Supplemental digital content is available in the text.
Abstract
Background
In kidney transplantation, nonimmunologic donor-recipient (D-R) pairing is generally not given the same consideration as immunologic matching. The aim of this study was to determine how nonimmunologic D-R pairing relates to independent donor and recipient factors, and to immunologic HLA match for predicting graft loss.
Methods
Seven D-R pairings (race, sex, age, weight, height, cytomegalovirus serostatus, and HLA match) were assessed for their association with the composite outcome of death or kidney graft loss using a Cox regression-based forward stepwise selection model. The best model for predicting graft loss (including nonimmunologic D-R pairings, independent D-R factors, and/or HLA match status) was determined using the Akaike Information Criterion.
Results
Twenty three thousand two hundred sixty two (29.9%) people in the derivation data set and 9892 (29.7%) in the validation data set developed the composite outcome of death or graft loss. A model that included both independent and D-R pairing variables best predicted graft loss. The c-indices for the derivation and validation models were 0.626 and 0.629, respectively. Size mismatch (MM) between donor and recipient (>30 kg [D < R} and >15 cm [D < R]) was associated with poor patient and graft survival even with 0 HLA MM, and conversely, an optimal D-R size pairing mitigated the risk of graft loss seen with 6 HLA MM.
Conclusions
D-R pairing is valuable in predicting patient and graft outcomes after kidney transplant. D-R size matching could offset the benefit and harm seen with 0 and 6 HLA MM, respectively. This is a novel finding.
Kidney transplantation is believed to be the optimal treatment strategy for patients with end-stage renal disease; however, graft survival is finite. Risk prediction models have been created which use donor, recipient, and transplant characteristics to quantify the risk of death and/or graft failure after kidney transplant.1-3 However, these models do not consider the particular pairing of a specific donor-recipient (D-R) and instead incorporate only individual donor and/or recipient factors.
Kidney graft failure has been independently associated with increasing degrees of mismatch (MM) in HLA between D-R,4 and the suboptimal pairing of D-R age,5 weight,6 height,7 sex,8,9 race,10,11 and cytomegalovirus (CMV) status.12 HLA MM between D-R is a widely accepted and well established risk for posttransplant rejection episodes and kidney graft loss, with a graduated increased risk as the number of HLA MMs increases from 0 to 6.13 For this reason, many organ allocation programs strive for a favorable HLA match between D-R with preference given to better immunologically matched recipients.13 Additionally, increasing D-R age has also been independently associated with graft failure. Although controversial, age matching between D-R (<10 years difference) has been associated with better graft survival after transplantation.14 Therefore, many deceased donor renal transplant allocation strategies also currently incorporate some degree of age matching between D-R directly or indirectly as a means of improving total graft years achieved for a given pool of donors.15,16 Size MM between D-R (using varying metrics including weight, height, and body surface area) has been associated with worse graft outcomes when the donor is significantly smaller than the recipient, reflecting nephron underdosing with resultant hyperfiltration injury and graft compromise.17 In terms of sex pairing, the highest risk D-R sex combination in unadjusted analyses is a female donor/male recipient, believed to be due to generally smaller female donors contributing to nephron underdosing (a surrogate for size MM between D-R).8,17 In weight-adjusted analyses, however, a male donor/female recipient pairing has also been shown to be at a high risk for kidney graft failure, presumed to be due to increased sensitization and subsequent graft rejection in female recipients due to an H-Y antigen on the Y chromosome.17,18 Race pairing between D-R has a less clear impact on transplant outcomes. A black donor/white recipient D-R pairing is at high risk for graft failure, hypothesized to be related to unrecognized HLA antigens in the black donor kidney with resultant increased sensitization and chronic allograft nephropathy ensuing in the white recipient.10 A white donor/black recipient D-R pairing is also associated with an increased risk of graft failure however, a finding at least partially due to increased presensitization in the black recipient.10 Lastly, CMV disease has been shown to be an independent risk factor for chronic allograft nephropathy,19 acute and chronic rejection,20,21 and kidney graft loss. CMV viremia and subsequent graft nephropathy occurs most commonly in a CMV-positive donor/CMV-negative recipient.22
The implications of D-R pairing have only recently garnered attention. The combined exposure of suboptimal weight and sex pairing has been associated with a 50% increased risk of graft failure.17 A more recent publication further explored the effects of D-R matching by creating a model including age, sex, weight ratio, height ratio, HLA MM, and ABO pairing as predictors of death or graft failure at 5 and 10 years.23 These D-R pairs were included in the model a priori given a suspected role in graft loss, but the relative impact of individual D-R factors versus their paired effect was not explored.
The collective impact of suboptimal nonimmunologic D-R pairing on posttransplant outcomes and how this compares with individual D-R factors, and to HLA MM has not been previously studied. Herein we create and subsequently validate a risk prediction model for the composite outcome of death or kidney graft failure considering the predictive capacity of specific D-R pairings compared with individual D-R factors and to HLA MM. We hypothesize that nonimmunologic D-R pairing will better predict patient and kidney graft failure than will independent donor and/or recipient variables and, in this modern era of immunosuppression, will also better predict outcome than HLA matching.
METHODS
Study Population and Assembly of Patients
A retrospective analysis was conducted of patients 16 years and older, who received a solitary deceased donor kidney transplant in the United States from January 1, 2000, to December 31, 2014. This study used the data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the Organ Procurement and Transplantation Network and SRTR contractors. Key inclusion criteria included any patient receiving a solitary deceased donor kidney transplant in the United States over the defined period. Exclusion criteria included those younger than 16 years and those receiving en bloc or sequential transplants.
Exposures and Their Measurement
The primary exposure was D-R pairing in 7 categories: race, sex, age, weight, height, CMV serostatus, and HLA match (Supplemental Table 1, http://links.lww.com/TXD/A163). The reference categories were white donor/white recipient, male donor/male recipient, 0/6 HLA MM, CMV-negative to negative serostatus, weight match (0 kg difference if linear, <10 kg difference if categorical), height match (0 m difference if linear, <0.1 m difference if categorical), and age match (0 year difference if linear, <10 year difference if categorical), based on a predicted risk profile extrapolated from earlier literature looking at isolated D-R pairings.4-6,8-12,17 Age, height, and weight MM were assessed as continuous variables (donor value minus recipient) if the differences were normally distributed and linearly associated with graft loss. If mean weight, height, or age difference was not near 0, data were centered. If the effect was nonlinear and could not be transformed or approximated with linear splines, age, height, and weight were categorized with cut points chosen based on prior literature and to divide the population into relatively even groups.1,17 The functional form of the variable with the lowest Akaike Information Criterion (AIC) from a series of competing univariate analyses was chosen. The AIC is used to test the relative fit of nonnested models for predicting an outcome in a given data set. It offers a trade-off between model fit and model simplicity (penalizing models for including covariates with little predictive capacity) with the best model having the lowest AIC among all other models.24 The best fit (lowest AIC) functional form for each variable was then verified in a multivariable model to ensure the fit was not appreciably worse after adjustment.
Confounders and Their Measurement
Other known literature predictors of graft loss were considered for inclusion in our model, such as D-R age, sex, race, weight, height, CMV serostatus, medical comorbidities (diabetes, hepatitis C virus infection, hypertension, cardiovascular disease), prior transplant, type of donation (expanded criteria, standard criteria or donation by cardiac death), etiology of recipient end stage renal disease, dialysis vintage, cold ischemia time, and peak panel reactive antibody level as per earlier studies of posttransplant graft failure.17
Outcomes and Their Measurement
The primary outcome for this study was time to the composite of death or kidney graft failure (defined as return to dialysis or preemptive retransplantation). The secondary outcome was time to death-censored kidney graft failure.
Primary Analysis
Descriptive statistics were used to report baseline characteristics for all patients enrolled in the study. Means and standard deviations and medians with first and third quartiles were used for continuous normal and continuous nonnormally distributed variables.
Model Derivation
The data set was divided 70/30 using a random number generator with model derivation in 70% and subsequent validation in 30% of the eligible cohort. A univariable Cox regression model was used to determine the relative hazard ratios for the composite of death or graft failure associated with each described D-R pairing variable (sex, race, weight, height, age, CMV serostatus, and HLA match) relative to the above noted reference categories, Supplemental Table 1, (http://links.lww.com/TXD/A163). Each of these factors as independent D-R variables, and all other potential confounders (above) were also assessed in univariable analyses. The functional form of each continuous variable was assessed using univariable Cox regression models fitted to test the assumption of linearity with outcome.
The goal of this multivariable analysis was to build a parsimonious predictive model using Cox regression to determine the relative hazard for the composite outcome of death or kidney graft loss as it relates to the D-R pairings discussed above. D-R pairing variables as well as the corresponding independent D-R variables (for example D-R weight pairing, donor weight, recipient weight) were considered for possible inclusion in our model and to maintain face validity, any additional variables which might confound the association between our exposure variables and outcome of interest were controlled for. Thus, a forward stepwise iterative process was used to include the remaining variables from the univariable models that associated with graft failure at a level of significance less than 0.001. Evaluation of AIC score for candidate models was employed to maximize model fit while maintaining a parsimonious model. Models for consideration included:
A model with independent D-R variables, but no D-R pairing variables aside from HLA MM (given its known strong association with outcome);
A model with D-R pairing variables but no independent D-R variables other than comorbidities unlikely to be influenced by D-R matching;
A model including both D-R pairing variables and independent D-R variables.
Proportionality of hazards was assessed graphically given the large sample size using visual examination of log-log plots. The log cumulative-hazard function and baseline survival functions were depicted in the derivation data set using fractional polynomials.25 The linear predictor from the generated Cox model formed a Prognostic Index (PI) which was centered on its mean and subsequently divided into 3 risk groups based on predetermined risk cutpoints, 0 to 25th percentile, greater than 25th to 75th percentile, and greater than 75th to 100th percentile.25 This was shown graphically with Kaplan-Meier failure curves for the 3 prognostic groups in the derivation data set against predicted event probabilities to visually demonstrate model calibration.
Model Validation
This model was validated in the remaining 30% of the original cohort. Model calibration was determined graphically as a function of observed and predicted death or graft loss by PI risk group in the validation data set. The Harrell's c-index was used to assess model discrimination and is defined as the proportion of all usable patient pairs with concordant observed and predicted outcomes.26 It was also calculated for the highest (>75%) and lowest (≤25%) PI risk groups,25 and for the highest and lowest deciles of risk (>90% and ≤10%).
Secondary and Sensitivity Analyses
The analysis was repeated using backwards stepwise selection to determine which of the 3 models (combined independent and pairing variables, pairing variables alone, independent variables alone) had the best predictive capacity for the composite outcome of death or graft loss. We also repeated the primary analysis using death-censored kidney graft loss as the outcome of interest.
To demonstrate the implications of nonimmunologic D-R pairing on patient and graft survival, data was presented graphically using Kaplan-Meier survival curves for optimal versus suboptimal nonimmunologic match (pairing factors identified in the primary analysis) stratified by optimal versus suboptimal immunologic (HLA) match. Statistically significant differences were assessed using the log-rank test.
Missing Data
Data were assumed to be missing at random and missing predictors were treated with case wise deletion given that imputation may be unreliable with model derivation. Any variable with greater than 7.5% missing data felt was treated with missing data as an indicator variable so as not to lose substantial amounts of data.
Institutional ethics approval to conduct this study was provided by the Nova Scotia Health Authority research ethics board.
RESULTS
The derivation cohort consisted of 77827 people; 23262 (29.9%) had a diagnosis of death or graft failure. The validation data set consisted of 33355 people; 9892 (29.7%) with death or graft failure. In total, 23485 (17.4%) people were dropped for missing data. Cohort derivation is shown in Supplemental Figure 1 (http://links.lww.com/TXD/A164). Baseline characteristics are shown in Table 1, stratified by derivation versus validation cohort.
TABLE 1.
Baseline characteristics of derivation and validation data sets
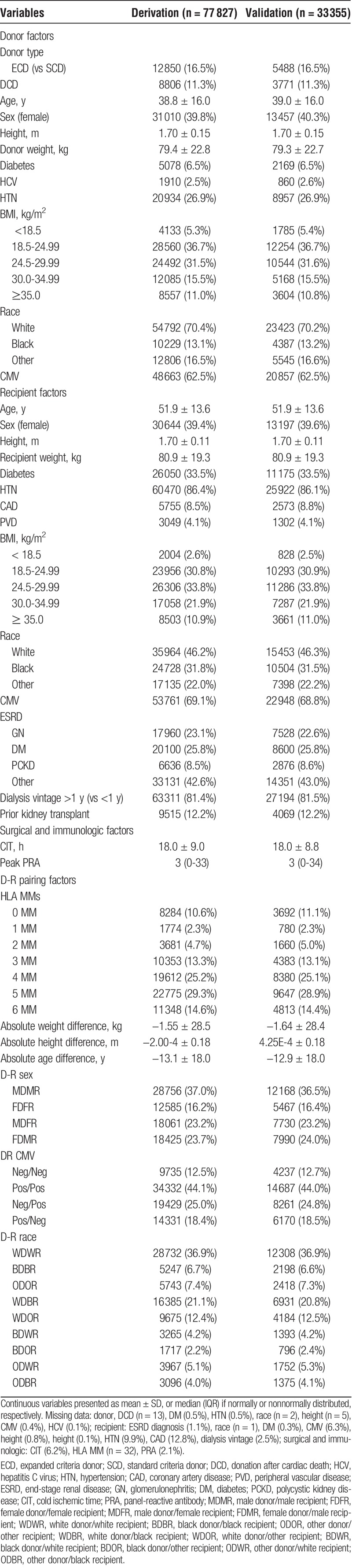
For continuous variables, the various functional forms assessed are depicted in Supplemental Table 2 (http://links.lww.com/TXD/A165). Using Cox proportional hazards regression, a univariable model was performed on the literature predictors of graft failure discussed above (Supplemental Table 3, http://links.lww.com/TXD/A166). Relative hazards were proportional based on graphical visualization.
To test the association of independent D-R factors with graft failure compared with specific D-R pairing variables, a number of potential candidate models were assessed for fit (AIC) and discrimination (Harrell's c-index) (Table 2).
TABLE 2.
Candidate risk prediction models for the composite of death or graft failure
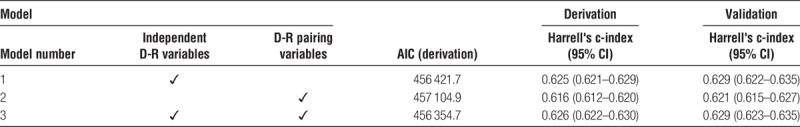
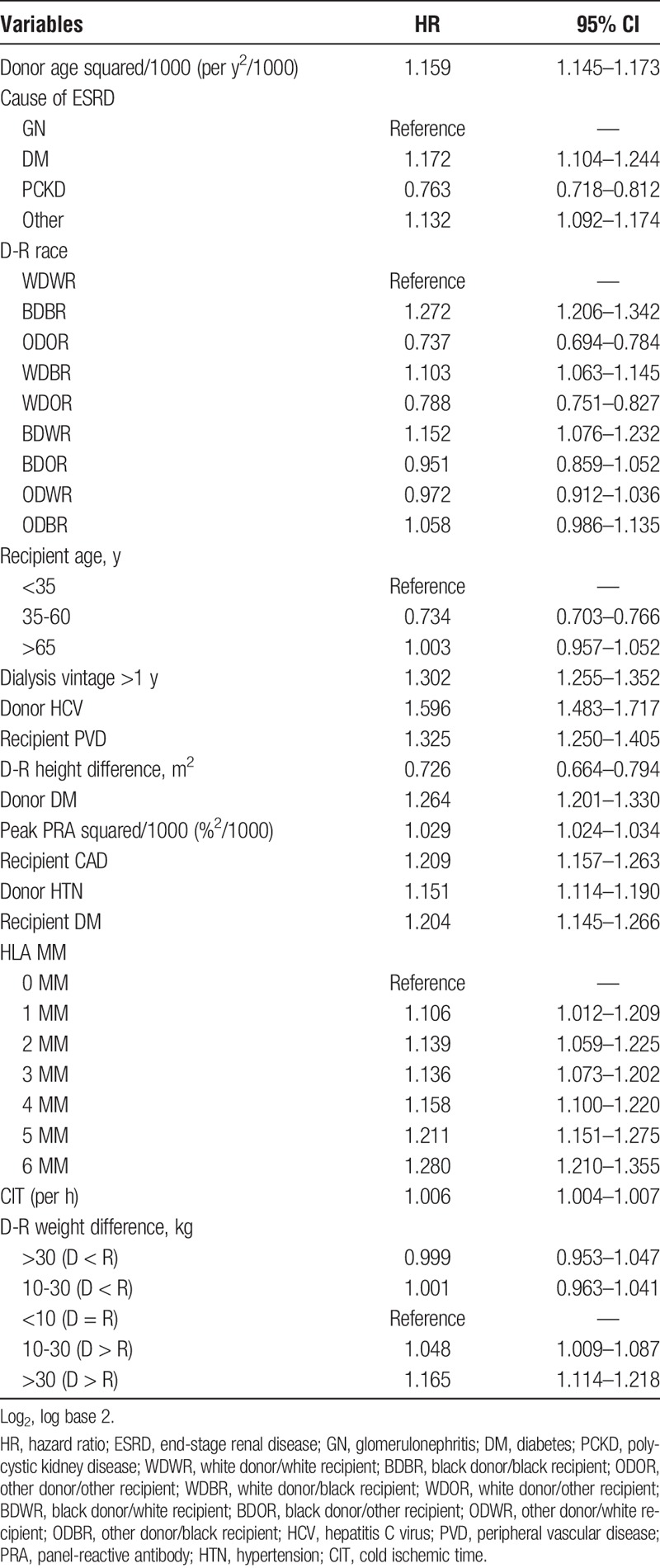
The best-fit model overall (lowest AIC) incorporated independent D-R factors as well as specific D-R pairings (model 3). Ultimately, 16 variables (including 4 of 7 possible D-R pairing variables) were included in the best-fit model, shown in Table 3 with relative hazard ratios and 95% confidence intervals. The variables included in models 1 and 2 are shown in Supplemental Table 4 (http://links.lww.com/TXD/A167) and Supplemental Table 5 (http://links.lww.com/TXD/A168), respectively.
TABLE 3.
Factors included in the final risk prediction model with associated hazard ratios
The Harrell's c-index for the best-fit derivation model (model 3) was 0.626 (95% confidence interval, 0.622–630), corresponding to reasonable discrimination. The Harrell's c-index for the validation data set was 0.629 (95% CI, 0.623–635). When comparing the highest (>75%) and lowest (≤25%) PI risk groups, the c-index was 0.663 in the derivation cohort and 0.662 in the validation cohort. The c-index was further increased to 0.698 and 0.690 in the derivation and validation cohorts, respectively, when comparing the highest (>90%) and lowest (≤10%) PI risk deciles, suggesting better discrimination at extremes of risk. It should be noted that the c-indices for models 1 and 3 do not differ significantly (Table 2), suggesting comparable discrimination. Calibration curves for both the derivation and validation data sets are shown in Supplemental Figure 2, (http://links.lww.com/TXD/A169). There was good correlation between observed and predicted outcomes in both the derivation and validation cohorts for all 3 PI categories.
Like for the primary analysis, using the secondary outcome of death-censored graft failure the best-fit model overall (lowest AIC) still incorporated independent D-R factors and specific D-R pairings (model 3; Supplemental Table 6, http://links.lww.com/TXD/A170). The same was true when we used backward stepwise selection for model derivation (Supplemental Table 7, http://links.lww.com/TXD/A171).
Based on the variables included in the best fit model (model 3; Table 3), a nested variable examining modifiable nonimmunologic and immunologic match was created. The nested variables consisted of: (i) optimal size match (>30 kg difference [D > R] and >15 cm [D > R]) with 0 HLA MM, (ii) optimal size match with 6 HLA-MM, (iii) suboptimal size match (>30 kg difference [D < R] and >15 cm [D < R]) with 0 HLA-MM, and (iv) suboptimal size match with 6 HLA-MM. Race match was not included in the nested variable given it largely reflects donor and/or recipient race status and is therefore not modifiable in a fixed pool of donors and recipients. The Kaplan Meier survival curves for the composite of death or graft loss are shown in Figure 1. Optimal size match was associated with superior patient and graft survival outcomes irrespective of HLA match status.
FIGURE 1.
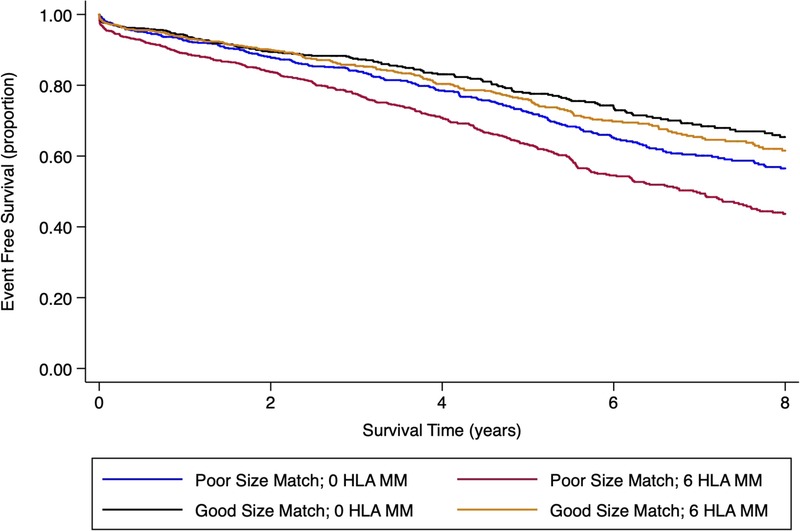
Kaplan Meier Curve for nonimmunologic size match versus immunologic HLA match between donors and recipients. (i) Optimal size match (>30 kg difference [D > R] and >15 cm [D > R]); suboptimal size match (>30 kg difference [D < R] and >15 cm [D < R]. Log-rank P value <0.001.
DISCUSSION
This study demonstrates that the combined analysis of individual D-R variables plus D-R pairing variables best predicts the composite outcome of death or kidney graft failure. Our model had excellent calibration and better discriminative ability than earlier models which have focused primarily on donor and/or recipient factors, but not D-R pairing factors.2,3 The implications of D-R pairing is only recently gaining recognition as an important consideration for predicting long-term graft survival. Recently, we explored the impact of combined weight and sex MM between kidney donors and recipients and demonstrated a 50% increased risk of graft loss if pairing was suboptimal.17 Subsequently, a prediction model in live and deceased kidney donors further explored the effects of D-R matching by including age, sex, weight ratio, height ratio, HLA MM, and ABO D-R pairing as predictors of death or graft failure at 5 and 10 years.23 These D-R pairs were included in their model a priori given a suspected role in graft loss, but the relative impact of individual D-R factors versus their potentially synergistic effect was not explored. Importantly, our model selected D-R variables (independently or as a pair) based on model fit and predictive ability.
The benefit of 0 HLA MMed kidneys (no antigen MMs at any of 6 HLA loci) is associated with better patient and graft survival, fewer rejection episodes, and reduced sensitization compared to higher degrees of HLA MM.26,27 Currently, other than centers which incorporate a degree of age matching, deceased donor kidney allocation protocols do not routinely consider nonimmunologic matching between donors and recipients,28-30 with kidneys allocated in most cases based on immunologic match and increasing time accrued on the renal transplant waitlist. Although there is no universally accepted allocation strategy for deceased donor kidney transplantation, the United Kingdom,31 Australia,32 New Zealand,32 members of Eurotransplant,33 members of Scandiatransplant,34 and most regional centers in Canada35,36 continue to prioritize immunologic matching in an effort to improve overall kidney graft survival. Even in the era of the new kidney allocation system in the United States37,38 there remains an emphasis on HLA matching, albeit to a lesser extent than previous. The focus has now shifted toward longevity matching (best kidneys for best recipients) at the forefront, with immunologic match only considered subsequent to this. Assume 2 donors become available for 2 possible recipients. Assume these donors have identical Kidney Donor Profile Index scores and the recipients have identical Estimated Posttransplant Survival scores. Assume the only difference between these 2 difference between these 2 recipients is their weight, height and HLA antigens. There are no differences in blood group, transplant wait time, panel-reactive antibody, or age. Current literature and the kidney allocation system would then suggest kidneys be allocated on the basis of HLA match.
In this study, we examined the relative importance of D-R size matching (defined as a composite of weight and height match) compared with HLA matching. Although both appear to be important in predicting patient and graft survival, we show that an optimal D-R size match can offset the increased risk associated with poor HLA matching, and conversely, suboptimal D-R size matching can mitigate the benefit seen with a 0 HLA-MM transplant, assuming all else is equal. This is the first time this observation has been demonstrated and may be a reflection of improved immunosuppressive regimens in a modern era which reduce the relative importance of immunologic matching.
Although D-R race pairing is also strongly associated with patient and graft survival, unlike size matching, these are not modifiable exposures. Globally, no kidney allocation programs formally consider size match between donors and recipients. Given that a recipient weighing greater than 30 kg more than a donor and standing 15 cm taller is associated with a greater hazard for death or graft loss than is 6 HLA MM, perhaps optimizing size match between donors and recipients should be prioritized above optimizing HLA MM. At the very least, it should be considered with potential absolute size MM thresholds incorporated into allocation programs such that beyond a certain cut point, transplant between a particular D-R pair is deferred. Additionally, when allocating organs, this study would suggest that overall D-R match should be analyzed as an aggregate that includes both immunologic (HLA) and nonimmunologic (weight, height, etc) match as a means of optimizing and improving long-term patient and graft survival.
Importantly, unlike donor, recipient and transplant factors in isolation, the intentional pairing of donors and recipients is a modifiable exposure. If strategic D-R pairing can improve graft longevity outcomes despite a fixed pool of donor kidneys, this practice should potentially be considered for future renal transplant allocation programs. However, given the overrepresentation of older, Caucasian males in the donor pool, strict D-R pairing would have the potential to disadvantage certain minority groups, for example, younger, nonwhite females. The risk of prolonged waitlist times in certain recipient subgroups would require further detailed exploration with a full analysis of the societal implications before a D-R matching strategy could be implemented. A medical decision analysis may be able to identify a threshold wait time before which D-R matching is a dominant strategy and after which organs should be allocated based primarily on wait times.
This study has a number of strengths. Importantly, this research study addresses a clinically relevant question with the potential to identify a modifiable intervention in an otherwise fixed pool of donors and recipients. Unlike earlier literature, our model makes no assumption that individual or paired D-R variables have superior predictive accuracy.23,39,40 Instead, we included both D-R individual and pairing variables for consideration in our analysis and chose the best predictors of graft failure through an iterative process whereby variables were selected (for example donor weight vs recipient weight vs D-R weight pair vs none of the above) based on which had the most significant association with the outcome of interest. Additionally, for continuous variables, we investigated the optimal functional form to be considered for inclusion in our model.
As with any study, however, there are limitations to this analysis. First, stepwise selection models have the potential to bias parameter estimates away from 0 and associated P values toward zero41; however model predictive accuracy was maintained in a separate validation cohort suggesting appropriate multivariable selection. Furthermore, with any predictive modeling, there is the potential that variables will be incorporated by chance alone; however, this risk was mitigated by use of a strict selection criteria (P < 0.001) to account for repeated testing. Missing data were treated with case wise deletion given that imputation may be unreliable for the creation of prediction models. If data were missing at random, as we assumed, then this is a reasonable strategy; however, if data were missing informatively, then case wise deletion could potentially bias our results. Lastly, there is no robust statistical test for calibration in time to event models, thus model calibration had to be assessed graphically.
In conclusion, optimizing nonimmunologic D-R pairing appears to mitigate the risk associated with suboptimal HLA matching. This study is exploratory, however, and it is important that the utility and equitability of nonimmunologically based organ allocation strategies be considered. To incorporate D-R pairing strategies into our current organ allocation protocols would require further research to ensure certain recipient subgroups would not be unfairly disadvantaged.
Supplementary Material
ACKNOWLEDGMENTS
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Footnotes
Published online 19 December, 2018.
R.B.D. and K.K.T. are co-senior authors.
The authors declare no funding or conflicts of interest.
All authors participated in the research design. A.V. did the initial data analysis with input from R.D. and K.T. throughout. B.K. provided feedback and suggestions to make the analysis more robust. A.V. wrote the initial article, and B.K., R.D. and K.T. provided feedback. This article was undertaken for A.V.'s Master's thesis through the Harvard Chan School of Public Health Master of Science in Epidemiology program.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Irish WD, Ilsley JN, Schnitzler MA, et al. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10:2279–2286. [DOI] [PubMed] [Google Scholar]
- 2.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Israni AK, Snyder JJ, et al. A simple tool to predict outcomes after kidney transplant. Am J Kidney Dis. 2010;56:947–960. [DOI] [PubMed] [Google Scholar]
- 4.Opelz G. Correlation of HLA matching with kidney graft survival in patients with or without cyclosporine treatment. Transplantation. 1985;40:240–243. [DOI] [PubMed] [Google Scholar]
- 5.Waiser J, Schreiber M, Budde K, et al. Age-matching in renal transplantation. Nephrol Dial Transplant. 2000;15:696–700. [DOI] [PubMed] [Google Scholar]
- 6.Giral M, Foucher Y, Karam G, et al. Kidney and recipient weight incompatibility reduces long-term graft survival. J Am Soc Nephrol. 2010;21:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho YW, Terasaki PI, Cecka JM, et al. Should excessive height and weight differences between the kidney donor and recipient be avoided? Transplant Proc. 1997;29:104–105. [DOI] [PubMed] [Google Scholar]
- 8.Kolonko A, Chudek J, Wiecek A. Nephron underdosing as a risk factor for impaired early kidney graft function and increased graft loss during the long-term follow-up period. Transplant Proc. 2013;45:1639–1643. [DOI] [PubMed] [Google Scholar]
- 9.McGee J, Magnus JH, Islam TM, et al. Donor-recipient gender and size mismatch affects graft success after kidney transplantation. J Am Coll Surg. 2010;210:718–725.e1, 725–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young CJ, Kew C. Health disparities in transplantation: focus on the complexity and challenge of renal transplantation in African Americans. Med Clin North Am. 2005;89:1003–1031, ix. [DOI] [PubMed] [Google Scholar]
- 11.Callender CO, Cherikh WS, Traverso P, et al. Effect of donor ethnicity on kidney survival in different recipient pairs: an analysis of the OPTN/UNOS database. Transplant Proc. 2009;41:4125–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arthurs SK, Eid AJ, Pedersen RA, et al. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008;46:840–846. [DOI] [PubMed] [Google Scholar]
- 13.Opelz G, Döhler B. Effect of human leukocyte antigen compatibility on kidney graft survival: comparative analysis of two decades. Transplantation. 2007;84:137–143. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Oh CK, Shin GT, et al. Age matching improves graft survival after living donor kidney transplantation. Transplant Proc. 2014;46:449–453. [DOI] [PubMed] [Google Scholar]
- 15.Lim WH, Chang S, Chadban S, et al. Donor–recipient age matching improves years of graft function in deceased-donor kidney transplantation. Nephrol Dial Transplant. 2010;25:3082–3089. [DOI] [PubMed] [Google Scholar]
- 16.Miller AJ, Kiberd BA, Alwayn IP, et al. Donor-recipient weight and sex mismatch and the risk of graft loss in renal transplantation. Clin J Am Soc Nephrol. 2017;12:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLeeuw J. Introduction to Akaike (1973) information theory and an extension of the maximum likelihood principle. In: Kotz S, Johnson NL, eds. Breakthroughs in Statistics I. : Springer-Verlag: New York; 1992:599–609. [Google Scholar]
- 18.Gratwohl A, Döhler B, Stern M, et al. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. Lancet. 2008;372:49–53. [DOI] [PubMed] [Google Scholar]
- 19.Sola R, Diaz JM, Guirado L, et al. Significance of cytomegalovirus infection in renal transplantation. Transplant Proc. 2003;35:1753–1755. [DOI] [PubMed] [Google Scholar]
- 20.Humar A, Gillingham KJ, Payne WD, et al. Association between cytomegalovirus disease and chronic rejection in kidney transplant recipients. Transplantation. 1999;68:1879–1883. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin K, Wu C, Fick G, et al. Cytomegalovirus seromismatching increases the risk of acute renal allograft rejection. Transplantation. 2002;74:813–816. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi K, Tokumoto T, Tanabe K, et al. Association of the outcome of renal transplantation with antibody response to cytomegalovirus strain-specific glycoprotein H epitopes. Clin Infect Dis. 2007;45:60–67. [DOI] [PubMed] [Google Scholar]
- 23.Ashby VB, Leichtman AB, Rees MA, et al. A kidney graft survival calculator that accounts for mismatches in age, sex, HLA, and body size. Clin J Am Soc Nephrol. 2017;12:1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 25.Royston P. Tools for checking calibration of a cox model in external validation: prediction of population-averaged survival curves based on risk groups. Stata J. 2015;15:275–291. [Google Scholar]
- 26.Takemoto SK, Terasaki PI, Gjertson DW, et al. Twelve years' experience with national sharing of HLA-matched cadaveric kidneys for transplantation. N Engl J Med. 2000;343:1078–1084. [DOI] [PubMed] [Google Scholar]
- 27.Opelz G, Dohler B. Association of HLA mismatch with death with a functioning graft after kidney transplantation: a collaborative transplant study report. Am J Transplant. 2012;12:3031–3038. [DOI] [PubMed] [Google Scholar]
- 28.Wu DA, Watson CJ, Bradley JA, et al. Global trends and challenges in deceased donor kidney allocation. Kidney Int. 2017;91:1287–1299. [DOI] [PubMed] [Google Scholar]
- 29.US Department of Health and Human Services. Organ procurement and transplantation network (OPTN) policies. Available at https://optn.transplant.hrsa.gov/learn/professional-education/kidney-allocation-system/. Updated July 7, 2016. Accessed on August 15, 2017.
- 30.The Transplantation Society of Australia and New Zealand. Clinical guidelines for organ transplantation from deceased donors. Version 1.1: May 2017.
- 31.Kidney Advisory Group on behalf of NHSBT. Kidney transplantation: deceased donor organ allocation. Available at https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/4967/kidney_allocation_policy.pdf. Accessed on August 15, 2017.
- 32.The Transplantation Society of Australia and New Zealand. Clinical guidelines for organ transplantation and deceased donors. Available at https://www.tsanz.com.au/organallocationguidelines/documents/ClinicalGuidelinesV1.1May2017.pdf. Accessed on August 15, 2017.
- 33.Eurotransplant Manual. ET kidney allocation systems (ETKAS/ESP/ESDP). Available at http://www.eurotransplant.org/cms/mediaobject.php?file=Chapter4_thekidney7.pdf. Accessed on August 15, 2017.
- 34.Scandiatransplant. Rules for exchange of kidneys from deceased donor within the Scandiatransplant cooperation. Available at http://www.scandiatransplant.org/organ-allocation/Kidney_exchange_14_dec_2016.pdf. Accessed on August 15, 2017.
- 35.The Canadian Council for Donation and Transplantation. Kidney allocation in Canada: a Canadian forum. Available at https://professionaleducation.blood.ca/sites/msi/files/Kidney_Allocation_FINAL.pdf. Accessed on August 15, 2017.
- 36.The Canadian Council for Donation and Transplantation. Kidney Allocation in Canada: A Canadian Forum. Report and Recommendations. Toronto, Ontario; 2006. [Google Scholar]
- 37.Organ Procurement and Transplantation Network (OPTN). Policies: Allocation of Kidneys. 2017:82–102.
- 38.Wang CJ, Wetmore JB, Israni AK. Old versus new: progress in reaching the goals of the new kidney allocation system. Hum Immunol. 2017;78:9–15. [DOI] [PubMed] [Google Scholar]
- 39.Vianello A, Calconi G, Amici G, et al. Importance of donor/recipient body weight ratio as a cause of kidney graft loss in the short to medium term. Nephron. 1996;72:205–211. [DOI] [PubMed] [Google Scholar]
- 40.Dick AA, Mercer LD, Smith JM, et al. Donor and recipient size mismatch in adolescents undergoing living-donor renal transplantation affect long-term graft survival. Transplantation. 2013;96:555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrell FE., Jr Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York, NY: Springer International Publishing; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


