. 2003;74:704–708. doi: 10.1080/00016470310018234. [DOI] [PubMed] [Google Scholar]
- 3.Wassall M.A., Santin M., Isalberti C., Cannas M., Denyer S.P. Adhesion of bacteria to stainless steel and silver-coated orthopedic external fixation pins. J Biomed Mater Res. 1997;36:325–330. doi: 10.1002/(sici)1097-4636(19970905)36:3<325::aid-jbm7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Moroni A., Faldini C., Marchetti S., Manca M., Consoli V., Giannini S. Improvement of the bone-pin interface strength in osteoporotic bone with use of hydroxyapatite-coated tapered external-fixation pins. A prospective, randomized clinical study of wrist fractures. J Bone Joint Surg Am. 2001;83:717–721. doi: 10.2106/00004623-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura H., Matsuno T., Hashimoto Y., Nakamura T., Mataga I. Comparison of a hydroxyapatite-coated and an anodic oxidized titanium implant for experimentally induced peri-implantitis: macroscopic and novel radiographic evaluations in a canine model. J Hard Tissue Biol. 2015;24:347–355. [Google Scholar]
- 6.Mutsuzaki H., Ito A., Sakane M., Sogo Y., Oyane A., Ochiai N. Fibroblast growth factor-2–apatite composite layers on titanium screw to reduce pin tract infection rate. J Biomed Mater Res B. 2008;86:365–374. doi: 10.1002/jbm.b.31029. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y., Tensho K., Nakaya H., Nawata M., Okabe T., Wakitani S. Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)–induced ectopic bone formation in mice. Bone. 2005;36:399–407. doi: 10.1016/j.bone.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Akita S., Akino K., Hirano A. Basic fibroblast growth factor in scarless wound healing. Adv Wound Care. 2013;2:44–49. doi: 10.1089/wound.2011.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsurushima H., Marushima A., Suzuki K. Enhanced bone formation using hydroxyapatite ceramic coated with fibroblast growth factor-2. Acta Biomater. 2010;6:2751–2759. doi: 10.1016/j.actbio.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Mutsuzaki H., Ito A., Sogo Y., Sakane M., Oyane A., Ochiai N. Enhanced wound healing associated with Sharpey's fiber-like tissue formation around FGF-2–apatite composite layers on percutaneous titanium screws in rabbits. Arch Orthop Trauma Surg. 2012;132:113–121. doi: 10.1007/s00402-011-1381-7. [DOI] [PubMed] [Google Scholar]
- 11.Aro H.T., Markel M.D., Chao E.Y. Cortical bone reactions at the interface of external fixation half-pins under different loading conditions. J Trauma. 1993;35:776–785. doi: 10.1097/00005373-199311000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Checketts R.G., Otterburn M., MacEachern G. Pin track infection: definition, incidence and prevention. Suppl. Int J Orthop Trauma. 1993;3:16–18. [Google Scholar]
- 13.Gustilo R.B., Anderson J.T. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58:453–458. [PubMed] [Google Scholar]
- 14.Kawaguchi H., Jingushi S., Izumi T. Local application of recombinant human fibroblast growth factor-2 on bone repair: a dose-escalation prospective trial on patients with osteotomy. J Orthop Res. 2007;25:480–487. doi: 10.1002/jor.20315. [DOI] [PubMed] [Google Scholar]
- 15.Sanden B., Olerud C., Johanson C., Larsson S. Improved extraction torque of hydroxyapatite-coated pedicle screw. Eur Spine J. 2009;9:534–537. doi: 10.1007/s005860000180. [DOI] [PMC free article] [PubMed] [Google Scholar]