Abstract
To date, the majority of MRS reproducibility studies have been conducted in healthy younger adults, with only a few conducted in older adults at 3T. With the growing interest in applying MRS methods to study the longitudinal course and effects of treatments in neurodegenerative disease, it is important to establish reproducibility in age-matched controls, especially in older individuals. In this study, spectroscopic data were acquired using a stimulated echo acquisition mode (STEAM) localization technique in two regions (anterior and posterior cingulate cortices; ACC, PCC, respectively) in ten healthy, cognitively normal older adults (64 ± 8.1 years). Reproducibility was assessed via mean coefficients of variation (CV) and relative difference (RD) calculated across two visits performed 2–3 months apart. Metabolites with high signal to noise ratio (SNR) such as NAA, tCho and Glu had mean CVs of 10% or less and mean RDs of 15% or less across both regions. Metabolites with lower SNR such as GABA and Gln had slightly higher mean CVs of 22% or less and mean RDs of 27% or less across both regions. These results demonstrate the feasibility of acquiring MRS data at 7T in older subjects, and establish that the spectroscopic data is reproducible in both the ACC and PCC in older, healthy subjects to the same extent as previous studies in young subjects.
Keywords: magnetic resonance spectroscopy, older adults, reproducibility, 7T, anterior cingulate cortex, posterior cingulate cortex
Graphical Abstract
MRS data were acquired in the anterior and posterior cingulate cortices in ten healthy older adults at 7T. Reproducibility was assessed across two visits via mean coefficients of variation (CV) and relative differences (RD). High signal to noise ratio (SNR) metabolites had mean CVs of ≤ 10% and RDs of ≤ 15% compared to low SNR metabolites that had slightly higher mean CVs and RDs. These results demonstrate the feasibility of acquiring MRS data at 7T in older subjects.
Introduction
The elderly are at increased risk for progressive cognitive impairment, most commonly Alzheimer’s dementia1, and the number of people with dementia and the costs associated with caring for them are expected to increase substantially1–3. In order to develop new treatments to slow or halt the progression of neurodegenerative disease, it is important to understand the underlying disease pathophysiology. In addition, it is useful to have non-invasive tools available for diagnostic and treatment monitoring purposes. The magnetic resonance spectroscopy (MRS) technique provides neurochemical information regarding neuronal and glial cell populations, energy metabolism, neurotransmission, and cellular turnover. Compared to other imaging modalities, MRS can assess this information non-invasively, simultaneously, and in certain cases uniquely (e.g. measures of oxidative stress). In recent years, 7 Tesla (T) MR scanners have become available and are currently installed in approximately fifty academic medical centers worldwide. The increased magnetic field strength (compared to conventional MR scanners operating at either 1.5 or 3T) has a number of technical advantages; in particular, for MRS of the brain, 7T yields increased spectral resolution, improved signal-to-noise ratio (SNR), and improved accuracy in the detection of more metabolites compared to lower field strengths4–7. 7T MR scanners are increasingly being used for clinical research8–10.
The ability to detect more relevant metabolites, especially those with low SNR, could prove informative for studies of Alzheimer’s disease and other disorders of aging. However, prior to conducting clinical studies, it is important to establish reproducibility of the proposed research methods in healthy older controls similar to those in the proposed study. Prior reproducibility studies at 7T using localization methods such as stimulated echo acquisition mode (STEAM)11 or semi-LASER12 have only established reproducibility in younger healthy populations6,13–15. Older adults are more likely to move during a MR scan than younger adults16–19, which could negatively impact reproducibility. In addition, older adults have greater medical co-morbidities20,21 such as high blood pressure that could possibly result in greater fluctuations in imaging measures. Establishing reproducibility in older participants is needed to inform subsequent prospective studies of neurobiological processes aimed at assessing changes longitudinally or to evaluate the impact of treatment. Thus, the goal of this study was to establish the reproducibility of the STEAM technique in the anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC) in a cohort of healthy, cognitively normal older (55–80 years old) adults.
Experimental
Ten healthy older adults (mean age: 64±8.1 years, 4 male/6 female) had two separate MRS scans approximately 2–3 months apart (average: 2.4±0.6 months). After a complete description of the study to participants, written informed consent was obtained as approved by the Johns Hopkins University Institutional Review Board. All subjects underwent psychiatric and neuropsychological evaluations, including a structured clinical interview by a clinical psychologist (SCID;22), clinical dementia rating scale (CDR; a score of 0 or normal was required;23), Mini Mental State Examination (MMSE;24), physical and neurological examination, laboratory testing (including complete blood count and blood chemistry), and toxicology screening (psychotropic drugs and drugs of abuse). Participants who had a history of or current neurological or current Axis I psychiatric disorder including substance abuse, who were not medically stable (including a current diagnosis of insulin dependent diabetes and/or poorly controlled hypertension), or who had used prescription or over-the-counter medications (e.g. antihistamines, cold medications) with potential central nervous system effects within the past two weeks were excluded from the study. None of the participants had current or a history of psychotropic drug use.
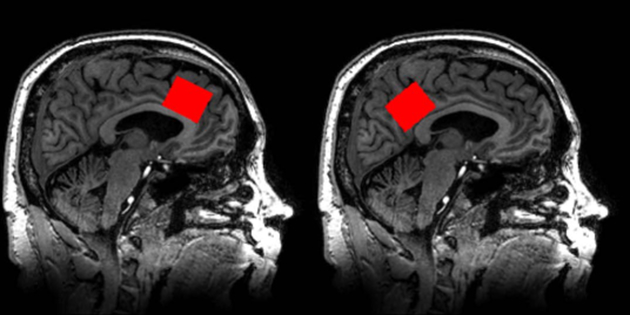
Data were acquired on a 7T MR scanner (Philips ‘Achieva’, Best, The Netherlands) equipped with a 32-channel receive head coil and a quadrature transmit coil (Nova Medical, Wilmington, Massachusetts). The peak B1 of the RF transmit coil was set at 15 μT. Padding was placed inside the head coil for patient comfort and to restrict motion. Prior to acquisition, localized power optimization and first and second order shimming were performed25. High resolution 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) images were acquired in the sagittal plane and reformatted into the coronal and axial planes in order to prescribe the approximately 9-cm3 spectroscopic voxels in the ACC (2.8 × 2.0 ×1.6 cm, anterior-posterior x left-right x superior-inferior) and the PCC (1.6 × 2.0 × 2.8 cm) as shown in Figure 1. For the ACC, the inferior edge of the voxel was placed perpendicular to the genu of the corpus callosum. For the PCC, the inferior edge of the voxel was placed perpendicular to the splenium of the corpus callosum. The ACC and PCC were chosen as these regions are implicated in the pathophysiology of affective and cognitive symptoms in the early stages of Alzheimer’s disease26–30, as well as other neurodegenerative diseases. A STEAM sequence was applied with the following parameters: TR/TM/TE=3000/25/14 ms, 96 averages, acquisition time: 4 min 48 sec, 2048 complex points, 3 kHz spectral width, VAPOR water suppression31, and average total scanner time ~ 45 minutes. A basis set was simulated in the VeSPA program32 that included 20 metabolites: alanine (Ala), aspartate (Asp), creatine (Cr), γ-aminobutyric acid (GABA), glucose (Glc), glutamate (Glu), glutamine (Gln), glycine (Gly), glycerophosphocholine (GPC), glutathione (GSH), lactate (Lac), myo-inositol (mI), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphocholine (PCh), phosphocreatine (PCr), phosphoethanolamine (PE), serine (Ser), scyllo-Inositol (sI), and taurine (Tau). The basis set was incorporated into LCModel (Version 6.3–0D) for spectroscopic quantification33. Macromolecules were handled by LCModel, which parameterizes the macromolecule background to remove its influence from concentration outputs. Data were apodized with a Chapman-Richards function using an in-house IDL program (Exelis Visual Information Solutions, Boulder, CO)14. All metabolites are reported as ratios to tCr (Cr+PCr), and only metabolites with Cramér–Rao Lower Bounds (CRLB) of 20% or less were utilized in further analyses. As described by LCModel, mean linewidth, a rough estimate of the linewidth in the in vivo spectrum, and mean SNR, a ratio of the maximum in the spectrum-minus the baseline to twice the RMS residuals, were reported for each visit in each region34. A t-test was performed on linewidth and SNR between visits to ensure comparable spectral quality. In addition, mean NAA linewidth and SNR were computed for each region using jMRUI35,36. MPRAGE images were segmented using SPM1237. In-house Matlab code based on the Gannet 3.0 toolbox38 was used to extract gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) tissue fractions. A t-test was performed on GM, WM, and CSF between visits to ensure consistent voxel placement. Using the metabolite ratios relative to tCr, reproducibility was assessed by calculating mean coefficients of variation (CV) and mean relative difference (RD) between visits. A participant CV was calculated as the standard deviation between visits divided by the mean between visits such that a CV was calculated for each participant. Then, a mean CV was computed from all participant CVs.. Similarly, mean RD was calculated as the absolute difference between visits divided by the first visit such that an RD was calculated for each participant. A mean RD was calculated from the individual RDs. Both mean CV and mean RD are reported as percentages. In order to be included in mean CV and RD analyses, the metabolite CRLB for both visits must be 20% or less; otherwise, the data were not included in the reproducibility analyses. To examine regional differences in metabolite ratios, a t-test was performed on mean metabolite ratios from each region with significance set to p<0.05.
Figure 1.
T1-weighted images showing voxel placement for the anterior cingulate cortex (left) and posterior cingulate cortex (right).
Results
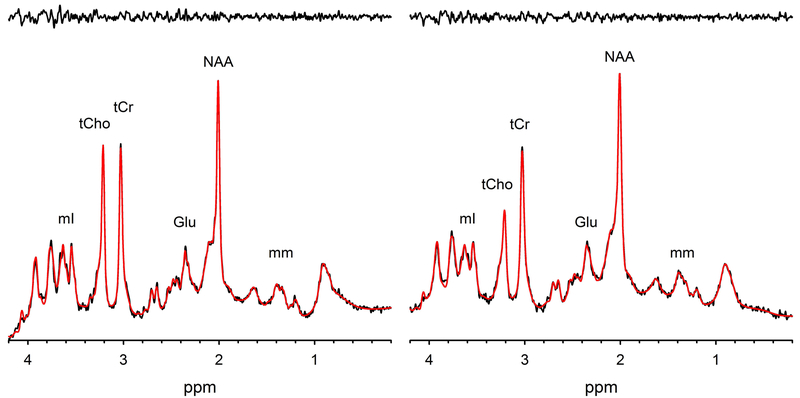
Representative spectra from the ACC and PCC with LCModel fits and residuals are shown in Figure 2. All spectra acquired in each region and each visit are shown overlaid in the Supplement (Figure S1). Using a CRLB cutoff of 20%, mean metabolite ratios and reproducibility assessments of mean CV and RD for the ACC and PCC are summarized in Tables 1 and 2, respectively. Due to the ongoing discussion regarding CRLB cutoff39, mean metabolite ratios and reproducibility assessments with a CRLB cutoff of 50% or higher are summarized in Tables S1 and S2 in the Supplement. Graphical representations of mean metabolite ratios, mean CVs, and mean RDs are shown in the Supplement (Figures S2–S4).
Figure 2.
Representative spectra from (a) the anterior cingulate and (b) the posterior cingulate shown with the LCModel fit (in red) and residuals shown above (in black).
Table 1.
ACC Metabolite Ratios to tCr and Reproducibility Measures
| Metabolites | Visit | Mean Ratio to tCr (StdDev) | Mean CRLB (%) | Mean CV (%) | Mean RD (%) | N |
|---|---|---|---|---|---|---|
| Cr | 1 | 0.49 (0.2) | 7.3 | 14.4 | 20.3 | 10 |
| 2 | 0.45 (0.07) | 7.6 | ||||
| GABA | 1 | 0.38 (0.1) | 8.4 | 17.7 | 21.9 | 10 |
| 2 | 0.34 (0.1) | 9.4 | ||||
| Gln | 1 | 0.25 (0.1) | 14.6 | 21.6 | 34.8 | 6 |
| 2 | 0.24 (0.07) | 12.9 | ||||
| Glu | 1 | 1.4 (0.2) | 2.9 | 7.9 | 10.2 | 10 |
| 2 | 1.29 (0.07) | 3.0 | ||||
| GSH | 1 | 0.24 (0.06) | 7.2 | 15.9 | 21.8 | 10 |
| 2 | 0.26 (0.02) | 7.4 | ||||
| mI | 1 | 0.71 (0.2) | 5.0 | 12.2 | 16.1 | 10 |
| 2 | 0.69 (0.1) | 5.0 | ||||
| NAA | 1 | 1.53 (0.1) | 1.9 | 5.3 | 7.4 | 10 |
| 2 | 1.46 (0.2) | 2.1 | ||||
| PCh | 1 | 0.26 (0.04) | 7.4 | 16.8 | 23 | 9 |
| 2 | 0.24 (0.06) | 7.1 | ||||
| PCr | 1 | 0.56 (0.07) | 5.6 | 8.4 | 11.7 | 9 |
| 2 | 0.55 (0.07) | 6.3 | ||||
| PE | 1 | 0.46 (0.07) | 7.1 | 10.2 | 14 | 10 |
| 2 | 0.45 (0.07) | 7.3 | ||||
| GPC+PCh | 1 | 0.28 (0.03) | 3.2 | 6.2 | 8.5 | 10 |
| 2 | 0.27 (0.05) | 3.5 | ||||
| NAA+NAAG | 1 | 1.63 (0.2) | 2.3 | 5.4 | 7.5 | 10 |
| 2 | 1.57 (0.2) | 2.3 | ||||
| Ins+Gly | 1 | 0.88 (0.2) | 3.5 | 10.2 | 13.2 | 10 |
| 2 | 0.81 (0.1) | 3.8 | ||||
| Glx | 1 | 1.63 (0.3) | 3.2 | 10.2 | 13.3 | 10 |
| 2 | 1.49 (0.1) | 3.6 |
ACC - anterior cingulate cortex; CV - coefficient of variation; CRLB - Cramer-Rao lower bound; RD - relative difference; StDev - standard deviation
Table 2.
PCC Metabolite Ratios to tCr and Reproducibility Measures
| Metabolites | Visit | Mean Ratio to tCr (StdDev) | Mean CRLB (%) | Mean CV (%) | Mean RD (%) | N |
|---|---|---|---|---|---|---|
| Cr | 1 | 0.50 (0.09) | 6.8 | 11.2 | 14.5 | 10 |
| 2 | 0.45 (0.07) | 6.5 | ||||
| GABA | 1 | 0.34 (0.09) | 8.1 | 18.4 | 27.2 | 10 |
| 2 | 0.36 (0.06) | 6.7 | ||||
| Gln | 1 | 0.21 (0.06) | 16.7 | 17.8 | 22.7 | 7 |
| 2 | 0.18 (0.02) | 13.9 | ||||
| Glu | 1 | 1.25 (0.1) | 2.7 | 6.2 | 8.2 | 10 |
| 2 | 1.16 (0.08) | 2.5 | ||||
| GSH | 1 | 0.24 (0.05) | 6.6 | 14.3 | 19.3 | 10 |
| 2 | 0.23 (0.03) | 5.7 | ||||
| mI | 1 | 0.71 (0.06) | 4.0 | 4.3 | 6.1 | 10 |
| 2 | 0.72 (0.07) | 3.5 | ||||
| NAA | 1 | 1.62 (0.2) | 2.1 | 6.4 | 8.6 | 10 |
| 2 | 1.58 (0.2) | 1.9 | ||||
| NAAG | 1 | 0.19 (0.06) | 12.3 | 24.8 | 30.6 | 9 |
| 2 | 0.17 (0.06) | 12.2 | ||||
| PCh | 1 | 0.18 (0.04) | 6.3 | 11.8 | 18.2 | 8 |
| 2 | 0.19 (0.03) | 4.7 | ||||
| PCr | 1 | 0.50 (0.09) | 7.4 | 11.1 | 18.2 | 10 |
| 2 | 0.55 (0.07) | 5.2 | ||||
| PE | 1 | 0.46 (0.07) | 6.0 | 8.6 | 11.7 | 10 |
| 2 | 0.46 (0.06) | 5.1 | ||||
| GPC+PCh | 1 | 0.19 (0.04) | 3.9 | 9.6 | 14.7 | 10 |
| 2 | 0.19 (0.03) | 3.4 | ||||
| NAA+NAAG | 1 | 1.80 (0.2) | 2.0 | 7.2 | 9.5 | 10 |
| 2 | 1.74 (0.2) | 1.9 | ||||
| Ins+Gly | 1 | 0.83 (0.09) | 2.9 | 7.3 | 9.9 | 10 |
| 2 | 0.83 (0.1) | 2.8 | ||||
| Glx | 1 | 1.44 (0.2) | 3.2 | 6.7 | 8.8 | 10 |
| 2 | 1.33 (0.09) | 3.1 |
CV - coefficient of variation; CRLB - Cramer-Rao lower bound; PCC - posterior cingulate cortex; RD - relative difference, StDev - standard deviation
In the ACC, mean linewidth and SNR were 0.032 ± 0.007 ppm and 26.4 ± 7.8 for visit 1 and 0.029 ± 0.007 ppm and 25.1 ± 6.9 for visit 2. Linewidth and SNR were not significantly different between visits (p > 0.05). Mean NAA linewidth and SNR for the ACC were 0.048 ppm and 40.0, respectively. Mean percentage of GM, WM, and CSF were 62.3 ± 6.4%, 9.5 ± 2.9%, and 28.2 ± 8.4% for visit 1 and 63.2 ± 6.8%, 9.9 ± 4.2%, and 26.9 ± 9.1% for visit 2. The proportions of GM, WM, and CSF within the voxel were not significantly different between visits (p’s>0.7).
In the ACC, the following metabolites were quantified with CRLBs ≤ 20% in all ten subjects at both visits: Cr, GABA, Glu, GSH, mI, NAA, PE, tCho, tNAA, mI+Gly, and Glx. PCh and PCr had CRLBs ≤ 20% in nine out of ten subjects whereas Gln had CRLBs ≤ 20% in six out of ten subjects. Ala, Asp, Glc, Gly, GPC, Lac, NAAG, Ser, sI, and Tau had CRLBs > 20% in at least seven subjects, so these data were not included in Table 1. All metabolites with CRLBs ≤ 20% in nine out of ten subjects had mean CVs and RDs of 17.7% and 21.9% or less, respectively. As expected, metabolites with high SNR showed excellent reproducibility with low mean CVs and RDs. NAA had the lowest CV at 5.4% and RD at 7.5% followed by tNAA, tCho, Glu, PCr, mI+Gly, Glx, PE, and mI. Metabolites with low SNR, such as GABA and GSH, had higher CVs that were still below 20% or slightly above at 21.6% for Gln. GABA and GSH had lower RDs of 22% or less compared to Gln with a mean RD of 34.8%.
In the PCC, mean linewidth and SNR were 0.033 ± 0.006 ppm and 31.6 ± 6.8 for visit 1 and 0.033 ± 0.004 ppm and 36.5 ± 5.1 for visit 2, respectively. Linewidths and SNR were not significantly different between visits (p>0.05). Mean NAA linewidth and SNR for the PCC were 0.060 ppm and 63.7, respectively. Mean percentage of GM, WM, and CSF were 65.8 ± 4.0%, 18.4 ± 5.1%, and 15.8 ± 5.5% for visit 1 and 64.8 ± 4.8%, 21.6 ± 4.6%, and 13.6 ± 4.1% for visit 2. The proportions of GM, WM, and CSF within the voxel were not significantly different between visits (p’s>0.2).
In the PCC, the following metabolites were quantified with CRLBs ≤ 20% in all ten subjects: Cr, GABA, Glu, GSH, mI, NAA, PCr, PE, tCho, tNAA, mI+Gly, and Glx. NAAG and PCh had CRLBs ≤ 20% in nine and eight out of ten subjects, respectively; whereas, Gln had CRLBs ≤ 20% in seven subjects. Similar to the ACC, in PCC, Ala, Asp, Glc, GPC, Gly, Lac, Ser, sI, and Tau had CRLBs > 20% in at least seven subjects, so these data were not included in Table 2. All metabolites found in nine out of ten subjects listed in Table 2 had mean CV and mean RDs of 24.8% and 30.6% or less, respectively. mI had the lowest mean CV and mean RD at 4.3% and 6.1%, respectively, followed by Glu, NAA, Glx, tNAA, mI+Gly, PE, and tCho such that high SNR metabolites had CVs of 9.6% or less and RDs of 14.7% or less. In terms of low SNR metabolites, CVs were slightly higher with mean CVs all less than 24.8% or less and mean RDs at 30.6% or less.
In terms of regional metabolite ratio differences, there were several significant differences. tCho/tCr and PCh/tCr were higher in the ACC compared to the PCC (p<0.001 for both). Glx/tCr was higher in the ACC compared to the PCC (p=0.005), and tNAA/tCr was lower in the ACC compared to the PCC (p=0.017). There were no significant regional differences for the other metabolite ratios.
Discussion
These results demonstrate both the feasibility and reproducibility of acquiring MRS data at 7T using the STEAM localization technique in both the ACC and PCC in older healthy adults. Metabolite ratios reported here are similar in magnitude to those from previous 7T studies in healthy young adults7,13,14. As expected, metabolites with high SNR had excellent reproducibility with mean CVs and RDs of 10% or less in both regions. Metabolites with low SNR due to lower concentrations and metabolites with signals that overlap with those of other metabolites had slightly higher mean CVs and RDs. These reproducibility data provide the foundation for utilizing MRS at 7T to study the ACC and PCC, two regions that play a major role in the affective and cognitive symptoms of AD26,28,29, in intervention or longitudinal studies. To date, there have been four other reproducibility studies at 7T in younger healthy adults6,13–15. The mean CVs and RDs reported here were on average slightly higher than those reported in previous studies, but many metabolites still had metrics under 10%. The slightly higher CVs and RDs may be due to acquisition or quantification factors, which affect spectral SNR and reliability metrics. Acquisition differences between studies may be due to different types of RF coils used (8-channel transmit-receive array15, 16-channel SENSE head and transmission through head volume coil13, 16-channel transceiver array coil6, and 32-channel receive head coil and quadrature transmit coil14), localization sequence used (semi-LASER6 and STEAM13–15), voxel size and location, scan length, and age. More specifically, two studies examined the ACC with voxel sizes of 9 cm3 13 and 27 cm3 14, respectively, while the other two studies examined the PCC with a voxel size of 8 cm3 6 and the occipital cortex with a voxel size of 8 cm3 15. In terms of scan length, the shortest acquisition time was 1 min 36 sec14, followed by two other studies with similar scan lengths of 4 min 48 sec15 and 5 min and 20 sec6, and finally a longer scan of 9 min 36 sec13. It appears that the reproducibility for scan lengths around 5 minutes had lower mean CVs compared to shorter and longer scan lengths. Finally, the previous four studies examined younger adults whereas this study examined older adults. Older adults have a tendency to move more during scans18,19, and this motion could potentially translate to increased spectral line widths and lower SNR. Here, we observed comparable linewidths to the younger cohort in14, but observed a reduction in SNR which can negatively impact fitting.
In terms of quantification factors, differing numbers of metabolites within the basis set and handling of macromolecular background may contribute to the differences observed between this study and previous reproducibility studies. Previous reproducibility studies at 7T have utilized basis sets of varying sizes (ten to twenty metabolites). A basis set should contain all metabolites that are present in vivo; otherwise, systematic bias and errors in fitting will be introduced into the data set40. However, including extra metabolites not expected to be present in the brain at detectable concentrations may negatively impact fitting accuracy. With respect to handling macromolecules, previous 7T reproducibility studies have differed on approaches to account for macromolecules by using the standard LCModel macromolecule basis set, suppressing macromolecules, or collecting a macromolecule spectrum. A recent study at 3T showed that using the simulated macromolecule spectrum provided by LCModel was sufficient41, and two other studies at 7T showed that the macromolecule spectrum is similar between white matter and gray matter42,43. To date, there has yet to be a study at 7T that examined quantification using measured or simulated macromolecule spectra. Overall, when fitting both metabolites and macromolecules, parsimonious fitting is most appropriate.
In this study, the majority of metabolites met the stringent criteria of CRLB ≤ 20%. In the ACC, there was one dataset each for PCr and PCh that had CRLB > 80%, and a similar finding was observed in PCC for PCh and NAAG. In contrast, Gln datasets were excluded in the ACC with CRLBs of 22, 23, 25, and 41%, respectively. Similarly, for the PCC, Gln was excluded in three instances where CRLBs were 22, 24, and 27%, respectively. At lower field strengths where Gln is more difficult to resolve from Glu, the CRLB cut-off is often adjusted to 25% or 30%44–46. At higher field strengths, a consensus on an appropriate cutoff for Gln has yet to be reached since there are a wide range of acceptable CRLBs from 20% for two studies6,14, 50% for one study15, and 100% for another study13. A recent study suggests that using a threshold of 20% or 50% CRLB for inclusion in further analyses creates a bias in the data, especially for metabolites with low concentrations that typically have higher CRLB39. When the CRLB cutoff was increased from 20% to 50% for this dataset, there were differences in Gln metrics and more metabolites became detectable. The Gln/tCr levels in both regions decreased on average and the variance increased. In addition, mean RD and CV values increased, suggesting less reproducibility. If we restricted the Gln CRLB to 30%, there were negligible differences between means and variances for a 30% and 50% cutoff. Increasing the threshold to 50% also led to detection of Asp, Gly, Lac, NAAG, and Tau in N ≥ 6 participants with worse reproducibility than major metabolites such as tNAA, Glx, tCho, etc. As a compromise between keeping these stringent criteria and providing valuable information on a metabolite that plays a vital role in the glutamate-glutamine cycle, Gln with CRLB of 20% was included in the tables as a reference for future studies and summary metrics with a CRLB of 50% are provided in the Supplement.
Despite acquisition and regional differences, the metabolite ratios presented in Tables 1 and 2 are similar to previous reports. For the ACC, metabolite ratios were similar to those reported by two studies13,14. Differences between the number of metabolites detected between this study and another study14 that used the same scanner and a similar voxel placement may be attributed to the lower SNR and broader linewidths reported here compared to those reported in a healthy young sample. Comparing the major metabolite ratios from these two age groups revealed comparable results reported in MRS aging studies in animals47, humans48, and a meta-analysis in humans49. However, compared to a healthy young sample14 that reported mean ratios of 1.8 for tNAA/Cr, 1.7 for Glu/Cr, and 0.25 for tCho/Cr, tNAA/Cr (mean ratio of 1.6) and Glu/Cr (mean ratio of 1.3) were lower and tCho/Cr (mean ratio of 0.28) was higher in the ACC in this older sample. For the PCC, metabolite ratios were similar to those reported by another study6 as well as those in a MRSI study of healthy elderly individuals50 in the posterior cingulate/precuneus region.
There are some differences when comparing metabolite ratios and reproducibility metrics between regions. There appears to be a regional gradient between the ACC and PCC with higher tNAA/tCr as well as lower tCho/tCr and Glx/tCr in the PCC compared to the ACC. The observed NAA/tCr and tCho/tCr gradient across the brain is similar to results from a study51 in a young adult cohort and another study with a broader age range52. While there have been many studies examining the effects of aging in specific brain regions48,50,52,53, to our knowledge, there have been no studies examining whether these regional metabolite gradients differ between younger and older populations. When comparing CRLBs between regions, it appears that, on average, CRLBs are lower from the PCC than the ACC. When comparing reproducibility regionally, the majority of metabolites had lower mean CVs and RDs in the PCC versus ACC. Of the major metabolites, tCho and tNAA had better reproducibility in the ACC compared to the PCC while Glx and mI+Gly had comparable reproducibility in the PCC and ACC. GABA reproducibility was better in the ACC, whereas Gln, Glu, Glx, mI+Gly, NAAG, and GSH had improved reproducibility in the PCC. Better reproducibility as reflected by lower mean CVs and RDs in the PCC compared to the ACC is expected. Due to its close proximity to the sinuses, the ACC is typically more difficult to shim and is more likely to contain spurious peaks from alternate coherence pathways than the PCC. This typically translates to broader linewidths and lower SNR, reflected by higher reproducibility metrics as seen in the ACC compared to the PCC.
Metabolite level normalization in MRS typically employs referencing to an internal standard such as creatine or water reference. Each method has its own advantages and disadvantages54,55,56. In this study, a water reference dataset was not acquired; thus, normalization to creatine was the only feasible option. Normalization to tCr does not require acquisition of a water reference and thus less scan time is needed. In addition, there is no need to correct for partial volume effects of CSF or differing water content or relaxation times of gray and white matter. There are also disadvantages to referencing to tCr. There is a loss of metabolite specificity since it is unclear whether differences observed by ratios are driven by a particular metabolite in the numerator or tCr in the denominator. This becomes problematic in disease populations where tCr has been observed to differ between groups57–59. Since this study focused only on a healthy population, normalization to total creatine is considered a valid referencing method. Nevertheless, we acknowledge that the lack of water reference scans is a limitation of the study. Despite referencing metabolites to tCr, this study still provides valuable information regarding STEAM reproducibility at 7T in healthy older adults.
There are several limitations to this study. Participant motion correction was not performed. While participant motion is an inherent concern with all imaging studies, precautions were taken to minimize its contribution to the data. To minimize motion, participants were made as comfortable as possible and padding was used within the head coil to restrict motion. Linewidths reported by LCModel were excellent in both regions and were not different between visits, suggesting that motion was minimal. Another factor that could contribute to reproducibility is that different technologists were involved in and ran the MR scans. To minimize operator effects, each technologist followed strict voxel placement guidelines and for the second visit, was given printouts of the voxel placement from visit one to ensure consistent placement. Further, metabolite levels were referenced to an internal reference, tCr and not to water. In summary, this test-retest study reveals that 7T MRS is reproducible in older healthy participants. Results indicate that the STEAM localization technique, applied to the ACC and PCC regions that are notably affected with aging, is suitable for longitudinal studies in older cohorts. In particular, this data suggests that increases in Cho and mI levels as well as decreases in NAA and Glu levels that are often reported in aging studies can be detected using STEAM based on the excellent CVs of 10.2% or less and RDs of 13.3% or less.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health: R01MH086881 (GSS), AG041633 (GSS), AG038893 (GSS), R01MH094520 (LMR), R21MH113182 (LMR), P41EB015909 (PBB), and R01MH096263 (PBB), and the Johns Hopkins University School of Medicine Brain Science Institute.
Abbreviations
- Ala
alanine
- ACC
anterior cingulate cortex
- Asp
aspartate
- CDR
clinical dementia rating
- Cr
creatine
- CRLB
Cramér–Rao Lower Bounds
- CV
coefficient of variation
- GABA
γ-aminobutyric acid
- Glc
glucose
- Gln
glutamine
- Glu
glutamate
- Glx
Glu + Gln
- Gly
glycine
- GPC
glycerophosphocholine
- GSH
glutathione
- Lac
lactate
- mI
myo-Inositol
- MMSE
Mini Mental State Exam
- NAA
N-acetylaspartate
- NAAG
N-acetylaspartylglutamate
- PCC
posterior cingulate cortex
- PCh
phosphocholine
- PCr
phosphocreatine
- PE
phosphoethanolamine
- ppm
parts per million
- RD
relative difference
- SCID
structured clinical interview
- Ser
serine
- sI
scyllo-Inositol
- SNR
signal-to-noise ratio
- STEAM
stimulated echo acquisition mode
- Tau
taurine
- tCho
total Choline (GPC+PCh)
- tCr
total Creatine (Cr+PCr)
- tNAA
total NAA (NAA+NAAG)
References
- 1.2015 Alzheimer’s disease facts and figures. Alzheimers Dement 2015;11:332–384. [DOI] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med 2013;368:1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med 2001;46:451–456. [DOI] [PubMed] [Google Scholar]
- 5.Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med 2009;62:868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terpstra M, Cheong I, Lyu T, Deelchand DK, Emir UE, Bednarik P, Eberly LE, Oz G. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magn Reson Med 2016;76:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pradhan S, Bonekamp S, Gillen JS, Rowland LM, Wijtenburg SA, Edden RA, Barker PB. Comparison of single voxel brain MRS AT 3T and 7T using 32-channel head coils. Magn Reson Imaging 2015;33:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Kolk AG, Hendrikse J, Zwanenburg JJ, Visser F, Luijten PR. Clinical applications of 7 T MRI in the brain. Eur J Radiol 2013;82(5):708–718. [DOI] [PubMed] [Google Scholar]
- 9.Gizewski ER, Monninghoff C, Forsting M. Perspectives of ultra-high-field MRI in neuroradiology. Clin Neuroradiol 2015;25:267–273. [DOI] [PubMed] [Google Scholar]
- 10.Kraff O, Fischer A, Nagel AM, Monninghoff C, Ladd ME. MRI at 7 Tesla and above: demonstrated and potential capabilities. J Magn Reson Imaging 2015;41:13–33. [DOI] [PubMed] [Google Scholar]
- 11.Frahm J, Merboldt KD, Hanicke W. Localized proton spectroscopy using stimulated echoes. J Magn Reson 1987;72:502–508. [DOI] [PubMed] [Google Scholar]
- 12.Scheenen TW, Heerschap A, Klomp DW. Towards 1H-MRSI of the human brain at 7T with slice-selective adiabatic refocusing pulses. Magma 2008;21:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephenson MC, Gunner F, Napolitano A, Greenhaff PL, Macdonald IA, Saeed N, Vennart W, Francis ST, Morris PG. Applications of multi-nuclear magnetic resonance spectroscopy at 7T. World J Radiol 2011;3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijtenburg SA, Rowland LM, Edden RA, Barker PB. Reproducibility of brain spectroscopy at 7T using conventional localization and spectral editing techniques. J Magn Reson Imaging 2013;38:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prinsen H, de Graaf RA, Mason GF, Pelletier D, Juchem C. Reproducibility measurement of glutathione, GABA, and glutamate: Towards in vivo neurochemical profiling of multiple sclerosis with MR spectroscopy at 7T. J Magn Reson Imaging 2017;45:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ooi MB, Krueger S, Thomas WJ, Swaminathan SV, Brown TR. Prospective real-time correction for arbitrary head motion using active markers. Magn Red Med 2009; 62:943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guglielmi G, Peh WCG, Guermazi A, editors. Geriatric Imaging. New York: Springer; 2013. [Google Scholar]
- 18.Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Pacheco J, Albert M, Killiany R, Blacker D, Maguire P, Rosas D, Makris N, Gollub R, Dale A, Dickerson BC, Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 2009;46:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wijtenburg SA, Fuchs KL, Simnad VI, Knight-Scott J. Motion detection in healthy young, middle-aged, and elderly adults using a water based navigator echo: A 1H MRS study. Proc Intl Soc Magn Reson Med 2010; 2007. [Google Scholar]
- 20.Karlamangla A, Tinetti M, Guralnik J, Studenski S, Wetle T, Reuben D. Comorbidity in older adults: nosology of impairment, diseases, and conditions. J Gerontol A Biol Sci Med Sci 2007; 62:296–300. [DOI] [PubMed] [Google Scholar]
- 21.Davis JW, Chung R, Juarez DT. Prevalence of comorbid conditions with aging among patients with diabetes and cardiovascular disease. Hawaii Med J 2011; 70:209–13. [PMC free article] [PubMed] [Google Scholar]
- 22.First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis 1 disorders-patient edition (SCID-I/P). New York: New York Psychiatric Institute; 1995. [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 24.Folstein M, Folstein S, McHugh P. Mini-mental state. J Psychiatr Res 1976;12:189–198. [DOI] [PubMed] [Google Scholar]
- 25.Automatic Gruetter R., localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med 1993;29:804–811. [DOI] [PubMed] [Google Scholar]
- 26.Donovan NJ, Hsu DC, Dagley AS, Schultz AP, Amariglio RE, Mormino EC, Okereke OI, Rentz DM, Johnson KA, Sperling RA, Marshall GA. Depressive symptoms and biomarkers of Alzheimer’s disease in cognitively normal older adults. J Alzheimers Dis 2015;46:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardo JV, Lee JT, Sheikh SA, Surerus-Johnson C, Shah H, Munch KR, Carlis JV, Lewis SM, Kuskowski MA, Dysken MW. Where the brain grows old: decline in anterior cingulate and medial prefrontal function with normal aging. Neuroimage 2007;35:1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheff SW, Price DA, Ansari MA, Roberts KN, Schmitt FA, Ikonomovic MD, Mufson EJ. Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer’s disease. J Alzheimers Dis 2015;43:1073–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanctot KL, Amatniek J, Ancoli-Israel S, Arnold SE, Ballard C, Cohen-Mansfield J, Ismail Z, Lyketsos CG, Miller DS. Neuropsychiatric signs and symptoms of Alzheimer’s disease: new treatment paradigms. Alzheimers Dement (NY) 2017;3:440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuda H Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Ann Nucl Med 2001;15:85–92. [DOI] [PubMed] [Google Scholar]
- 31.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 1999;41:649–656. [DOI] [PubMed] [Google Scholar]
- 32.Soher BJ, Semanchuk P, Todd D, Steinberg J, Young K. VeSPA: Integrated application for RF pulse design, spectral simulation and MRS data analysis. Proc Intl Soc Magn Reson Med 2011;19:1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–679. [DOI] [PubMed] [Google Scholar]
- 34.Provencher SW. LCModel Manual. 2018. http://s-provencher.com/pub/LCModel/manual/manual.pdf
- 35.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, Beer RD, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. Magma. 2001; 12: 141–152. [DOI] [PubMed] [Google Scholar]
- 36.Stefan D, Cesare FD, Andrasescu A, Popa E, Lazariev A, Vescovo E, Strbak O, Williams S, Starcuk Z, Cabanas M, van Ormondt D, Graveron-Demilly D. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas Sci Technol. 2009; 20: 104035. [Google Scholar]
- 37.Friston KJ. Statistical Parametric Mapping the Analysis of Funtional Brain Images. Boston: Elsevier/Academic Press; 2007. [Google Scholar]
- 38.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreis R The trouble with quality filtering based on relative Cramer-Rao lower bounds. Magn Reson Med 2016;75:15–18. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann L, Slotboom J, Jung B, Maloca P, Boesch C, Kreis R. Quantitative 1H-magnetic resonance spectroscopy of human brain: Influence of composition and parameterization of the basis set in linear combination model-fitting. Magn Reson Med 2002;48:440–453. [DOI] [PubMed] [Google Scholar]
- 41.Schaller B, Xin L, Cudalbu C, Gruetter R. Quantification of the neurochemical profile using simulated macromolecule resonances at 3 T. NMR Biomed 2013;26:593–599. [DOI] [PubMed] [Google Scholar]
- 42.Schaller B, Xin L, Gruetter R. Is the macromolecule signal tissue-specific in healthy human brain? A (1)H MRS study at 7 Tesla in the occipital lobe. Magn Reson Med 2014;72:934–940. [DOI] [PubMed] [Google Scholar]
- 43.Snoussi K, Gillen JS, Horska A, Puts NA, Pradhan S, Edden RA, Barker PB. Comparison of brain gray and white matter macromolecule resonances at 3 and 7 Tesla. Magn Reson Med 2015;74:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shirayama Y, Obata T, Matsuzawa D, Nonaka H, Kanazawa Y, Yoshitome E, Ikehira H, Hashimoto K, Iyo M. Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: a preliminary study. Neuroimage 2010;49:2783–2790. [DOI] [PubMed] [Google Scholar]
- 45.Bustillo JR, Chen H, Jones T, Lemke N, Abbott C, Qualls C, Canive J, Gasparovic C. Increased glutamine in patients undergoing long-term treatment for schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. JAMA Psychiatry 2014;71:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rowland LM, Summerfelt A, Wijtenburg SA, Du X, Chiappelli JJ, Krishna N, West J, Muellerklein F, Kochunov P, Hong LE. Frontal glutamate and gamma-aminobutyric acid levels and their associations with mismatch negativity and digit sequencing task performance in schizophrenia. JAMA Psychiatry 2016;73:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris JL, Yeh HW, Swerdlow RH, Choi IY, Lee P, Brooks WM. High-field proton magnetic resonance spectroscopy reveals metabolic effects of normal brain aging. Neurobiol Aging 2014;35:1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Scyllo-inositol in normal aging human brain: 1H magnetic resonance spectroscopy study at 4 Tesla. NMR Biomed 2005;18:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging 2009;30:353–363. [DOI] [PubMed] [Google Scholar]
- 50.Schreiner SJ, Kirchner T, Wyss M, Van Bergen JMG, Quevenco FC, Steininger SC, Griffith EY, Meier I, Michels L, Gietl AF, Leh SE, Brickman AM, Hock C, Nitsch RM, Pruessmann KP, Henning A, Unschuld PG. Low episodic memory performance in cognitively normal elderly subjects is associated with increased posterior cingulate gray matter N-acetylaspartate: a 1H MRSI study at 7 Tesla. Neurobiol Aging 2016;48:195–203. [DOI] [PubMed] [Google Scholar]
- 51.Bracken BK, Jensen JE, Prescot AP, Cohen BM, Renshaw PF, Ongur D. Brain metabolite concentrations across cortical regions in healthy adults. Brain Res 2011;1369:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiu PW, Mak HK, Yau KK, Chan Q, Chang RC, Chu LW. Metabolic changes in the anterior and posterior cingulate cortices of the normal aging brain: proton magnetic resonance spectroscopy study at 3 T. Age 2014;36:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eylers VV, Maudsley AA, Bronzlik P, Dellani PR, Lanfermann H, Ding XQ. Detection of Normal Aging Effects on Human Brain Metabolite Concentrations and Microstructure with Whole-Brain MR Spectroscopic Imaging and Quantitative MR Imaging. Am J Neuroradiol 2016;37:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jansen JFA, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology 2006; 240:318–332. [DOI] [PubMed] [Google Scholar]
- 55.Buonocore MH and Maddock RJ. Magnetic resonance spectrocopy of the brain: a reivew of physical priniciples and technical methods. Rev Neurosci 2015; 26:609–32. [DOI] [PubMed] [Google Scholar]
- 56.de Graaf RA. In vivo NMR spectroscopy: priniciples and techniques. New York: John Wiley & Sons. New York; 2002. [Google Scholar]
- 57.Ongur D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res 2009; 172:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibasaki J, Aida N, Morisaki N, Tomiyasu M, Nishi Y, Toyoshima K. Changes in brain metabolite concentrations after neonatal hypoxic-ischemic encephalopathy. Radiology 2018; 288:840–8. [DOI] [PubMed] [Google Scholar]
- 59.Adanyeguh IM, Monin ML, Rinaldi D, Freeman L, Durr A, Lehericy S, PG Henry, Mochel F. Expanded neurochemical profile in the early stage of Huntington disease using proton magnetic resonance spectroscopy. NMR Biomed 2018; 31:e3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.