Abstract
Background
Simultaneous heart-liver (SHL) transplantation is an efficacious therapeutic modality for patients with combined heart and liver failure. However, the extent to which heart transplantation followed by sequential liver transplantation (LAH) can match the benefit of simultaneous transplantation has not previously been examined. Our objective was to determine if LAH offers comparable survival to SHL.
Methods
The Organ Procurement and Transplantation Network/United Network for Organ Sharing Standard Transplant Analysis and Research file was queried for adult recipients waitlisted for both heart and liver transplantation. The United Network for Organ Sharing thoracic and liver databases were linked to facilitate examination of waitlist and transplant characteristics for simultaneously listed patients. Univariate survival analysis was used to determine overall survival.
Results
Of the 236 patients meeting inclusion criteria, 200 underwent SHL, 7 sequentially underwent LAH, and 29 received heart transplantation only (isolated orthotopic heart transplantation [iOHT]). Recipients of SHL were less likely to have an episode of acute rejection before discharge (LAH, 14.2%; SHL, 2.4%; iOHT, 3.6%; P = .019) or be treated for acute rejection within 1 year after transplantation (LAH, 14.3%; SHL, 2.5%; iOHT, 13.8%; P = .007). Otherwise, postoperative hospital length of stay, stroke, need for dialysis, and need for pacemaker placement were comparable across groups. Ten-year survival similarly favored both LAH and SHL over iOHT (LAH: 100%, 71.4%, 53.6%; SHL: 87.1%, 80.4%, 52.1%, iOHT: 70.1%, 51.6%, 27.5% for 1-, 5-, and 10-year survivals, respectively, P = .003). However, median time between heart and liver transplant was 302 days in patients undergoing sequential transplantation.
Conclusions
Although transplantation in a simultaneous or sequential fashion yields equivalent outcomes, a high fraction of patients undergoing initial heart transplant alone fail to proceed to subsequent liver transplantation. Therefore, in patients with combined heart and liver failure with a projected need for 2 allografts, simultaneous transplantation is associated with maximum benefit.
Since first described by Starzl and colleagues in 1984, simultaneous heart-liver (SHL) transplant has been increasingly utilized as a viable therapeutic option for patients with concomitant end-stage liver and heart disease.1 Although experience with SHL remains limited overall, several single-institution studies demonstrate its safety and efficacy in treating patients with heart and liver failure from a variety of etiologies.2-6 Further, SHL offers comparable patient and graft survival to orthotopic liver transplant (OLT) or heart transplant (OHT) alone.7,8 Thus, SHL may offer the best option for these critically ill patients with both heart and liver failure.
Although SHL is effective, there remains controversy regarding the best way to allocate allografts to these dual-organ transplant candidates for the purposes of just and efficacious distribution.9,10 Simultaneous heart-liver transplantation involves the simultaneous transplantation of both a heart and liver allograft during a single operation and current guidelines allow for potential SHL candidates to be included on the waitlists for both heart transplant and liver transplant concurrently. Thus, if an SHL candidate is offered a matching heart or liver, the additional organ is also allocated to the patient for simultaneous transplantation of both organs regardless of the independent waitlist priority of the second organ. Consequently, patients with disease severe enough to warrant higher status on the waitlist for 1 organ may have a higher likelihood of being allocated the other organ than a patient on the waitlist for isolated transplant of that organ.11
Others have posited that this system remains tenable if it can satisfy the principles of justice and utility.12 As defined by the ethics committee for the United Network for Organ Sharing (UNOS), justice describes fairness in distribution, whereas utility refers to the greatest aggregate good that can be achieved through distribution of a scarce resource to the whole population.13 Wolf and colleagues12 demonstrated that patients listed for SHL exhibited greater medical urgency as they were at greater risk for waitlist mortality than heart only or liver only transplant candidates. Consequently, SHL offered a significant survival advantage compared with remaining on the waitlist while producing similar outcomes to both OHT and OLT alone.
Although it may be argued that SHL satisfies the principle of justice based on medical urgency, it remains unclear whether it confers the greatest aggregate good. A potential alternative means of providing both needed organs to patients with combined heart and liver failure is sequential heart-liver transplant or liver after heart (LAH) transplantation.14 This sequential organ allocation approach would also serve to resolve the conflicting aims of justice and utility in this context, as patients could be waitlisted for both organs and allocated those organs per their independent waitlist status. Consequently, patients in need of multiple organs would be able to receive them without impeding access to a scarce medical good for patients in need of a single organ.
Although previous studies have examined outcomes for the reception of both needed organs compared with isolated organ transplant in SHL candidates,7,8,12 no studies to date have examined whether sequential allocation of 2 organs is an acceptable alternative approach to SHL. We, therefore, conducted this analysis to test the hypothesis that, for the treatment of patients in need of both heart and liver transplantation, LAH is noninferior with respect to mortality and morbidity when compared with SHL.
MATERIALS AND METHODS
Data Source
The institutional review board at Duke University approved this retrospective cohort analysis of the UNOS Standard Analysis and Research (STAR) database before data collection. The Organ Procurement and Transplantation Network (OPTN) is administered by UNOS under contract with the United States Department of Health and Human Services. The OPTN/UNOS database is comprised of data on all transplant candidates listed for organ allotransplantation in the United States since October 1987. Since 1999, all data in this database are collected and entered by transplant professionals into an Internet-based database application. The electronic data are subsequently validated and on-site audits are performed intermittently to ensure data quality.
Study Design
The OPTN/UNOS STAR file was queried to identify all first-time heart transplant candidates who were colisted for liver transplantation before their heart transplantation. Exclusion criteria included pediatric candidates (younger than 18 years), those undergoing simultaneous lung, kidney, or other abdominal organ transplantation, and those with missing survival data. All liver allografts were procured from deceased donors. As no patients in this cohort received an isolated liver allograft before heart transplantation, recipients meeting appropriate criteria were subsequently divided into those that underwent SHL transplantation or an initial heart transplant alone (HTA). Differential survival was then examined between these groups to determine the potential benefit of simultaneous liver transplantation in this cohort of heart transplant recipients. Recipients that had undergone isolated heart transplantation (HTA group) were subsequently separated into recipients who later underwent liver transplantation (LAH) and those who did not (iOHT) (Figure 1).
FIGURE 1.
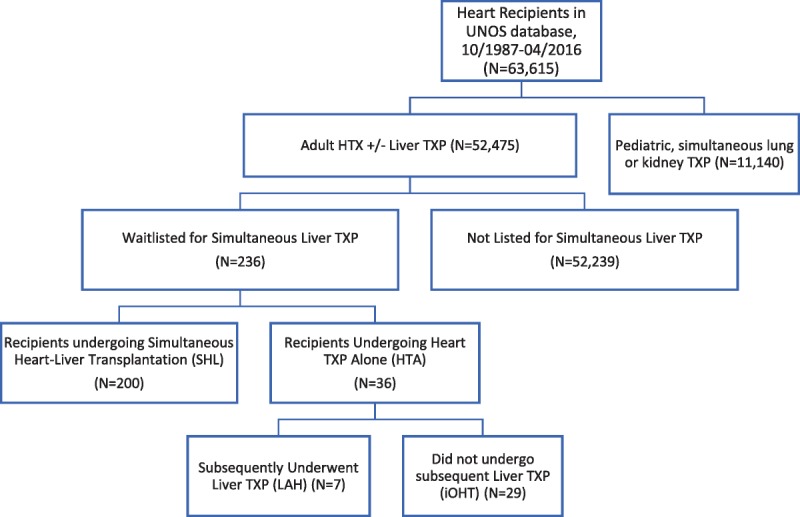
STROBE diagram of recipients analyzed. STROBE, Strengthening The Reporting of Observational studies in Epidemiology.
Statistical Analysis
Patient baseline demographic characteristics and outcomes were described and compared between treatment groups. Analysis was performed using the Kruskal-Wallis test for continuous variables and χ2 for categorical variables. The primary outcome was overall survival (defined as the date of initial transplantation to the date of death from any cause and censored at date of most recent alive follow-up which is recorded in the STAR file). Patient survival was estimated using the Kaplan-Meier method and compared with log-rank testing. An intention-to-treat survival analysis was performed to ascertain the survival benefit of the second (liver) allograft in SHL transplantation in candidates listed for both organs, regardless of subsequent sequential transplantation. Univariate Cox proportional hazards models were developed to estimate the hazard ratio associated with simultaneous compared to isolated transplantation strategies. Secondary outcomes included hospital length of stay, acute rejection before discharge, stroke, perioperative need for dialysis or pacemaker implantation, and treatment for rejection within 1 year of discharge from transplant hospitalization. Secondary outcomes were defined according to the STAR database definitions. Results are reported as median (Interquartile Range), proportions (%) and odds ratios (OR, 95% Confidence Interval) as applicable. All comparisons were 2-tailed, and a P value less than .05 was used to indicate statistical significance. We controlled for type I error at the level of the comparison. All statistical analyses were performed using R (The R Foundation for Statistical Computing, version 3.3.2, Vienna, Austria).
RESULTS
Transplant Candidate Demographic and Baseline Information
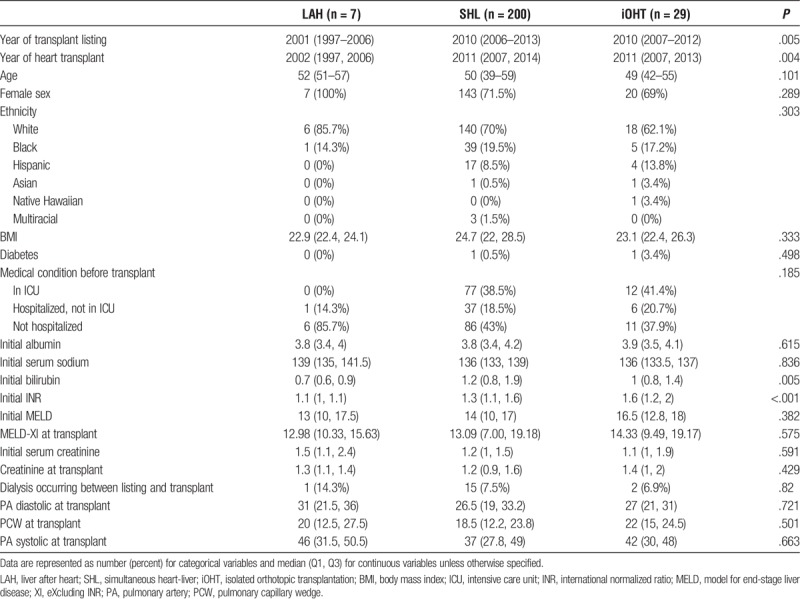
A total of N = 236 OHT recipients were identified as meeting criteria for study inclusion. Of these, 200 (84%) recipients underwent SHL and 36 (16%) recipients underwent HTA. Of the HTA recipients, 7 (19.5%) later received LAH, whereas 29 (80.5%) patients never underwent subsequent liver transplantation, and consequently only received the initial, iOHT. The year of transplant listing was significantly earlier for our LAH cohort (LAH, 2001; SHL, 2010; iOHT, 2010; P = .005). Similarly, year of heart transplantation was also significantly earlier in our LAH cohort (LAH, 2002; SHL, 2011; iOHT, 2011; P = .004). Recipients across the 3 groups had similar age, sex, ethnicity, body mass index (BMI), diabetic status, dialysis requirement, and hospitalization status before transplantation. In comparing recipient hepatic function at the time of listing, initial bilirubin (LAH, 0.7; SHL, 1.2; iOHT, 1.0; P = .005) and International Normalized Ratio (INR) (LAH, 1.1; SHL, 1.3; iOHT, 1.6; P < .001) were significantly lower in LAH patients compared with patients in the SHL and iOHT groups. However, initial model for end-stage liver disease (MELD) (LAH, 13; SHL, 14; iOHT, 16.5; P = .382) and baseline albumin (LAH: 3.8, SHL: 3.8, iOHT: 3.9, P = .615) were comparable between groups. As MELD is not recorded in the UNOS thoracic database, MELD-XI (MELD eXcluding INR) was calculated using values available in the thoracic database. There was no significant difference in MELD-XI at the time of heart transplantation between groups (LAH, 12.98; SHL, 13.09; iOHT, 14.33; P = .575) (Table 1).
TABLE 1.
Baseline characteristics for adult transplant candidates, UNOS/OPTN 2005–2016
Donor Characteristics
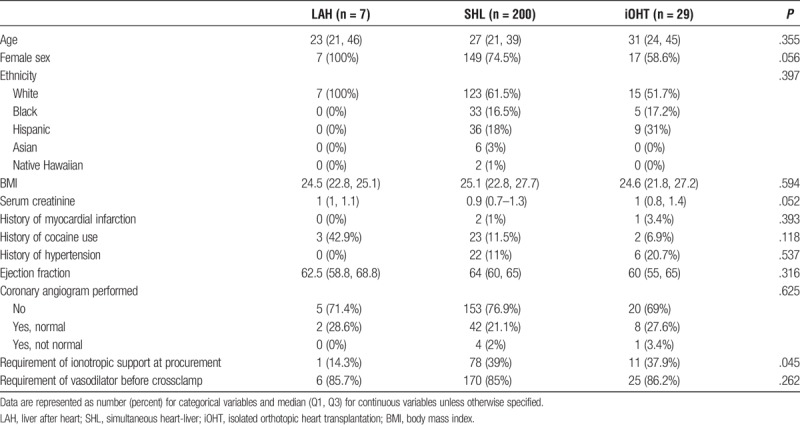
Donors to recipients were also similar with respect to age, sex, and ethnicity. History of hypertension (LAH, 0%; SHL, 11%; iOHT, 20.7% yes; P = .537), history of myocardial infarction (LAH, 0%; SHL, 1%; iOHT, 3.4% yes; P = .393), and ejection fraction (LAH, 62.5; SHL, 64.0; iOHT, 60.0; P = .316) were comparable as well. However, donors to SHL recipients were more likely to require inotropes during procurement than donors to LAH or iOHT recipients (LAH, 37.9%; SHL, 39%; iOHT, 14.3%; P = .045) (Table 2).
TABLE 2.
Baseline characteristics for adult heart transplant donors, UNOS/OPTN 2005–2016
Postoperative Outcomes
Recipients from the 3 groups had a similar length of stay for transplant hospitalization (LAH, 23.5; SHL, 21.0; iOHT, 19.0 days; P = .178), postoperative need for dialysis (LAH, 0.0%; SHL, 21.0%; iOHT, 27.6%; P = .604), need for pacemaker (LAH, 14.3%; SHL, 7.0%; iOHT, 0.0%; P = .505), and stroke (LAH, 0.0%; SHL, 6.5%; iOHT, 0.0%; P = .999). Recipients of SHL, however, were less likely to have an episode of acute rejection before discharge (LAH, 14.2%; SHL, 2.4%; iOHT, 3.6%; P = .019) or be treated for acute rejection within 1 year after transplantation (LAH, 14.3%; SHL, 2.5%; iOHT, 13.8%; P = .007) (Table 3). There was a significant increase in overall survival between patients who initially received SHL compared with those that underwent HTA (SHL, 87.1%, 80.4%, 52.1%; HTA, 76.4%, 55.8%, 36.2% for 1-, 5- and 10-year survival, respectively, hazard ratio, 0.43; 0.26-0.73; P = .002) (Figure 2). For patients that did proceed to sequential liver transplantation, the median time between heart and liver transplant was 302 days (77-1970 days). After accounting for eventual sequential liver transplantation among patients in the HTA group, there was a significant difference in 10-year survival based on transplantation strategy favoring both LAH and SHL over iOHT (LAH: 100%, 71.4%, 53.6%, SHL: 87.1%, 80.4%, 52.1%, iOHT: 70.1%, 51.6%, 27.5%, for 1-, 5- and 10-year survivals, respectively, P = .003) (Figure 3).
TABLE 3.
Posttransplant outcomes, UNOS/OPTN 2005–2016
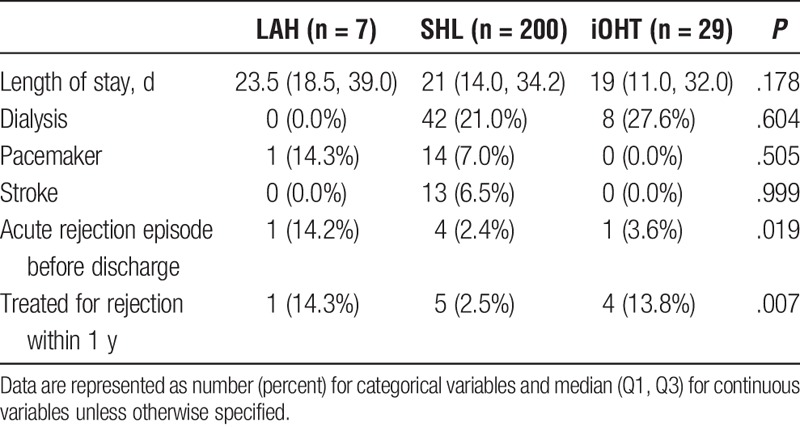
FIGURE 2.
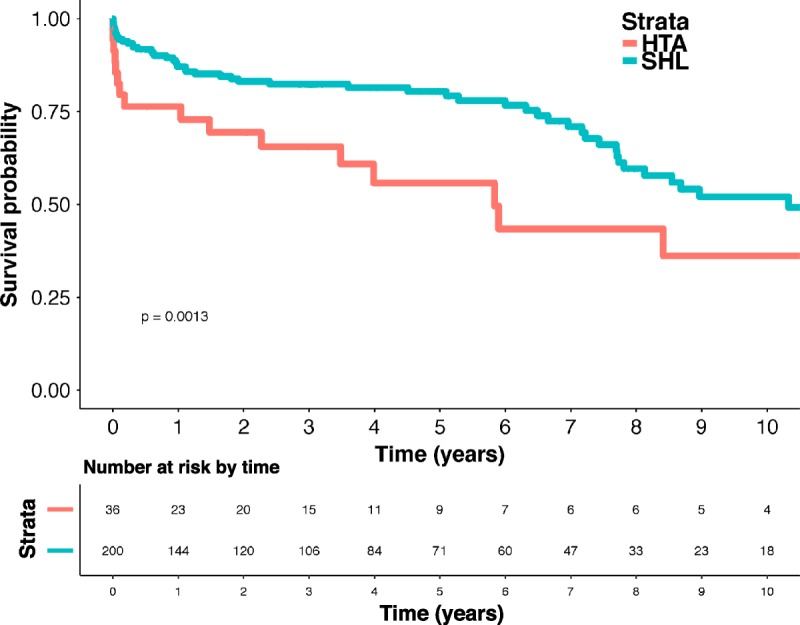
Ten-year unadjusted overall survival for heart transplant by simultaneous heart-liver (SHL) versus Initial Heart Only, UNOS/OPTN 2005 to 2016. Ten-year survival estimated via a univariate Cox proportional hazards regression model and represented as a percentage of the transplantation strategy cohort. The estimates were compared through calculation of a χ2. HTA, heart transplant alone.
FIGURE 3.
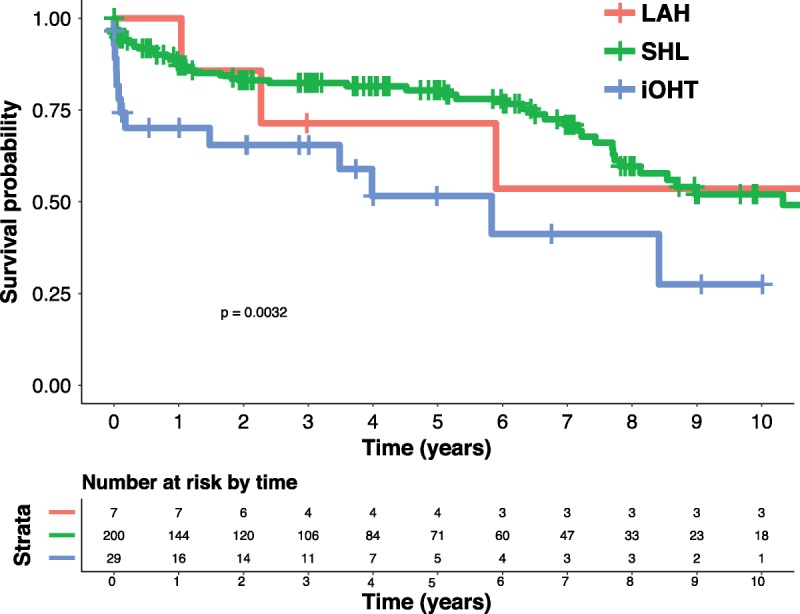
Ten-year unadjusted overall survival for heart transplant by simultaneous heart-liver (SHL) versus heart subsequent liver, UNOS/OPTN 2005 to 2016. Ten-year survival estimated via the Kaplan-Meier method and represented as a percentage of the transplantation strategy cohort. The estimates were compared with log-rank testing. LAH, liver after heart; iOHT, isolated orthotopic heart transplantation.
iOHT Subgroup Analysis
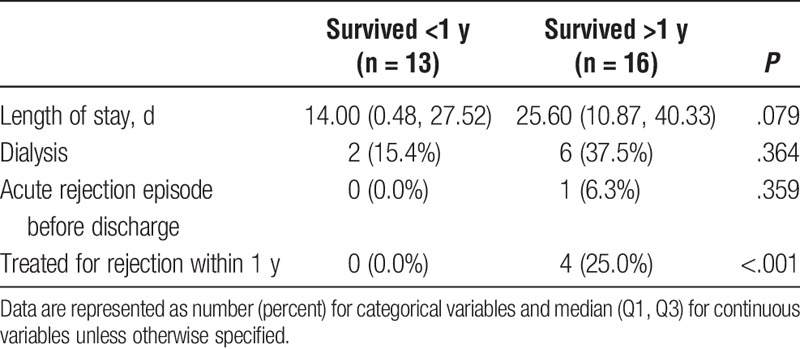
Of the 29 patients that received isolated Orthotopic Heart Transplant, 13 patients survived less than 1 year, whereas 16 patients survived greater than 1 year. In comparing survivors (S) to nonsurvivors (NS), both groups were similar in year of transplant listing, year of heart transplant, age, sex, ethnicity, baseline liver function, and other medical comorbidities (Table 4). Patients in our survivor group were significantly more likely to have diabetes, however (S, 6.2%; NS, 0.0%; P = .046). Both groups were similar in all donor characteristics including age, sex, ethnicity, BMI, renal function, and cardiovascular history and current function (Table 5). With regard to postoperative outcomes, survivors were significantly more likely to be treated for acute rejection within 1 year (S, 25.0%; NS, 0.0%; P < .001). Otherwise, there were no significant differences between survivors and nonsurvivors in postoperative length of stay, need for dialysis, or acute rejection (Table 6).
TABLE 4.
Baseline characteristics for Isolated Orthotopic Heart Transplant survivors compared to nonsurvivors—recipients, UNOS/OPTN 2005–2016
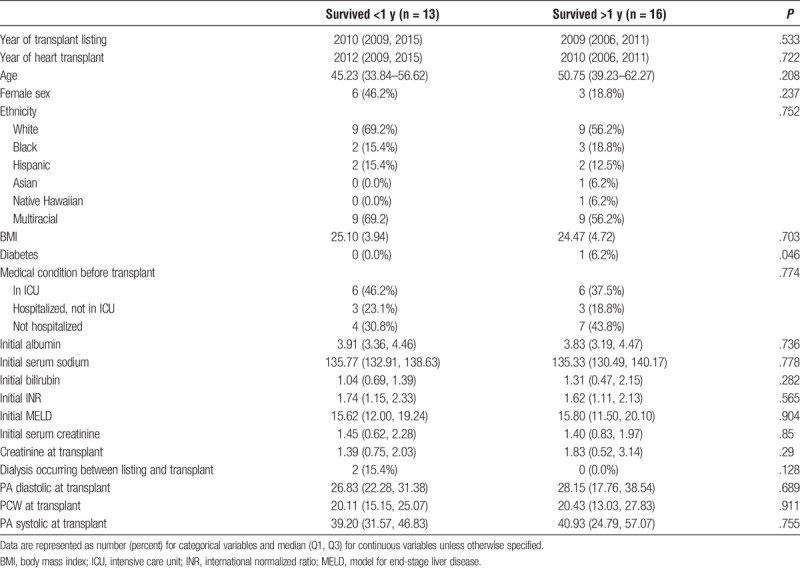
TABLE 5.
Baseline donor characteristics for Isolated Orthotopic Heart Transplant survivors compared to nonsurvivors - Donors, UNOS/OPTN 2005–2016
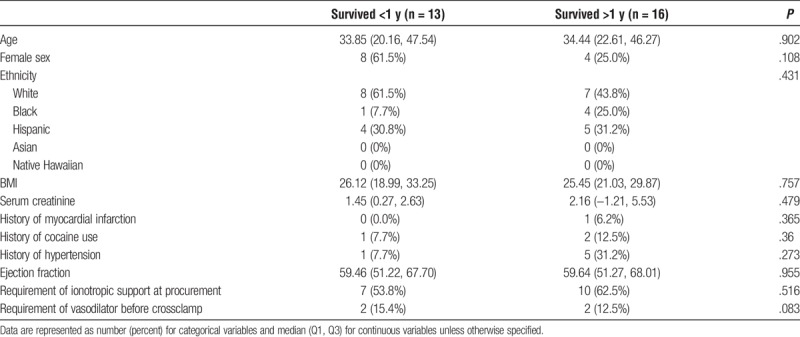
TABLE 6.
Posttransplant perioperative outcomes - Isolated Orthotopic Heart Transplant survivors compared with nonsurvivors, UNOS/OPTN 2005–2016
DISCUSSION
Since 1984, SHL transplantation has been performed in the United States in just over 200 patients with concurrent end-stage heart and liver disease.15 Although this population is small in comparison to the total number of all transplant candidates, they represent a critically ill cohort who uniquely challenges current strategies for organ allocation. Although the majority of these patients have heart and liver failure from related etiologies,7,8,16 this strategy has also been used in OHT candidates with less severe liver dysfunction to protect the cardiac allograft.6 Regardless of the indication, these patients have diminished waitlist survival compared with isolated OHT or OLT candidates and SHL transplantation has been demonstrated to be effective in improving survival in this cohort while providing otherwise comparable outcomes to single-organ transplantation.12
Most broadly, SHL permits the treatment of candidates with advanced dual-organ failure that would otherwise contraindicate isolated organ transplantation. Although the benefits of SHL in this patient population are clear, how to best incorporate the needs of this group into organ allocation policy more generally is less evident. Critics of the current strategy for multiple (simultaneous) organ allocation argue that allowing the waitlist status of 1 organ to drive the allocation of a second organ unfairly advantages dual-organ transplant candidates over patients in need of a single organ in their access to a scarce medical good. Indeed, this concern has manifested in this analysis by the relatively low degree of liver failure (as evidenced by MELD score) in the overall population of candidates listed for combined organ transplantation. Liver after heart transplantation (LAH) offers an alternative to SHL for patients in need of both heart and liver transplantation that could potentially rectify the conflicting aims of the organ allocation system to provide these transplant candidates both needed organs while not hindering organ access for isolated OHT or OLT candidates.
Our study was designed to ascertain whether LAH offered similar mortality and morbidity when compared with SHL and could therefore be established as a viable alternative to SHL for patients listed for both OHT and OLT before heart transplantation. With respect to mortality, our analysis supports our hypothesis and demonstrated that both LAH and SHL each offered improved survival compared with undergoing iOHT alone. Although LAH and SHL were similar in their superiority to iOHT with regard to overall survival, a significant portion of patients who did not receive SHL did not survive long enough to receive their liver allograft given the substantial median time between transplantations (302 days). Therefore, the survival of the LAH group, then, is influenced highly by the survivorship bias inherent in this form of analysis.
Despite this, we do acknowledge that waitlist mortality does not completely explain the failure of the majority of the HTA cohort to proceed to subsequent liver transplant, given that our survival curves show a greater number of HTA patients survived beyond 302 days than were in our LAH group (Figure 3). We are limited in our ability to comment on the specific etiology of this discrepancy given the data available in UNOS. However, current methods to clinically assess which heart transplant candidates with concurrent liver failure will ultimately also need a liver transplant remain imperfect. Consequently, it is possible that some of the patients in the HTA cohort may not have needed a liver allograft despite being listed for both heart and liver transplantation, and that while listed for both organs, their liver dysfunction was resolved by isolated heart transplantation.
To gain insight into this question, we performed a subgroup analysis in which we divided patients in our iOHT cohort into those that survived greater than 1 year and those that did not. There were no significant differences between these groups in baseline recipient and donor characteristics. We did find that patients who survived longer than 1 year with iOHT were more likely to have an episode of acute rejection within a year posttransplant. However, this finding is more likely a consequence of enhanced survival leading to greater opportunity for rejection to manifest and be diagnosed than differences in acute rejection resulting in disparate survival outcomes. Otherwise, we found no significant differences in postoperative outcomes between survivors and nonsurvivors. Given that relatively few patients in our study received iOHT, it is possible that our analysis had insufficient power to detect differences between these groups, especially given the limited data available in national registries. Thus, further studies are needed to determine the characteristics of heart-liver candidates who would be treated successfully with HTA, thus providing a means to better define the population truly in need of combined heart-liver transplantation.
Additionally, we found that patients in our LAH cohort received their heart transplant significantly earlier than patients in our other 2 groups. This may be a consequence of our sampling methodology as patients in our LAH group were required to undergo HTA first and then spend additional time on the waitlist before receiving OLT. As such, it is possible that some patients in our iOHT cohort may later crossover into LAH. However, this finding also likely indicates a change in practice patterns. Specifically, it suggests that we have grown more technically adept with increasing experience in the transplantation of multiple organs. That in combination with the growing literature espousing the safety of multiorgan transplantation when compared with single organ transplant, there is now greater comfort in offering the more challenging simultaneous operation. Alternatively, it may mean that while selection criteria remain imperfect, with time we have grown better at identifying patients in need of simultaneous transplant from the onset. Given the small size of our LAH cohort, we were limited in our ability to perform any further subgroup analysis based on year of transplant. Consequently, future studies are needed to better identify if and how listing patterns for concurrent heart-liver transplant have changed over time. This would provide critical insight into patient populations that would benefit most from allocation of both organs as compared to those that might have comparable survival with isolated organ transplantation.
These data also support the conclusion that except for episodes of acute rejection, there were no significant differences between our LAH and SHL cohorts in our secondary outcomes, which included postoperative length of stay, need for dialysis, need for pacemaker, and stroke. Our choice of secondary outcomes was determined by the postoperative variables available in UNOS. Despite this, our analysis does provide insight into potential differences in morbidity based on transplantation strategy for heart-liver transplant candidates. In aggregate, our analysis suggests that LAH may produce comparable outcomes to SHL in the treatment of select patients with concomitant heart and liver failure in need of dual organ transplantation. However, the challenge remains in identifying the patients for which this strategy is appropriate in the long term.
Previous studies demonstrate that patients listed for SHL have both higher incidence of mortality and decreased incidence of transplant 1 year after listing compared with patients listed for either heart or liver transplantation alone.17,18 In contrast, studies examining survival outcomes for candidates on the liver transplant waitlist who were bypassed for organ allocation by heart-liver transplant candidates have found that although liver-alone candidates experienced longer times on the waitlist, they did not have excess mortality in comparison to matched controls.9,10 These studies taken together with our findings demonstrate the greater medical urgency of heart-liver transplant candidates while contradicting the previous hypotheses regarding their detrimental impact on survival outcomes for single-organ transplant candidates.
Additionally, consistent with other studies of heart-liver transplant candidates, patients in our analysis that received liver allografts did so at relatively low MELD scores (LAH, 13; SHL, 14).8,12 Liver-only candidates likely would not have received transplantation until they reached higher MELD scores or achieved 1A status due to fulminant liver failure.19 However, recent data assessing the use of MELD-XI scores to predict morbidity and mortality in OHT candidates indicate that performing SHL in patients at lower MELD scores may be justified. As MELD data are not recorded in UNOS for isolated heart transplant candidates, MELD-XI is often used as a surrogate in these patients. Grimm and colleagues found that OHT candidates with MELD-XI scores greater than 16.4 experienced greater mortality at 30 days, 1 year, and 5 years compared with OHT candidates with lower scores.20 Deo et al21 similarly found greater mortality in OHT candidates in the highest quartile for MELD-XI (>14.4) in addition to increased risk for posttransplant stroke, infection, need for dialysis, prolonged hospitalization, and acute rejection. These findings would suggest that earlier receipt of the liver allograft in heart-liver transplant candidates may help in providing maximal utility of the cardiac allograft.
Our analysis found that patients undergoing SHL were less likely to be treated for acute rejection both during their index hospitalization and at 1 year posttransplant. This corroborates findings of low rates of acute rejection in SHL seen previously in single-center studies.4,22 There is a growing literature supporting that transplant of a liver allograft in a multiorgan transplant may provide a degree of immunoprotection to the simultaneously transplanted organ and reduce the risk of long-term alloimmune-mediated injury. For example, when simultaneous combined liver-kidney transplant was compared to kidney after liver transplant rejection-free survival of the renal allograft was inferior in the kidney after liver transplant cohort at 1 and 3 years.23 Further, an analysis comparing liver-kidney transplantation with kidney-pancreas transplantation and kidney transplantation alone found that rejection-free survival was highest in liver-kidney recipients.24 An analysis examining rates of rejection in various multiorgan transplants, found lower rejection rates for patients who received cotransplant of a donor-specific heart, kidney, and liver allografts.25 Although the specific mechanism of this phenomenon has not been completely elucidated, it is believed that the liver allograft may produce soluble class I human leukocyte antigens that can neutralize alloantibodies and cytotoxic T lymphocytes that exist in systemic circulation.26,27 In other contexts, protection from rejection is theorized to be at least partially attributable to increased maintenance immunosuppression due to the cotransplanted allograft (eg, heart and kidney) or increased antigen load overwhelming the recipient's immune system leading to immune paralysis (eg, double lung and double kidney).25
In conclusion, our findings suggest that for patients with concurrent heart and liver failure requiring transplantation of both organs, LAH and SHL each offer improved mortality when compared with iOHT while having largely comparable morbidity. However, given the substantial time between OHT and the receipt of a liver allograft in our LAH cohort, in combination with the high risk of waitlist mortality in our HTA cohort, we are reticent to suggest that LAH is truly noninferior to SHL. Further, the small sample size in our LAH group hinders our ability to truly discern the noninferiority of LAH compared with SHL. Despite this, given the dearth of studies comparing these approaches in the literature, our findings do provide supporting insight into how SHL and LAH may be used as a treatment strategy for combined heart-liver transplant candidates.
Our study has several limitations. For one, due to the rarity of sequential heart-liver transplantation, the number of patients in our LAH group was small and as a consequence some of the comparisons between our 3 cohorts may be underpowered. Additionally, being in our LAH group required patients to survive a prolonged time after OHT before receiving a liver allograft. Thus, while we show similar 10-year survival between LAH and SHL in our analysis, we understand that inherent survivor bias may limit our ability to make inferences from these data. An additional ramification of our LAH selection criteria is that patients who received LAH did so in an earlier era than patients in our SHL and iOHT cohorts. Therefore, we cannot definitely exclude year of transplant is a potential confounder in our analysis. Additionally, as in all retrospective studies using large national databases, there is potential that unmeasured confounders exist in our analysis that we cannot appropriately account for. This study was also somewhat limited by the granularity of the data provided in UNOS. Consequently, it was not possible for our study to discern the specific reasons why patients who initially received heart transplant alone did not also receive their liver allograft concurrently. Future studies are needed to better understand how factors like poor liver allograft quality or complications at the time of heart transplantation may contribute to this phenomenon. Similarly, although there appears to be some sub-population of heart-liver transplant candidates who would survive with HTA, we were limited in our ability to determine predictive patient factors that would be useful in identifying these patients. Therefore, we are restricted in our ability to draw conclusions about ideal patient characteristics or the optimal care of heart transplants candidates with concurrent liver dysfunction. Future studies using UNOS data would be improved by provision of more detailed information regarding the nature of liver dysfunction in these heart transplant candidates. With these data, we may be able to better identify patient characteristics that predict improvement in morbidity and mortality with HTA.
ACKNOWLEDGMENTS
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government.
Footnotes
Published online 19 December, 2018.
The authors declare no conflicts of interest.
Funding Sources: Institutional Funding was the primary funding source for this study. In addition, B.A.Y. receives research support from a cooperative agreement (U01 HL088942) by the National Institute of Health/National Heart Lung and Blood Institute and the National Institute of Neurological Disorders and Stroke of the NIH and the Canadian Institutes of Health Research.
A.J.R. wrote the article. K.L.A. wrote the article. M.S.M. analyzed data. B.A.Y. analyzed the data. A.S.B. designed the research study. M.G.H. designed the research study.
REFERENCES
- 1.Starzl T, Bilheimer D, Bahnson H, et al. Heart-liver transplantation in a patient with familial hypercholesterolaemia. Lancet. 1984;1:1382–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagpal AD, Chamogeorgakis T, Shafii AE, et al. Combined heart and liver transplantation: the Cleveland Clinic experience. Ann Thorac Surg. 2013;95:179–182. [DOI] [PubMed] [Google Scholar]
- 3.Raichlin E, Daly RC, Rosen CB, et al. Combined heart and liver transplantation: a single-center experience. Transplantation. 2009;88:219–225. [DOI] [PubMed] [Google Scholar]
- 4.Atluri P, Gaffey A, Howard J, et al. Combined heart and liver transplantation can be safely performed with excellent short- and long-term results. Ann Thorac Surg. 2014;98:858–862. [DOI] [PubMed] [Google Scholar]
- 5.Careddu L, Zanfi C, Pantaleo A, et al. Combined heart–liver transplantation: a single-center experience. Transpl Int. 2015;28:828–834. [DOI] [PubMed] [Google Scholar]
- 6.Barbara DW, Rehfeldt KH, Heimbach JK, et al. The perioperative management of patients undergoing combined heart-liver transplantation. Transplantation. 2015;99:139–144. [DOI] [PubMed] [Google Scholar]
- 7.Te HS, Anderson AS, Millis JM, et al. Current state of combined heart-liver transplantation in the United States. J Heart Lung Transplant. 2008;27:753–759. [DOI] [PubMed] [Google Scholar]
- 8.Cannon RM, Hughes MG, Jones CM, et al. A review of the United States experience with combined heart-liver transplantation. Transpl Int. 2012;25:1223–1228. [DOI] [PubMed] [Google Scholar]
- 9.Sulewski ME, Wolf JH, Hasz R, et al. Combined heart-liver transplantation; implications for liver-alone wait list mortality. Transplantation. 2014;98:e45–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg DS, Reese PP, Amaral S, et al. Reframing the impact of combined heart-liver allocation on liver transplant wait-list candidates. Liver Transpl. 2014;20:1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Organ Procurement and Transplantation Network. Section 5:10 organ offers, acceptance, and verification: allocation of multi-organ combinations. Policies. [Google Scholar]
- 12.Wolf JH, Sulewski ME, Cassuto JR, et al. Simultaneous thoracic and abdominal transplantation: can we justify two organs for one recipient? Am J Transplant. 2013;13:1806–1816. [DOI] [PubMed] [Google Scholar]
- 13.OPTN/UNOS Ethics Committee. Ethical principles to be considered in the allocation of human organs. 2015.
- 14.Nelson LM, Penninga L, Sander K, et al. Long-term outcome in patients treated with combined heart and liver transplantation for familial amyloidotic cardiomyopathy. Clin Transplant. 2013;27:203–209. [DOI] [PubMed] [Google Scholar]
- 15.Beal EW, Mumtaz K, Hayes D, Jr., et al. Combined heart-liver transplantation: indications, outcomes and current experience. Transplant Rev (Orlando). 2016;30:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barshes NR, Udell IW, Joyce DL, et al. A pooled analysis of posttransplant survival following combined heart-liver transplantation. Transplantation. 2007;83:95–98. [DOI] [PubMed] [Google Scholar]
- 17.Porrett PM, Desai SS, Timmins KJ, et al. Combined orthotopic heart and liver transplantation: the need for exception status listing. Liver Transpl. 2004;10:1539–1544. [DOI] [PubMed] [Google Scholar]
- 18.Schaffer JM, Chiu P, Singh SK, et al. Combined heart-liver transplantation in the MELD era: do waitlisted patients require exception status? Am J Transplant. 2014;14:647–659. [DOI] [PubMed] [Google Scholar]
- 19.Organ Procurement and Transplantation Network. Section 9: allocation of livers and liver-intestines. https://optn.transplant.hrsa.gov/governance/policies. Updated October 18, 2018.
- 20.Grimm JC, Shah AS, Magruder JT, et al. MELD-XI score predicts early mortality in patients after heart transplantation. Ann Thorac Surg. 2015;100:1737–1743. [DOI] [PubMed] [Google Scholar]
- 21.Deo SV, Al-Kindi SG, Altarabsheh SE, et al. Model for end-stage liver disease excluding international normalized ratio (MELD-XI) score predicts heart transplant outcomes: evidence from the registry of the United Network for Organ Sharing. J Heart Lung Transplant. 2016;35:222–227. [DOI] [PubMed] [Google Scholar]
- 22.Wong TW, Gandhi MJ, Daly RC, et al. Liver allograft provides Immunoprotection for the cardiac allograft in combined heart-liver transplantation. Am J Transplant. 2016;16:3522–3531. [DOI] [PubMed] [Google Scholar]
- 23.Simpson N, Cho YW, Cicciarelli JC, et al. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: analysis of UNOS database. Transplantation. 2006;82:1298–1303. [DOI] [PubMed] [Google Scholar]
- 24.Fong TL, Bunnapradist S, Jordan SC, et al. Analysis of the United Network for Organ Sharing database comparing renal allografts and patient survival in combined liver-kidney transplantation with the contralateral allografts in kidney alone or kidney-pancreas transplantation. Transplantation. 2003;76:348–353. [DOI] [PubMed] [Google Scholar]
- 25.Rana A, Robles S, Russo MJ, et al. The combined organ effect: protection against rejection? Ann Surg. 2008;248:871–879. [DOI] [PubMed] [Google Scholar]
- 26.Sumimoto R, Kamada N. Specific suppression of allograft rejection by soluble class I antigen and complexes with monoclonal antibody. Transplantation. 1990;50:678–682. [DOI] [PubMed] [Google Scholar]
- 27.Davies HS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524–527. [DOI] [PubMed] [Google Scholar]


