Abstract
The turquoise killifish, Nothobranchius furzeri, is a promising vertebrate model in ageing research and an emerging model organism in genomics, regenerative medicine, developmental biology and ecotoxicology. Its lifestyle is adapted to the ephemeral nature of shallow pools on the African savannah. Its rapid and short active life commences when rains fill the pool: fish hatch, grow rapidly and mature in as few as two weeks, and then reproduce daily until the pool dries out. Its embryos then become inactive, encased in the dry sediment and protected from the harsh environment until the rains return. This invertebrate-like life cycle (short active phase and long developmental arrest) combined with a vertebrate body plan provide the ideal attributes for a laboratory animal.
Introduction
The African savannah is dotted with ephemeral freshwater pools known as water pans, which form during the rainy season. Killifishes of the genus Nothobranchius, colloquially called annual fishes, have adapted to live in these pools. Popular with specialist aquarium hobbyists for their stunning colouration, most of 75 described species are available in captivity (Neumann, 2008), which is how Nothobranchius furzeri first made its way into research laboratories worldwide. Alessandro Cellerino, a physiologist from Scuola Normale Superiore in Pisa, Italy, was intrigued by an incredibly short-lived population of the annual fish that his friend Stefano Valdesalici bred at home. Experimental investigation of its lifespan revealed that the fish matured after just four weeks, after which their mortality increased sharply from the age of six weeks and all the fish died within 10 weeks (Valdesalici and Cellerino, 2003). The fish of this strain were named GRZ after Gona Re Zhou National Park in Zimbabwe where the fish were originally collected in 1970 (Jubb, 1971). They were later found to live slightly longer as housing conditions developed (Cellerino et al., 2016), though it remains the shortest-lived population of N. furzeri yet recorded. Collection trips to Mozambique and Zimbabwe between 2004 and 2016 assembled a set of populations that vary in lifespan and possess a variable level of inbreeding (Terzibasi et al., 2008; Cellerino et al., 2016; Reichard et al., 2017a).
The remarkably short lifespan of N. furzeri recapitulates the hallmarks of vertebrate ageing (Harel et al., 2015), condensed into few weeks and responsive to pharmacological, dietary and lifestyle interventions (Cellerino et al., 2016). Its genome has been sequenced and assembled (Valenzano et al., 2015; Reichwald et al., 2015), and there are transcriptomes for a number of its tissues (Petzold et al., 2013; Baumgart et al., 2014; Baumgart et al., 2017). Several inbred lines are also available (Hu and Brunet, 2018) and genome-editing techniques are widely adopted to rapidly produce stable transgenic lines too (Harel et al., 2016). With this background, N. furzeri has reached outside the world of ageing research and has become valuable for studies in such disparate branches of investigation as evolutionary genomics (Reichwald et al., 2015), regenerative medicine (Wendler et al., 2015), developmental biology (Hu and Brunet, 2018) and ecotoxicology (Philippe et al., 2018). Here, we explore how our understanding of the natural history of the species makes a key contribution to the development of this species as a laboratory model.
Natural life cycle and longevity
The life cycle of N. furzeri is adapted to the transient nature of its habitat and is strictly separated into two distinct phases. The first phase starts when fish hatch after their pool is inundated with rainwater and immediately start feeding. Explosive juvenile growth brings them to sexual maturity in as little as two weeks, growing from the initial size of 5 mm to 30–50 mm over that period (Vrtílek et al., 2018a). Growth slows thereafter as resources are diverted to reproduction, but a body size of 75 mm can be reached in males after 10–15 weeks (Blažek et al., 2013; Vrtílek et al., 2018a). Importantly, their growth rate is strongly dependent on population density and food availability; fish in dense populations are typically small and sexually immature at an age of 3–5 weeks, both in the wild and in the laboratory (Graf et al., 2010; Grégoir et al., 2018; Philippe et al., 2018; Vrtílek et al., 2018a). Individual fish vary in growth rates (Blažek et al., 2013), perhaps reflecting variation in personality traits (Thoré et al., 2018). Upon sexual maturity, females lay eggs daily. A typical fecundity of 20–120 eggs per day was estimated in wild populations, with more variation among populations than among females within a sample (Vrtílek and Reichard, 2016; Vrtílek et al., 2018b), seemingly in response to resource availability.
All adult fish die when their pool desiccates, but mortality is strong over the entire lifespan (Vrtílek et al., 2018c). In longer-lasting pools (persisting more than four months), fish often disappear despite the habitat still appearing capable of supporting them. The fish likely succumb to a combination of predation by birds, bouts of extremely high water temperatures, decreased water quality from organic input by visiting animals, low dissolved oxygen levels, exhaustion of resources, and physiological deterioration as a trade-off to rapid juvenile development (Reichard, 2015). While wild-derived, outbred N. furzeri populations have a maximum lifespan (defined as 90% survival at the population level) of 25–42 weeks in captivity (Terzibasi et al., 2008; Tozzini et al., 2013; Blažek et al., 2017), only one of 13 N. furzeri populations monitored in the wild survived beyond the age of 17 weeks and most disappeared at the age of 3–10 weeks as their habitat dried up (Vrtílek et al., 2018c). After desiccation, dry sediment retains the eggs in a dormant state (diapause); an adaptation to the harsh environmental conditions, over the entire dry season that lasts 5–11 months (Cellerino et al., 2016; Vrtílek et al., 2018b).
Distribution
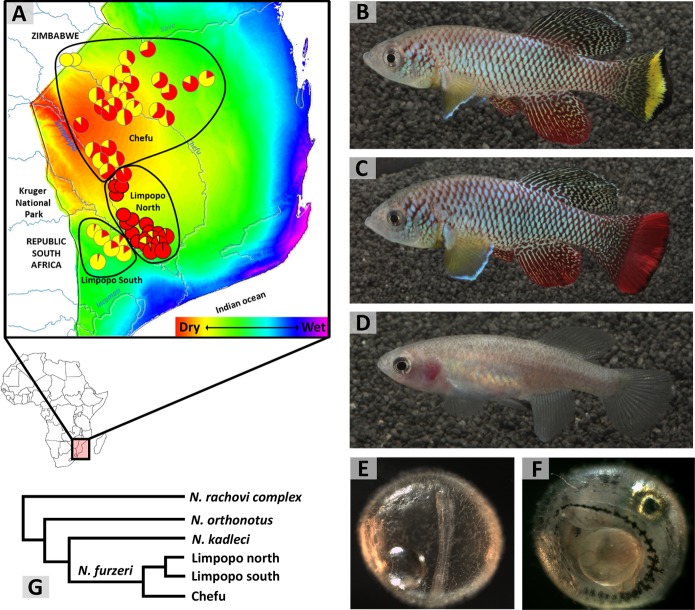
N. furzeri is distributed in southern Mozambique, with a few populations extending to adjacent parts of southern Zimbabwe, only few kilometres across the border (Figure 1A). Interestingly, the range of N. furzeri lies on a gradient in aridity associated with distance from the coast. Evaporation, the amount of rainfall and its predictability are major environmental factors that vary across that gradient (Terzibasi et al., 2008; Tozzini et al., 2013; Polačik et al., 2018), with the driest and least predictable conditions furthest from the ocean (and at the highest altitude) (Figure 1A). N. furzeri is absent in the wettest region, where another two Nothobranchius species, that otherwise coexist with N. furzeri, maintain viable populations. In contrast, N. furzeri is the most common Nothobranchius species in the dry region, 90–400 km from the coast and 19–220 m above sea level (Reichard et al., 2017a). Two isolated Zimbabwean populations are found even higher, at 325–340 m above sea level. To date, we have located and georeferenced 90 N. furzeri populations over its entire range and their geographic coordinates are accessible at the Figshare repository (doi: 10.6084/m9.figshare.7017167). Our sampling was limited by accessibility from roads and there are many more N. furzeri populations scattered throughout the Mozambican mopane and miombo woodlands and Acacia savannah.
Figure 1. Turquoise killifish phenotypes and their distribution.
(A) Map of N. furzeri distribution across its entire range overlaid on a gradient of aridity (wet to dry: blue to red) and with the proportion of male colour morphs visualised by pie charts and the geographic distribution of intra-specific clades delineated by black lines. (B) Adult yellow morph male, (C) red morph male, and (D) female N. furzeri. (E) Embryo at diapause II, representing the longest interval of its lifespan in natural habitats. (F) Embryo at diapause III, fully developed and awaiting hatching cues. (G) Simplified schematic phylogeny of the Southern clade of Nothobranchius, with details on N. furzeri intra-specific lineages (simplified from Bartáková et al., 2015). Image credits: Martin Reichard (1A, 1G), Radim Blažek (1B–1D), Matej Polačik (1E, 1F).
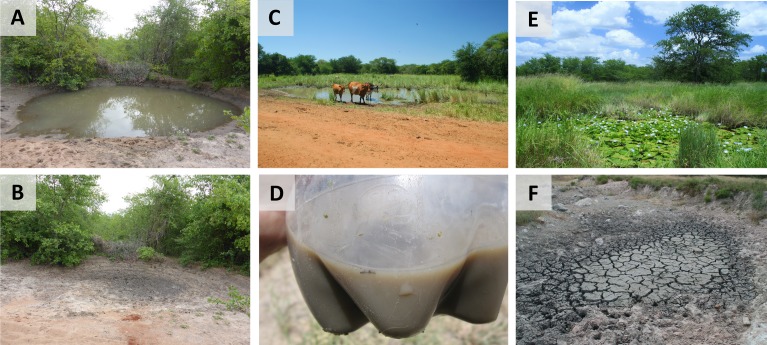
Local pools vary greatly in size and shrink gradually (Vrtílek et al., 2018b) (Figure 2A–B). Environmental conditions in the pool may be harsh; water temperature can fluctuate between 20 and 36°C from early morning to late afternoon (Reichard et al., 2009; Žák et al., 2018). Late in the season (in May), morning water temperature can drop to 15°C. Fine sediment is repeatedly disturbed by cattle (which have functionally replaced the wild African megafauna) (Figure 2C) and partially dissolves in water, producing extremely turbid conditions (Figure 2D). However, in cases when the pool is thickly overgrown with vegetation (Figure 2E) and undisturbed by domestic cattle, the water can be transparent.
Figure 2. Turquoise killifish habitats.
(A) A structurally simple habitat one week after filling with water. (B) The same habitat three weeks after filling, fully desiccated. (C) Domestic cattle that commonly visits killifish habitats. (D) Turbid water from a structurally simple killifish habitat discoloured by dissolved fine sediment particles. (E) A structurally complex habitat with abundant aquatic vegetation. (F) Desiccated pool sediment, with typical deep cracks. Image credits: Milan Vrtílek (2A, 2B), Martin Reichard (2C, 2E, 2F), Matej Polačik (2D).
Male colour morphs
Two distinct colour morphs of male N. furzeri occur, often coexisting in the same population (Reichard et al., 2009). The "yellow" morph (Figure 1B) possesses a yellow crescent marking along the outer margin of the caudal fin, outlined by a black margin. "Red" morph males (Figure 1C) have red caudal fins. The extent of colouration on the caudal fin varies widely among wild populations, but the two morphs are clearly discrete (Valenzano et al., 2009; Ng'oma et al., 2014). The black margin of the caudal fin is also present in some populations of red males and exceptionally absent in yellow males. Female colouration is dull and brownish and shows no indication of polymorphism (Figure 1D).
The geographic distribution of the morphs suggests a link between male colouration and adaptations to different habitat conditions. The yellow morph dominates dry, marginal parts of the range, with exclusively yellow males in the driest region of Zimbabwe. Red males dominate populations in the wet part of the range (Figure 1A) (Reichard et al., 2009; Dorn et al., 2011). This contrast is mirrored in the colouration of the most common laboratory strains, with the short-lived GRZ comprising exclusively yellow males while the longer-lived MZM0403 are exclusively red (Cellerino et al., 2016). Interestingly, most natural populations are polymorphic (Figure 1A) and no differences in life history traits between red and yellow males have been established where they coexist. While it is tempting to associate male colouration with adaptation to dry and wet conditions, there is no evidence of any causal link so far.
Genetic structure
Populations of N. furzeri are divided into two main phylogeographic clades (named Chefu and Limpopo), with limited secondary contact (Terzibasi et al., 2008; Bartáková et al., 2013; Figure 1A). The populations are strongly structured, with rare dispersal between them. The population genetic signature reveals that the Chefu clade has recently experienced dramatic population expansion (Dorn et al., 2011) and demonstrated linkage between populations along the small rivulet basins (Bartáková et al., 2015). Hence, large floods, occurring in exceptionally wet years, are assumed to provide the main mode of dispersal between populations (Reichard, 2015). Surprisingly for a fish, large rivers form boundaries in the distribution of clades and species, including a division of the Limpopo clade to two subclades (Figure 1G) and a boundary between the range of N. furzeri and its sister species, N. kadleci (Bartáková et al., 2015).
Diet
N. furzeri are opportunistic and generalist predators of small invertebrates. In general, the diet reflects prey availability in particular pools. Small crustaceans (Cladocera, Copepoda, and Ostracoda) typically constitute more than 70% of digested prey, with insect larvae also being a common prey item (Polačik and Reichard, 2010). Backswimmers (nothonectid bugs) are common in the pools but underrepresented in the diet. Chironomid larvae, the most common diet of N. furzeri in the laboratory, are also consumed by wild N. furzeri (Polačik and Reichard, 2010). The diet of N. furzeri differs from the two coexisting species (N. orthonotus and N. pienaari) when prey is abundant though all species resort to any available food of a suitable size when choice is limited (Polačik et al., 2014a). The diet is reflected in the intestinal microbiome and wild N. furzeri populations differ in the microbial composition of their guts (Smith et al., 2017). Interestingly, laboratory fish possess the core microbiota of wild N. furzeri and reconstitution of high microbial diversity in old fish extends their lifespan in the laboratory (Smith et al., 2017).
Biotic interactions
Predators
Potential predators include wading and diving birds, such as herons, hammerheads, storks and kingfishers. These birds prey on killifish, tadpoles and, perhaps, larger invertebrates. Large predatory waterbugs are abundant in many pools and readily consume adult killifish in captivity. These sit-and-wait predators may hunt killifish, especially in pools with dense vegetation. Lungfish (Protopterus annectens) commonly coexist with N. furzeri and may also prey on them, though they are not their typical predator (Reichard et al., 2014). The eggs of N. furzeri are likely predated by a range of invertebrates. For instance, freshwater crabs can be abundant in N. furzeri habitats and probably extract killifish eggs from the sediment.
Parasites
The parasites of wild N. furzeri have been well characterised (Nezhybová et al., 2017). All recorded animal parasites were endoparasites, infecting muscles and internal organs. Perhaps most notably, killifish predominantly hosted intermediate stages of the parasites, forming an important link in the transmission cascade to the final hosts: fish-eating birds. The metacercariae larval stage of parasitic trematodes was by far the most abundant parasite, typically with tens of them infecting the muscle tissue. A surprisingly high diversity of flukes (Trematoda), roundworms (Nematoda) and tapeworms (Cestoda) also reside in N. furzeri muscle, intestine, cerebral and abdominal cavities, gallbladder and gills. It is clear that N. furzeri are challenged by a multitude of infections over their short lifespan and capable of encysting parasites that penetrated their bodies, providing a potential to study their immune responses.
One fluke species deserves particular attention. Larvae of Apatemon sp. infect the cerebral cavity (Nezhybová et al., 2017) and manipulate host behaviour. When attacked by a bird, infected killifish stay close to the water surface, even jumping onto water lily pads and thereby exposing themselves to predators. In contrast, uninfected fish quickly seek refuge when exposed to potential predator attack.
Reproduction and mating behaviour
All Nothobranchius species are sexually dimorphic (Sedláček et al., 2014). Male colouration and mating behaviour appear strongly sexually selected (Haas, 1976a). Males compete aggressively for access to females and large males are dominant (Polačik and Reichard, 2009). Females express mate choice by approaching a displaying male. Males actively pursue females and may coerce spawning (Polačik and Reichard, 2011). The elaborate male colouration is puzzling, as N. furzeri often live in extremely turbid water where visibility is minimal. Indeed, male colouration fades in turbid water. Courtship is brief; the male approaches a female and stops to perform lateral displays with his unpaired fins extended. A receptive female allows the male to fold her in his dorsal fin and the female lays an egg into the sediment. During oviposition, the pair jerks and the sediment is disturbed. With the aid of stiffened rays of the anal fin, the egg is deposited slightly into the substrate (Passos et al., 2015). Most eggs are found at pool margins (M. Polačik, unpublished data). There is no consistent choice of red or yellow males by individual N. furzeri females (Reichard and Polačik, unpublished data) and mate choice appears to be based on other traits.
Reproduction occurs primarily in the morning. The eggs are ovulated just after sunrise (07:00-08:00) (Haas, 1976b) and by 10:00 some females have already spawned all their ovulated eggs. Most females are spent by 15:00 and a new batch of eggs is ovulated next morning (Vrtílek and Reichard, 2016). Daily fecundity is strongly contingent upon long-term (the effect of body mass: Vrtílek and Reichard, 2016) and short-term (the effect of ration on the preceding day: Vrtílek and Reichard, 2015) access to resources.
Embryo ecology
The embryonic phase of the N. furzeri life cycle represents the longest but least understood part of the life cycle in the wild. All Nothobranchius populations are limited to pools with a special type of substrate composed of an alkaline swelling clay (Watters, 2009), the physical and chemical properties of which are crucial for the survival of N. furzeri eggs. Annual killifish possess three dormancy stages occurring at defined developmental points (Wourms, 1972) (Box 1). Watters, 2009 proposed a model of natural embryo development of Nothobranchius that links embryo developmental with three phases of incubation conditions (wet, dry and humid) (Box 1).
Box 1.
Embryo development
Embryonic diapause is a distinctive feature of annual fish. It occurs through a developmental arrest coupled with a marked depression in metabolic rate (Podrabsky and Hand, 1999) at three well-defined developmental points called facultative diapauses, all of which can be entered or skipped. Diapause I occurs after an early stage in embryonic development called epiboly, when some cells are distributed across the yolk. It can be induced by a lack of oxygen (anoxia), low temperature and possibly by other conditions (Wourms, 1972). Diapause II occurs in the long embryo, midway through embryo development (at the 38 somite stage; Figure 1E). Some rudimentary organs are visible but the circulatory system is inactive (Wourms, 1972; Podrabsky et al., 2017). At diapause III (Figure 1F), the embryo has completed its development but awaits appropriate hatching cues at a decreased metabolic rate (Podrabsky et al., 2010; Furness et al., 2015a; Furness, 2016). While this developmental pathway appears strikingly congruent across all annual killifish clades (Furness et al., 2015b), it remains to be determined whether it also fully applies to N. furzeri. Watters, 2009 proposed a model of natural embryo development of Nothobranchius that links three embryo developmental phases with the three phases of incubation conditions (wet, dry and humid). During the wet phase, the eggs are deposited into the upper substrate layer. Anoxic conditions in the sediment apparently trigger the onset of diapause I (Watters, 2009). Any embryo development beyond diapause I in aquatic conditions in the wild is rare (Domínguez-Castanedo et al., 2013) and likely linked to embryo position outside the anoxic substrate. The dry incubation phase starts with desiccation (Figure 2B), when the substrate loses its moisture and breaks along deep cracks (Figure 2E). This oxygenates the substrate and embryos resume their development to reach diapause II (Watters, 2009), a stage most resistant to water loss (Podrabsky et al., 2001). The egg banks in dry sediment are exclusively composed of diapause II embryos (Domínguez-Castanedo et al., 2017). Specific physical features of the clay-rich substrate ensure that some moisture is retained even in the upper substrate layer (Watters, 2009). The humid phase starts when the first rains moisten the substrate. Increased moisture and a decrease in oxygen availability supposedly trigger development from diapause II to diapause III (Watters, 2009). Embryos at diapause III await a hatching stimulus that is probably associated with torrential rains and inundation of the pool. Hatching is synchronous within and among populations in years when a cyclone-associated rainfall inundates the pools (Polačik et al., 2011), but may be asynchronous in dry years (Reichard et al., 2017b), perhaps because the substrate is only gradually wetted.
Large variability in embryo developmental dynamics is a key feature for the long-term persistence of N. furzeri populations in their highly unpredictable environment (Furness, 2016), serving as an effective bet-hedging strategy against false hatching cues, such as insufficient rainfall (Polačik et al., 2017). Erratic rains and mid-season desiccation generate conditions for two (or perhaps even more) generations or cohorts in some years (Reichard et al., 2017b).
Laboratory research in N. furzeri and other annual killifishes has suggested that developmental disparity is achieved through maternal effects, perhaps based on RNA or protein products. Interestingly, embryos of young females tend to develop rapidly while embryos of older females tend to halt development at diapause (Podrabsky et al., 2010; Polačik et al., 2017); a seeming adaptation to the possibility of a second inundation in a single rainy season that the eggs of young females experience more often than the eggs of older females (Polačik et al., 2014b). However, in the wild the beginning and end of diapause, at least in other killifish species, appears to be controlled by environmental factors (Matias and Markofsky, 1978; Podrabsky et al., 2010; Domínguez-Castanedo et al., 2017) and developmental variability may be achieved through habitat heterogeneity rather than intrinsic control. Gradual desiccation and vertical distribution of embryos create a cline of incubation conditions that generate a staggered embryo development.
The course of embryo development has marked impacts on post-hatching lifespan. Rapidly developing embryos produce phenotypes with a rapid post-hatching strategy, typified by a smaller size at hatching but more rapid growth and sexual maturation, and smaller final size and shorter lifespan (Polačik et al., 2014b). It remains to be identified whether embryonic diapause is causally responsible for the limited rapid development later in life, representing one of the outstanding questions to be addressed (Box 2). Intriguingly, in a Neotropical annual killifish, insulin-like growth factor signalling regulates developmental trajectories (Woll and Podrabsky, 2017), representing candidate regulatory pathway for killifish life history.
Box 2.
Outstanding questions about the natural history of N. furzeri
Do N. furzeri senesce in natural populations? In the wild, the life of N. furzeri is often brief but may be longer than four months, especially in wet years. How much do wild N. furzeri suffer from ageing-related declines?
How do N. furzeri embryos develop under natural conditions? Current knowledge of the embryo development comes from laboratory conditions. As incubation in the laboratory largely differs from natural conditions, we need to know how much it diverges from the natural course of development.
Which genes underlie lifespan and ageing? With natural populations that predictably vary in lifespan, can we dissect genetic and regulatory pathways associated with differences in ageing?
Is rapid juvenile development traded off against rapid deterioration later in life? When comparing different Nothobranchius species, it appears so, but no longitudinal study has compared life history traits within a single cohort, be it in the laboratory or in the wild.
Do rapid and slow pace of life coexist within the same N. furzeri populations? There is impressive variability in the speed of juvenile development among individuals within the same pool (and laboratory cohort). Is this variation determined by the individual’s genetic background or primarily affected by environmental and developmental conditions that an individual experiences during early life?
Do regulatory pathways that control diapause and ageing overlap? Regulatory networks associated with growth and development are involved in both diapause and ageing in non-vertebrate models (Frézal and Félix, 2015). Could that link help us to understand the prevention of ageing-associated damage?
What are the sources of persistent male-biased mortality in wild populations? Combining data from wild populations with experiments in the laboratory may elucidate why male N. furzeri, like many other species including humans, die younger than females.
Why do males coexist in two colour morphs? Existence of two male morphs is often associated with discrete reproductive tactics or with speciation through adaptation to a divergent environment or from strong female choice.
Key advantages of N. furzeri for the model species role
The key advantage of N. furzeri as a model species stems from the combination of its rapid post-hatching lifestyle and long phase of embryonic arrest that can be altered by manipulating environmental conditions. Having a model with a vertebrate body plan and invertebrate-like life history and lifespan (i.e. short and rapid active lifespan and dormant stage) is a valuable characteristic beyond the field of ageing research, not least because researchers are often constrained by the duration of grant funding. The arrested stage makes the species ideal for storing and shipping and permits flexible time management when working with this 'instant' fish (Polačik et al., 2016). In addition, given harsh and variable conditions in natural habitats, captive N. furzeri does not require any precise conditions for water quality and housing, perhaps except access to abundant and nutrient rich food (Polačik et al., 2016; Dodzian et al., 2018).
Information on wild N. furzeri populations, and particularly the environmental gradient in its range, have been especially instrumental in explaining differences in lifespan among captive strains (Tozzini et al., 2013; Blažek et al., 2017) and in validating that its rapid life history is not an artefact of the laboratory (Vrtílek et al., 2018a; Vrtílek et al., 2018c). Field studies have also strengthened conclusions on the role of microbiota on ageing (Smith et al., 2017) and genomic insights into the evolution of short lifespan (Valenzano et al., 2015; Reichwald et al., 2015). Formulating a standardised artificial diet that would be readily consumed by captive N. furzeri now appears critical to increase experimental repeatability across laboratories. Detailed understanding of the natural diet of N. furzeri (Polačik and Reichard, 2010; Polačik et al., 2014a) should help the community to accomplish this goal.
Conclusions
Compared to other fish model systems in biomedical research (e.g. Parichy, 2015), our understanding of the natural history of N. furzeri is substantial (Cellerino et al., 2016). Indeed, N. furzeri may, uniquely, be a model that bridges the interests of ecological and biomedical research (Cellerino et al., 2016). Still, we know relatively little about some features of the natural history of N. furzeri. While some pressing issues are outlined in Box 2, we highlight that the greatest gap in our knowledge of the natural life cycle of N. furzeri is in understanding embryonic development under natural conditions. Data from wild populations demonstrated that high phenotypic plasticity of N. furzeri, manifested in the laboratory by individually disparate growth rates, timing of sexual maturation and fecundity, is natural. While perhaps complicating some experimental designs, it raises a multitude of questions on the underlying mechanisms, from gene expression to individual behaviour (Box 2). With much more to be discovered on the natural history of the species, we anticipate that N. furzeri will continue to be instrumental in showing how conceptual and methodological insights from ecological and biomedical research can be integrated within a single research agenda (Reichard et al., 2015; Cellerino et al., 2016).
Acknowledgements
We thank R. Blažek, M. Vrtílek, J. Žák, V. Nezhybová and P. Vallo for support during our long-term research on wild killifish populations, R. Blažek, M. Vrtílek, J. Žák, C. Smith, A. Furness, D. R. Valenzano and an anonymous referee for constructive comments on the manuscript, and the Czech Science Foundation for financial support that has kept our killifish research ongoing through several successive projects awarded since 2006.
Biographies
Martin Reichard is at the Czech Academy of Sciences, Institute of Vertebrate Biology, Brno, Czech Republic
Matej Polačik is at the Czech Academy of Sciences, Institute of Vertebrate Biology, Brno, Czech Republic
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Competing interests
No competing interests declared.
Contributor Information
Martin Reichard, Email: reichard@ivb.cz.
Stuart RF King, eLife, United Kingdom.
Peter A Rodgers, eLife, United Kingdom.
Funding Information
This paper was supported by the following grants:
Grantová Agentura České Republiky 16-00291S to Martin Reichard.
Grantová Agentura České Republiky 18-26284S to Matej Polačik.
References
- Bartáková V, Reichard M, Janko K, Polačik M, Blažek R, Reichwald K, Cellerino A, Bryja J. Strong population genetic structuring in an annual fish, Nothobranchius furzeri, suggests multiple savannah refugia in southern Mozambique. BMC Evolutionary Biology. 2013;13:196. doi: 10.1186/1471-2148-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartáková V, Reichard M, Blažek R, Polačik M, Bryja J. Terrestrial fishes: rivers are barriers to gene flow in annual fishes from the African savanna. Journal of Biogeography. 2015;42:1832–1844. doi: 10.1111/jbi.12567. [DOI] [Google Scholar]
- Baumgart M, Groth M, Priebe S, Savino A, Testa G, Dix A, Ripa R, Spallotta F, Gaetano C, Ori M, Terzibasi Tozzini E, Guthke R, Platzer M, Cellerino A. RNA-seq of the aging brain in the short-lived fish N. furzeri - conserved pathways and novel genes associated with neurogenesis. Aging Cell. 2014;13:965–974. doi: 10.1111/acel.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart M, Barth E, Savino A, Groth M, Koch P, Petzold A, Arisi I, Platzer M, Marz M, Cellerino A. A miRNA catalogue and ncRNA annotation of the short-living fish Nothobranchius furzeri. BMC Genomics. 2017;18:693. doi: 10.1186/s12864-017-3951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blažek R, Polačik M, Reichard M. Rapid growth, early maturation and short generation time in African annual fishes. EvoDevo. 2013;4:24. doi: 10.1186/2041-9139-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blažek R, Polačik M, Kačer P, Cellerino A, Řežucha R, Methling C, Tomášek O, Syslová K, Terzibasi Tozzini E, Albrecht T, Vrtílek M, Reichard M. Repeated intraspecific divergence in life span and aging of African annual fishes along an aridity gradient. Evolution. 2017;71:386–402. doi: 10.1111/evo.13127. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Valenzano DR, Reichard M. From the bush to the bench: the annual Nothobranchius fishes as a new model system in biology. Biological Reviews. 2016;91:511–533. doi: 10.1111/brv.12183. [DOI] [PubMed] [Google Scholar]
- Dodzian J, Kean S, Seidel J, Valenzano DR. A protocol for laboratory housing of turquoise killifish (Nothobranchius furzeri) Journal of Visualized Experiments. 2018;134:57073. doi: 10.3791/57073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Castanedo O, MÁ M-C, Valdesalici S. First observations of annualism in (Cyprinodontiformes: Rivulidae) Ichthyological Exploration of Freshwaters. 2013;24:15–20. [Google Scholar]
- Domínguez-Castanedo O, Uribe MC, Rosales-Torres AM. Life history strategies of annual killifish Millerichthys robustus (Cyprinodontiformes:Cynolebiidae) in a seasonally ephemeral water body in Veracruz, México. Environmental Biology of Fishes. 2017;100:995–1006. doi: 10.1007/s10641-017-0617-y. [DOI] [Google Scholar]
- Dorn A, Ng'oma E, Janko K, Reichwald K, Polačik M, Platzer M, Cellerino A, Reichard M. Phylogeny, genetic variability and colour polymorphism of an emerging animal model: the short-lived annual Nothobranchius fishes from southern Mozambique. Molecular Phylogenetics and Evolution. 2011;61:739–749. doi: 10.1016/j.ympev.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Frézal L, Félix M-A. C. elegans outside the Petri dish. eLife. 2015;4:e05849. doi: 10.7554/eLife.05849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness AI, Lee K, Reznick DN. Adaptation in a variable environment: Phenotypic plasticity and bet-hedging during egg diapause and hatching in an annual killifish. Evolution. 2015a;69:1461–1475. doi: 10.1111/evo.12669. [DOI] [PubMed] [Google Scholar]
- Furness AI, Reznick DN, Springer MS, Meredith RW. Convergent evolution of alternative developmental trajectories associated with diapause in African and South American killifish. Proceedings of the Royal Society B: Biological Sciences. 2015b;282:20142189. doi: 10.1098/rspb.2014.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness AI. The evolution of an annual life cycle in killifish: adaptation to ephemeral aquatic environments through embryonic diapause. Biological Reviews. 2016;91:796–812. doi: 10.1111/brv.12194. [DOI] [PubMed] [Google Scholar]
- Graf M, Cellerino A, Englert C. Gender separation increases somatic growth in females but does not affect lifespan in Nothobranchius furzeri. PLoS One. 2010;5:e11958. doi: 10.1371/journal.pone.0011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grégoir AF, Thoré ESJ, Philippe C, Pinceel T, Brendonck L, Vanschoenwinkel B. Squeezing out the last egg-annual fish increase reproductive efforts in response to a predation threat. Ecology and Evolution. 2018;8:6390–6398. doi: 10.1002/ece3.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R. Sexual selection in Nothobranchius guentheri (Pisces: Cyprinodontidae) Evolution. 1976a;30:614–622. doi: 10.1111/j.1558-5646.1976.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Haas R. Behavioral Biology of the Annual Killifish, Nothobranchius guentheri. Copeia. 1976b;1976:80–91. doi: 10.2307/1443776. [DOI] [Google Scholar]
- Harel I, Benayoun BA, Machado B, Singh PP, Hu CK, Pech MF, Valenzano DR, Zhang E, Sharp SC, Artandi SE, Brunet A. A platform for rapid exploration of aging and diseases in a naturally short-lived vertebrate. Cell. 2015;160:1013–1026. doi: 10.1016/j.cell.2015.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel I, Valenzano DR, Brunet A. Efficient genome engineering approaches for the short-lived African turquoise killifish. Nature Protocols. 2016;11:2010–2028. doi: 10.1038/nprot.2016.103. [DOI] [PubMed] [Google Scholar]
- Hu CK, Brunet A. The African turquoise killifish: A research organism to study vertebrate aging and diapause. Aging cell. 2018;17:e12757. doi: 10.1111/acel.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubb RA. A new Nothobranchius (Pisces, Cyprinodontidae) from Southeastern Rhodesia. Journal of American Killifish Association. 1971;8:12–19. [Google Scholar]
- Matias JR, Markofsky J. The survival of embryos of the annual fish Nothobranchius guentheri exposed to temperature extremes and the subsequent effects on embryonic diapause. Journal of Experimental Zoology. 1978;204:219–227. doi: 10.1002/jez.1402040209. [DOI] [Google Scholar]
- Neumann W. Eierlegende Zahnkarpfen. Marktheidenfeld, Germany: Schleunungdruck GmbH; 2008. Prachtgrunkärpflinge; p. 128. [Google Scholar]
- Nezhybová V, Reichard M, Blažek R, Ondračková M. Metazoan parasites of African annual killifish (Nothobranchiidae): abundance, diversity, and their environmental correlates. Biotropica. 2017;49:229–238. doi: 10.1111/btp.12396. [DOI] [Google Scholar]
- Ng'oma E, Groth M, Ripa R, Platzer M, Cellerino A. Transcriptome profiling of natural dichromatism in the annual fishes Nothobranchius furzeri and Nothobranchius kadleci. BMC Genomics. 2014;15:754. doi: 10.1186/1471-2164-15-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parichy DM. Advancing biology through a deeper understanding of zebrafish ecology and evolution. eLife. 2015;4:e05635. doi: 10.7554/eLife.05635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos C, Tassino B, Rosenthal GG, Reichard M. Reproductive behavior and sexual selection in annual fishes. In: Berois N, García G, de Sá R, editors. Annual Fishes: Life History Strategy, Diversity, and Evolution. CRC Press; 2015. pp. 207–229. [Google Scholar]
- Petzold A, Reichwald K, Groth M, Taudien S, Hartmann N, Priebe S, Shagin D, Englert C, Platzer M. The transcript catalogue of the short-lived fish Nothobranchius furzeri provides insights into age-dependent changes of mRNA levels. BMC Genomics. 2013;14:185. doi: 10.1186/1471-2164-14-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe C, Hautekiet P, Grégoir AF, Thoré ESJ, Pinceel T, Stoks R, Brendonck L, Boeck G. Combined effects of cadmium exposure and temperature on the annual killifish (Nothobranchius furzeri) Environmental Toxicology and Chemistry. 2018;37:2361–2371. doi: 10.1002/etc.4182. [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Carpenter JF, Hand SC. Survival of water stress in annual fish embryos: dehydration avoidance and egg envelope amyloid fibers. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2001;280:R123–R131. doi: 10.1152/ajpregu.2001.280.1.R123. [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Garrett ID, Kohl ZF. Alternative developmental pathways associated with diapause regulated by temperature and maternal influences in embryos of the annual killifish Austrofundulus limnaeus. Journal of Experimental Biology. 2010;213:3280–3288. doi: 10.1242/jeb.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podrabsky JE, Hand SC. The bioenergetics of embryonic diapause in an annual killifish, Austrofundulus limnaeus. Journal of Experimental biology. 1999;202:2567–2580. doi: 10.1242/jeb.202.19.2567. [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Riggs CL, Romney AL, Woll SC, Wagner JT, Culpepper KM, Cleaver TG. Embryonic development of the annual killifish Austrofundulus limnaeus: An emerging model for ecological and evolutionary developmental biology research and instruction. Developmental Dynamics. 2017;246:779–801. doi: 10.1002/dvdy.24513. [DOI] [PubMed] [Google Scholar]
- Polačik M, Reichard M. Indirect fitness benefits are not related to male dominance in a killifish. Behavioral Ecology and Sociobiology. 2009;63:1427–1435. doi: 10.1007/s00265-009-0798-2. [DOI] [Google Scholar]
- Polačik M, Reichard M. Diet overlap among three sympatric African annual killifish species Nothobranchius spp. from Mozambique. Journal of Fish Biology. 2010;77:754–768. doi: 10.1111/j.1095-8649.2010.02717.x. [DOI] [PubMed] [Google Scholar]
- Polačik M, Donner MT, Reichard M. Age structure of annual Nothobranchius fishes in Mozambique: is there a hatching synchrony? Journal of Fish Biology. 2011;78:796–809. doi: 10.1111/j.1095-8649.2010.02893.x. [DOI] [PubMed] [Google Scholar]
- Polačik M, Reichard M. Asymmetric reproductive isolation between two sympatric annual killifish with extremely short lifespans. PLoS One. 2011;6:e22684. doi: 10.1371/journal.pone.0022684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polačik M, Harrod C, Blažek R, Reichard M. Trophic niche partitioning in communities of African annual fish: evidence from stable isotopes. Hydrobiologia. 2014a;721:99–106. doi: 10.1007/s10750-013-1652-0. [DOI] [Google Scholar]
- Polačik M, Blažek R, Režucha R, Vrtílek M, Terzibasi Tozzini E, Reichard M. Alternative intrapopulation life-history strategies and their trade-offs in an African annual fish. Journal of Evolutionary Biology. 2014b;27:854–865. doi: 10.1111/jeb.12359. [DOI] [PubMed] [Google Scholar]
- Polačik M, Blažek R, Reichard M. Laboratory breeding of the short-lived annual killifish Nothobranchius furzeri. Nature Protocols. 2016;11:1396–1413. doi: 10.1038/nprot.2016.080. [DOI] [PubMed] [Google Scholar]
- Polačik M, Smith C, Reichard M. Maternal source of variability in the embryo development of an annual killifish. Journal of Evolutionary Biology. 2017;30:738–749. doi: 10.1111/jeb.13038. [DOI] [PubMed] [Google Scholar]
- Polačik M, Reichard M, Vrtílek M. Local variation in embryo development rate in annual fish. Journal of Fish Biology. 2018;92:1359–1370. doi: 10.1111/jfb.13591. [DOI] [PubMed] [Google Scholar]
- Reichard M, Polacik M, Sedlácek O. Distribution, colour polymorphism and habitat use of the African killifish Nothobranchius furzeri, the vertebrate with the shortest life span. Journal of Fish Biology. 2009;74:198–212. doi: 10.1111/j.1095-8649.2008.02129.x. [DOI] [PubMed] [Google Scholar]
- Reichard M, Polačik M, Blažek R, Vrtílek M. Female bias in the adult sex ratio of African annual fishes: interspecific differences, seasonal trends and environmental predictors. Evolutionary Ecology. 2014;28:1105–1120. doi: 10.1007/s10682-014-9732-9. [DOI] [Google Scholar]
- Reichard M, Cellerino A, Valenzano DR. Turquoise killifish. Current Biology. 2015;25:R741–R742. doi: 10.1016/j.cub.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Reichard M. The evolutionary ecology of African annual fishes. In: Berois N, García G, de Sá R, editors. Annual Fishes: Life History Strategy, Diversity, and Evolution. CRC Press; 2015. pp. 133–158. [Google Scholar]
- Reichard M, Janáč M, Polačik M, Blažek R, Vrtílek M. Community assembly in Nothobranchius annual fishes: Nested patterns, environmental niche and biogeographic history. Ecology and Evolution. 2017a;7:2294–2306. doi: 10.1002/ece3.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard M, Blažek R, Polačik M, Vrtílek M. Hatching date variability in wild populations of four coexisting species of African annual fishes. Developmental Dynamics. 2017b;246:827–837. doi: 10.1002/dvdy.24500. [DOI] [PubMed] [Google Scholar]
- Reichwald K, Petzold A, Koch P, Downie BR, Hartmann N, Pietsch S, Baumgart M, Chalopin D, Felder M, Bens M, Sahm A, Szafranski K, Taudien S, Groth M, Arisi I, Weise A, Bhatt SS, Sharma V, Kraus JM, Schmid F, Priebe S, Liehr T, Görlach M, Than ME, Hiller M, Kestler HA, Volff JN, Schartl M, Cellerino A, Englert C, Platzer M. Insights into sex chromosome evolution and aging from the genome of a short-lived fish. Cell. 2015;163:1527–1538. doi: 10.1016/j.cell.2015.10.071. [DOI] [PubMed] [Google Scholar]
- Sedláček O, Baciaková B, Kratochvíl L. Evolution of body colouration in killifishes (Cyprinodontiformes: Aplocheilidae, Nothobranchiidae, Rivulidae): Is male ornamentation constrained by intersexual genetic correlation? Zoologischer Anzeiger - A Journal of Comparative Zoology. 2014;253:207–215. doi: 10.1016/j.jcz.2013.12.004. [DOI] [Google Scholar]
- Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, Valenzano DR. Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. eLife. 2017;6:e27014. doi: 10.7554/eLife.27014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzibasi E, Valenzano DR, Benedetti M, Roncaglia P, Cattaneo A, Domenici L, Cellerino A. Large differences in aging phenotype between strains of the short-lived annual fish Nothobranchius furzeri. PLoS One. 2008;3:e3866. doi: 10.1371/journal.pone.0003866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoré ESJ, Steenaerts L, Philippe C, Grégoir A, Brendonck L, Pinceel T. Individual behavioral variation reflects personality divergence in the upcoming model organism Nothobranchius furzeri. Ecology and Evolution. 2018;8:8448–8457. doi: 10.1002/ece3.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzini ET, Dorn A, Ng'oma E, Polačik M, Blažek R, Reichwald K, Petzold A, Watters B, Reichard M, Cellerino A. Parallel evolution of senescence in annual fishes in response to extrinsic mortality. BMC Evolutionary Biology. 2013;13:77. doi: 10.1186/1471-2148-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdesalici S, Cellerino A. Extremely short lifespan in the annual fish Nothobranchius furzeri. Proceedings of the Royal Society B: Biological Sciences. 2003;270 Suppl 2:S189–S191. doi: 10.1098/rsbl.2003.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano DR, Kirschner J, Kamber RA, Zhang E, Weber D, Cellerino A, Englert C, Platzer M, Reichwald K, Brunet A. Mapping loci associated with tail color and sex determination in the short-lived fish Nothobranchius furzeri. Genetics. 2009;183:1385–1395. doi: 10.1534/genetics.109.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano DR, Benayoun BA, Singh PP, Zhang E, Etter PD, Hu CK, Clément-Ziza M, Willemsen D, Cui R, Harel I, Machado BE, Yee MC, Sharp SC, Bustamante CD, Beyer A, Johnson EA, Brunet A. The african turquoise killifish genome provides insights into evolution and genetic architecture of lifespan. Cell. 2015;163:1539–1554. doi: 10.1016/j.cell.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtílek M, Reichard M. Highly plastic resource allocation to growth and reproduction in females of an African annual fish. Ecology of Freshwater Fish. 2015;24:616–628. doi: 10.1111/eff.12175. [DOI] [Google Scholar]
- Vrtílek M, Reichard M. Female fecundity traits in wild populations of African annual fish: the role of the aridity gradient. Ecology and Evolution. 2016;6:5921–5931. doi: 10.1002/ece3.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtílek M, Žák J, Pšenička M, Reichard M. Extremely rapid maturation of a wild African annual fish. Current Biology. 2018a;28:R822–R824. doi: 10.1016/j.cub.2018.06.031. [DOI] [PubMed] [Google Scholar]
- Vrtílek M, Žák J, Blažek R, Polačik M, Cellerino A, Reichard M. Limited scope for reproductive senescence in wild populations of a short-lived fish. The Science of Nature. 2018b;105:68. doi: 10.1007/s00114-018-1594-5. [DOI] [PubMed] [Google Scholar]
- Vrtílek M, Žák J, Polačik M, Blažek R, Reichard M. Longitudinal demographic study of wild populations of African annual killifish. Scientific Reports. 2018c;8:4774. doi: 10.1038/s41598-018-22878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters BR. The ecology and distribution of Nothobranchius fishes. The Journal of the American Killifish Association. 2009;42:37–76. [Google Scholar]
- Wendler S, Hartmann N, Hoppe B, Englert C. Age-dependent decline in fin regenerative capacity in the short-lived fish Nothobranchius furzeri. Aging Cell. 2015;14:857–866. doi: 10.1111/acel.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll SC, Podrabsky JE. Insulin-like growth factor signaling regulates developmental trajectory associated with diapause in embryos of the annual killifish Austrofundulus limnaeus. The Journal of Experimental Biology. 2017;220:2777–2786. doi: 10.1242/jeb.151373. [DOI] [PubMed] [Google Scholar]
- Wourms JP. The developmental biology of annual fishes. 3. Pre-embryonic and embryonic diapause of variable duration in the eggs of annual fishes. Journal of Experimental Zoology. 1972;182:389–414. doi: 10.1002/jez.1401820310. [DOI] [PubMed] [Google Scholar]
- Žák J, Reichard M, Gvoždík L. Limited differentiation of fundamental thermal niches within the killifish assemblage from shallow temporary waters. Journal of Thermal Biology. 2018;78:257–262. doi: 10.1016/j.jtherbio.2018.10.015. [DOI] [PubMed] [Google Scholar]