Abstract
Lamotrigine (LTG), a phenyltriazine derivative and anti-epileptic drug, has emerged as an effective first-line treatment for bipolar mood disorder. Like the other mood stabilizers lithium and valproate, LTG also has neuroprotective properties but its exact mechanisms remain poorly defined. The present study utilized rat cerebellar granule cells (CGCs) to examine the neuroprotective effects of LTG against glutamate-induced excitotoxicity and to investigate potential underlying mechanisms. CGCs pretreated with LTG were challenged with an excitotoxic dose of glutamate. Pretreatment caused a time-and concentration-dependent inhibition of glutamate excitotoxicity with nearly full protection at higher doses (⩾100 μM), as revealed by cell viability assays and morphology. LTG treatment increased levels of acetylated histone H3 and H4 as well as dose- and time-dependently enhanced B-cell lymphoma-2 (Bcl-2) mRNA and protein levels; these changes were associated with up-regulation of the histone acetylation and activity of the Bcl-2 promoter. Importantly, lentiviral-mediated Bcl-2 silencing by shRNA reduced both LTG-induced Bcl-2 mRNA up-regulation and neuroprotection against glutamate excitotoxicity. Finally, the co-presence of a sub-effective concentration of LTG (10 μM) with lithium or valproate produced synergistic neuroprotection. Together, our results demonstrate that the neuroprotective effects of LTG against glutamate excitotoxicity likely involve histone deacetylase inhibition and downstream up-regulation of anti-apoptotic protein Bcl-2. These underlying mechanisms may contribute to the clinical efficacy of LTG in treating bipolar disorder and warrant further investigation.
Keywords: Bcl-2, glutamate, histone deacetylase, lamotrigine, neuroprotection
Introduction
Lamotrigine [LTG; 3,5-diamino-6-(2,3-dichlorphenyl)- 1,2,4-triazine] is an anticonvulsant and first-line treatment for bipolar disorder (Bowden, 2002). It is primarily successful in controlling depressive episodes (Calabrese et al. 1999) and can be used either alone or in combination with additional mood stabilizers such as lithium or valproate (VPA), which are also effective against manic states (Redmond et al. 2006; Walden et al. 1996). LTG is well-characterized as a voltage-gated sodium (Na+) channel inhibitor and subsequently stabilizes neuronal membranes and suppresses post-synaptic release of excitatory amino acids, notably glutamate (Lee et al. 2008; Lees & Leach, 1993; Wang et al. 2001; Zona & Avoli, 1997). Abnormal Na+ influx and voltage-gated sodium channel activity may play a critical role in the pathophysiology of neurological conditions including stroke, amytrophic lateral sclerosis, traumatic brain injury and multiple sclerosis (for review, see Mantegazza et al. 2010). The neuroprotective potential of LTG has therefore been investigated in a number of cellular and animal models, where it demonstrated efficacy against insults such as status epilepticus (Halonen et al. 2001), malonate (Connop et al. 1997), 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine (Lagrue et al. 2007), rotenone (Kim et al. 2007), oxygen-glucose deprivation (Calabresi et al. 2000), neonatal hypoxic-ischaemia (Papazisis et al. 2008) and adult ischaemia (Shuaib et al. 1995; Smith & Meldrum, 1995; Wiard et al.1995). Despite its widespread clinical use and putative neuroprotective effects, the molecular mechanisms underlying the therapeutic actions of LTG remain elusive.
Interestingly, the other mood stabilizers, lithium and VPA, also have well-documented neuroprotective effects in vivo and in vitro. Lithium is a direct inhibitor of pro-apoptotic glycogen synthase kinase-3 (GSK-3; Klein & Melton, 1996; Ryves & Harwood, 2001) and an indirect inhibitor through enhancing phosphorylation of GSK-3α(Ser21)/β(Ser9) (Chalecka-Franaszek & Chuang, 1999). The neuroprotective effects of lithium have been demonstrated in diverse models including glutamate excitotoxicity, endoplasmic reticulum stress, stroke, Parkinson’s disease, Huntington’s disease and spinal cord injury, among others (for review, see Chiu & Chuang, 2010). Structurally dissimilar to both lithium and LTG, VPA is an anticonvulsant and histone deacetylase (HDAC) inhibitor (Phiel et al. 2001). By inducing histone hyperacetylation and subsequent chromatin remodelling, VPA and other HDAC inhibitors such as phenylbutyrate, sodium bu-tyrate and trichostatin A can regulate the expression of neuroprotective and neurotrophic factors (for review, see Chuang et al. 2009). VPA is protective against in vitro glutamate excitotoxicity (Kanai et al. 2004; Leng & Chuang, 2006) and therapy with HDAC inhibitors may slow or prevent the neurodegeneration seen in animal models of Huntington’s disease (Chiu et al. 2011), cerebral ischaemia (Wang et al. 2011), intracerebral hemorrhage (Sinn et al. 2007) and Parkinson’s disease (Monti et al. 2010).
Given that the therapeutics used to treat bipolar disorder enhance neuroprotection, it is not surprising that accumulating evidence links mood disorders to the dysregulation of neuronal resilience, survival and plasticity cascades (for review, see Schloesser et al. 2008). For example, structural imaging and post-mortem studies suggest that bipolar disorder is associated with enlarged ventricles, reduced grey matter and cortical volume and decreased neuronal/glial density (for review, see Manji et al. 2000). Long-term lithium treatment increases total grey matter volume (Moore et al. 2000 b) and enhances levels of N-acetyl-aspartate, a marker of neuronal viability, in the brains of bipolar patients (Moore et al. 2000a); these structural changes correlate with its clinical efficacy (Lyoo et al. 2010). In light of this phenomenon, the anti-apoptotic protein B-cell lymphoma-2 (Bcl-2) has garnered close attention. Part of a protein family that tightly regulates cellular resilience and apoptosis, Bcl-2 is robustly up-regulated by treatment with lithium and VPA (Chen & Chuang, 1999; Chen et al. 1999). Furthermore, behavioural data suggest that Bcl-2 deficiency is associated with symptoms of bipolar disorder, which are attenuated by chronic lithium pretreatment (Lien et al. 2008). It was recently reported that Bcl-2 expression increases in rat frontal cortex following chronic treatment with LTG (Chang et al. 2009), raising the intriguing possibility that it is also involved in the therapeutic mechanism of this mood stabilizer.
In this study, we investigated the protective effects of LTG against glutamate excitotoxicity in primary neuronal cerebellar granule cells (CGCs). We first examined LTG-induced neuroprotection and identified two underlying mechanisms: chromatin remodelling via HDAC inhibition and up-regulation of anti-apop-totic Bcl-2. Since combined treatment with mood stabilizers is a common clinical strategy to control monotherapy-resistant bipolar disorder (Redmond et al. 2006; Walden et al. 1996; Zarate & Quiroz, 2003), we then investigated the interaction between LTG and lithium or VPA. For the first time, our results show that a sub-effective concentration of LTG has synergistic neuroprotective effects against glutamate excitotoxicity in the co-presence of either lithium or VPA.
Materials and method
Primary rat CGC cultures
CGCs were prepared from 8-d-old Sprague–Dawley rats as described previously (Nonaka et al. 1998) using animal procedures approved by the NIH Animal Care and Use Committee. Dissociated cells were resuspended in serum-free B27/neurobasal medium and plated at 1.6 × 106 cells/ml on 0.01% poly-L-lysine pre-coated plates, dishes or glass chamber slides. Cytosine arabinofuranoside (10 μM) was added 24 h later to arrest non-neuronal cell growth. Cultures were pretreated with indicated concentrations of LTG for 1–6 d, starting from day 6 in vitro, and then exposed to 100 μM glutamate for 24 h to induce neurotoxicity. During experimentation, >92% of cells represented neurons.
Measurement of cell viability
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction via mitochondrial dehydrogenase was used to quantify cell survival in a colorimetric assay, as previously described (Nonaka et al. 1998). CGCs were incubated with MTT (125 μg/ml) for 1 h at 37 °C. After media aspiration, the for-mazan product was dissolved in dimethylsulfoxide and quantified spectrophotometrically at 540 nm.
Results were expressed as percentage of control culture viability. For several experiments, MTT staining was analysed prior to media aspiration by fluorescent microscopy. A lactate dehydrogenase (LDH) cytotoxicity detection kit was also used to assay cell viability as per manufacturer’s instructions (Roche Applied Science, USA). Briefly, culture medium from CGCs grown on 96-well plates was incubated with the provided substrates. LDH released by treated cells was expressed as a percentage of total LDH released from lysed control cells. In the LTG-lithium synergy experiment, cell viability was assessed using the membrane-permeable dye calcein-AM (Sigma, USA), which is hydrolysed in viable cells to yield a green fluorescent product. 2 μM calcein-AM was added to living cells and incubated for 2 min at 37 °C before microscopic examination.
Analysis of chromatin condensation
CGCs grown on 35-mm dishes were washed with ice-cold phosphate-buffered saline (PBS), fixed with ice-cold methanol for 2 min and stained with Hoechst dye 33258 (5 μg/ml; Sigma) for 5 min at 4 °C. Nuclei were visualized under a fluorescent microscope at 360 nm.
Western blotting
CGCs were harvested and sonicated in lysis buffer as previously described (Leng et al. 2008). Sample aliquots containing 10 μg protein were dissolved in an equal volume of Nupage LDS sample buffer (Invitrogen, USA), loaded onto a 4–12% Nupage Bis-Tris gel (Invitrogen) and subjected to electrophoresis. After separation, proteins were transferred to a nitrocellulose membrane (Invitrogen), which was blocked and incubated overnight at 4 °C with the primary antibody against acetylated histone H3 (AH3) at Lys9 and Lys14, acetylated histone H4 (AH4) at Lys12 (1:3000; Milli-pore, USA); Bcl-2 (1:500; Santa Cruz Biotechnology, USA); p-GSK-3α(Ser21)/β(Ser9) (1:2000; Millipore); β-actin (1:5000; Sigma); or GAPDH (1:10000; Advanced Immunochemical Inc., USA) in 0.01% Tween- 20/PBS and then with a fluorescent dye-labelled secondary antibody (1:10000; LI-COR Biosciences, USA). Reactive bands were scanned by Odyssey Infrared Imaging System (LI-COR) and analysed with ImageJ (NIH, USA).
Immunofluorescence
CGCs cultured on glass chamber slides (Nalge Nunc International, USA) were treated with 100 μM LTG from 6–7 d in vitro (DIV), washed with PBS, fixed with methanol and blocked with 3% goat serum for 15 min. Cells were washed again and incubated overnight at 4 °C with anti-AH3 antibody (1:200). After PBS washes, cells were incubated for 1 h with Alexa Fluor 488 (1:200; Invitrogen), washed and mounted. Nuclei were stained with propidium iodide (1.5 μg/ml) included in Vectashield mounting medium (Vector Laboratories, USA) to yield red fluorescent nuclear images, which were examined by confocal microscopy.
HDAC/histone acetyltransferase (HAT) activity assays
CGCs were treated with 100 μM LTG at 6 DIV and harvested after 24 h for colorimetric HDAC/HAT activity assays (Biovision Research Products, USA) as per manufacturer’s instructions. To measure HDAC activity, 85 μl (50 μg) nuclear extracts, 10 μl HDAC assay buffer and 5 μl HDAC colorimetric substrate were mixed and incubated for 2 h at 37 °C. Then, 10 μl lysine developer was added, mixed and incubated for 30 min. Samples were read at 405 nm in a plate reader and HDAC activity expressed as percentage optical density (OD) vs. vehicle-treated controls. To measure HAT activity, 40 μl (50 μg) nuclear extracts were mixed with 50 μl HAT assay buffer, 5 μl HAT substrates I and II and 8 μl NADH-generating enzyme. After 2 h incubation at 37 °C, samples were read at 450 nm and HAT activity expressed as percentage OD vs. vehicle-treated controls.
Total RNA extraction and quantitative real-time polymerase chain reaction (PCR)
CGC total RNA was obtained using the RNeasy® Mini-kit (Qiagen, USA) as per instructions provided. Then, 1 μg of each RNA sample was reverse transcribed with the High-Capacity cDNA Archive kit (Applied Biosystems, USA). Reverse transcription reactions were run at 25 °C for 10 min and 37 °C for 120 min. Quantitative PCR was performed using TaqMan universal PCR mastermix and Taqman Bcl-2 and β-actin gene expression assays (Applied Biosystems). Each assay included negative controls without reverse transcriptase. Triplicate PCR reactions were run at 50 °C for 2 min, 95 °C for 10 min and for 40 cycles of 95 °C for 15 s and 60 °C for 1 min. mRNA expression in each sample was quantified relative to matched vehicle-treated controls with β-actin internal reference via the 2−ΔΔCt method, where Ct = cycle threshold number, ΔCt=β-actin Ct−target gene Ct and ΔΔCt = ΔCt control−ΔCt treated cells.
Semi-quantitative RT-PCR
CGC total RNA (0.1 μg) was used as a template with the MyTaq One-Step RT-PCR kit (Bioline, USA) as per manufacturer’s instructions. Rat primers used were: Bcl-2,5′-TGGACAACATCGCTCTGTGGATGA-3′ (forward) and 5′-TGTGTGTGTGTGTGTGTGTTCT-GC-3′ (reverse); GAPDH 5′-TGATGCTGGTGCTGAG-TATGTCGT-3′ (forward) and 5′-TTGTCATTGAGAG-CAATGCCAGCC-3′ (reverse); and β-actin, 5′-CCAC-AGCTGAGAGGGAAATCG-3′ (forward) and 5′-AG-TAACAGTCCGCCTAGAAGCA-3′ (reverse). Primers, reverse transcriptase, 2 × MyTaq mix, RNase inhibitor, and template were mixed and incubated at 45 °C for 20 min, 95 °C for 1 min and for 35 cycles of 95 °C for 10 s, 60 °C for 10 s and 72 °C for 30 s with a Biometra T-gradient thermoblock (Germany). RT-PCR products were analysed by 1.2% agarose gel electrophoresis and visualized with ethidium bromide staining.
Bcl-2 promoter activity assay
A 0.3-kb human Bcl-2 promoter gene fragment located 1640 bp upstream from the translation start site was cloned into a luciferase reporter plasmid (Bcl-2-Luc) as described previously (Creson et al. 2009) and kindly supplied by Dr Peixiong Yuan (NIMH, NIH). CGCs were transfected with this construct immediately before plating via electroporation (Nucleofector-II; Lonza, Switzerland) as per manufacturer’s instructions. Human epithelial kidney HEK293 cells and human neuroblastoma SH-SY5Y cells were cultured as described (Leng & Chuang, 2006) and transfected with Bcl-2-Luc using lipofectamine-2000 (Invitrogen). Twenty-four hours later, cells were treated with LTG (10–200 μM) for 24 h, and then harvested, lysed and assayed for luciferase activity (Luciferase Reporter Assay; Promega, USA) with a LumiCount microplate luminometer (Packard BioScience, USA).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using the ChIP-IT Express assay kit (Active Motif, USA), using the instructions provided and as described (Tsai et al. 2010). Briefly, CGCs were treated with LTG (100 μM) or vehicle at 6 DIV. After 24 h incubation, cells were crosslinked with 1 % formaldehyde at 37 °C for 10 min, repeatedly washed with ice-cold PBS, lysed and centrifuged. Nuclear pellets were resuspended in shearing buffer and sonicated (Sonics and Materials, USA) at 30% power, 6 × 10 s, to shear DNA into 200–1000 bp fragments. Then, 10% of the protein/DNA complex was used for ‘input DNA’ analysis. Another 10% was incubated with anti-AH3 antibody (2 μg) at 4 °C overnight. Cross-linking was reversed and DNA purified. The Bcl-2 promoter region was PCR-amplified using primers 5′-TCCTTGCCTGCATTTAGCAAGCTG-3′ (forward) and 5-CCAATTTACACTCGCGCACACA-CA-3′ (reverse). After 36 PCR cycles, samples were electrophoresed and photographed under UV light (FOTODYNE, USA).
Lentiviral Bcl-2-shRNA knockdown of Bcl-2 mRNA
A Bcl-2 lentiviral small hairpin RNA (shRNA) plasmid and control non-targeting scramble lentiviral shRNA plasmid (Sigma-Aldrich, USA) were used for Bcl-2 knockdown studies. Sequences of control shRNA and Bcl-2 shRNA were 5′-CCGGCAACAAGATGAAGA-GCACCACTCGAGTTGGTGCTCTTCATCTTGTTGT-TTTT-3′ and 5′-CCGGCATGCGACCTCTGTTTGAT-TTCTCGAGAAATCAAACAGAGGTCGCATGT TT-TTGGTTTTT-3′, respectively. HEK 293T/17 cells were plated at 1 × 105 cells/well on six-well plates in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% foetal bovine serum. The next day, cells were transfected with shRNA plasmids using a reagent mixture [0.5 μg plasmid DNA, 5 μl lentiviral packaging mix (Sigma-Aldrich) and 3 μl FuGENE transfection reagent (Roche, USA)] in 2 ml DMEM/ well. Culture medium containing lentiviral particles was collected 2 d after transfection and added to six-well plates followed by immediate CGC plating. DMEM was replaced with neurobasal medium the following day for the Bcl-2 mRNA and neuroprotection studies.
Statistical analysis
Data are expressed as mean ± S.E.M. from at least three independent experiments. Statistical significance was analysed by one-way analysis of variance and Bonferroni’s post-hoc test, except as specified (p⩽0.05 was considered significant).
Results
LTG pretreatment provides dose- and time-dependent protection against glutamate excitotoxicity in rat CGCs
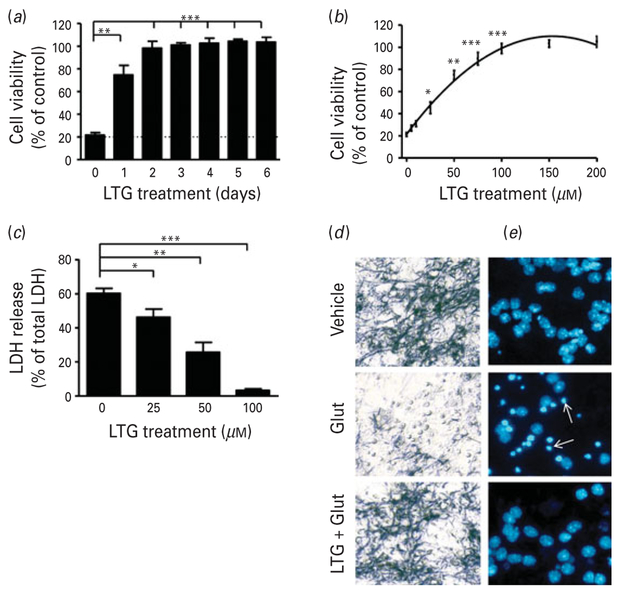
CGCs at 6 DIV were pretreated with 100 μM LTG for 1–6 d and then exposed to 100 μM glutamate for 24 h to induce excitotoxic death. Glutamate exposure resulted in about 80 % neuronal death as measured by MTT assay. Twenty-four hour pretreatment with LTG was sufficient to achieve 75% cell survival and full protection was observed after pretreatment for 3 or 4 d (Fig. 1a). Cultures were pretreated for 3 d (6–9 DIV) with incremental concentrations of LTG (5–200 μM and then exposed to 100 μM glutamate for 24 h. Excitotoxicity was blocked by LTG pretreatment in a concentration-dependent manner, confirmed by both MTT (Fig. 1b) and LDH (Fig. 1c) assays. Furthermore, cultures pretreated with 100 μM LTG from 6–9 DIV contained fewer small, round dead cells unable to metabolize MTT after 24 h of 100 μM glutamate exposure (Fig. 1d). When Hoechst 33258 dye was used to identify neurons undergoing chromatin condensation, a hallmark of apoptosis, these neuroprotective effects were also evident (Fig. 1e).
Fig. 1.
Pretreatment with lamotrigine (LTG) produced time and concentration-dependent protection against glutamate-induced excitotoxicity in cerebellar granule cells (CGCs). (a) Cells were pretreated with 100 μM LTG for the indicated number of days, prior to treatment with 100 μM glutamate (Glut) on 12 d in vitro (DIV) for 24 h. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and expressed as % viability vs. vehicle-treated controls. (b) CGCs were treated with the indicated concentrations of LTG for 3 d, starting from 6 DIV, and then exposed to 100 μM Glut for 24 h. Cell viability was assessed by MTT assay and (c) lactate dehydrogenase (LDH) release expressed as %total LDH from lysed control cells. (d) Cells were stained with MTT for phase-contrast microscopy imaging or (e) Hoechst dye 33258 to detect chromatin condensation; arrows, apoptotic cells. Cell viability and LDH release are expressed as mean±S.E.M. from six independent cultures.* p<0.05, ** p<0.01, *** p<0.001 compared to Glut-only group.
LTG increases levels of AH3 and AH4 in CGCs
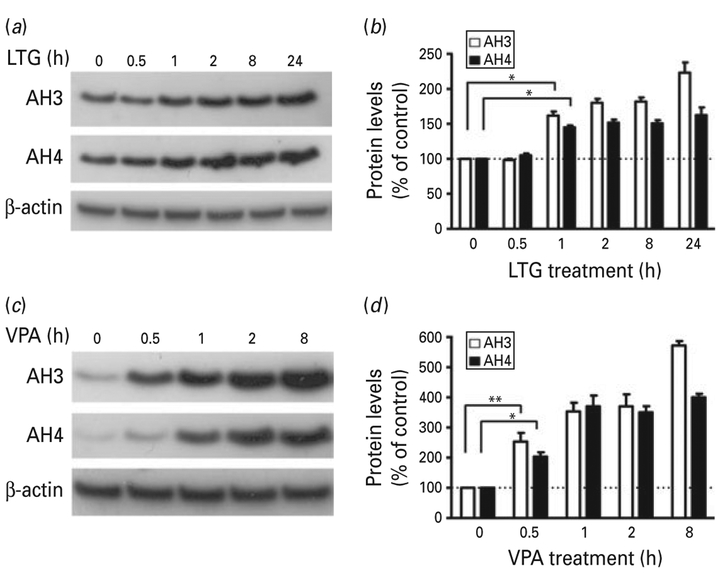
HDAC inhibition is well-characterized as a primary mechanism of VPA-induced neuroprotection, and HDAC inhibitors including sodium butyrate and tri-chostatin A have been shown to regulate the expression of neuroprotective factors through histone hyperacetylation (for review, see Chuang et al. 2009). Therefore, we investigated whether the observed neuroprotective effects of LTG against glutamate excitotoxicity were related to the regulation of histone acetylation. Treatment of CGC cultures with LTG for different time intervals starting from 6 DIV caused a time-dependent increase in the levels of AH3 and AH4 proteins (Fig. 2a, b). This increase was moderate compared to the rapid and robust increase in AH3 and AH4 induced by similar treatment with VPA (Fig. 2c, d).
Fig. 2.
Lamotrigine (LTG) induced a time-dependent increase in the levels of histone H3 and H4 acetylation : comparison with valproate (VPA). Cerebellar granule cells (CGCs) were treated with (a, b) 100 μM LTG or (c, d) 1 mM VPA starting at 6 d in vitro for the indicated time. Cells lysates were subjected to Western blot analysis for AH3, AH4 and β-actin. Quantified data are expressed as mean±S.E.M. from three independent cultures. * p<0.05, ** p<0.01 compared to the indicated groups.
LTG increases nuclear localization of AH3 in CGCs and inhibits HDAC activity
Immunocytochemical staining revealed that the intensity of AH3 immunofluorescence in CGCs was markedly increased by LTG treatment (Fig. 3a). We then used in vitro HDAC and HAT activity assays to further investigate the regulation of histone acety-lation by LTG. CGCs were treated with 100 μM LTG at 6 DIV and after incubation for 24 h cell nuclei were harvested and assayed. LTG pretreatment induced a moderate (20%) decrease in HDAC activity without affecting HAT activity (Fig. 3b, c).
Fig. 3.
Lamotrigine (LTG) increased nuclear localization of acetylated histone H3 (AH3) and inhibited histone deacetylase (HDAC) activity. (a) Cerebellar granule cells (CGCs) were treated with vehicle or 100 μM LTG for 1 d, starting from 6 d in vitro (DIV), followed by immunocytochemistry to confirm up-regulation of AH3. DIC: phase contract images; green AH3 immunostaining and red nuclear staining by propidium iodide (PI) are shown as individual and overlay images. Scale bar, 20 μm. CGCs were treated with 100 μM LTG at 6 DIV. After incubation for 24 h, cell nuclei were harvested for (b) HDAC and (c) histone acetyltransferase (HAT) activity assays. Optical density (OD) values were normalized to vehicle-treated controls for three independent experiments. * p<0.05 compared to control using an unpaired t test.
LTG increases levels of Bcl-2 mRNA and protein in CGCs
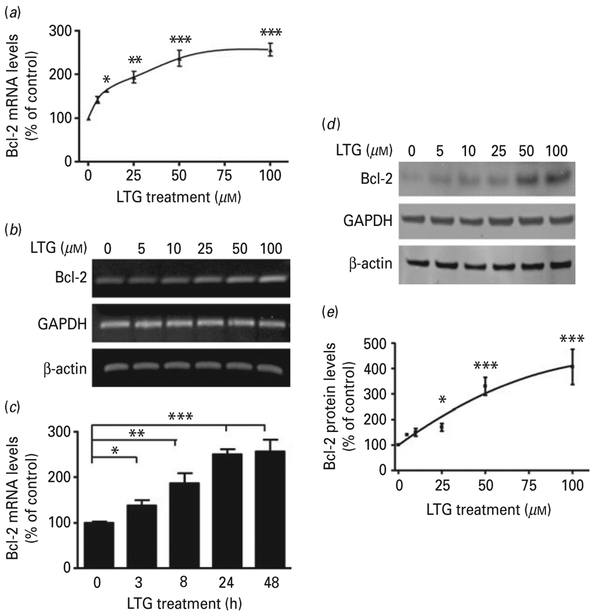
Next, we sought to determine the effects of LTG on the key anti-apoptotic protein Bcl-2. CGC cultures were treated with incremental doses of LTG (0–100 μM) for 2 d, starting from 6 DIV. This caused a concentration-dependent increase in the levels of Bcl-2 mRNA, as measured by real-time quantitative PCR (Fig. 4a) and confirmed by semi-quantitative RT-PCR (Fig. 4b). There was no change in the mRNA levels of Bcl-xl and Bax under the same conditions (data not shown). Quantitative PCR also revealed a time-dependent increase in Bcl-2 mRNA levels after LTG treatment (Fig. 4c). The LTG-induced increase in Bcl-2 mRNA was associated with an increase in the levels of Bcl-2 protein, with a significant effect at 25 μM, as determined by Western blotting (Fig. 4 d, e).
Fig. 4.
Lamotrigine (LTG) treatment increased cerebellar granule cell (CGC) B-cell lymphoma-2 (Bcl-2) mRNA and protein levels in a dose- and time-dependent manner. (a) CGCs were treated with the indicated concentrations of LTG for 2 d, starting from 6 d in vitro (DIV) and analysed for Bcl-2 mRNA expression level by quantitative polymerase chain reaction (qPCR) or (b) semiquantitative reverse transcription-PCR. (c) CGCs were treated with LTG for the indicated length of time starting from 6 DIV and analysed for Bcl-2 mRNA expression level by qPCR. (d) CGCs were treated with the indicated concentrations of LTG for 3 d starting from 6 DIV and analysed by Western blot; quantified results of Bcl-2 protein levels are shown in (e). Quantified data are normalized to respective controls and expressed as mean±S.E.M. from three independent cultures. * p<0.05, ** p<0.01, *** p<0.001 compared to the indicated groups.
LTG increases the activity of the Bcl-2 promoter and up-regulates its association with AH3
To assess whether enhanced levels of Bcl-2 mRNA and protein were triggered by an increase in Bcl-2 promoter activity, CGCs, HEK 293 cells and SH-SY5Y cells were transfected with a luciferase reporter driven by a 0.3-kb fragment of the human Bcl-2 promoter. Twenty-four hours after transfection, CGCs, HEK 293 cells and SH-SY5Y cells were treated with LTG (10–200 μM) for 24 h. Treatment with 100 μM LTG induced an approximately five-fold maximum increase in Bcl-2 promoter activity in CGCs (Fig. 5a) and an even more robust increase in HEK 293 and SH-SY5Y cells (Fig. 5b), as quantified by a luciferase reporter assay system. Next, a ChIP assay was utilized to investigate whether LTG-induced Bcl-2 expression was related to increased association between the Bcl-2 promoter region and AH3. CGCs were treated with 100 μM LTG for 24 h and then harvested for ChIP analysis. PCR results showed that LTG treatment caused a significant increase in the AH3 × Bcl-2 promoter interaction (Fig. 5c, d).
Fig. 5.
Lamotrigine (LTG) induced a robust increase in B-cell lymphoma-2 (Bcl-2) promoter activity and association with acetylated histone H3 (AH3). (a) Cerebellar granule cells (CGCs), (b) HEK293 cells and SH-SY5Y cells were transfected with a luciferase reporter driven by a 0.3-kb fragment of the human Bcl-2 promoter. Twenty-four hours after transfection, cells were treated for 24 h with 10–200 μM LTG. Bcl-2 promoter activity was assayed using the luciferase reporter system. Data are mean±S.E.M. from three independent experiments. (c) CGCs were treated with 100 μM LTG or vehicle for 24 h and subjected to chromatin immunoprecipitation (ChIP) analysis to detect levels of AH3 associated with the Bcl-2 promoter by semi-quantitative polymerase chain reaction (PCR). (d) Quantified data are expressed as mean±S.E.M. of the relative optical density ratio of Bcl-2 promoter PCR product bands vs. input lane bands and normalized to vehicle-treated controls from three independent experiments. * p<0.05 compared to vehicle-treated control cultures using an unpaired t test.
Bcl-2 shRNA suppresses the increase in Bcl-2 mRNA and neuroprotection induced by LTG
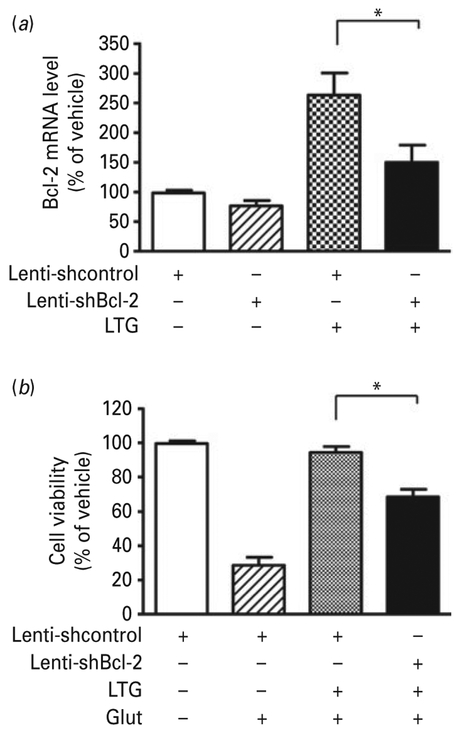
To confirm the importance of Bcl-2 in LTG-induced neuroprotection, CGCs were transduced with lentivirus-driven Bcl-2 shRNA (lenti-shBcl-2) or scramble control shRNA immediately after plating. LTG was added for 24 h beginning at 6 DIV and cells were harvested for mRNA detection. Bcl-2 shRNA markedly suppressed the robust LTG-induced increase in Bcl-2 mRNA (Fig. 6a). When transduced CGCs were incubated with LTG from 6 to 9 DIV followed by glutamate treatment for 24 h, the neuroprotective effects of LTG were significantly attenuated (Fig. 6b).
Fig. 6.
Bcl-2 shRNA suppressed lamotrigine (LTG)-induced increase in B-cell lymphoma-2 (Bcl-2) mRNA and neuroprotection. Cerebellar granule cells (CGCs) were transduced with lentivirus-driven Bcl-2 shRNA (lenti-shBcl-2) and scramble control shRNA (lenti-shcontrol) at the time of plating. (a) At 6 d in vitro (DIV), CGCs were treated with 100 μM LTG or vehicle and harvested 24 h later for Bcl-2 mRNA quantification by quantitative polymerase chain reaction (qPCR). (b) Transduced CGCs were treated with 100 μM LTG at 6 DIV and after 3 d incubation 100 μM glutamate was added to induce neurotoxicity. Cell viability was detected via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. Data are mean±S.E.M. from three independent experiments. * p<0.05 compared to the indicated groups, unpaired t test.
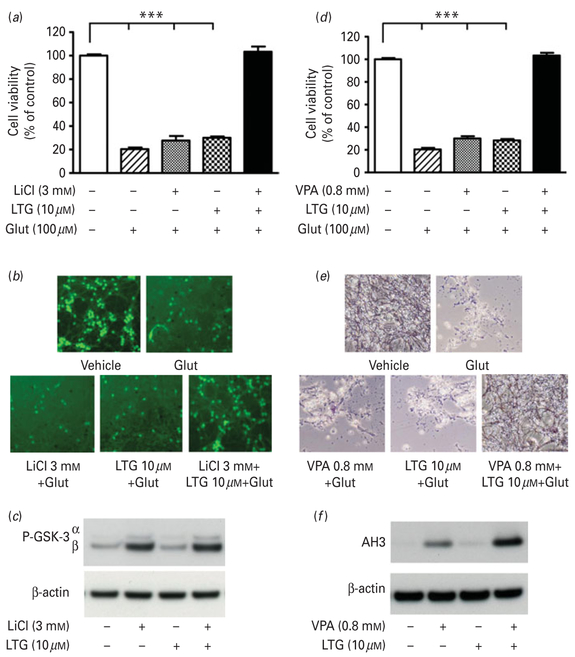
Synergistic neuroprotection by co-treatment with low doses of LTG and lithium or VPA in CGC cultures
Finally, we examined the potential synergistic neuroprotection following pretreatment with a sub-effective concentration of LTG in the co-presence of lithium or VPA. Combined treatment with 10 μM LTG and 3 mM LiCl from 6–12 DIV, although individually ineffective, had robust protective effects against 24 h of 100 μM glutamate exposure in aging CGCs (Fig. 7a, b). This was not associated with a synergistic increase in levels of phospho-GSK-3α(Ser21) or β(Ser9) (Fig. 7c). In addition, 10 μM LTG exhibited synergistic protection against glutamate excitotoxicity when combined with 0.8 mM VPA, as demonstrated by cell viability quantification (Fig. 7d) and MTT staining for preserved morphology (Fig. 7e). LTG + VPA co-treatment was associated with a robust increase in levels of AH3 compared to either monotherapy (Fig. 7f).
Fig. 7.
Synergistic neuroprotective effects of low dose lamotrigine (LTG) with lithium or valproate (VPA). Cerebellar granule cells (CGCs) were treated with 10 μM LTG in the presence or absence of 3.0 mM LiCl from 6–12 d in vitro (DIV) followed by 100 μM glutamate for 24 h. (a) Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)–2,5-diphenyl tetrazolium bromide (MTT) assay and expressed as mean±S.E.M. of % viability compared to vehicle-treated control cultures from five independent experiments. (b) Cells were stained with calcein-AM and examined under an inverted fluorescence microscope. (c) CGCs at 6 DIV were treated with 10 μM LTG and 3.0 mM LiCl, individually and in combination, for 24 h. Cell lysates were analysed by Western blot for levels of p-GSK-3α(Ser21)/β(Ser9) and β-actin. CGCs were treated with 10 μM LTG in the presence or absence of 0.8 mM VPA from 6–12 DIV followed by 100 μM glutamate for 24 h. Cell viability was assessed by (d) MTT assay expressed as mean±S.E.M. of % viability compared to vehicle-treated control cultures from five independent experiments and (e) MTT staining. (f) CGCs at 6 DIV were treated with 10 μM LTG and 0.8 mM VPA, individually and in combination, for 24 h. Cell lysates were analysed by Western blot for levels of acetylated histone H3 (AH3) and β-actin. *** p<0.001 compared to the indicated groups.
Discussion
For the first time, our study showed that pretreatment with LTG protected CGCs against glutamate excitotoxicity in a time- and dose-dependent manner. Thus, significant protection was observed with 25μM pretreatment and virtually complete protection was obtained at 100 μM. This compares to LTG’s therapeutic target range of 10–60 μM in the serum (Johannessen et al. 2003), although higher doses are often well tolerated by patients (Morris et al. 2004). Moreover, pretreatment with a low dose of LTG combined with lithium or VPA resulted in synergistic neuroprotection as assessed biochemically and morphologically. HDAC inhibition and Bcl-2 induction were closely associated with the neuroprotective effects of LTG.
Glutamate-induced excitotoxicity in the hippocampus has been implicated in stress-related depression (for review, see Lee et al. 2002). Glutamate overflow and excitotoxicity has also been linked to a number of neurological conditions, including Huntington’s disease, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, cerebral ischaemia and traumatic brain injury, among others (for review, see Doble, 1999). The mood stabilizers lithium and VPA protect against glutamate excitotoxicity in vitro (Chalecka-Franaszek & Chuang, 1999; Leng & Chuang, 2006; Marinova et al. 2009; Nonaka et al. 1998) and demonstrate promising therapeutic effects in many preclinical models of brain injury and neurodegeneration (Chiu & Chuang, 2010; Chuang et al. 2009; Roh et al. 2005). LTG, presumably through inhibition of voltage-gated sodium channels, has been shown to protect against experimental cerebral ischaemia in multiple reports (Shuaib et al.1995; Smith & Meldrum, 1995; Wiard et al.1995). In this study, we extended these findings by demonstrating that LTG had remarkable neuroprotective effects against glutamate excitotoxicity in vitro, where it both induced chromatin remodelling via HDAC inhibition and robustly increased Bcl-2 expression. Therefore, the beneficial effects of LTG merit further investigation in models of a wide range of glutamate-related neurological disorders.
Our results indicate that HDAC inhibition by LTG, as measured by changes in the levels of AH3 and AH4, followed a slower time-course and was less robust compared to that of VPA. In an in vitro HDAC activity assay, LTG only inhibited HDAC activity by approximately 20 %, whereas VPA has been shown to inhibit activity by up to 70% (Leng & Chuang, 2006). Moreover, and again in contrast to VPA, addition of LTG directly to the CGC lysates failed to inhibit HDAC activity (data not shown). Nevertheless, Western blotting and immunocytochemistry showed a striking up-regulation and nuclear localization of AH3 following LTG pretreatment. We therefore hypothesize that LTG may act by indirect mechanisms to inhibit HDAC activity, such as through downstream signalling cascades or metabolic products, as opposed to the direct inhibitory actions of VPA (Phiel et al. 2001). A number of endogenous HDAC inhibitors have recently been identified. For example, the lipid sphingosine-1-phosphate binds specifically to HDAC1 and 2 to inhibit enzymatic activity (Hait et al. 2009). In addition, two members of a novel family of proteins termed ′KCTD containing, Cullin3 adaptor, suppressor of Hedgehog′ (KCASH) proteins, KCASH2 and 3, have been detected in rodent cerebellum and shown to promote HDAC1 ubiquitination and degradation (de Smaele et al. 2011). These mechanisms warrant further investigation in the context of LTG neuroprotection. To our knowledge, this is the first time that LTG has been observed to inhibit HDAC activity. Different HDAC inhibitors including VPA, trichostatin A, suberoylanilide hydroxamic acid and butyrates have robust neuroprotective effects in cellular and animal models of neurodegeneration, primarily through regulation of gene expression (for review, see Chuang et al.2009). Thus, the observed increase in AH3 and AH4 protein levels may be closely tied to the neuroprotective effects of LTG.
For more than a decade, Bcl-2 protein family members have been identified as prominent players in cellular apoptotic cascades. A key neuroprotective protein, Bcl-2 levels decrease as early as 6 h after glutamate challenge in neurons (Liu & Zhu, 1999) and its over-expression is associated with protection against apoptosis in multiple cellular and animal models of neuronal injury (Rohn et al. 2008; Vukosavic et al. 2000; Yuan et al.2003). Mood stabilizers including lithium, VPA, carbamazepine and LTG have been reported to induce Bcl-2 mRNA and protein levels (Chang et al. 2009; Chen & Chuang, 1999; Chen et al. 1999). Notably, Bcl-2 dysregulation has recently been linked to the behavioural symptoms of mood disorders. Both repeated unpredictable stress and chronic restraint stress, rodent models of depressive-like behaviour, result in down-regulation of Bcl-2 mRNA in key brain regions (Bravo et al. 2009; Kosten et al. 2008). In addition, enhanced Bcl-2 function through pharmacological inhibition of its antagonist, BH3-interacting domain death agonist, exerts antidepressant-like effects in mice (Malkesman et al.2011). Clinical studies have shown that a Bcl-2 single nucleotide polymorphism can affect Bcl-2 expression levels and calcium homeostasis in bipolar patient lymphoblasts, suggesting that the Bcl-2 dysregulation observed in animal models has direct clinical relevance (Machado-Vieira et al. 2011; Uemura et al. 2011).
In this study, we showed that LTG increased the activity and histone acetylation levels of the Bcl-2 promoter, as well as Bcl-2 mRNA and protein levels.
This paralleled the dose-dependent neuroprotective effects of pretreatment, which were significantly attenuated when the LTG-induced increase in Bcl-2 was suppressed by its shRNA. It is worth noting that our lenti-shBcl-2 did not completely abolish Bcl-2 expression, which was still moderately up-regulated by LTG pretreatment in transduced CGCs. Therefore, the attenuated protection shown in Fig. 6b likely does not represent the full contribution of Bcl-2 to the neuro-protective effects of LTG. We cannot exclude the importance of additional undefined mechanisms in the actions of LTG against glutamate excitotoxicity. Nevertheless our data support a significant role for Bcl-2, which may also be linked to the therapeutic effects of this mood stabilizer. Specifically, the accumulation of data connecting glutamate and glutamate receptors to depression (Paul & Skolnick, 2003) suggests that the shared anti-glutamatergic properties of lithium, VPA, and LTG – all involving up-regulation of Bcl-2 – may contribute to their efficacy against depressive episodes in bipolar disorder. In contrast, only lithium and VPA, but not LTG, are successful in controlling manic/mixed states. These anti-manic effects may require either GSK-3β inhibition or robust, direct HDAC inhibition, actions that we observed only for lithium and VPA, respectively.
Interestingly, previous studies have implicated several microRNAs in the mechanism of action of mood stabilizers (Zhou et al. 2009) as well as the post-transcriptional regulation of Bcl-2 (e.g. Cimmino et al. 2005; Wang et al. 2009). Under our conditions, 24 h and 3 d of LTG pretreatment did not significantly affect the levels of two of these non-coding RNAs, rno-miR-34a and rno-miR-16 (data not shown). Given the growing body of evidence linking psychiatric disorders and mood stabilizers to alterations in microRNA expression (for review, see Hunsberger et al. 2009), investigation of LTG-mediated microRNA regulation may merit consideration.
We have previously shown that co-treatment with lithium and VPA has robust synergistic neuroprotective effects in an aging CGC model, where monotherapy with either drug is ineffective (Leng et al. 2008). Excitingly, combined pretreatment with subeffective doses of LTG and lithium also produced synergistic neuroprotection against glutamate excitotoxicity in CGCs. However, whereas co-treatment with VPA potentiated the lithium-induced increase in phospho-GSK-3α(Ser21) and β(Ser9) isoforms (Leng et al. 2008), LTG did not have any additional effect on GSK-3α(Ser21)/β(Ser9) phosphorylation. Mechanisms underlying the marked neuroprotective effects of combined LTG-lithium treatment remain to be elucidated. Synergistic neuroprotection was also evident in cultures co-treated with low concentrations of LTG and VPA. This may be due to the robust increase in levels of AH3 observed compared to monotherapy. In the clinic, LTG is often used together with lithium or VPA to treat bipolar disorder (Redmond et al. 2006; van der Loos et al. 2009; Walden et al. 1996; Zarate & Quiroz, 2003). Therefore, the observed drug interactions may offer clues to the therapeutic efficacy of these combinations. One important caveat is the additional pharmacokinetic interaction between LTG and VPA: the elimination half-life of LTG is significantly prolonged when co-administered with VPA, carrying a risk of adverse side-effects and limiting the clinical utility of this treatment regimen (Faught et al. 1999; Gidal et al. 2003).
In summary, our novel results revealed the strong neuroprotective effects of LTG against glutamate excitotoxicity in CGC cultures. This was associated with an up-regulation of AH3 and AH4, as well as a robust increase in Bcl-2 mRNA promoter activity and Bcl-2 mRNA and protein levels. Moreover, the copresence of a low dose of LTG with lithium or VPA showed remarkable synergistic neuroprotection. The relationship between the neuroprotective mechanisms of lithium, VPA and LTG, particularly regulation of Bcl-2, and their efficacy in treating mood disorders warrants further investigation. Finally, based on our data, we would strongly recommend follow-up studies to explore the therapeutic potential of LTG, notably in combination with lithium or VPA, in preclinical models of glutamate-related neurological disorders.
Acknowledgements
This study was supported by the Intramural Research Program of NIMH, NIH and a gift fund from the Hsu Family Foundation. The authors thank members in the Molecular Neurobiology Section, NIMH, NIH, notably Dr Chi-Tso Chiu, Dr Joshua Hunsberger and Peter Leeds, for their helpful discussions and assistance.
Footnotes
Statement of Interest
None.
References
- Bowden CL (2002). Lamotrigine in the treatment of bipolar disorder. Expert Opinion on Pharmacotherapy 3, 1513–1519. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Diaz-Veliz G, Mora S, Ulloa JL, et al. (2009). Desipramine prevents stress-induced changes in depressive-like behavior and hippocampal markers of neuroprotection. Behavioural Pharmacology 20, 273–285. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Bowden CL, Sachs GS, Ascher JA, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group . Journal of Clinical Psychiatry 60, 79–88. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Saulle E, Centonze D, et al. (2000). Is pharmacological neuroprotection dependent on reduced glutamate release? Stroke 31, 766–772; discussion 773. [DOI] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Chuang DM (1999). Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proceedings of the National Academy of Sciences USA 96, 8745–8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Rapoport SI, Rao JS (2009). Chronic administration of mood stabilizers upregulates BDNF and bcl-2 expression levels in rat frontal cortex. Neurochemical Research 34, 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zeng WZ, Yuan PX, Huang LD, et al. (1999). The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. Journal of Neurochemistry 72, 879–882. [DOI] [PubMed] [Google Scholar]
- Chen RW, Chuang DM (1999). Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. Journal of Biological Chemistry 274, 6039–6042. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Chuang DM (2010). Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacology and Therapeutics 128, 281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CT, Liu G, Leeds P, Chuang DM (2011). Combined treatment with the mood stabilizers lithium and valproate produces multiple beneficial effects in transgenic mouse models of Huntington’s disease. Neuropsychopharmacology 36, 2406–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DM, Leng Y, Marinova Z, Kim HJ, et al. (2009). Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends in Neurosciences 32, 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, et al. (2005). miR-15 and miR-16 induce apoptosis by targeting BCL2. Proceedings of the National Academy of Sciences USA 102, 13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connop BP, Boegman RJ, Beninger RJ, Jhamandas K (1997). Malonate-induced degeneration of basal forebrain cholinergic neurons: attenuation by lamotrigine, MK-801, and 7-nitroindazole. Journal of Neurochemistry 68, 1191–1199. [DOI] [PubMed] [Google Scholar]
- Creson TK, Yuan P, Manji HK, Chen G (2009). Evidence for involvement of ERK, PI3K, and RSK in induction of Bcl-2 by valproate. Journal of Molecular Neuroscience 37, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smaele E, Di Marcotullio L, Moretti M, Pelloni M, et al. (2011). Identification and characterization of KCASH2 and KCASH3, 2 novel Cullin3 adaptors suppressing histone deacetylase and Hedgehog activity in medulloblastoma. Neoplasia 13, 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble A (1999). The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacology and Therapeutics 81, 163–221. [DOI] [PubMed] [Google Scholar]
- Faught E, Morris G, Jacobson M, French J, et al. (1999). Adding lamotrigine to valproate: incidence of rash and other adverse effects. Postmarketing Antiepileptic Drug Survey (PADS) Group . Epilepsia 40, 1135–1140. [DOI] [PubMed] [Google Scholar]
- Gidal BE, Sheth R, Parnell J, Maloney K, et al. (2003). Evaluation of VPA dose and concentration effects on lamotrigine pharmacokinetics : implications for conversion to lamotrigine monotherapy. Epilepsy Research 57, 85–93. [DOI] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, et al. (2009). Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen T, Nissinen J, Pitkänen A (2001). Effect of lamotrigine treatment on status epilepticus-induced neuronal damage and memory impairment in rat. Epilepsy Research 46, 205–223. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Austin DR, Chen G, Manji HK (2009). MicroRNAs in mental health: from biological underpinnings to potential therapies. Neuro Molecular Medicine 11,173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen SI, Battino D, Berry DJ, Bialer M, et al. (2003). Therapeutic drug monitoring of the newer antiepileptic drugs. Therapeutic Drug Monitoring 25, 347–363. [DOI] [PubMed] [Google Scholar]
- Kanai H, Sawa A, Chen RW, Leeds P, et al. (2004). Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharmacogenomics Journal 4, 336–344. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Ko HH, Han ES, Lee CS (2007). Lamotrigine inhibition of rotenone- or 1-methyl-4-phenylpyridinium-induced mitochondrial damage and cell death. Brain Research Bulletin 71, 633–640. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA (1996). A molecular mechanism for the effect of lithium on development. Proceedings of the National Academy of Sciences USA 93, 8455–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Galloway MP, Duman RS, Russell DS, et al. (2008). Repeated unpredictable stress and antidepressants differentially regulate expression of the bcl-2 family of apoptotic genes in rat cortical, hippocampal, and limbic brain structures. Neuropsychopharmacology 33, 1545–1558. [DOI] [PubMed] [Google Scholar]
- Lagrue E, Chalon S, Bodard S, Saliba E, et al. (2007). Lamotrigine is neuroprotective in the energy deficiency model of MPTP intoxicated mice. Pediatric Research 62, 14–19. [DOI] [PubMed] [Google Scholar]
- Lee AL, Ogle WO, Sapolsky RM (2002). Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disorders 4, 117–128. [DOI] [PubMed] [Google Scholar]
- Lee CY, Fu WM, Chen CC, Su MJ, et al. (2008). Lamotrigine inhibits postsynaptic AMPA receptor and glutamate release in the dentate gyrus. Epilepsia 49, 888–897. [DOI] [PubMed] [Google Scholar]
- Lees G, Leach MJ (1993). Studies on the mechanism of action of the novel anticonvulsant lamotrigine (Lamictal) using primary neurological cultures from rat cortex. Brain Research 612, 190–199. [DOI] [PubMed] [Google Scholar]
- Leng Y, Chuang DM (2006). Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. Journal of Neuroscience 26, 7502–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Liang MH, Ren M, Marinova Z, et al. (2008). Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons : roles of glycogen synthase kinase-3 inhibition. Journal of Neuroscience 28, 2576–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien R, Flaisher-Grinberg S, Cleary C, Hejny M, et al. (2008). Behavioral effects of Bcl-2 deficiency: implications for affective disorders. Pharmacological Reports 60, 490–498. [PubMed] [Google Scholar]
- Liu X, Zhu XZ (1999). Roles of p53, c-Myc, Bcl-2, Bax and caspases in glutamate-induced neuronal apoptosis and the possible neuroprotective mechanism of basic fibroblast growth factor. Molecular Brain Research 71, 210–216. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Dager SR, Kim JE, Yoon SJ, et al. (2010). Lithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: a longitudinal brain imaging study. Neuropsychopharmacology 35, 1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Pivovarova NB, Stanika RI, Yuan P, et al. (2011). The Bcl-2 gene polymorphism rs956572AA increases inositol 1,4,5-trisphosphate receptor-mediated endoplasmic reticulum calcium release in subjects with bipolar disorder. Biological Psychiatry 69, 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman O, Austin DR, Tragon T, Henter ID, et al. (2011). Targeting the BH3-interacting domain death agonist to develop mechanistically unique antidepressants. Molecular Psychiatry. Published online: 5 July 2011. doi: 10.1038/mp.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G (2000). Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biological Psychiatry 48, 740–754. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Curia G, Biagini G, Ragsdale DS, et al. (2010). Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurology 9, 413–424. [DOI] [PubMed] [Google Scholar]
- Marinova Z, Ren M, Wendland JR, Leng Y, et al. (2009). Valproic acid induces functional heat-shock protein 70 via Class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. Journal of Neurochemistry 111, 976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti B, Gatta V, Piretti F, Raffaelli SS, et al. (2010). Valproic acid is neuroprotective in the rotenone rat model of Parkinson’s disease: involvement of alpha-synuclein. Neurotoxicity Research 17, 130–141. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Hasanat K, Chen G, et al. (2000a). Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2’s neurotrophic effects? Biological Psychiatry 48, 1–8. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, et al. (2000b). Lithium-induced increase in human brain grey matter. Lancet 356, 1241–1242. [DOI] [PubMed] [Google Scholar]
- Morris RG, Lee MY, Cleanthous X, Black AB (2004). Longterm follow-up using a higher target range for lamotrigine monitoring. Therapeutic Drug Monitoring 26, 626–632. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Hough CJ, Chuang DM (1998). Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proceedings of the National Academy of Sciences USA 95, 2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazisis G, Kallaras K, Kaiki-Astara A, Pourzitaki C, et al. (2008). Neuroprotection by lamotrigine in a rat model of neonatal hypoxic–ischaemic encephalopathy. International Journal of Neuropsychopharmacology 11, 321–329. [DOI] [PubMed] [Google Scholar]
- Paul IA, Skolnick P (2003). Glutamate and depression: clinical and preclinical studies. Annals of the New York Academy of Sciences 1003, 250–272. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, et al. (2001). Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. Journal of Biological Chemistry 276, 36734–36741. [DOI] [PubMed] [Google Scholar]
- Redmond JR, Jamison KL, Bowden CL (2006). Lamotrigine combined with divalproex or lithium for bipolar disorder: a case series. CNS Spectrums 11, 915–918. [DOI] [PubMed] [Google Scholar]
- Roh MS, Eom TY, Zmijewska AA, De Sarno P, et al. (2005). Hypoxia activates glycogen synthase kinase-3 in mouse brain in vivo : protection by mood stabilizers and imipramine. Biological Psychiatry 57, 278–286. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, et al. (2008). Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2. Journal of Neuroscience 28, 3051–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryves WJ, Harwood AJ (2001). Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochemical and Biophysical Research Communications 280, 720–725. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Huang J, Klein PS, Manji HK (2008). Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology 33,110–133. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Mahmood RH, Wishart T, Kanthan R, et al. (1995). Neuroprotective effects of lamotrigine in global ischemia in gerbils. A histological, in vivo microdialysis and behavioral study. Brain Research 702,199–206. [DOI] [PubMed] [Google Scholar]
- Sinn DI, Kim SJ, Chu K, Jung KH, et al. (2007). Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiology of Disease 26, 464–472. [DOI] [PubMed] [Google Scholar]
- Smith SE, Meldrum BS (1995). Cerebroprotective effect of lamotrigine after focal ischemia in rats. Stroke 26,117–122. [DOI] [PubMed] [Google Scholar]
- Tsai LK, Leng Y, Wang Z, Leeds P, et al. (2010). The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology 35, 2225–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Green M, Corson TW, Perova T, et al. (2011). Bcl-2 SNP rs956572 associates with disrupted intracellular calcium homeostasis in bipolar I disorder. Bipolar Disorders 13, 41–51. [DOI] [PubMed] [Google Scholar]
- van der Loos ML, Mulder PG, Hartong EG, Blom MB, et al. (2008). Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: a multicenter, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 70, 223–231. [DOI] [PubMed] [Google Scholar]
- Vukosavic S, Stefanis L, Jackson-Lewis V, Guegan C, et al. (1999). Delaying caspase activation by Bcl-2: a clue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. Journal of Neuroscience 20, 9119–9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden J, Hesslinger B, van Calker D, Berger M (1996). Addition of lamotrigine to valproate may enhance efficacy in the treatment of bipolar affective disorder. Pharmacopsychiatry 29, 193–195. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Sihra TS, Gean PW (2001). Lamotrigine inhibition of glutamate release from isolated cerebrocortical nerve terminals (synaptosomes) by suppression of voltage-activated calcium channel activity. Neuroreport 12, 2255–2258. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu P, Zhu H, Xu Y, et al. (2009). miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer’s disease, inhibits bcl2 translation. Brain Research Bulletin 80, 268–273. [DOI] [PubMed] [Google Scholar]
- Wang Z, Leng Y, Tsai LK, Leeds P, et al. (2011). Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. Journal of Cerebral Blood Flow and Metabolism 31, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiard RP, Dickerson MC, Beek O, Norton R, et al. (1995). Neuroprotective properties of the novel antiepileptic lamotrigine in a gerbil model of global cerebral ischemia. Stroke 26, 466–472. [DOI] [PubMed] [Google Scholar]
- Yuan J, Lipinski M, Degterev A (2003). Diversity in the mechanisms of neuronal cell death. Neuron 40, 401–413. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Quiroz JA (2003). Combination treatment in bipolar disorder: a review of controlled trials. Bipolar Disorders 5, 217–225. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yuan P, Wang Y, Hunsberger JG, et al. (2009). Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology 34, 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zona C, Avoli M (1997). Lamotrigine reduces voltage-gated sodium currents in rat central neurons in culture. Epilepsia 38, 522–525. [DOI] [PubMed] [Google Scholar]