Abstract
The classic treatment of chronic osteomyelitis is usually a two-stage surgery combined with systemic antibiotic therapy for several months. We report the case of a patient presenting a chronic osteomyelitis caused by methicillin-resistant Staphylococcus aureus who was treated with a one-stage surgery using an antibiotic-loaded ceramic. We used a porous alumina ceramic loaded with gentamicin to reconstruct the bone removed during débridement and to avoid its colonization. All bacteriological samples performed before and during the surgery revealed the presence of a methicillin-resistant S aureus. Because of the local release of the antibiotic, very high concentrations (more than 50 times the concentration needed) were administered in the surgical wound, thus helping to cure the infection. Owing to the strength of the ceramic, the patient was allowed to walk 10 days after the surgery. After a follow-up at 14 months, the patient is well-being, without any relapse of the infection. The CT-scan follow-up shows an osseointegration of the ceramic. Even, if it is too early to tell that infection is completely cured, these first results are encouraging for the use, in the future, of this antibiotic-loaded ceramic for complex bone infection.
To date, the benchmark technique for chronic osteomyelitis is the combination of surgical débridement and an antibiotic therapy for several months.1,2 The surgical technique used is usually a two-stage surgery. During the second stage, a bone graft is harvested from the iliac bone. In this context, implantation of foreign body is usually contraindicated because there is a high risk of device infection. However, one of the main problems for antibiotic therapy of bone infection is bacteria biofilm development, which protects them from immune system and from antibiotic.3 One consequence of this biofilm is that the minimal inhibitory concentration (MIC) greatly increases (up to 1,000-folds the one of a planktonic bacteria),4 thus needing a high dosage of antibiotic, which sometimes cannot be reached with conventional administration routes. In this context and to avoid a two-stage surgery, one solution could be to locally release antibiotic via the implanted device. Interestingly, because of its porous nature, porous alumina ceramic can be loaded with active molecules such as antibiotics. This loaded device has been developed recently (I.Ceram). We report the case of a patient presenting a chronic osteomyelitis caused by methicillin-resistant Staphylococcus aureus (MRSA) who was surgically treated with a one-stage surgery using a gentamicin-loaded ceramic.
Case Report
A 17-year-old male patient presented with a chronic fistula at the distal extremity of his right femur. Several months before, he experienced an open fracture after a fall at 7 m. An external fixator was used, but an infection occurred and was not properly treated. After fixator removal, he moved to France and came to the outpatient consultation with a chronic bone infection lasting for 18 months. Clinically, he presented with a chronic skin fistula with pus at the lateral aspect of the thigh. He did not have temperature. The C-reactive protein was <5 mg/L. CT scan revealed bone destruction on the distal diaphyseal-metaphyseal part of the femur (Figure 1). Microbiological samples (percutaneous core bone biopsy) revealed an MRSA sensitive, among other, to gentamicin, vancomycin, ofloxacin, and rifampicin.
Figure 1.
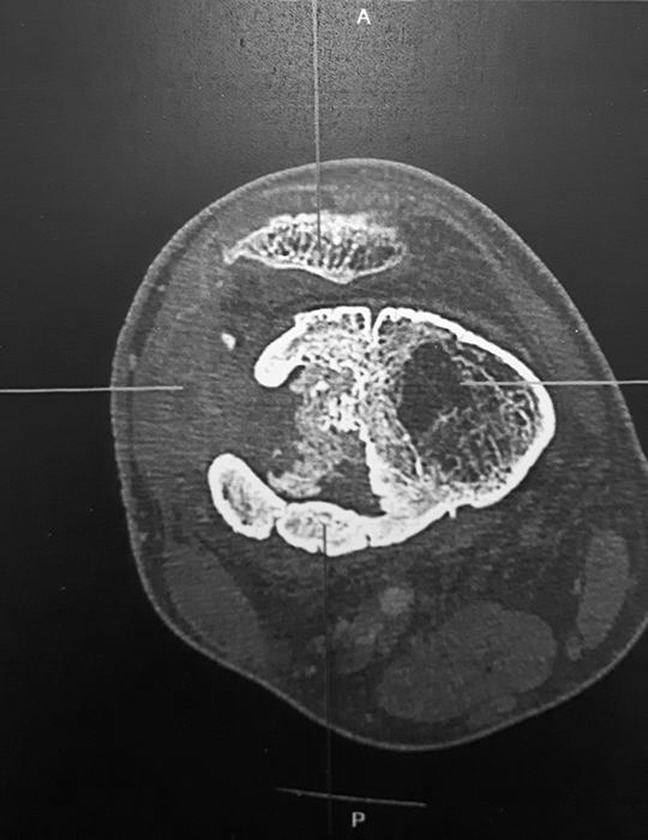
Axial CT scan of the lower extremity of the femur showing bone loss and bone reorganization because of the chronic infection.
To avoid a two-stage surgery, we used an antibiotic-loaded porous alumina ceramic to (1) preserve the strength of the bone, performing only a one-stage surgery without bone graft, (2) deliver locally a high dose of antibiotic to complete the surgical débridement, and (3) prevent device infection.
The surgery was approved by the Ethic Committee of the Limoges University Hospital and the French Agency for Health Security (Agence Nationale de la Sécurité du Médicament et des produits de santé, ANSM). The patient and his tutor gave their written consent.
The ceramic used for this patient is porous alumina (I.Ceram) and has been implanted for more than 20 years as, for example, tibial wedges for osteotomy.5 In addition to its great biocompatibility, porous alumina has resistance strength of threefolds the cancellous bone. It is nonabsorbable, and it has a great osseointegration.5 Because of its porosity, it can be loaded with active molecules. The device was tailored for this patient with a shape designed after 3D CT-scan analysis. The device was loaded afterward with gentamicin, an antibiotic from aminoglycosides class. This antibiotic is already locally administered via cement6 or collagen sponges.7 The loaded dose in the ceramic was 160 mg (roughly half the daily intravenous dose for this patient). Three sizes of the same shape were produced to allow the surgeon choosing the best fit after surgical débridement. The loaded device was delivered as ready to use and proposed in a dry form requiring no additional step before or after implantation.
The six samples tested during débridement were microbiologically positive with the same MRSA found on biopsy. After débridement, the ceramic was impacted in the cortical bone and anchored with absorbable suture threats to ensure implant stability during the first stages of bone healing (Figure 2). After muscle plane closure, a Redon drain was placed about 4 cm away from the ceramic.
Figure 2.
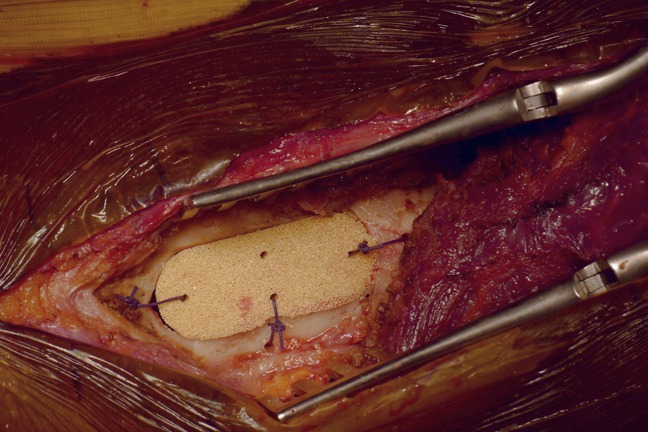
The porous alumina ceramic device stuck in the femoral cortical bone after débridement. Suture threads are used to maintain the device during the first step of healing.
Local determinations of gentamicin, performed through Redon drain, 5 and 24 hours after the implantation were 184 and 13 mg/L respectively. In comparison to the MIC of the MRSA (<0.5 mg/L), the local gentamicin concentration was greater than 50-folds the one needed to be efficient after 5 hours and was still greater by 4-folds after 24 hours. Indeed, to be efficient, the concentration of gentamicin needs to be almost eightfolds greater than the MIC. Noteworthy, the Redon drain was 4 cm away from the ceramic implant, meaning that there should have been a greater concentration in contact with the implant and that there is a good local diffusion of the antibiotic. Meanwhile, blood samples from 1 hour to 48 hours did not reveal any gentamicin (concentration <0.5 mg/L).
After the surgery, the patient was treated with conventional antibiotic treatment. This treatment was defined by the Reference Center for Complex Bone and Joint Infections (CRIOAC) of the Limoges University Hospital. He first received vancomycin, and after 10 days, a combination of ofloxacin (200 mg three times a day) and rifampicin (600 mg two times a day) for a total course of 3 months.
After a follow-up of more than 14 months, there is no relapse of skin fistula or signs of bone infection recurrence. The patient is well-being without temperature. CT scan revealed a tight contact between bone and ceramic (Figure 3). Biologically, no sign of inflammation is observed (C-reactive protein <5 mg/L).
Figure 3.
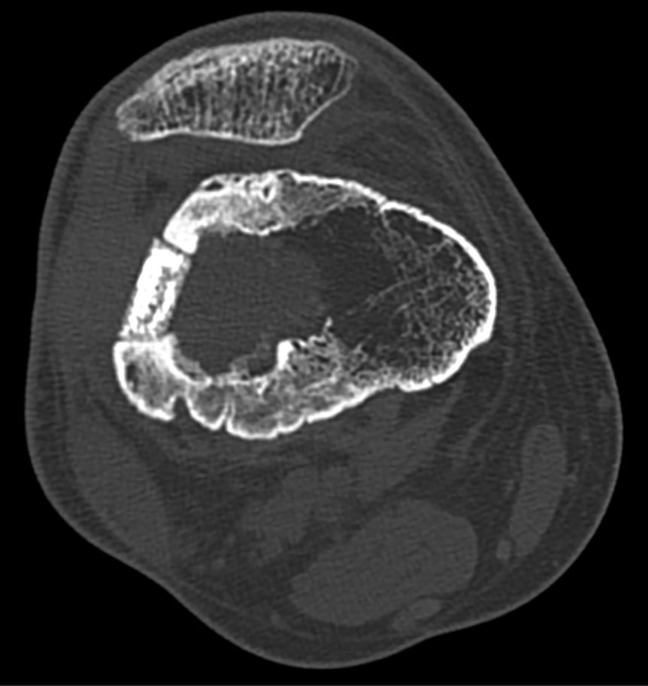
Axial CT scan after a follow-up of 11 months. A tight contact between the bone and the ceramic testifies biocompatibility and osseointegration.
Discussion
It is usually not recommended to implant foreign bodies when an infection is not controlled. Indeed, bacteria colonize the implant and produce a biofilm, making them nonremovable from the device, which leads to a chronic infection. Thus, one way to treat bone infection is to perform a two-stage surgery. During the first stage, the bone is cleaned, sequestra are removed, and cement loaded with antibiotics can be placed to replace removed bone. During the second stage, usually 6 weeks later, the cement is removed to avoid a foreign body effect with the risk of reinfection. Then, bone graft, usually from the iliac crest, is used to restore bone integrity and strength. After the first stage, an antibiotic treatment targeting bacteria found in bone samples is administered. The main drawbacks of this technic are the need for two surgeries and the iliac pain induced by bone graft harvest.8
The use of the ceramic allows reinforcement of bone after its débridement. But as a foreign body, it needs to be protected to avoid its colonization by bacteria, which could remain in the surgical wound. This is the role of the loaded antibiotic. With its local release and its high concentration, it protects the implant by killing the remaining bacteria. The 100% release and the local concentration, largely exceeding the dose needed, allow this protection. The absence of blood diffusion decreases the risk of adverse effects, which are well known with gentamicin. The loaded dose is the same as the one used in Cerament G (Bonesupport, Lund, Sweden) for example.9 The local observed concentrations are about the same,9 and no local toxicity is reported. However, this bone substitute does not have the strength to replace the bone right after its implantation contrarily to alumina.
Before using this therapeutic option, it is mandatory to identify the bacteria involved in osteomyelitis, as antibiotic must target them. Gentamicin was used for this patient. But ceramic loaded with vancomycin or the combination of vancomycin and gentamicin are already available (I.Ceram). All these options allow targeting a wide range of bacteria implicated in bone infection.
One restriction to use this loaded ceramic is the need to know in advance the shape and the size of the bone to be replaced. Indeed, this ceramic cannot be modified in the theatre room with usual surgical tools. However, using three-dimensional CT-scan reconstruction, the shape can easily be decided before the surgery. For practicality reason, three sizes of the chosen shape could be provided like it was provided for this patient. This allows the surgeon to choose the best fitting size during the surgery. Indeed, there can be some discrepancy between the CT scan and the bone removed during the surgery. Furthermore, an ancillary could be delivered to cut the bone at the right size for a perfect impaction of the ceramic in the bone.
Conclusion
Because it is the first case, it has to be confirmed by other identical surgeries, but the use of this loaded ceramic seems to be a reliable option to treat osteomyelitis with bone loss. The local release of high antibiotic concentration allows implant's protection against bacteria in such infected area. A follow-up of more than 12 months is short regarding the time scale of bone infection, but usually, device infection occurs quickly after its implantation when colonization occurs during the surgery, which was not the case here.
Footnotes
Dr. Fiorenza or an immediate family member serves as an unpaid consultant to I.CERAM. Dr. El Balkhi or an immediate family member is an employee of I.CERAM. Dr. Denes or an immediate family member is an employee of and has stock or stock options held in I.CERAM. Neither Dr. Durox nor any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article.
Dr. Fiorenza: performed the surgery and follow-up, and revised the manuscript. Dr. Denes: developed the loading, drafted the manuscript, and interpreted gentamicin dosages. Dr. El Balkhi: performed gentamicin dosages and interpretation, and revised the manuscript. Dr. Durox: managed antibiotic therapy and revised the manuscript.
References
- 1.Panteli M, Giannoudis PV: Chronic osteomyelitis: What the surgeon needs to know. EFORT Open Rev 2017;1:128-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winkler H: Treatment of chronic orthopaedic infection. EFORT Open Rev 2017;2:110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S: Biofilms: An emergent form of bacterial life. Nat Rev Microbiol 2016;14:563-575. [DOI] [PubMed] [Google Scholar]
- 4.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O: Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 2010;35:322-332. [DOI] [PubMed] [Google Scholar]
- 5.Denes E, Barrière G, Poli E, Lévêque G: Commentary: Bioceramics and scaffolds: A winning combination for tissue engineering. Front Bioeng Biotechnol 2017;5:35-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y, Tai CL, Hsieh PH, Ueng SWN: Gentamicin in bone cement: A potentially more effective prophylactic measure of infection in joint arthroplasty. Bone Joint Res 2013;2:220-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang WK, Srinivasa S, MacCormick AD, Hill AG: Gentamicin-collagen implants to reduce surgical site infection. Ann Surg 2013;258:59-65. [DOI] [PubMed] [Google Scholar]
- 8.Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV: Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011;42(suppl 2):S3-S15. [DOI] [PubMed] [Google Scholar]
- 9.Stravinskas M, Horstmann P, Ferguson J, et al. : Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: In vitro and clinical release studies. Bone Joint Res 2016;5:427-435. [DOI] [PMC free article] [PubMed] [Google Scholar]


