Abstract
Background:
Low diastolic blood pressure (DBP) is associated with increased risk of cardiovascular events. In patients with coronary artery disease (CAD), limitations in coronary blood flow and immune activity are implicated mechanisms, but evidence is lacking. We investigated the association between DBP, biomarkers of myocardial injury, inflammation, immune activation and incident events in patients with CAD.
Methods:
We studied 2448 adults (mean age 65 ± 12 years, 68% male, median follow-up 4.5 years) with CAD. DBP was categorized into 10 mm Hg increments. Biomarkers of myocardial injury (high sensitivity cardiac troponin-I [hs-cTnI]) and immune activity/inflammation (soluble urokinase plasminogen activator receptor [suPAR]) were dichotomized at their median values. DBP 70–79 mm Hg was used as the referent group, and individuals were followed prospectively for adverse outcomes.
Results:
After adjusting for demographic and clinical covariates, individuals with DBP <60 mm Hg had increased odds of elevated levels of hs-cTnI (OR = 1.68; 95% CI = 1.07, 2.65) and suPAR (OR = 1.71; 95% CI = 1.10, 2.65) compared to the referent group. Additionally, DBP <60 mm Hg was associated with increased adjusted risk of cardiovascular death or MI (HR = 2.04; 95% CI = 1.32, 3.16) and all-cause mortality (HR = 2.41; 95% CI = 1.69, 3.45).
Conclusion:
In patients with CAD, DBP <60 mm Hg is associated with subclinical myocardial injury, immune/inflammatory dysregulation and incident events. Aggressive BP control may be harmful in these patients, and further investigation is warranted to determine appropriate BP targets in patients with CAD.
Keywords: Blood pressure, high-sensitivity troponin, soluble urokinase plasminogen activator receptor, coronary artery disease, J-curve
1. Introduction
Hypertension is one of the most prevalent and modifiable risk factors for cardiovascular disease, stroke and mortality [1]. However, optimal blood pressure (BP) targets in patients with hypertension continue to be debated [2, 3]. Lower systolic blood pressure (SBP) is associated with improved outcomes, but aggressive lowering of SBP will inherently lead to a lower diastolic blood pressure (DBP) [2]. Observational data from cohort studies and post-hoc analyses from clinical trials have identified a “J-curve” relation with clinical vascular events, whereby lower DBP (particularly ≤60 mmHg) is associated with an increased risk of adverse outcomes [4, 5]. The mechanisms underlying this increased risk at lower DBP remain unclear. One hypothesis is that in patients with coronary artery disease (CAD), low DBP is associated with reduced coronary perfusion pressure and subsequent myocardial injury and damage [6].
High-sensitivity cardiac troponins (hs-cTn) are markers of myocardial injury and elevated levels are associated with adverse cardiovascular outcomes in patients with and without cardiovascular disease [7, 8]. Soluble urokinase plasminogen activator receptor (suPAR), a marker of immune activation, inflammation, thrombogenesis and endothelial dysfunction [9], is associated with adverse cardiovascular and renal outcomes in patients with CAD and provides independent and additive risk prediction beyond high sensitivity C-reactive protein (hsCRP), a commonly-used biomarker of inflammation and risk in patients with CAD [10–15].
Circulating biomarkers representing pathways of myocardial injury, inflammation/immune activation and endothelial dysfunction may provide mechanistic insights into the J-curve of DBP and adverse outcomes. Therefore, we examined the association between DBP, hs-cTnI, suPAR and adverse outcomes in patients with CAD. Our hypothesis was that these pathologic pathways are activated in CAD patients with low DBP, and this would at least partially explain the increased risk observed in patients with CAD and low DBP.
2. Methods
2.1. Study Design and Population
We studied 2448 adults, aged 18 years and older enrolled in the Emory Cardiovascular Biobank, a prospective cohort of patients undergoing left heart catheterization for suspected or confirmed CAD at three Emory Healthcare sites in Atlanta, GA, between 2004 and 2014. Participants were interviewed to collect demographic characteristics, medical history, medication use and behavioral habits, as previously described [16]. All patients with CAD, defined as at least one epicardial vessel with ≥30% obstruction, were included. Those presenting with acute coronary syndrome or with a history of heart failure with reduced ejection fraction were excluded. Patients with valvular heart disease, those who had undergone or were under evaluation for cardiac transplant, and those with active cancer were also excluded. All participants provided written informed consent at the time of enrollment, and the study was approved by the institutional review board at Emory University (Atlanta, GA).
2.2. Baseline Characteristics
Individuals enrolled in the Emory Cardiovascular Biobank underwent a detailed baseline evaluation using standardized self-report questionnaires and medical records review. Age (years), sex (male/female), race (white/black), and smoking (current/former/never) were obtained by self-report. Additionally, medical history and/or medication use were obtained by self-report and confirmed by evaluation of medical records for the following conditions: hypertension, diabetes, hyperlipidemia, heart failure with preserved ejection fraction (HFpEF), and prior myocardial infarction (MI). Anthropometric data were measured by trained staff and included SBP (in mm Hg), DBP (in mm Hg), pulse pressure (SBP – DBP, in mm Hg) and body mass index (in kg/m2). Blood pressure was obtained using calibrated, non-invasive automated blood pressure machines with appropriate cuff size after at least 5 minutes of rest during pre-procedural evaluation for left heart catheterization, and all participants were instructed to take home medications as usual and to abstain from caffeine, nicotine, alcohol and food for at least 8 hours prior to evaluation.
Routine laboratory data included fasting values of low-density lipoprotein cholesterol (LDL-C, in mg/dL) and high-density lipoprotein cholesterol (HDL-C, in mg/dL), as well as baseline white blood cell count (WBC, in 103 cells/L). Serum creatinine (mg/dL) was used to calculate the estimated glomerular filtration rate(eGFR, in ml/min/1.73 m2) [17]. Measurement of hs-cTnI, suPAR and hsCRP were performed on fasting arterial blood samples collected at the time of cardiac catheterization and stored at −80°C. Additional details on the assays used for biomarker measurement are available in the Online Supplement. All participants underwent a detailed medication questionnaire to document use of the following: angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ACEi/ARB), beta blockers, aspirin, and statins. Burden of CAD was assessed using modified AHA/ACC classifications for luminal narrowing seen on coronary angiogram [18]. Obstructive CAD was defined as ≥ 50% stenosis at least 1 major epicardial vessel.
2.3. Outcomes and Follow-up
Follow-up was conducted by phone, electronic medical record review, and review of the social security death index and state records to identify cardiovascular (CV) death or non-fatal myocardial infarction (MI), and all-cause mortality. CV death was further defined as death from MI, heart failure, sudden death, stroke, or pulmonary embolization. MI was defined according to relevant medical history, diagnostic cardiac enzymes, and/or electrocardiogram tracing consistent with myocardial infarction. Event adjudication was conducted by two independent, board-certified cardiologists blinded to baseline characteristics.
2.4. Statistical Analysis
Analysis of variance (continuous, normally-distributed variables), Mann-Whitney U test (continuous, non-normally distributed variables) and the chi-square test (categorical variables) were used to compare baseline clinical characteristics between 5 categories of DBP: <60, 60 to 69, 70 to 79 (referent), 80 to 89, and ≥90 mm Hg.
To test the association between DBP categories and biomarkers of subclinical myocardial injury (hs-cTnI), immune/inflammatory activity (suPAR) and inflammation (hsCRP), fullyadjusted multivariable linear and logistic regression models were constructed using the following covariates: age, sex, BMI, SBP, smoking, hyperlipidemia, HFpEF, diabetes, hypertension, ACEi/ARB use, beta blocker use, statin use, aspirin use, and eGFR. Log-transformed hs-cTnI, suPAR and hsCRP were used as outcome measures in linear regression models. Median cutoffs (hs-cTnI >5 pg/mL, suPAR >3 ng/mL) or clinically-appropriate cutoffs[19] (hsCRP > 3 mg/dL) were used to define biomarker elevations (high vs low) in logistic regression models. Finally, we modeled the association between elevated biomarkers and DBP as a continuous variable using restricted cubic splines with 4 knots (referent, 75 mm Hg), adjusting for the previously-defined covariates.
The cumulative incidence of CV death or MI and all-cause mortality was plotted as (1-survival) for each DBP category. Cox proportional hazards models were used to model the prospective association between all-cause death and DBP categories (referent, DBP = 70–79 mm Hg), and all models were adjusted for the previously-defined covariates. There were no violations to the proportional hazards assumption. Follow-up time was defined as the time from enrollment until one of the following: CV death or MI, death, loss to follow-up, or end of followup. We performed competing risk analyses for CV death or MI using Fine and Gray’s method [20], treating non-CV death as a competing risk. DBP was again modeled as a continuous variable using restricted cubic splines to construct distributions of adjusted hazard ratios for allcause mortality, and subdistribution hazard ratios for CV death or MI.
To determine if the effect of low DBP on all-cause death and CV death or MI was attenuated by elevations in biomarkers, we analyzed stepwise Cox regression models: Model 1: crude; Model 2: adjusted for age, sex, BMI, SBP, smoking, hyperlipidemia, HFpEF, diabetes, hypertension, ACEi/ARB use, beta blocker use, statin use, aspirin use, and eGFR; Model 3: adjusted for Model 2 plus hs-cTnI or suPAR; and Model 4: adjusted for Model 2 plus hs-cTnI and suPAR.
Finally, we performed several sensitivity analyses to determine if the relationship between DBP <60 mmHg and outcomes differed by: elevated hs-cTnI (>5 pg/mL vs ≤5 pg/mL), elevated suPAR (>3 ng/mL vs ≤3 ng/mL), diabetes (yes/no), HFpEF (yes/no), SBP (<120 vs 120 to 139 vs ≥140 mm Hg), obesity (BMI <30 vs ≥30 kg/m2), chronic kidney disease (eGFR <60 vs ≥60 ml/min/1.73 m2), CAD burden (non-obstructive vs obstructive), race (black/white), sex (male/female), or age (<60 years vs. ≥60 years) using multiplicative interaction terms. Statistical significance, including interaction terms, was defined as p <0.05 (2-sided). SAS Version 9.4 (Cary, NC) was used for all analyses.
3. Results
Individuals with lower DBP were more likely to be older, female and white, with lower LDL-C levels, compared to those with higher DBP (Table 1). Additionally, the prevalence of hypertension and higher SBP levels were greater in those with higher DBP; however, there were no differences in antihypertensive medication use or pulse pressure across DBP groups (Table 1).
Table 1.
Baseline characteristics of the cohort
| Characteristic | Diastolic blood pressure groups |
P-value | |||||
|---|---|---|---|---|---|---|---|
| Overall (n=2,448) |
<60 mm Hg (n=168) |
60–69 mm Hg (n=577) |
70–79 mm Hg (n=794) |
80–89 mm Hg (n=616) |
>90 mm Hg (n=293) |
||
| Age, years | 64.5 (11.6) | 68.2 (11.9) | 66.8 (11.4) | 64.9 (11.1) | 62.7 (11.3) | 60.3 (11.9) | <0.001 |
| Female | 785 (32) | 81 (48) | 237 (41) | 257 (32) | 152 (25) | 58 (20) | <0.001 |
| Black | 478 (20) | 24 (14) | 98 (17) | 145 (18) | 134 (22) | 77 (26) | 0.002 |
| Hypertension | 2004 (82) | 129 (78) | 474 (82) | 633 (80) | 516 (84) | 252 (87) | 0.04 |
| SBP, mm Hg | 139.3 (21.7) | 117.9 (17.2) | 129.3 (17.4) | 138.5 (19.2) | 146.2 (19.9) | 159.0 (20.2) | <0.001 |
| Pulse pressure, mm Hg | 63.6 (18.6) | 63.3 (17.9) | 64.4 (17.0) | 64.2 (18.8) | 62.4 (19.6) | 62.9 (19.1) | 0.29 |
| Antihypertensive use | |||||||
| ACEi/ARB | 1403 (57) | 86 (51) | 339 (59) | 460 (58) | 348 (57) | 170 (58) | 0.49 |
| Beta blocker | 1660 (68) | 128 (76) | 399 (69) | 526 (66) | 413 (67) | 194 (66) | 0.12 |
| Smoking status | |||||||
| Never smoker | 785 (32) | 48 (29) | 200 (35) | 240 (30) | 198 (32) | 99 (34) | 0.36 |
| Current/former smoker | 1663 (68) | 120 (71) | 377 (65) | 554 (70) | 418 (68) | 194 (66) | |
| Diabetes | 947 (39) | 73 (44) | 240 (42) | 302 (38) | 230 (38) | 102 (35) | 0.22 |
| BMI, kg/m2 | 29.7 (6.2) | 28.9 (6.1) | 29.7 (6.5) | 29.6 (5.9) | 30.1 (6.1) | 29.9 (6.5) | 0.29 |
| Hyperlipidemia | 1877 (77) | 119 (71) | 450 (78) | 619 (78) | 470 (76) | 171 (76) | 0.47 |
| LDL-C, mg/dL | 92.6 (35.3) | 87.9 (32.8) | 89.6 (32.7) | 91.6 (36.1) | 95.3 (35.5) | 98.0 (38.1) | 0.003 |
| HDL-C, mg/dL | 43.0 (13.1) | 43.8 (13.2) | 44.0 (13.2) | 42.8 (13.2) | 42.5 (12.6) | 42.3 (13.3) | 0.26 |
| WBC, 103 cells/μL | 7.1 (2.2) | 7.0 (2.1) | 7.1 (2.2) | 7.1 (2.2) | 7.1 (2.2) | 7.0 (2.1) | 0.79 |
| Statin Use | 1856 (76) | 129 (77) | 436 (76) | 607 (77) | 471 (77) | 213 (73) | 0.74 |
| Aspirin Use | 1970 (81) | 142 (85) | 471 (82) | 638 (80) | 492 (80) | 227 (77) | 0.40 |
| HFpEF | 678 (28) | 56 (33) | 154 (27) | 211 (27) | 176 (29) | 81 (28) | 0.45 |
| Prior MI | 665 (27) | 38 (23) | 146 (25) | 229 (29) | 168 (28) | 84 (29) | 0.35 |
| Obstructive CAD | 1601 (77) | 108 (73) | 392 (78) | 534 (79) | 392 (76) | 175 (74) | 0.38 |
| eGFR, mL/min/1.73 m2 | 70.5 (24.3) | 67.0 (24.8) | 69.2 (23.0) | 71.4 (23.7) | 71.1 (24.9) | 71.5 (26.3) | 0.13 |
Values are n (%), mean (standard deviation), median [25th-75th percentile]
P-value represents results of analysis of variance, Mann-Whitney U, or chi-square tests of comparison across DBP groups.
Abbreviations: DBP = diastolic blood pressure; SBP = systolic blood pressure; ACEi/ARB = angiotensin-converting enzyme inhibitor/angiotensin
II receptor blocker; BMI = body mass index; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; HFpEF = heart failure with preserved ejection fraction; MI = myocardial infarction; CAD = coronary artery disease; eGFR = estimated glomerular filtration rate
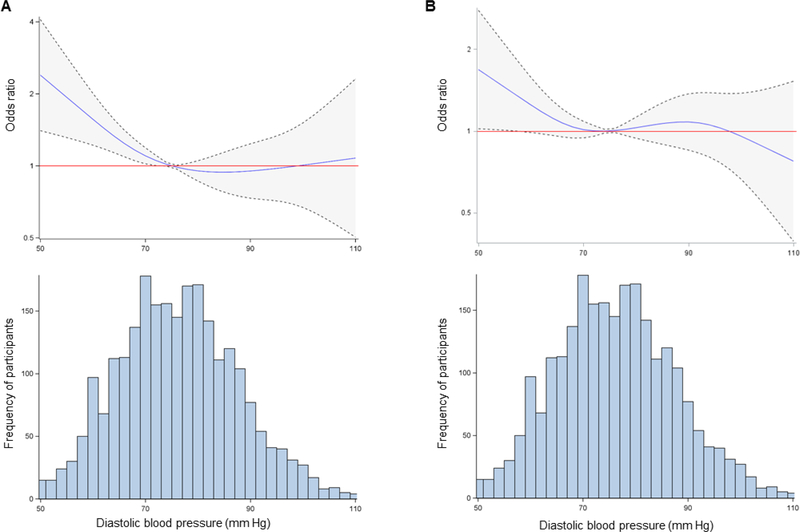
Compared with the referent group (DBP 70 to 79 mm Hg), those with DBP <60 mm Hg had increased odds of elevated hs-cTnI (odds ratio [OR] = 1.68; 95% confidence interval [CI] = 1.07, 2.65) and suPAR (OR = 1.71; 95% CI = 1.10, 2.65), Supplemental Table 1. Similar results were obtained from generalized linear models using continuous, log-transformed hs-cTnI and suPAR (Supplemental Table 2). There was no association between DBP and elevated hsCRP (Supplemental Table 3). Using restricted cubic splines, there was an inverse linear relationship between DBP and the odds of elevated hs-cTnI and suPAR levels (Figure 1), but not hsCRP levels (Supplemental Figure 1), such that hs-cTnI and suPAR were higher at lower levels of DBP.
Figure 1. Association between diastolic blood pressure and biomarkers of myocardial injury.
Restricted cubic spline regression with 4 knots modeling odds ratio estimates for (A) hscTnI >5 pg/mL and (B) suPAR >3 ng/mL along a continuous spectrum for diastolic blood pressure (DBP) from the 1st to the 99th percentile of DBP. Dotted lines and shaded areas represent the 95% confidence interval. Histograms of the distribution of DBP are presented below each spline. Models are adjusted for age, race, sex, body mass index, systolic blood pressure, smoking, hyperlipidemia, heart failure with preserved ejection fraction, diabetes, ACEi/ARB use, beta blocker use, statin use, aspirin use, and estimated eGFR. Models for suPAR are additionally adjusted for white blood cell count.
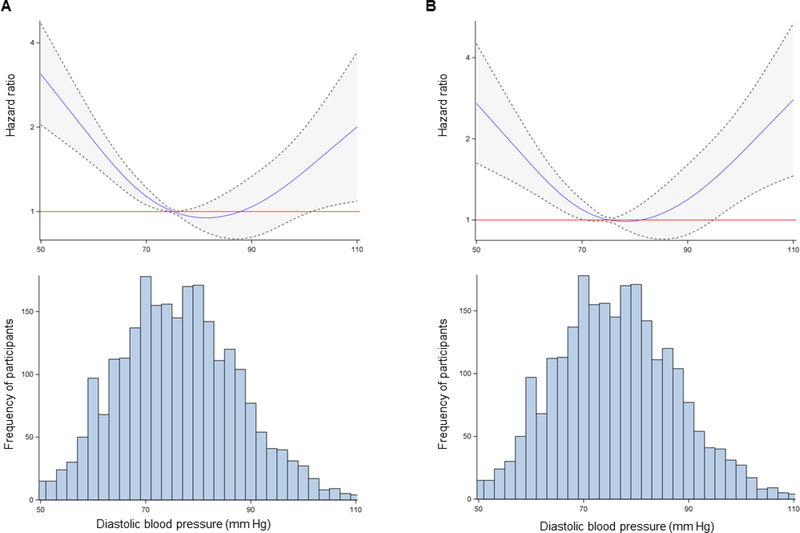
Over a median follow-up period of 4.5 years (25th-75th percentiles = 1.8–6.9 years), a total of 120 MIs, 234 CV deaths and 420 all-cause deaths occurred. The cumulative incidence for allcause death and the composite of CV death or MI events are shown in Supplemental Figure 2. DBP <60 mm Hg was associated with an increased cumulative risk of all-cause death (hazard ratio [HR] = 2.21; 95% CI = 1.60, 3.08) and CV death or MI (HR = 2.14; 95% CI = 1.45, 3.17) compared to the referent group of DBP 70 to 79 mm Hg. After adjustment for risk factors, DBP <60 mm Hg remained associated with an increased cumulative risk of all-cause death (HR 2.41; 95% CI 1.69, 3.45) and CV death or MI (HR 2.04; 95% CI 1.32, 3.16), Table 2. Results were mildly attenuated but remained significant after further adjustment for WBC count. When modeling the association between continuous DBP and risk of events using restricted cubic splines, a J-shaped relationship was evident for both all-cause death and CV death or MI, as shown in Figure 2.
Table 2:
Risk of incident events by DBP category
| Diastolic BP | All-Cause Death |
CV Death or MI |
||||
|---|---|---|---|---|---|---|
| n/N | HR* (95% CI) | P-value | n/N | sHR* (95% CI) | P-value | |
| <60 mm Hg | 50/168 | 2.41 (1.69–3.45) | <0.001 | 36/168 | 2.04 (1.32–3.16) | 0.001 |
| 60–69 mm Hg | 105/577 | 1.27 (0.96–1.69) | 0.10 | 75/577 | 1.16 (0.83–1.62) | 0.40 |
| 70–79 mm Hg | 118/794 | 1.00 (reference) | -- | 85/794 | 1.00 (reference) | -- |
| 80–89 mm Hg | 101/616 | 1.11 (0.84–1.46) | 0.48 | 65/616 | 1.02 (0.72–1.43) | 0.92 |
| >90 mm Hg | 46/293 | 1.10 (0.76–1.59) | 0.63 | 40/293 | 1.53 (0.98–2.37) | 0.06 |
Adjusted for age, race, sex, body mass index, systolic blood pressure (continuous), smoking, hyperlipidemia, HFpEF, diabetes, ACEi/ARB use, beta blocker use, statin use, aspirin use, and eGFR. Abbreviations: DBP = diastolic blood pressure; CV = cardiovascular; MI = non-fatal myocardial infarction; n = number of events; N = number at risk; HR = hazard ratio; CI = confidence interval; sHR = subdistribution hazard ratio; HFpEF = heart failure with preserved ejection fraction; ACEi/ARB = angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; eGFR = estimated glomerular filtration rate
Significant values are indicated in bold.
Figure 2. Association between diastolic blood pressure and incident events.
Restricted cubic spline regression with 4 knots modeling hazard ratio estimates for (A) all-cause death and (B) cardiovascular death or non-fatal MI along a continuous spectrum for DBP, from the 1st to the 99th percentile of DBP. Dotted lines and shaded areas represent the 95% confidence interval. Histograms of the distribution of DBP are presented below each spline. Models are adjusted for age, race, sex, body mass index, systolic blood pressure, smoking, hyperlipidemia, heart failure with preserved ejection fraction, diabetes, ACEi/ARB use, beta blocker use, statin use, aspirin use, and estimated eGFR.
In fully-adjusted Cox regression models, the association between DBP <60 mm Hg and all-cause death (HR 1.87; 95% CI 1.22, 2.87) as well as CV death or MI (HR 1.73; 95% CI 1.03, 2.92) were attenuated but maintained statistical significance after adjustment for hs-cTnI and suPAR levels, Supplemental Table 4.
In sensitivity analyses, the association between DBP <60 mm Hg and all-cause death was greater in patients without diabetes (HR = 2.79; 95% CI = 1.69, 4.43) than in those who had diabetes (HR = 1.52; 95% CI = 0.83, 2.77; P-interaction = 0.003); similarly, the association between DBP <60 mm Hg and CV death or MI was greater in patients without diabetes (HR = 2.39; 95% CI = 1.25, 4.59) than in those who had diabetes (HR = 1.61; 95% CI = 0.77, 3.36; Pinteraction = 0.01) (Supplemental Tables 5 and 6). The risks for all-cause death and CV death or MI were otherwise similar by age, sex, race, HFpEF, SBP, antihypertensive medication use, obesity, chronic kidney disease, and CAD burden (Supplemental Tables 5 and 6).
Specifically, while there were no significant interactions between SBP and DBP <60 mm Hg for all-cause death (P-interaction = 0.25, Supplemental Table 5) or CV death and MI (Pinteraction = 0.33, Supplemental Table 6), DBP <60 mm Hg was generally associated with an increased risk of events at all levels of SBP; however, these associations were only significant at SBP <140 mm Hg (Supplemental Tables 5 and 6).
Lastly, regarding the relationship between SBP, biomarker elevations and outcomes, low SBP was not associated with elevations in hs-cTnI, suPAR, or hsCRP; however, SBP ≥160 mm Hg was independently associated with elevated hs-cTnI and suPAR (Supplemental Table 7), as well as increased risk of all-cause death (Supplemental Table 8).
4. Discussion
The 2017 American guidelines for the management of high blood pressure in adults recommend a BP target of <130/80 mm Hg for adults with hypertension and prevalent CV disease, which is more aggressive than the previous guideline recommendation of <140/90 [2, 21], and our results have potential implications for the management of BP in these patients. We have shown that individuals with CAD and DBP <60 mm Hg had increased prevalence of subclinical myocardial injury and evidence of immune/inflammatory activation, demonstrated as elevated levels of hs-cTnI and suPAR. Furthermore, we showed that the association between low DBP and events was explained in part by prevalent elevations in biomarkers of myocardial injury (hs-cTnI) and immune/inflammatory activation (suPAR). The combination of these findings begins to address several key proposed mechanisms for the J-curve phenomenon and supports recommendations for caution against significantly reducing DBP (especially below 60 mm Hg) while targeting lower SBP in patients with CAD [22].
Observational data from cohort studies, post-hoc analyses of randomized clinical trials and meta-analyses have shown a J-curve relationship for DBP and cardiovascular events [4, 5, 23–27]. While many have included patients at increased cardiovascular risk, few have focused specifically on BP targets in patient with CAD. Vidal-Petiot et al. reported on cardiovascular event rates and DBP from the CLARIFY registry, a large multinational cohort of patients with CAD. Subjects with DBP <60 mm Hg had double the risk of a composite outcome of CV death, MI or stroke compared to a referent group of DBP 70–79 mm Hg [28]. Our findings are in agreement with these previous studies and show an approximate doubling of risk for all-cause death and CV death or MI at DBP <60 mm Hg, even after rigorous adjustment for several clinical risk factors, antihypertensive treatment, and CAD burden.
Beyond simply demonstrating the J-curve phenomenon in our population with CAD, we investigated potential mechanisms for its existence. Because coronary blood flow occurs during diastole, low DBP may lead to decreased coronary perfusion pressure and result in CV events, especially in CAD, where autoregulatory mechanisms for coronary perfusion are compromised [26]. Using hs-cTnT as a marker of myocardial ischemia or injury, McEvoy et al explored the association between DBP, hs-cTnT and incident events in 11,565 participants from the ARIC (Atherosclerosis Risk In Communities) study. Despite the relatively low-risk population, the authors demonstrated that patients with DBP <60 mm hg had higher hs-cTnT values and higher rates of coronary heart disease events [6]. For the first time in a population with CAD, we found similar associations between elevated hs-cTnI levels and low DBP. Importantly, this may not be simply a “supply and demand” issue, as the degree of epicardial CAD burden did not augment the association between low DBP, hs-cTnI and outcomes. While seemingly counterintuitive, this is not unexpected based on what is known regarding determinants of coronary flow at the lower extremes of perfusion pressure; specifically, subendocardial capillary networks appear to be more responsible for perfusion pressure drops than the epicardial arteries [29, 30].
Immune activation and inflammation also play an integral role in the development and progression of CAD [12]. In patients with CAD, plaque stability and eventual rupture is related to inflammation, and several biomarkers have been identified to help risk-stratify patients with CAD [12]. Plasma suPAR is an emerging biomarker associated with inflammatory and immune cell proliferation, migration and adhesion [11]. suPAR is only modestly associated with hsCRP and is less sensitive to acute changes in inflammation, suggesting that suPAR and hsCRP may represent different pathways along the inflammatory cascade [31]. Furthermore, in patients with known atherosclerosis, elevated suPAR may additionally represent significant endothelial dysfunction associated with CAD, thus providing a more specific marker that incorporates several disease pathways, as opposed to hsCRP [9]. While elevations in suPAR have been associated with adverse outcomes in a wide variety of disease processes [11, 13, 32–34], it has more recently shown promise as a predictor of incident cardiovascular events [10, 15, 35]. Both serum and intraplaque levels of suPAR were elevated in patients with symptomatic carotid atherosclerosis, suggesting its potential utility in identifying a vulnerable plaque phenotype [36]. Little is known regarding the associations between blood pressure, CAD and suPAR; however, Sun et al recently showed that low DBP was independently associated with plaque vulnerability, defined as intraplaque hemorrhage [37]. Given our findings that DBP <60 mm Hg was associated with elevations in plasma suPAR and a higher risk of CV events, there may be a potential pathophysiological link between low DBP, high plasma suPAR and vulnerable plaque phenotypes leading to CV events in patients with CAD. Further studies are needed to determine the mechanisms by which low DBP leads to endothelial dysfunction, immune activation and ultimately, adverse events.
When we included hs-cTnI and suPAR into our fully-adjusted survival models, DBP <60 mm Hg remained an independent predictor of both all-cause mortality and CV death or MI; however, the associations were attenuated by approximately 40%. These findings suggest that the observed increase in cardiovascular events for individuals with DBP <60 mm Hg is at least partially explained through mechanisms of subclinical myocardial damage, immune activation and inflammation.
Finally, no interpretation of the associations between DBP, biomarkers and outcomes is complete without consideration of SBP. Low DBP may simply be a marker of low SBP, which has also been shown to be associated with adverse cardiovascular outcomes in patients with CAD [28]. Additionally, pulse pressure, or the difference between systolic and diastolic blood pressure, has been identified as an independent predictor of adverse outcomes and has been proposed as the primary mechanism by which low DBP leads to cardiovascular morbidity and mortality [38]. Although we found an expected stepwise increase in SBP across categories of DBP, we found no differences in pulse pressure. Furthermore, low SBP was not associated with biomarker elevations or increased risk of events. And while DBP <60 mm Hg was associated with an increased risk of all-cause death or CV death and MI at all levels of SBP, these associations were only significant at SBP <140 mm Hg. While pulse pressure may be a significant predictor of events and a potential explanation for the J-curve phenomenon in the general population, its importance in CAD remains unclear [39].
Our study is the first to identify mechanistic plausibility for the J-curve of DBP in patients with CAD using biomarkers of myocardial injury and immune/inflammatory activity. Several limitations merit discussion to place our findings in the appropriate context. Notably, BP was measured and obtained at a single time point, prior to left heart catheterization. Although patients were instructed to fast, avoid caffeine and nicotine, and take medications as prescribed, this single BP reading may not reliably indicated true ambulatory BP for all patients. This single baseline outpatient BP measurement precludes adjustment for BP fluctuations during follow-up, and changes in clinical status, including medication adherence and comorbidities, are unknown. As is the case in all observational cohort studies, additional residual confounding and misclassification bias are possible.
In conclusion, we have shown that in patients with CAD, low DBP <60 mm Hg is associated with increased risk of all-cause death and CV death or MI. This risk is partially explained by elevations in biomarkers of subclinical myocardial injury and immune/inflammatory activation. Although further studies are needed to identify optimal BP targets in this high-risk population, caution regarding aggressive DBP lowering in patients with CAD is warranted [22].
Supplementary Material
Highlights.
Low DBP is associated with adverse outcomes in patient with coronary artery disease
Biomarkers of myocardial injury and immune activation are associated with both low
DBP and risk of events
Associations between DBP, biomarkers and events are not modified by SBP
Acknowledgements:
We would like to thank all participants of the Emory Cardiovascular Biobank. Additional, we thank Joy Hartsfield for her tireless work in maintaining the integrity of patient follow-up.
Sources of Funding: MLT is supported by the National Institutes of Health (NIH) T32 THL130025A and the Abraham J. & Phyllis Katz Foundation grant (Atlanta, GA). AAQ is supported by grants 5P01HL101398–02, 1P20HL113451–01, 1R56HL126558–01, 1RF1AG051633–01, R01 NS064162–01, R01 HL89650–01, HL095479–01, 1U10HL110302–01, 1DP3DK094346–01, 2P01HL086773–06A1.
Footnotes
Conflicts of Interest: The authors report no relationships that could be construed as a conflict of interest
Statement of Authorship: All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ford ES. Trends in mortality from all causes and cardiovascular disease among hypertensive and nonhypertensive adults in the United States. Circulation. 2011;123(16):1737–44. [DOI] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20. [DOI] [PubMed] [Google Scholar]
- 3.Peterson ED, Gaziano JM, Greenland P. Recommendations for treating hypertension: what are the right goals and purposes? JAMA. 2014;311(5):474–6. [DOI] [PubMed] [Google Scholar]
- 4.Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, et al. J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J. 2010;31(23):2897–908. [DOI] [PubMed] [Google Scholar]
- 5.Rahman F, McEvoy JW. The J-shaped Curve for Blood Pressure and Cardiovascular Disease Risk: Historical Context and Recent Updates. Curr Atheroscler Rep. 2017;19(8):34. [DOI] [PubMed] [Google Scholar]
- 6.McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, et al. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. J Am Coll Cardiol. 2016;68(16):1713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford I, Shah AS, Zhang R, McAllister DA, Strachan FE, Caslake M, et al. High-Sensitivity Cardiac Troponin, Statin Therapy, and Risk of Coronary Heart Disease. J Am Coll Cardiol. 2016;68(25):2719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61(12):1240–9. [DOI] [PubMed] [Google Scholar]
- 9.Hodges GW, Bang CN, Wachtell K, Eugen-Olsen J, Jeppesen JL. suPAR: A New Biomarker for Cardiovascular Disease? Can J Cardiol. 2015;31(10):1293–302. [DOI] [PubMed] [Google Scholar]
- 10.Eapen DJ, Manocha P, Ghasemzadeh N, Patel RS, Al Kassem H, Hammadah M, et al. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc. 2014;3(5):e001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuhrman B The urokinase system in the pathogenesis of atherosclerosis. Atherosclerosis. 2012;222(1):8–14. [DOI] [PubMed] [Google Scholar]
- 12.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. [DOI] [PubMed] [Google Scholar]
- 13.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N Engl J Med. 2015;373(20):1916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyngbaek S, Sehestedt T, Marott JL, Hansen TW, Olsen MH, Andersen O, et al. CRP and suPAR are differently related to anthropometry and subclinical organ damage. Int J Cardiol. 2013;167(3):781–5. [DOI] [PubMed] [Google Scholar]
- 15.Samman Tahhan A, Hayek SS, Sandesara P, Hajjari J, Hammadah M, O’Neal WT, et al. Circulating soluble urokinase plasminogen activator receptor levels and peripheral arterial disease outcomes. Atherosclerosis. 2017;264:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, et al. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013;62(4):329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(4 Suppl):5–40. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–9. [DOI] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 21.Beddhu S, Chertow GM, Cheung AK, Cushman WC, Rahman M, Greene T, et al. Influence of Baseline Diastolic Blood Pressure on Effects of Intensive Compared to Standard Blood Pressure Control. Circulation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation. 2015;131(19):e435–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JFE, et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet. 2017;389(10085):2226–37. [DOI] [PubMed] [Google Scholar]
- 24.Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA. 1991;265(4):489–95. [PubMed] [Google Scholar]
- 25.Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144(12):884–93. [DOI] [PubMed] [Google Scholar]
- 26.Messerli FH, Panjrath GS. The J-curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? J Am Coll Cardiol. 2009;54(20):1827–34. [DOI] [PubMed] [Google Scholar]
- 27.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290(21):2805–16. [DOI] [PubMed] [Google Scholar]
- 28.Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388(10056):2142–52. [DOI] [PubMed] [Google Scholar]
- 29.Harrison DG, Barnes DH, Hiratzka LF, Eastham CL, Kerber RE, Marcus ML. The effect of cardiac hypertrophy on the coronary collateral circulation. Circulation. 1985;71(6):1135–45. [DOI] [PubMed] [Google Scholar]
- 30.Harrison DG, Florentine MS, Brooks LA, Cooper SM, Marcus ML. The effect of hypertension and left ventricular hypertrophy on the lower range of coronary autoregulation. Circulation. 1988;77(5):1108–15. [DOI] [PubMed] [Google Scholar]
- 31.Eugen-Olsen J, Andersen O, Linneberg A, Ladelund S, Hansen TW, Langkilde A, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268(3):296–308. [DOI] [PubMed] [Google Scholar]
- 32.Donadello K, Scolletta S, Covajes C, Vincent JL. suPAR as a prognostic biomarker in sepsis. BMC Med. 2012;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langkilde A, Hansen TW, Ladelund S, Linneberg A, Andersen O, Haugaard SB, et al. Increased plasma soluble uPAR level is a risk marker of respiratory cancer in initially cancer-free individuals. Cancer Epidemiol Biomarkers Prev. 2011;20(4):609–18. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen LJ, Knudsen A, Katzenstein TL, Gerstoft J, Obel N, Jorgensen NR, et al. Soluble urokinase plasminogen activator receptor (suPAR) is a novel, independent predictive marker of myocardial infarction in HIV-1-infected patients: a nested case-control study. HIV Med. 2016;17(5):3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koller L, Stojkovic S, Richter B, Sulzgruber P, Potolidis C, Liebhart F, et al. Soluble Urokinase Type Plasminogen Activator Receptor Improves Risk Prediction in Patients With Chronic Heart Failure. JACC Heart Fail. 2017;5(4):268–77. [DOI] [PubMed] [Google Scholar]
- 36.Edsfeldt A, Nitulescu M, Grufman H, Gronberg C, Persson A, Nilsson M, et al. Soluble urokinase plasminogen activator receptor is associated with inflammation in the vulnerable human atherosclerotic plaque. Stroke. 2012;43(12):3305–12. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Canton G, Balu N, Hippe DS, Xu D, Liu J, et al. Blood Pressure Is a Major Modifiable Risk Factor Implicated in Pathogenesis of Intraplaque Hemorrhage: An In Vivo Magnetic Resonance Imaging Study. Arterioscler Thromb Vasc Biol. 2016;36(4):743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selvaraj S, Steg PG, Elbez Y, Sorbets E, Feldman LJ, Eagle KA, et al. Pulse Pressure and Risk for Cardiovascular Events in Patients With Atherothrombosis: From the REACH Registry. J Am Coll Cardiol. 2016;67(4):392–403. [DOI] [PubMed] [Google Scholar]
- 39.Bangalore S, Messerli FH, Franklin SS, Mancia G, Champion A, Pepine CJ. Pulse pressure and risk of cardiovascular outcomes in patients with hypertension and coronary artery disease: an INternational VErapamil SR-trandolapril STudy (INVEST) analysis. Eur Heart J. 2009;30(11):1395–401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.