Abstract
Background
Gastroesophageal reflux disease (GERD) is very common. Salivary pepsin detection has previously been considered as a method for GERD diagnosis. We performed a meta-analysis to investigate the utility of salivary pepsin assay as a diagnostic tool of GERD.
Material/Methods
PubMed, Web of Science, the Cochran Library, and EMBASE (from January 1980 to 23 October 2018) were searched for pepsin in saliva for GERD diagnosis. We summarized the retrieved specificity, sensitivity, negative likelihood ratio (NLR), positive likelihood ratio (PLR), diagnostic odds ratio (DOR), and receiver operating characteristic (ROC) curves data in the meta-analysis.
Results
In final analysis, a total of 5 studies were included. The summary sensitivity, specificity, NLR, and PLR were 0.60 (95% CI 0.41–0.76), 0.71 (95% CI 0.51–0.86), 0.56 (95% CI 0.34–0.93), and 2.1 (95% CI 1.1–4.1), respectively. The pooled DOR was 4 (95% CI 1.0–11.0) and area under the ROC was 0.70 (95% CI 0.66–0.74).
Conclusions
The meta-analysis showed that pepsin in saliva has moderate diagnostic value for GERD, and is not as helpful as previously thought.
MeSH Keywords: Gastroesophageal Reflux, Laryngopharyngeal Reflux, Meta-Analysis as Topic, Pepsin A, Sensitivity and Specificity
Background
Gastroesophageal reflux disease (GERD) is one of the most common esophageal diseases, and its prevalence is increasing worldwide [1,2]. GERD is the retrograde movement of gastric contents, resulting in mucosal damage and pathologic changes [3,4].
Diagnostic methods of GERD include empirical PPIs treatment, questionnaires, endoscopy, and ambulatory reflux monitoring. Ambulatory reflux monitoring can provide confirmatory evidence of GERD, such as 24-h pH and multichannel intraluminal impedancometry (24-h pH-MII). However, it is invasive, expensive, and uncomfortable for the patient [5]. Empirical PPIs treatment, also called “PPI test”, lack sensitivity and specificity for GERD diagnosis, as shown by a systematic review [6]. When compared with objective evidence of GERD as defined by pH-metry or endoscopy, questionnaires such as the reflux disease questionnaire (RDQ) and gastroesophageal reflux disease questionnaire (GERDQ) lack sensitivity and specificity [7]. Endoscopy is performed with patients with alarm signs or in refractory GERD patients. However, non-erosive reflux disease (NERD) and most extra-esophageal reflux (EER) have no endoscopic evidence of mucosa damage [8,9].
Pepsin is proteolytic enzyme of pepsinogen, which is released solely by gastric chief cells. There is no production of pepsin in the airway or esophagus, thus its presence in the esophagus or more proximally (pharynx or airways) suggests gastro-oesophageal reflux (GOR) [10,11]. Salivary pepsin detection has previously been considered as a method for GERD diagnosis. Many studies have assessed the value of salivary pepsin assay for GERD diagnosis, but the diagnostic values differed [12–26].
In the present study, a meta-analysis was performed to investigate the diagnostic value of pepsin in saliva for diagnosis of GERD.
Material and Methods
Search strategy and study inclusion
The present research was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline [27]. Two investigators (Guo and Wu) independently searched the published articles in multiple databases: PubMed, Web of Science, the Cochran Library, and EMBASE (from January 1980 to 23 October 2018). The search strategies in PubMed, Web of Science, the Cochran Library, and EMBASE were “(saliva[Title/Abstract]) OR salivary[Title/Abstract]) OR spit[Title/Abstract])) OR “Saliva”[Mesh])) AND (pepsin[Title/Abstract])”, “TS=(saliva AND pepsin)”, “saliva in Title Abstract Keyword AND pepsin in Title Abstract Keyword” and “‘saliva’: ab,ti AND ‘pepsin’: ab,ti”, respectively. References of review articles or previously published meta-analyses were also searched manually.
Inclusion and exclusion criteria
Studies were included if they: (1) provided sufficient information to construct a 2×2 contingency table, (2) assessed the accuracy of salivary pepsin assay for diagnosis of GERD, and (3) only involved adult patients. We only included original articles written in English. Reviews, animal experiments, case reports, conference abstract, correspondences, and editorials were excluded.
Procedures
The data was extracted and the methodological quality was assessed independently by 2 investigators (GZH and WH). The extracted data included year, methodology characteristics, country of origin, study population characteristics (adults or pediatric), details of the pepsin assays, and cut-off value. The Quality Assessment of Diagnostic Accuracy Studies-2(QUADAS-2) was used to assess the methodological quality of the studies [28]. If there were discrepancies between the 2 investigators, a consensus meeting was held by referral to a third investigator.
Statistical analysis
All the extracted data were inputted in STATA ver. 12.0 (Stata Corp, College Station, TX, USA) and Review Manager (version 5.2 of Windows; Cochrane Collaboration, Oxford, UK). The specificity, sensitivity, pooled negative likelihood ratio (NLR), pooled positive likelihood ratio (PLR), corresponding 95% confidence intervals (CIs), and diagnostic odds ratio (DOR) were calculated from false positive, true positive, true negative and false negative, which was extracted from each study before data pooling. The overall performance of salivary pepsin assay in GERD patients were evaluated by the summary receiver operator characteristic (SROC) curve.
The I2 statistic were used to assess the statistical heterogeneity among the studies. P≤0.10 indicated significant heterogeneity, while P>0.10 indicated no significant heterogeneity for the Q statistic [29]. A random-effect models was used when the heterogeneity was high (P<0.05, I2 >50%). Meta-regression analyses were conducted on the basis of sample size, cut-off value, and region [30]. Potential publication bias was evaluated by Deeks’ asymmetry test [31].
Results
Study characteristics
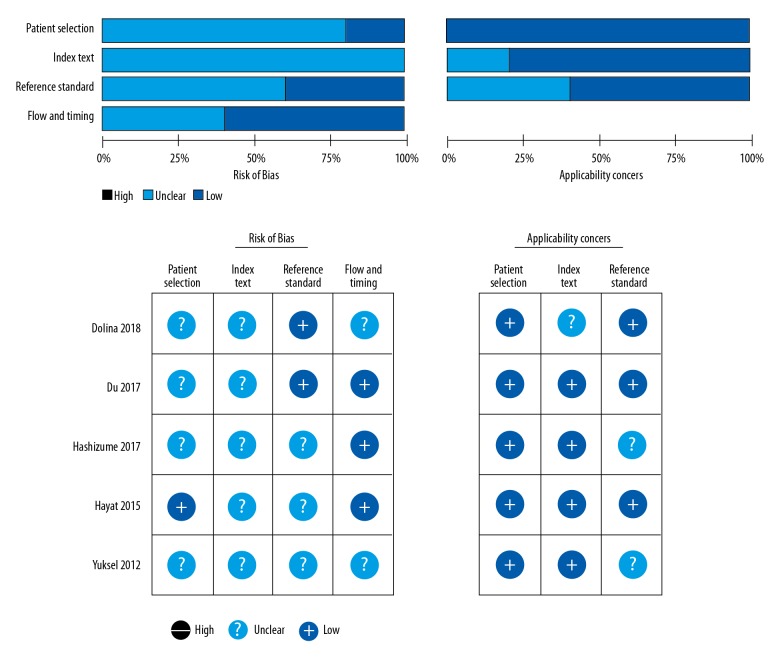
Detailed search steps are described in Figure 1. A total of 420 potentially relevant articles were identified for retrieval. After screening the titles and abstracts, 152 were excluded because they were irrelevant. No more relevant articles were identified by searching of the reference lists of previous systematic reviews [32,33]. After detailed full-article inspection, 2 articles for pediatrics were excluded [16,17], and 1 article using Western blotting analysis without cut-off value of pepsin for GERD was excluded [24]. Finally, 5 articles met our predefined inclusion criteria and were included in the final analysis [12,14,15,19,23]. Because diagnostic accuracy in 1 study was reported separately for different diagnostic criteria [12], the study was divided into 2 parts; as the result, 6 datasets were analyzed. Details of these studies are shown in Table 1. The risk of bias for the enrolled studies is presented in Figure 2.
Figure 1.

Retrieval flowchart to obtain study data for meta-analysis.
Table 1.
Characteristics of studies included in the meta-analysis.
| No. | Author | Year | Country | Sample size | Age | Diagnostic criteria | Pepsin assay method | Sample time/total sample number | Cut-off (ng/ml) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Dolina(a) [12] | 2018 | Czech | 32GERD/11 HC | Adult | 24h pH-MII (acid reflux) | PepTest | Waking up/1 | 36 |
| Dolina(b) | Weakly acid reflux | 36 | |||||||
| 2 | Du [15] | 2017 | China | 122GERD/128SC/ 35HC | Adult | 24h pH-MII | PepTest | Waking, 1–2 h after lunch and dinner/3 | 76 |
| 3 | Hashizume [14] | 2017 | Japan | 15GERD/11SC | Adult | 24h pH-MII | PepTest | Fasting | 16 |
| 4 | Hayat [19] | 2015 | UK | 111 GERD symptoms/100HC | Adult | 24h pH-MII | PepTest | Waking, 1 h after lunch and dinner/3 | 16 |
| 5 | Yuksel [23] | 2012 | UK | 22GERD/25SC | Adult | Wireless 48 h pH-monitoring | PepTest | Not mention | 16 |
GERD – gastroesophageal reflux disease; HC – health control; SC – symptomatic control.
Figure 2.
Quality of the studies as assessed by the Quality Assessment of Diagnostic Accuracy Studies questionnaire. Deep blue indicates absence of bias, black indicates the presence of bias, and light blue indicates unclear.
Meta-analysis results on diagnostic value of salivary pepsin assay for GERD
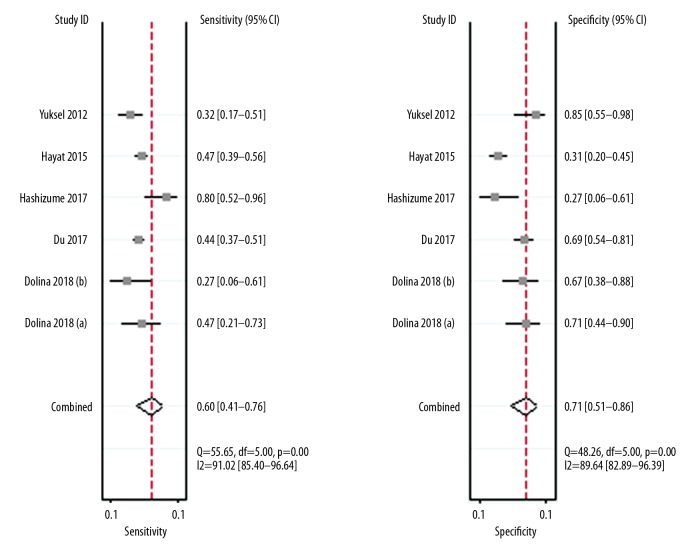
The 5 studies (6 datasets) were pooled for assessment of diagnostic accuracy. As shown in Figure 3, pooled specificity was 0.71 (95% CI 0.51–0.86) and pooled sensitivity was 0.60 (95% CI 0.41–0.76). The pooled negative likelihood ratio and pooled positive likelihood ratio were 0.56 (95% CI 0.34–0.93) and 2.1(95% CI 1.1–4.1), respectively. The diagnostic odds ratio (DOR) was 4 (95% CI 1.0–11.0). The area under the receiver operating characteristic curve was 0.70 (95% CI 0.66–0.74; Figure 4). The likelihood ratio plot showed the saliva pepsin assay for GERD neither confirmed nor excluded its utility (Figure 5).
Figure 3.
Sensitivity and specificity of saliva pepsin assay for diagnosis of GERD.
Figure 4.
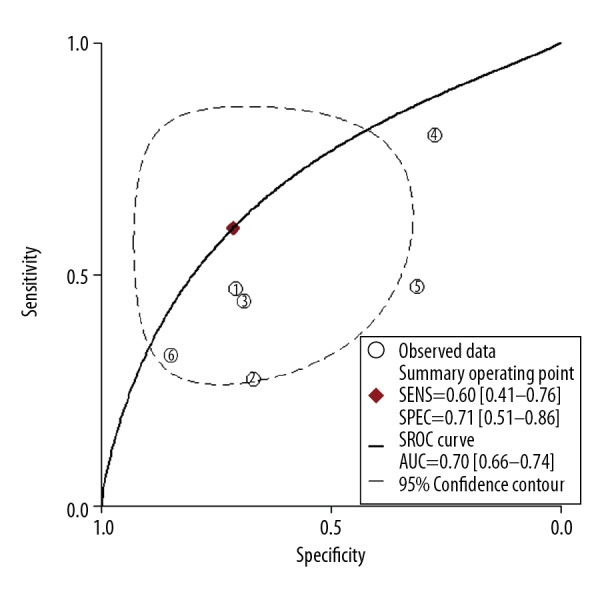
Summary receiver operating characteristic curve with 95% confidence contour and 95% prediction contour.
Figure 5.
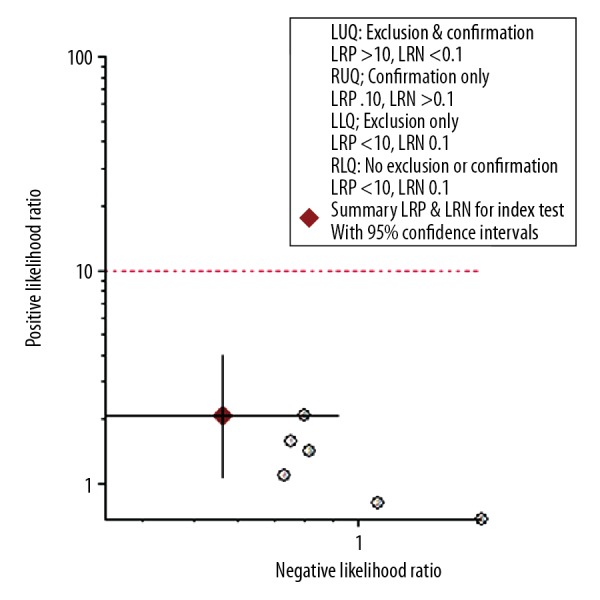
Likelihood ratio plot for saliva pepsin assay for diagnosis of GERD.
Heterogeneity, subgroup analysis, and publication bias
The proportion of heterogeneity probably was small (sensitivity: I2=91.02%, p=0.00; specificity: I2=89.64%, p=0.00). We did a meta-regression analyses to identify the source of heterogeneity. There was lower heterogeneity when subgroup analysis (Table 2) was conducted according to sample size (≥50 versus <50) (I2=2.15%, p=0.34), research region (Asia or Western countries) (I2=2.58%, p=0.28), and cut-off value (≥25 ng/ml or <25 ng/ml) (I2=2.15%, p=0.34), which indicated that they were not the heterogeneity source. No evidence of publication bias was shown by the Deeks’ asymmetry test (p=0.44; Figure 6).
Table 2.
Subgroup analysis.
| Subgroup (number of studies) | Sensitivity | p | Specificity | p |
|---|---|---|---|---|
| Region | 0.25 | 0.74 | ||
| Western country (n=4) | 0.51 [0.31–0.71] | 0.73 [0.52–0.94] | ||
| Asia (n=2) | 0.77 [0.57–0.97] | 0.68 [0.35–1.00] | ||
| Sample size | 0.16 | 0.78 | ||
| ≥50 (n=3) | 0.48 [0.24–0.72] | 0.75 [0.53–0.97] | ||
| <50 (n=3) | 0.71 [0.52–0.90] | 0.67 [0.41–0.93] | ||
| Cut-off value | 0.16 | 0.78 | ||
| ≥25 ng/ml (n=3) | 0.48 [0.24–0.72] | 0.75 [0.53–0.97] | ||
| <25 ng/ml (n=3) | 0.71 [0.52–0.90] | 0.67 [0.41–0.93] |
Figure 6.
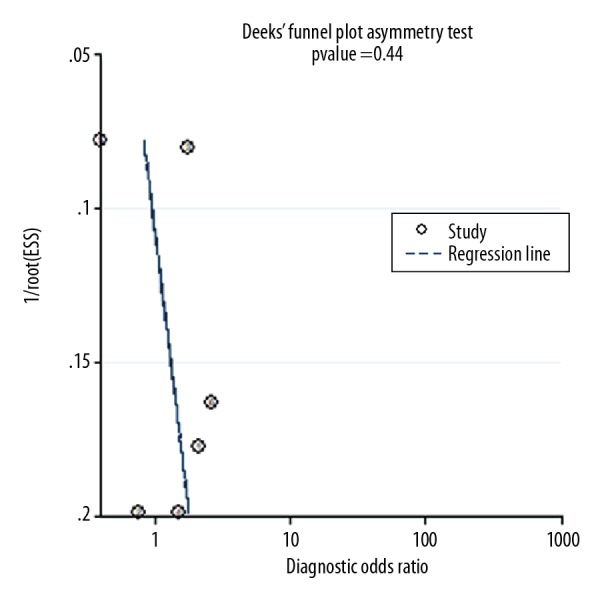
Deeks’ plot for saliva pepsin assay for diagnosis of GERD.
Discussion
Pepsin is only produced in the stomach, so it is a specific biomarker for gastric reflux and can be detected in saliva, sputum, secretary otitis media, and even in tears [34,35]. Detecting pepsin in saliva is a noninvasive and convenient diagnostic tool compared with endoscopy and 24-h pH-MII monitoring. In 2003, Potluri et al. used the fibrinogen digestion method to assay saliva/sputum pepsin for detection of gastric reflux, which was the first study on use of the salivary pepsin assay for GERD [26]. Western blot and enzyme-linked immunosorbent assay (ELISA) have also been used for pepsin assay [22–25]. In 2012, a rapid lateral flow test (Peptest) to detect pepsin in saliva/sputum was developed by Saritas Yuksel et al. [23], which has shortened time needed for the salivary pepsin assay to several minutes and offers a strong predictive value for GERD diagnosis. In our study, the summary sensitivity, specificity, NLR, and PLR were 0.60 (95% CI 0.41–0.76), 0.71 (95% CI 0.51–0.86), 0.56(95% CI 0.34–0.93), and 2.1(95% CI 1.1–4.1), respectively. Furthermore, the area under the ROC was 0.70 (95% CI 0.66–0.74). The likelihood ratio plot showed the saliva pepsin assay for GERD neither confirmed nor excluded its utility in diagnosis. Thus, these results suggest that the salivary pepsin assay does not have high utility in diagnosis of GERD.
The reported salivary pepsin for GERD diagnosis data, such as sensitivity and specificity, are highly variable among the research groups. The variation may be due to lack of a standard protocol in each study, which leaves unanswered questions such as: When should the saliva sample be collected? At the time of waking up, or after meals, or at the symptoms, or before bedtime, or before catheter placement? How many samples should be collected? Should samples be collected once or several times? Dolina et al. showed that the Peptest reaction was not finished at 15 min, as mentioned in previous articles, and the intensity continued to increase further until 40 min. The relative standard deviation of measurement was good when using the same device, but the reproducibility of 10 different individual devices was poor [12]. The subgroup analysis in our study showed that sample size (≥50 versus <50), research region (Asia or Western country), and the cut-off value (≥25 ng/ml or <25 ng/ml) had low heterogeneity. As a result, differences in study design and different enrollment criteria might be the source of heterogeneity. Some studies compared diagnosed GERD patients with health controls [12], while some compared GERD patients with symptomatic controls [14,15,19,23]. The study of Hayat et al. provided detailed diagnosis of non-GERD group (healthy controls, hypersensitive oesophagus, and functional heartburn) [19], but other studies just divided the GERD and non-GERD according to 24-h pH-MII or wireless 48-h pH monitoring, not giving the detailed diagnostic information of non-GERD patients [12,14,15,23].
The present study differs in several ways from previously meta-analyses and systematic reviews [3,32]. Firstly, we excluded studies on laryngopharyngeal reflux disease (LPRD) [13,18,20–22,25,26]. LPRD is a supra-esophageal form of GERD, which is defined as the back flow of gastric or gastroduodenal contents into the laryngopharynx. Although LPR and GERD are both caused by the reflux of stomach contents, it has been reported that LPR is distinct from GERD, with differing presenting symptoms and diagnostic criteria [36]. As a result, we excluded the studies on saliva pepsin assay for LPR patients. Secondly, the studies on pediatric patients were excluded because this study included infant GERD patients around 1 year old who cannot complain of symptoms. These entities are completely different from adult GERD. Thirdly, only the results of Peptest were included. There were several methods for pepsin assay, such as ELISA, Western blot, fibrinogen digestion, and Peptest, among which Peptest is now most widely used. To reduce heterogeneity, a study with 9 GERD patients and 31 symptomatic controls was excluded based on the Western blot method used [24]. Finally, ambulatory reflux monitoring was used as the criterion standards of GERD diagnosis for all the studies included, which reduces heterogeneity compared with the meta-analyses including studies using symptoms as diagnostic criteria.
The strengths of this study are that a comprehensive search strategy was used and a standard protocol was followed. In addition, the findings of a study with a large pooled sample are more robust than any individual study.
There are several limitations to our study. Firstly, the differences in study design and groups of each studies might be a source of heterogeneity. Secondly, as we only included published studies, publication bias is an inevitable problem. Finally, the summarized data in some articles limited our ability to conduct a more detailed analysis.
Conclusions
This meta-analysis shows that pepsin in saliva has only moderate diagnostic value for GERD, and is not as helpful as previously believed. More standard protocols for salivary pepsin detection are needed, and further large-scale studies with clear subgroup information should be done to verify the diagnostic value.
Footnotes
Conflict of interest
None.
Source of support: Beijing Municipal Administration of Hospitals Foundation for Clinical Medicine (ZYLX201612) and the Scientific Foundation of Tongren Hospital (TRYY-KYJJ-2015-020)
References
- 1.El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut. 2014;63:871–80. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: A meta-analysis. Gut. 2018;67(3):430–40. doi: 10.1136/gutjnl-2016-313589. [DOI] [PubMed] [Google Scholar]
- 3.Hammer HF. Reflux-associated laryngitis and laryngopharyngeal reflux: A gastroenterologist’s point of view. Dig Dis. 2009;27:14–17. doi: 10.1159/000210098. [DOI] [PubMed] [Google Scholar]
- 4.Campagnolo AM, Priston J, Heidrich Thoen R, et al. Laryngopharyngeal reflux: Diagnosis, treatment and latest research. Int Arch Otorhinolaryngol. 2014;18:184–91. doi: 10.1055/s-0033-1352504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt R, Armstrong D, Katelaris P, et al. World Gastroenterology Organisation Global Guidelines: GERD global perspective on gastroesophageal reflux disease. J Clin Gastroenterol. 2017;51(6):467–78. doi: 10.1097/MCG.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 6.Numans ME, Lau J, de Wit NJ, et al. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: A meta-analysis of diagnostic test characteristics. Ann Intern Med. 2004;140:518–27. doi: 10.7326/0003-4819-140-7-200404060-00011. [DOI] [PubMed] [Google Scholar]
- 7.Bolier EA, Kessing BF, Smout AJ, et al. Systematic review: Questionnaires for assessment of gastroesophageal reflux disease. Dis Esophagus. 2015;28:105–20. doi: 10.1111/dote.12163. [DOI] [PubMed] [Google Scholar]
- 8.Savarino E, Zentilin P, Savarino V. NERD: An umbrella term including heterogeneous subpopulations. Nat Rev Gastroenterol Hepatol. 2013;10:371–80. doi: 10.1038/nrgastro.2013.50. [DOI] [PubMed] [Google Scholar]
- 9.Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc. 2010;71:28–34. doi: 10.1016/j.gie.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Kim TH, Lee KJ, Yeo M, et al. Pepsin detection in the sputum/saliva for the diagnosis of gastroesophageal reflux disease in patients with clinically suspected atypical gastroesophageal reflux disease symptoms. Digestion. 2008;77:201–6. doi: 10.1159/000143795. [DOI] [PubMed] [Google Scholar]
- 11.Grabowski M, Kasran A, Seys S, et al. Pepsin and bile acids in induced sputum of chronic cough patients. Respir Med. 2011;105:1257–61. doi: 10.1016/j.rmed.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Dolina J, Konečný Š, Ďurč P, et al. Evaluation of important analytical parameters of the peptest immunoassay that limit its use in diagnosing gastroesophageal reflux disease. J Clin Gastroenterol. 2018 doi: 10.1097/MCG.0000000000001066. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Barona-Lleo L, Barona-De Guzman R, Krstulovic C. The diagnostic usefulness of the salivary pepsin test in symptomatic laryngopharyngeal reflux. J Voice. 2018 doi: 10.1016/j.jvoice.2018.07.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Hashizume N, Fukahori S, Asagiri K, et al. The characteristics of salivary pepsin in patients with severe motor and intellectual disabilities. Brain Dev. 2017;39(8):703–9. doi: 10.1016/j.braindev.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Du X, Wang F, Hu Z, et al. The diagnostic value of pepsin detection in saliva for gastro-esophageal reflux disease: A preliminary study from China. BMC Gastroenterol. 2017;17(1):107. doi: 10.1186/s12876-017-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortunato JE, D’Agostino RB, Jr, Lively MO. Pepsin in saliva as a biomarker for oropharyngeal reflux compared with 24-hour esophageal impedance/pH monitoring in pediatric patients. Neurogastroenterol Motil. 2017;29(2) doi: 10.1111/nmo.12936. [DOI] [PubMed] [Google Scholar]
- 17.Dy F, Amirault J, Mitchell PD, Rosen R. Salivary pepsin lacks sensitivity as a diagnostic tool to evaluate extraesophageal reflux disease. J Pediatr. 2016;177:53–58. doi: 10.1016/j.jpeds.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadlapati R, Adkins C, Jaiyeola DM, et al. Abilities of oropharyngeal pH tests and salivary pepsin analysis to discriminate between asymptomatic volunteers and subjects with symptoms of laryngeal irritation. Clin Gastroenterol Hepatol. 2016;14(4):535–42. doi: 10.1016/j.cgh.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayat JO, Gabieta-Somnez S, Yazaki E, et al. Pepsin in saliva for the diagnosis of gastro-oesophageal reflux disease. Gut. 2015;64(3):373–80. doi: 10.1136/gutjnl-2014-307049. [DOI] [PubMed] [Google Scholar]
- 20.Ocak E, Kubat G, Yorulmaz İ. Immunoserologic pepsin detection in the saliva as a non-invasive rapid diagnostic test for laryngopharyngeal reflux. Balkan Med J. 2015;32(1):46–50. doi: 10.5152/balkanmedj.2015.15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spyridoulias A, Lillie S, Vyas A, Fowler SJ. Detecting laryngopharyngeal reflux in patients with upper airways symptoms: Symptoms, signs or salivary pepsin? Respir Med. 2015;109(8):963–69. doi: 10.1016/j.rmed.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Hayat JO, Yazaki E, Moore AT, et al. Objective detection of esophagopharyngeal reflux in patients with hoarseness and endoscopic signs of laryngeal inflammation. J Clin Gastroenterol. 2014;48(4):318–27. doi: 10.1097/MCG.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 23.Saritas Yuksel E, Hong SK, Strugala V, et al. Rapid salivary pepsin test: Blinded assessment of test performance in gastroesophageal reflux disease. Laryngoscope. 2012;122(6):1312–16. doi: 10.1002/lary.23252. [DOI] [PubMed] [Google Scholar]
- 24.Kim TH, Lee KJ, Yeo M, et al. Pepsin detection in the sputum/saliva for the diagnosis of gastroesophageal reflux disease in patients with clinically suspected atypical gastroesophageal reflux disease symptoms. Digestion. 2008;77(3–4):201–6. doi: 10.1159/000143795. [DOI] [PubMed] [Google Scholar]
- 25.Printza A, Speletas M, Triaridis S, Wilson J. Is pepsin detected in the saliva of patients who experience pharyngeal reflux? Hippokratia. 2007;11(3):145–49. [PMC free article] [PubMed] [Google Scholar]
- 26.Potluri S, Friedenberg F, Parkman HP, et al. Comparison of a salivary/sputum pepsin assay with 24-hour esophageal pH monitoring for detection of gastric reflux into the proximal esophagus, oropharynx, and lung. Dig Dis Sci. 2003;48(9):1813–17. doi: 10.1023/a:1025467600662. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 29.Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 2002;9:1237–56. doi: 10.1002/sim.1099. [DOI] [PubMed] [Google Scholar]
- 30.Altman DG, Bland JM. Interaction revisited: The difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–93. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Calvo-Henríquez C, Ruano-Ravina A, Vaamonde P, et al. Is pepsin a reliable marker of laryngopharyngeal reflux? A systematic review. Otolaryngol Head Neck Surg. 2017;157(3):385–91. doi: 10.1177/0194599817709430. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Zhao Y, Ren J, Xu Y. Pepsin in saliva as a diagnostic biomarker in laryngopharyngeal reflux: A meta-analysis. Eur Arch Otorhinolaryngol. 2018;275(3):671–78. doi: 10.1007/s00405-017-4845-8. [DOI] [PubMed] [Google Scholar]
- 34.Doğru M, Kuran G, Haytoğlu S, et al. Role of laryngopharyngeal reflux in the pathogenesis of otitis media with effusion. J Int Adv Otol. 2015;11(1):66–71. doi: 10.5152/iao.2015.642. [DOI] [PubMed] [Google Scholar]
- 35.Iannella G, Di NG, Plateroti R, et al. Investigation of pepsin in tears of children with laryngopharyngeal reflux disease. Int J Pediatr Otorhinolaryngol. 2015;79(12):2312–15. doi: 10.1016/j.ijporl.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 36.Vavricka SR1, Storck CA, Wildi SM, et al. Limited diagnostic value of laryngopharyngeal lesions in patients with gastroesophageal reflux during routine upper gastrointestinal endoscopy. Am J Gastroenterol. 2007;102(4):716–22. doi: 10.1111/j.1572-0241.2007.01145.x. [DOI] [PubMed] [Google Scholar]