Abstract
Background:
Adiposity has been linked to both risk and prognosis of colorectal cancer; however, the impact of different fat areas [visceral (VFA) vs. subcutaneous fat area (SFA)] is unclear. We investigated associations between adiposity and biomarkers of inflammation and angiogenesis among patients with colorectal cancer.
Methods:
Preoperative serum samples and computed tomography scans were obtained from 188 patients diagnosed with primary invasive stage I-IV colorectal cancer enrolled in the ColoCare Study. Adiposity was assessed by area-based quantification of VFA, SFA, and VFA:SFA ratio on spinal levels L3/L4 and L4/L5. Circulating levels of inflammation (CRP, SAA, sICAM-1, sVCAM-1) and angiogenesis (VEGF-A, VEGF-D) were assessed from patient sera on the Meso-Scale-Discoveries platform. Partial correlations and regression analyses, adjusted for age, sex, and tumor stage, were performed.
Results:
VFA was moderately correlated with CRP and SAA (CRP: L3/L4 and L4/L5:r=0.21, p=0.01; SAA: L3/L4:r=0.17, p=0.04). The correlation between SFA and the measured biomarkers were weak (r≤0.13, not significant). The ratio of VFA:SFA at L3/L4 was moderately correlated with VEGF-A (r=0.28, p=0.0008), and SAA (r=0.24, p=0.006), and less so with CRP (r=0.18, p=0.04) and sICAM-1 (r=0.18, p=0.04). Similar correlations were found for VFA:SFA ratio at L4/L5.
Conclusions:
We observed an association between visceral adiposity and biomarkers of inflammation and angiogenesis in colorectal cancer patients. In particular, the VFA:SFA ratio was correlated with circulating levels of pro-angiogenic biomarker VEGF-A.
Impact:
Our findings support a direct association of visceral adipose tissue with inflammatory and angiogenic processes, which play fundamental roles in the development and progression of colorectal cancer.
INTRODUCTION
The prevalence of obesity (defined as body mass index (BMI) >30 kg/m2) among American adults aged 20 to 74 years has more than doubled since 1979 (1), and is estimated to increase by 65 million more obese adults in the United States alone from 2011 to 2030 (2). Accumulating evidence identifies obesity as a factor of colorectal cancer risk and prognosis (3,4). The International Agency for Cancer Research (IARC) has reported that individuals with a BMI greater than or equal to 25 kg/m2 have an increased risk of developing colorectal cancer relative to those with a normal BMI (defined as 18.5–24.9 kg/m2) (5). The recently published report of World Cancer Research Fund (WCRF) on nutrition, physical activity, and colorectal cancer shows that this association is non-linear with a stronger observed risk increase above a BMI of 27 kg/m2 (6). A review of the literature has further demonstrated poorer clinical outcomes (e.g., survival rates) for obese colorectal cancer patients compared to non-obese patients (7). However, the term “obesity-paradox” has risen from accumulating evidence that shows improved survival among overweight or early obese patients compared to patients with a BMI below 22.5 kg/m2 or over 30 kg/m2(8). Given that the prevalence of obesity among individuals with a history of colorectal cancer increases annually by about 3.5% (9), there is an increasing need to define the biological mechanisms underlying the obesity-colorectal cancer link.
In the obese host-tumor microenvironment, adipocytes and secreted mediators, inflammatory cells, and colonocytes generate a quartet that promotes carcinogenesis (10). In particular, non-tumor cells such as macrophages and adipocytes are suggested to increase inflammatory processes (e.g., production and secretion of inflammatory biomarkers, recruitment of inflammatory cells) that lead to a reprogramming of cancer cell metabolism, as well as to perturbation of additional cancer hallmarks, including invasion, metastasis, and immune clearance (4,10).
With respect to energy storage, white adipose tissue (WAT) is the key adipose tissue compartment (4). WAT and its related inflammatory secretion are together hypothesized to play a key role in the obesity-cancer link (10). Further subdivision of WAT into distinct body fat compartments, visceral (VAT) and subcutaneous adipose tissue (SAT), is based upon anatomical location, cellular structure, molecular composition, and secretome (10). Profiling the metabolome, lipidome, and transcriptome of distinct body fat compartments has further demonstrated that VFA consists of higher levels of tumor-promoting molecules (e.g., inflammation-related lipid metabolites, free arachidonic acid, phospholipases, and prostaglandin synthesis-related enzymes) compared to SFA (11). Together, these results are consistent with reports that increased visceral adiposity is associated with poorer outcomes, such as postoperative complications, survival, and recurrence, in the short- and long-term (12,13).
The objective of this study was to investigate associations between different dimensions of body fatness and inflammation-related as well as angiogenesis-related biomarkers. We analyzed the associations between specific fat areas (VFA and SFA), and the (VFA:SFA) ratio, and circulating biomarkers to unravel the impact of VFA and SFA on processes involved in the colorectal carcinogenesis and the progression of colorectal cancer.
MATERIALS AND METHODS
Study population
This study population includes patients from the international prospective ColoCare Study cohort (Clinicaltrials.gov.Identifier:NCT02328677), that has been described in detail in prior publications (11,12,14–16). The ColoCare Study cohort includes men and women aged 18–89 years who were diagnosed with a primary invasive colorectal cancer (stages I-IV) undergoing surgery at clinics and sites internationally. Electronic medical charts, including pathological reports, were reviewed to document other clinical characteristics (e.g., age at surgery, sex, tumor stage and site, treatment regimen). Anthropometric measurements (height, waist and hip circumference) were taken at the clinic visit, and data on lifestyle (e.g., smoking status) and drug use (e.g., non-steroidal anti-inflammatory drug (NSAIDs)) were obtained from questionnaires collected at baseline, prior to surgery. Out of 407 patients enrolled in the ColoCare Study site in Heidelberg, Germany between October 2010 and December 2014, 290 patients had blood draws prior to undergoing surgery. Patients were excluded if biomarker levels were outside of the detectable range (n=12), CT scans were not available (n=86), or patients who were classified with stage 0 or “no malignancy” post-surgery (n=4). In total, 188 men and women were included in this study. The study was approved by the Ethics Committee of the University of Heidelberg, and all subjects provided written informed consent.
Blood processing and biomarker assays
Non-fasting blood samples were collected from patients prior to surgery (baseline) at the University Hospital of Heidelberg. Serum was extracted within four hours after blood-draw and stored in aliquots at −80ºC until analysis. 500μl of each patient’s serum was shipped on dry ice to Huntsman Cancer Institute (HCI, Salt Lake City, Utah, USA) for analysis.
Serum-based assays for multiplexed vascular endothelial growth factor A (VEGF-A), vascular endothelial growth factor D (VEGF-D), C reactive protein (CRP), serum amyloid A (SAA), soluble intracellular adhesion molecule 1 (sICAM-1), and soluble vascular cell adhesion molecule 1 (sVCAM-1) have previously been established on the Mesoscale Discovery Platform (MSD, Rockville, MD, USA) in the Ulrich laboratory at HCI (17). Biomarkers were selected based upon i) clinical and epidemiological relevance in colorectal carcinogenesis and progression, as well as direct links to body fatness (e.g., CRP, SAA), and ii) high relevance in the stimulation and promotion of angiogenesis and metastases of colorectal cancer (e.g., sICAM-1, sVCAM-1, VEGF-A/D). Blinded patient samples plus three intraplate and interplate quality control samples (QC) were assayed for CRP, SAA, sICAM-1 and sVCAM-1 (V-PLEX Vascular Injury Plate 2), and for VEGFA and VEGFD (V-PLEX Angiogenesis Panel 1). Assays were conducted on the Sector 2400A (MSD, Rockville, MD, USA). Blinded serum samples were run at dilutions of 1:1000 (Vascular Injury panel) and 1:8 (Angiogenesis panel), and the serum was freeze-thawed only once. Data was analyzed with MSD Discovery Workbench 4.0 software (Meso Scale Diagnostics, Rockville, MD). The overall interplate coefficient of variability (CV) was 9.9% and intraplate CV was 4.6%.
Area-based computed tomography (CT) quantification of abdominal adipose tissue
Abdominal CT scans conducted between August 2010 and December 2014 were assessed retrospectively using Centricity RIS 4.1i and GE PACS (GE Medical Systems, Buckinghamshire, UK). CT scans were predominantly performed before surgery (mean time, before: 42 days, after: 41 days). A prior study that used this data from the same population showed that pre- and post-surgical CT scans were similar, and thus, could be combined for statistical analyses (18). The quantification of visceral (VFA) and subcutaneous fat area (SFA) based on diagnostic CT data was performed using a dedicated post-processing software (Syngo Volume tool, MMPW, Siemens Healthcare, Munich, Berlin, Germany).
An area-based quantification of adipose tissue compartments was performed on two spinal levels most representative of the abdominal adipose tissue distribution (L3/L4, L4/L5). The quantity of adipose tissue measured on levels L3/L4 has been reported (e.g., in the Framingham Heart Study) to best reflect the volume-based quantification of abdominal adipose tissue compartments including in age- and sex-subgroups (19). Spinal level L4/L5 has been observed to be strongly correlated with diabetes and hypertension (20). By manually tracing specific regions of interest at L3/L4 and L4/L5, total fat area (TFA, whole circumference), VFA (along the fascial plane tracing the abdominal wall) (Supplemental Figure 1) were measured (volumetric quantification of selected slice, divided by slice thickness)(18). Adipose tissue was selected by limiting the measurements to a lower attenuation limit of −190 Hounsfield units (HU) and an upper attenuation limit of −30 HU. (21,22) SFA was determined by subtracting VFA from TFA. The visceral to subcutaneous fat ratio was calculated as VFA:SFA (18).
Statistical analysis
Mean and standard deviation (SD) values were calculated for continuous variables (age, BMI, VFA, SFA, VFA:SFA ratio, and biomarker measurements) and compared among men and women using the Wilcoxon sign-rank test. Frequencies and percentages were calculated for categorical variables (sex, smoking status, neoadjuvant treatment, tumor stage, and tumor site).
Continuous data were tested for normal distributions by performing the Shapiro-Wilk test and investigating the q-q-plot distributions for each biomarker. All biomarker levels were log2-transformed to prevent heteroscedasticity. Potential confounding by age at surgery (years), sex (male, female), body mass index (BMI, kg/m2), clinical tumor stage (I-IV), NSAID use prior to blood draw (yes, no), and neoadjuvant chemotherapy (yes, no) was assessed. Final analyses were adjusted for patient age, sex, and tumor stage.
Pearson’s partial correlation coefficients adjusted for age, sex, and tumor stage, were calculated to address the link between adiposity (including VFA, SFA, and VFA:SFA ratio on both levels L3/L4 and L4/L5, and BMI) and inflammation- and angiogenesis-related biomarkers. We additionally assessed associations between adiposity (exposure) and biomarkers of inflammation and angiogenesis (outcome) computing multiple linear regression models adjusted for age, sex, tumor stage. Sensitivity analyses were performed, excluding i) patients who underwent a CT scan greater than 6 months prior to or 6 months after the blood draw, and ii) patients with stage IV disease. Statistical analyses were performed using SAS 9.4 (2008, SAS Institute, Cary, USA). All tests were considered to be statistically significant at ρ<0.05.
RESULTS
A total of 188 individuals diagnosed with clinical stage I-IV colorectal cancer with available diagnostic CT scan measurements and preoperative blood samples were identified from the ColoCare Study over the four-year study period (December 2010 to May 2014) (Table 1). Mean age at surgery among individuals was 62 years. Sixty-eight percent of individuals were men. Over 50% of the population was overweight with mean BMI at 26.2 kg/m2 (23). Approximately two-thirds of individuals reported being ever smokers. Among all cases, 62% of cancers were located in the rectum. Assessment of treatment history demonstrated that 45% of study participants had received neoadjuvant therapy. Overall, 27% of colorectal cancer patients were diagnosed with advanced stage IV disease (Table 1).
Table 1.
Baseline clinicopathologic and demographic characteristics among n=188 patients enrolled in the ColoCare Study.
| Age at surgery* (years) | 62.7 ± 12.0 |
| Sex, n (%) | |
| Female | 59 (31.4) |
| Male | 129 (68.6) |
| Body mass index* (kg/m2) | 26.2 ± 4.16 |
| Smoking history, n (%) | |
| Never smoker | 61 (32.4) |
| Ever smoker | 116 (61.7) |
| Unknown | 11 (5.9) |
| Neoadjuvant treatment, n (%) | |
| None | 103 (54.8) |
| Yes | 85 (45.2) |
| Tumor stage, n (%) | |
| I | 32 (17.0) |
| II | 54 (28.7) |
| III | 51 (27.1) |
| IV | 51 (27.1) |
| Tumor site, n (%) | |
| Colon | 71 (38) |
| Rectum | 117 (62) |
Mean ± standard deviation; AJCC: American Joint Committee on Cancer; kg: kilogram; m: meters
The geometric mean concentrations of inflammation and angiogenesis-related biomarkers and adiposity measurements are summarized in Table 2. Mean VEGF-A (827±583 pg/L) and VEGF-D (859±304 pg/L) levels were increased compared to reference levels (~500 pg/mL and ~300 pg/ml, respectively) (24,25). Elevated mean CRP (12±20 mg/L) and SAA (18±34 mg/L) biomarker levels were indicative of activated systemic inflammatory processes (26). VFA (L3/L4: 112.1±73.18 cm2 vs. 198.2±73.18 cm2, ρ<0.001; VFA (L4/L5): 114.8.±68.21 cm2 vs. 159.6±70.71 cm2, ρ<0.001) statistically significantly differed between men and women. We also observed sex specific differences of VFA:SFA ratios on L3/L4 and L4/L5 (VFA:SFA (L3/L4): 0.5±0.35 vs. 1.0±0.47, ρ<0.001; VFA:SFA (L4/L5): 0.4±0.24 vs. 0.7±0.32, ρ<0.001, respectively) (Supplemental Table 1) (18). SFA measured on L4/L5 was statistically significantly larger in men compared to women (260.8±97.57 cm2 vs. 224.8±84.26 cm2, ρ=0.011, respectively) (18).
Table 2.
Summary of adiposity and inflammation- and angiogenesis-related biomarker measurements (geometric mean ± SD) among 188 colorectal cancer patients.
| Adiposity (mean ± SD) | |
| Subcutaneous fat area, L3/L4 (cm2) | 203 ± 89.5 |
| Subcutaneous fat area, L4/L5 (cm2) | 237 ± 93.3 |
| Visceral fat area, L3/L4 (cm2) | 173 ± 100 |
| Visceral fat area, L4/L5 (cm2) | 148 ± 78.9 |
| Ratio 1: VFA:SFA at L3/L4 | 0.91 ± 0.54 |
| Ratio 2: VFA:SFA at L4/L5 | 0.66 ± 0.35 |
| Inflammation and angiogenesis-related biomarkers (mean ± SD) | |
| VEGF-A (pg/L) | 827 ± 583 |
| VEGF-D (pg/L) | 859 ± 304 |
| CRP (mg/L) | 12.0 ± 20.6 |
| SAA (mg/L) | 18.4 ± 34.7 |
| sICAM-1 (mg/L) | 0.50 ± 0.22 |
| sVCAM-1 (mg/L) | 066 ± 0.34 |
Abbreviations: SD, standard deviation; cm, centimeters; VFA: visceral fat area; SFA: subcutaneous fat area; mg: milligrams; L: liters; VEGF-A: vascular endothelial growth factor A; VEGF-D: vascular endothelial growth factor D; CRP: C reactive protein; SAA: serum amyloid A; sICAM-1: soluble intracellular cell adhesion molecule 1; sVCAM-1: soluble vascular cell adhesion molecule 1.
Pearson’s partial correlation coefficients, adjusted for age at surgery, sex, and tumor stage, are presented in Figure 1 and Table 3. Moderately positive correlations were observed between CRP-VFA and SAA-VFA measured on both, L3/L4 and L4/L5, (r=0.21, ρ=0.01; r=0.17, ρ=0.04, respectively). No significant correlations were observed between SFA and inflammation- or angiogenesis-related biomarkers. The ratio of VFA:SFA on L3/L4 showed a moderate positive correlation with SAA (r=0.24, ρ=0.006) and VEGF-A (r=0.28, ρ=0.0008). The correlations of VFA:SFA with CRP (r=0.18, ρ=0.04) and sICAM-1 (r=0.18, ρ=0.04) were modest (Figure 1). The correlations of VFA:SFA ratio with measured biomarkers at L4/L5 were similar, with the strongest correlation with VEGF-A (r=0.27, ρ=0.001). Similar results were observed in the multiple linear regression analyses (Table 4). Given the noted differences of body compositions between men and women (Supplemental Table 1), we performed correlation and regression analyses stratified by sex (Supplemental Table 2 and 3). We observed consistent results among men and women across CRP, SAA, SICAM-1, and sVCAM-1 levels, with exception of the correlation between SFA on level L3/L4 and SAA among women, which presented a strong correlation (r=0.41, p=0.02) compared to no observed correlation among men. However, there were marked differences for VEGF-A levels in correlation with VFA on level L4/L5 within the sex-specific subgroups. The positive moderate correlation was limited to men (r=0.20, p=0.04) versus an inverse moderate correlation noted among women (r=−0.20, p=0.19). The analyses also identified an interaction between VFA on L4/L5 and sex (p<0.03).
Figure 1. Heat map of partial Pearson correlation coefficients between adiposity and inflammation- and angiogenesis-related biomarker measurements.
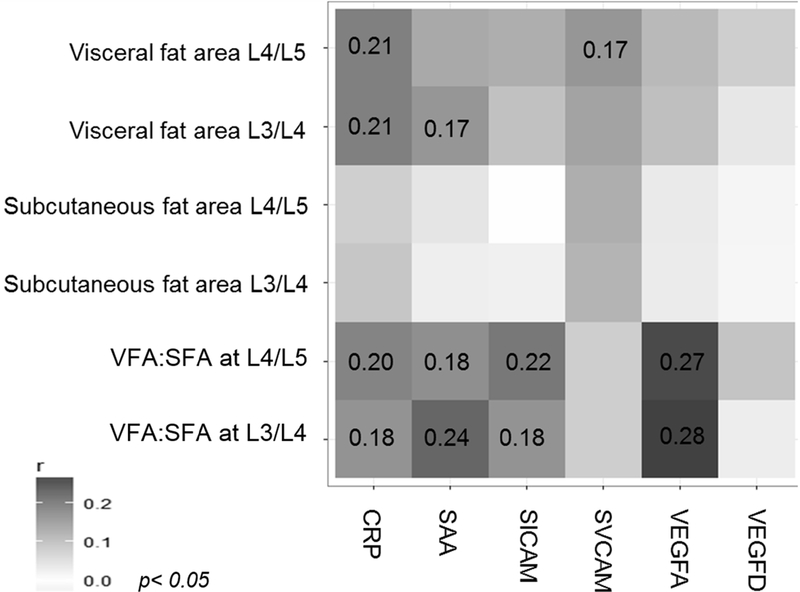
Correlation coefficients are presented for associations that reached the significance threshold of p< 0.05. Analyses were adjusted for age, sex, and tumor stage. L, level; VFA, visceral fat area; SFA, subcutaneous fat area; CRP, C reactive protein; SAA, serum amyloid A; SICAM, soluble intracellular adhesion molecule 1; SVCAM, soluble vascular adhesion molecule 1; VEGFA, vascular endothelial growth factor A; VEGFD, vascular endothelial growth factor D.
Table 3.
Pearson partial correlation coefficients between adiposity and inflammation- and angiogenesis-related biomarker measurements adjusted for age, sex, and tumor stage
| VEGF-A (pg/L) |
VEGF-D (pg/L) |
CRP (mg/L) |
SAA (mg/L) |
sICAM-1 (mg/L) |
sVCAM-1 (mg/L) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| BMI (kg/m2) | −0.09 | 0.25 | −0.03 | 0.69 | 0.12 | 0.16 | 0.00 | 0.98 | 0.09 | 0.32 | 0.23 | 0.006 |
| Subcutaneous fat area, L3/L4 (cm2) | −0.04 | 0.66 | 0.02 | 0.86 | 0.10 | 0.27 | 0.03 | 0.74 | 0.03 | 0.78 | 0.13 | 0.15 |
| Subcutaneous fat area, L4/L5 (cm2) | −0.04 | 0.64 | −0.02 | 0.84 | 0.08 | 0.36 | 0.04 | 0.62 | −0.00 | 0.99 | 0.13 | 0.12 |
| Visceral fat area, L3/L4 (cm2) | 0.11 | 0.17 | 0.04 | 0.62 | 0.21 | 0.01 | 0.17 | 0.037 | 0.10 | 0.21 | 0.15 | 0.07 |
| Visceral fat area, L4/L5 (cm2) | 0.12 | 0.14 | 0.08 | 0.31 | 0.21 | 0.01 | 0.14 | 0.09 | 0.114 | 0.10 | 0.17 | 0.036 |
| Ratio 1: VFA:SFA at L3/L4 | 0.28 | 0.0008 | 0.030 | 0.72 | 0.18 | 0.04 | 0.24 | 0.006 | 0.18 | 0.04 | 0.08 | 0.34 |
| Ratio 2: VFA:SFA at L4/L5 | 0.27 | 0.001 | 0.10 | 0.23 | 0.20 | 0.02 | 0.18 | 0.036 | 0.22 | 0.01 | 0.08 | 0.34 |
Abbreviations: cm, centimeters; VFA: visceral fat area; SFA: subcutaneous fat area; mg: milligrams; L: liters; VEGF-A: vascular endothelial growth factor A; VEGF-D: vascular endothelial growth factor D; CRP: C reactive protein; SAA: serum amyloid A; sICAM-1: soluble intracellular cell adhesion molecule 1; sVCAM-1: soluble vascular cell adhesion molecule 1; BMI: body mass index
Table 4.
Multiple regression analyses between adiposity and inflammation- and angiogenesis-related biomarker measurements adjusted for age, sex, and tumor stage
| VEGF-A (pg/L) |
VEGF-D (pg/L) |
CRP (mg/L) |
SAA (mg/L) |
sICAM-1 (mg/L) |
sVCAM-1 (mg/L) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | β | p | β | p | |
| BMI (kg/m2) | −0.02 | 0.28 | −0.002 | 0.73 | 0.04 | 0.18 | −0.0001 | 1.00 | 0.01 | 0.32 | 0.03 | 0.006 |
| Subcutaneous fat area, L3/L4 (cm2) | −0.0003 | 0.65 | 0.000007 | 0.82 | 0.001 | 0.31 | 0.0004 | 0.79 | 0.0001 | 0.78 | 0.005 | 0.15 |
| Subcutaneous fat area, L4/L5 (cm2) | −0.0003 | 0.64 | −0.00003 | 0.92 | 0.001 | 0.38 | 0.001 | 0.66 | 0.000001 | 1.00 | 0.005 | 0.13 |
| Visceral fat area, L3/L4 (cm2) | 0.0001 | 0.16 | 0.001 | 0.68 | 0.003 | 0.02 | 0.00 | 0.04 | 0.10 | 0.21 | 0.15 | 0.07 |
| Visceral fat area, L4/L5 (cm2) | 0.001 | 0.13 | 0.0004 | 0.35 | 0.0039 | 0.02 | 0.003 | 0.09 | 0.001 | 0.10 | 0.001 | 0.036 |
| Ratio 1: VFA:SFA at L3/L4 | 0.43 | 0.0006 | 0.015 | 0.80 | 0.53 | 0.053 | 0.66 | 0.007 | 0.15 | 0.04 | 0.06 | 0.33 |
| Ratio 2: VFA:SFA at L4/L5 | 0.62 | 0.001 | 0.09 | 0.30 | 0.89 | 0.41 | 0.76 | 0.04 | 0.27 | 0.01 | 0.10 | 0.32 |
Abbreviations: cm, centimeters; VFA: visceral fat area; SFA: subcutaneous fat area; mg: milligrams; L: liters; VEGF-A: vascular endothelial growth factor A; VEGF-D: vascular endothelial growth factor D; CRP: C reactive protein; SAA: serum amyloid A; sICAM-1: soluble intracellular cell adhesion molecule 1; sVCAM-1: soluble vascular cell adhesion molecule 1; BMI: body mass index
Results from sensitivity analyses excluding patients who underwent a CT scan over 6 months prior to or 6 months post-blood draw (n = 8, 4.3%) remained unchanged. Sensitivity analyses excluding stage IV colorectal cancer patients (n = 51, 27.1%) resulted in stronger correlations across all biomarker levels (Supplemental Table 4).
DISCUSSION
Our study of 188 patients diagnosed with colorectal cancer enrolled in the prospective, international ColoCare Study quantified area-based adipose tissue compartments via diagnostic CT scans and systemic biomarkers from patient sera to elucidate associations between the distribution of adipose tissue and circulating inflammation- and angiogenesis-related biomarker levels in colorectal cancer patients. Serum-based levels of CRP and SAA were modestly correlated with VFA and showed moderate correlations with the VFA:SFA ratio, but not with SFA. Correlations with sICAM-1 and VEGF-A were uniquely associated with the VFA:SFA ratio. This study is the first to investigate correlations between CT-based adipose tissue measurements and systemic levels of inflammatory and angiogenesis biomarkers among patients diagnosed with colorectal cancer.
Evidence from clinical and translational studies has led to the consensus that thirteen types of cancer are convincingly associated with body fatness, including cancers of the colon and rectum (5,27–29). In particular, visceral fat has been identified as the main driver of the obesity-cancer link. Compared to BMI, visceral adiposity has been reported to be a clinically significant predictor of short-term post-operative surgical complications, as well as long-term clinical outcomes (including recurrence and survival) among colorectal cancer patients (12,30,31). Increased metabolic processes leading to the production and secretion of tumor-promoting markers in visceral adipose tissue (VAT) (11), corroborates the distinct role of visceral fatness as a substantial component of the obesity-cancer link (11,12,30–34). Our results show a weaker correlation of pro-inflammatory biomarkers with BMI than with VAT. This observation supports the quantification of adipose tissue compartments as an improved predictor of tumor-promoting processes relative to BMI metrics.
The crosstalk between a tumor and its adipose tissue microenvironment is a complex interplay that includes heterogeneous cells as well as local and systemic secreted mediators (10,35). These adiposity-driven inflammatory processes can enhance carcinogenesis and tumor progression, including via the provision of cytokines to the tumor microenvironment (36). Visceral adipose tissue is directly adjacent to the colon and thus, part of the developing tumor microenvironment (10,35). In our study, we demonstrated moderately strong correlations between VFA and VFA:SFA, and inflammation-related biomarkers CRP and SAA.
Inflammatory processes driven by adipose tissue also sustain pro-angiogenic signals to the tumor microenvironment, as the oxygen and nutrient needs of tumor cells are supplied via the establishment of tumor-associated angiogenesis (36,37). Key mediators involved in angiogenesis, including VEGF and platelet-derived growth factor (PDGF), may also be driven by the obesity-related imbalance of adipokines (38) and are important targets for therapeutic development (39). Consistent with these findings, body fatness has been hypothesized as a predictive marker of anti-VEGF agents’ efficacy (e.g. bevacizumab, ramucirumab), particularly among individuals diagnosed with metastatic colorectal cancer (40–42). However, studies have reported conflicting evidence on the predictive role of body fatness in therapeutic efficacy, presenting none or a negative association between excess adiposity and outcomes of anti-VEGF agents (40–43). Intervention studies among cancer-free individuals with excess body fat have also reported a reduction in circulating VEGF levels associated with diet- and/or exercise-induced weight loss (44–47). These results suggest that the weight loss-induced reduction of adipose tissue does lead to alterations in the production and availability of angiogenesis-related mediators. Yet whether adiposity and angiogenesis-related pathways are linked among colorectal cancer patients remains unknown.
We observed associations between VFA, but not SFA, and angiogenesis biomarkers among colorectal cancer patients. In particular, systemic levels of VEGF-A were moderately correlated with the VFA:SFA ratio. These correlations were stronger in sensitivity analyses after excluding individuals with advanced colorectal cancer. This observation further lends support to our hypothesis that VFA may be correlated with circulating angiogenesis and inflammation biomarkers, particularly since patients with metastatic disease often have considerable cachexia and extensive depletion of adipose tissue, particularly VFA (48). Given our observed findings from sex stratified analysis, further sex-specific research regarding body fatness and angiogenesis biomarkers is warranted. Together, our results support an important role for visceral adiposity in the activation of inflammation and pro-angiogenic pathways, and warrant future studies that seek to evaluate the effectiveness of circulating angiogenesis-related biomarkers as predictive markers of targeted therapeutics of angiogenic signals among colorectal cancer patients.
Our study has several strengths and limitations: to our knowledge, this study is the first to assess correlations between adipose tissue compartments and systemic biomarker levels of inflammation and angiogenesis among colorectal cancer patients. The use of paired CT scan data to quantify specific adipose tissue compartments, medical records, and sera from colorectal cancer patients enrolled in the prospective ColoCare Study provided a well-characterized cohort of patients to examine these associations. Our study was limited in that inclusion of patients required available, pre-existing CT scan measurements from standard diagnostics for retrospective evaluation due to radiation protection. Since we evaluated correlations between biomarkers of inflammation and angiogenesis with adiposity in a cross-sectional design, our results do not allow for temporal interpretations. Finally, our results should be interpreted in the context of our study population of cancer patients, which additionally comprised a high proportion of smokers and rectal cancer patients, and are not generalizable to the general population.
In conclusion, visceral adiposity is associated with systemic biomarker levels of inflammation and angiogenesis among colorectal cancer patients. While no such associations were reported for subcutaneous adipose tissue, identification of this link between visceral fat and circulating biomarkers supports the impact of visceral adiposity on carcinogenesis and angiogenesis-promoting processes in colorectal cancer. Given the rise of the obesity epidemic among adults, our results emphasize the critical need to evaluate components of body fatness among colorectal cancer patients at time of cancer diagnosis and to understand the unique contributions of adipose tissue compartments to colorectal carcinogenesis.
Supplementary Material
ACKNOWLEDGEMENTS
ColoCare Study protocols, questionnaires, and procedures were developed in collaboration with ColoCare investigators at the Fred Hutchinson Cancer Research Center. We thank our collaborators involved in ColoCare Study patient recruitment, particularly Stephanie Skender, Clare Abbenhardt-Martin, and Verena Widmer. We also thank laboratory technicians Anette Brendel, Marita Wenzel and Renate Skatula.
This work was supported by grants from the National Institute of Health/National Cancer Institute (U01 CA206110, R01 CA189184, R01 CA211705, and R01 CA207371 to C.M.U.; R35 CA197627 to S.D.H.), the German Consortium of Translational Cancer Research (DKTK) and the German Cancer Research Center, the Matthias Lackas Foundation, Stiftung LebensBlicke, Claussen-Simon Stiftung (Germany), and the Huntsman Cancer Foundation. A.N.H. was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HG008962 from the National Human Genome Research Institute. The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA042014.
FUNDING AND MANUSCRIPT DISPOSITION
This work was supported by grants from the National Institute of Health/National Cancer Institute (U01 CA206110, R01 CA189184, R01 CA211705, and R01 CA207371 to C.M.U.; R35 CA197627 to S.D.H.), the German Consortium of Translational Cancer Research (DKTK) and the German Cancer Research Center, the Matthias Lackas Foundation, Stiftung LebensBlicke, Claussen-Simon Stiftung (Germany), and the Huntsman Cancer Foundation. A.N.H. was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HG008962 from the National Human Genome Research Institute. The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA042014.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest with this work.
REFERENCES
- 1.Obesity Trends in the United States. Journal of the National Cancer Institute 2016;108(10) doi 10.1093/jnci/djw246. [DOI] [PubMed] [Google Scholar]
- 2.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet (London, England) 2011;378(9793):815–25 doi 10.1016/s0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund. Diet, nutrition, physical activity and colorectal cancer. 2017.
- 4.Ulrich CM, Himbert C, Holowatyj AN, Hursting SD. Energy balance and gastrointestinal cancer: risk, interventions, outcomes and mechanisms. Nature reviews Gastroenterology & hepatology 2018. doi 10.1038/s41575-018-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. The New England journal of medicine 2016;375(8):794–8 doi 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Cancer Research Fund. Diet, nutrition, physical activity and colorectal cancer. American Institute for Cancer Research; 2018.
- 7.Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematology/oncology clinics of North America 2015;29(1):1–27 doi 10.1016/j.hoc.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lennon H, Sperrin M, Badrick E, Renehan AG. The Obesity Paradox in Cancer: a Review. Current oncology reports 2016;18(9):56 doi 10.1007/s11912-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34(26):3133–40 doi 10.1200/jco.2016.66.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himbert C, Delphan M, Scherer D, Bowers LW, Hursting S, Ulrich CM. Signals from the Adipose Microenvironment and the Obesity-Cancer Link-A Systematic Review. Cancer prevention research (Philadelphia, Pa) 2017;10(9):494–506 doi 10.1158/1940-6207.capr-16-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liesenfeld DB, Grapov D, Fahrmann JF, Salou M, Scherer D, Toth R, et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: the ColoCare study. The American journal of clinical nutrition 2015;102(2):433–43 doi 10.3945/ajcn.114.103804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozoya OO, Siegel EM, Srikumar T, Bloomer AM, DeRenzis A, Shibata D. Quantitative Assessment of Visceral Obesity and Postoperative Colon Cancer Outcomes. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2017;21(3):534–42 doi 10.1007/s11605-017-3362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark W, Siegel EM, Chen YA, Zhao X, Parsons CM, Hernandez JM, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg 2013;216(6):1070–81 doi 10.1016/j.jamcollsurg.2013.01.007(S1072-7515(13)00045-8[pii]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gigic B, Boeing H, Toth R, Bohm J, Habermann N, Scherer D, et al. Associations Between Dietary Patterns and Longitudinal Quality of Life Changes in Colorectal Cancer Patients: The ColoCare Study. Nutr Cancer 2018;70(1):51–60 doi 10.1080/01635581.2018.1397707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liesenfeld D, Habermann N, Toth R, Owen R, Frei E, Böhm J, et al. Changes in urinary metabolic profiles of colorectal cancer patients enrolled in a prospective cohort study (ColoCare). Metabolomics : Official journal of the Metabolomic Society 2015;11(4):998–1012 doi 10.1007/s11306-014-0758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skender S, Bohm J, Schrotz-King P, Chang-Claude J, Siegel EM, Steindorf K,@ et al. Plasma 25-Hydroxyvitamin D3 Levels in Colorectal Cancer Patients and Associations with Physical Activity. Nutr Cancer 2017;69(2):229–37 doi 10.1080/01635581.2017.1265131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holowatyj AN, Haffa M, Lin J, Gigic B, Ose J, Warby C, et al. Abstract 5249: Crosstalk between visceral adipose and tumor tissue in colorectal cancer patients: Molecular signals driving host-tumor interaction. Cancer research 2018(AACR Annual Meeting 2018) doi DOI: 10.1158/1538-7445.AM2018-5249. [DOI] [Google Scholar]
- 18.Nattenmueller J, Hoegenauer H, Boehm J, Scherer D, Paskow M, Gigic B, et al. CT-based compartmental quantification of adipose tissue versus body metrics in colorectal cancer patients. European radiology 2016;26(11):4131–40 doi 10.1007/s00330-016-4231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond) 2010;34(4):781–7 doi 10.1038/ijo.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balentine CJ, Marshall C, Robinson C, Wilks J, Anaya D, Albo D, et al. Validating quantitative obesity measurements in colorectal cancer patients. J Surg Res 2010;164(1):18–22 doi 10.1016/j.jss.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 21.Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol 1986;250(6 Pt 1):E736–45. [DOI] [PubMed] [Google Scholar]
- 22.Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, et al. Abdominal fat: standardized technique for measurement at CT. Radiology 1999;211(1):283–6 doi 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 23.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series; 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 24.Larsson A, Skoldenberg E, Ericson H. Serum and plasma levels of FGF-2 and VEGF in healthy blood donors. Angiogenesis 2002;5(1–2):107–10. [DOI] [PubMed] [Google Scholar]
- 25.Sallinen H, Heikura T, Koponen J, Kosma VM, Heinonen S, Yla-Herttuala S, et al. Serum angiopoietin-2 and soluble VEGFR-2 levels predict malignancy of ovarian neoplasm and poor prognosis in epithelial ovarian cancer. BMC Cancer 2014;14:696 doi 10.1186/1471-2407-14-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burtis CA, Ashwood ER, Bruns DE. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics London: Elsevier Health Sciences; 2012. [Google Scholar]
- 27.Berriel Diaz M, Herzig S, Schafmeier T. Biological Mechanisms for the Effect of Obesity on Cancer Risk: Experimental Evidence. Recent Results Cancer Res 2016;208:219–42 doi 10.1007/978-3-319-42542-9_12. [DOI] [PubMed] [Google Scholar]
- 28.Westbrook AM, Szakmary A, Schiestl RH. Mouse models of intestinal inflammation and cancer. Arch Toxicol 2016;90(9):2109–30 doi 10.1007/s00204-016-1747-2. [DOI] [PubMed] [Google Scholar]
- 29.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015;12(10):584–96 doi 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe J, Tatsumi K, Ota M, Suwa Y, Suzuki S, Watanabe A, et al. The impact of visceral obesity on surgical outcomes of laparoscopic surgery for colon cancer. International journal of colorectal disease 2014;29(3):343–51 doi 10.1007/s00384-013-1803-9. [DOI] [PubMed] [Google Scholar]
- 31.Esemuede IO, Murray AC, Lee-Kong SA, Feingold DL, Kiran RP. Obesity, regardless of comorbidity, influences outcomes after colorectal surgery-time to rethink the pay-for-performance metrics? Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2014;18(12):2163–8 doi 10.1007/s11605-014-2672-4. [DOI] [PubMed] [Google Scholar]
- 32.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Journal of the National Cancer Institute 2006;98(13):920–31. [DOI] [PubMed] [Google Scholar]
- 33.Vongsuvanh R, George J, Qiao L, van der Poorten D. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett 2013;330(1):1–10 doi 10.1016/j.canlet.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 34.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev 2006;27(7):762–78 doi 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 35.Ulrich CM, Himbert C, Holowatyj AN, Hursting SD. Energy balance and gastrointestinal cancer: risk, interventions, mechanisms and outcomes Nature Reviews Gastroenterology and Hepatology Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–74 doi 10.1016/j.cell.2011.02.013S0092-8674(11)00127-9[pii]. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Adjei AA. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015;20(6):660–73 doi 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nature reviews Endocrinology 2014;10(8):455–65 doi 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park DJ, Thomas NJ, Yoon C, Yoon SS. Vascular endothelial growth factor a inhibition in gastric cancer. Gastric Cancer 2015;18(1):33–42 doi 10.1007/s10120-014-0397-4. [DOI] [PubMed] [Google Scholar]
- 40.Faruk Aykan N, Yildiz I, Sen F, Kilic L, Keskin S, Ciftci R, et al. Effect of increased body mass index (BMI) on time to tumour progression (TTP) in unresectable metastatic colorectal cancer (mCRC) patients treated with bevacizumab-based therapy. Med Oncol 2013;30(3):679 doi 10.1007/s12032-013-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil JP, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 2010;59(3):341–7 doi 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto Y, Oki E, Emi Y, Tokunaga S, Shimokawa M, Ogata Y, et al. Low Visceral Fat Content Is a Negative Predictive Marker for Bevacizumab in Metastatic Colorectal Cancer. Anticancer Res 2018;38(1):491–9 doi 10.21873/anticanres.12249. [DOI] [PubMed] [Google Scholar]
- 43.Jubb AM. Visceral fat and bevacizumab in metastatic colorectal cancer. Gut 2010;59(10):1449–50; author reply 50 doi 10.1136/gut.2010.213777. [DOI] [PubMed] [Google Scholar]
- 44.Duggan C, Tapsoba JD, Wang CY, Foster-Schubert KE, McTiernan A. Long-term Effects of Weight Loss & Exercise on Biomarkers Associated with Angiogenesis. Cancer Epidemiol Biomarkers Prev 2017. doi 10.1158/1055-9965.epi-17-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duggan C, Xiao L, Wang CY, McTiernan A. Effect of a 12-month exercise intervention on serum biomarkers of angiogenesis in postmenopausal women: a randomized controlled trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2014;23(4):648–57 doi 10.1158/1055-9965.epi-13-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duggan C, Tapsoba Jde D, Wang CY, McTiernan A. Dietary Weight Loss and Exercise Effects on Serum Biomarkers of Angiogenesis in Overweight Postmenopausal Women: A Randomized Controlled Trial. Cancer research 2016;76(14):4226–35 doi 10.1158/0008-5472.can-16-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cullberg KB, Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Effect of weight loss and exercise on angiogenic factors in the circulation and in adipose tissue in obese subjects. Obesity (Silver Spring) 2013;21(3):454–60 doi 10.1002/oby.20060. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. Journal of cachexia, sarcopenia and muscle 2013;4(2):95–109 doi 10.1007/s13539-012-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


