Abstract
Background:
There is a substantial racial/ethnic disparity in female breast cancer mortality in Chicago between Non-Hispanic Black (NHBlack) and Hispanic patients compared to their Non-Hispanic White (NHWhite) counterparts. This observation prompted a multilevel examination of factors that might account for the disparity, with the goal of identifying potential policy interventions that might meaningfully address it
Methods:
In the Breast Cancer Care in Chicago study, 411 NHBlack, 397 NHWhite and 181 Hispanic patients diagnosed between the ages of 30–79 were interviewed, and medical records were abstracted information on screening and diagnostic follow-up. We conducted a multilevel analysis to assess the role of neighborhood context, patient resources, facility characteristics and mode of detection in determining the disparity in later stage at diagnosis.
Results:
After adjustment for neighborhood context, mode of detection and facility accreditation/resources, there was no significant disparity in later stage breast cancer diagnosis between NHBlack or Hispanic patients compared to NHWhite patients.
Conclusion:
The results suggest that racial/ethnic differences in mode of detection and facility accreditation/resources account for most of the disparity in stage at diagnosis. Understanding the causes of differential screen-detection and access to highly accredited facilities could inform interventions to meaningfully address this disparity.
Impact:
Multilevel approaches to studying health disparities are becoming the research standard for understanding and addressing health disparities. Optimal design of multilevel interventions addressing disparities in later stage diagnosis would benefit from enhanced understanding of pathways to detection and diagnosis available to patients in medically underserved communities.
Keywords: multilevel, breast cancer, breast cancer disparity, neighborhood context, patient resources, health care facilities
Background.
Despite improvements in overall survival from breast cancer, racial and ethnic disparities in survival in the United States remain.1–3 Access to mammography and diagnostic follow-up of abnormal screens, which permit identification of breast cancer at early stages when prognosis is most favorable, play an important role in these disparities In particular, minority and socioeconomically disadvantaged women are more likely to be screened at lower resourced and non-accredited facilities4, 5, experience delays in care,6 and are less likely to be referred to comprehensive care centers for follow-up.4, 5 For disadvantaged women the processes of referral for screening, getting screened and receiving high quality follow-up care in a timely manner is complex. The process is affected at multiple levels, ranging from national level policies, to neighborhood context to patient and facility characteristics.
Using data from a study conducted in Chicago, we conducted an analysis in a multilevel framework to further understand how disparities in later stage at diagnosis among Hispanic and Non-Hispanic Black patients (NHBlack) compared to Non-Hispanic White patients (NHWhite) come about and might be reduced.7–9 Our ultimate goal was to identify potential policy interventions that might meaningfully address the problem.
At the time of data collection for this study (2005–2008), Chicago was one of the most racially and ethnically segregated cities in the United States.10-12 Low income and racial and ethnic minority patients, largely residing in medically underserved communities on the south and west sides of the city, often received their care in under resourced safety net hospitals and public health clinics.4, 5, 13 Residents of the more racially, ethnically, and socioeconomically diverse north and east sides were more likely to receive their care at academic and high volume health centers primarily located there.4, 5 Breast screening and diagnosis at safety net hospitals was often fragmented. Referral for guideline-concordant specialty care to accredited facilities usually required travel outside the patient’s area of residence. Choice was further constrained by whether the patient’s provider network, established by third party payers, included accredited facilities4, 5, 14 Reimbursement policy, distance, and the primary care provider’s preferences often resulted in patient referral for diagnostic follow-up of an abnormal mammogram to a local safety net hospital. These hospitals in turn, often lack the full range of diagnostic and treatment options requiring that patients be referred elsewhere for diagnostic resolution of an abnormal mammogram..4, 5, 15
Conceptual Model
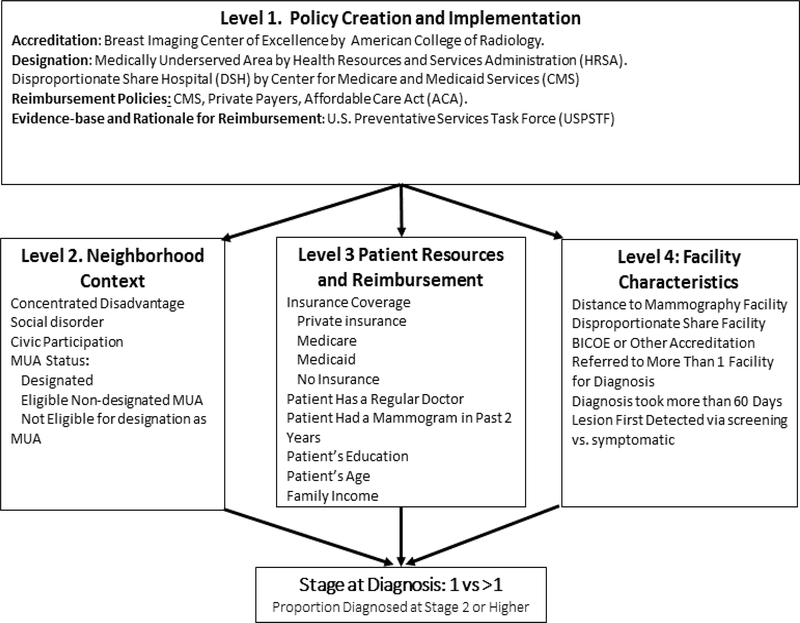
Models by Taplin, Zapka, and Warnecke7,16, 17 inform our multi-level logic model focused on the influence of policy implementation on patient outcomes during mammography screening and follow-up. Details of the model are shown in Figure 1.
Figure 1.
Multilevel model of hypothesized pathways of influence on disparity in stage of breast cancer diagnosis.Figure 1 represents a multilevel model for the analysis of the influence of policy on stage at diagnosis. Level 1 includes policies that could influence the outcome stage at diagnosis. Levels 2, 3 and 4 represent the locations of potential policy influence.
Level 1, Policy Creation and Implementation, refers to agencies, institutions and professional associations which create policy. The American College of Radiology (ACR) defines current standards for radiologic screening and follow-up and accredits high-performing facilities as Breast Cancer Centers of Excellence (BICOE).18, 19 Federal and state agencies including Medicare and Medicaid (CMS) determine criteria for designation of disproportionate share (DSH) hospitals. The Centers for Disease Control and Prevention (CDCP), the Illinois Breast and Cervical Cancer Program (IBCCP), create and define reimbursement criteria and eligibility for free services. The Health Resources and Service Administration’s Bureau of Primary Care designates communities as medically underserved areas or populations (MUA/MUP). The U.S. Preventive Services Task Force (USPSTF) defines the evidence base for reimbursement and provides a rationale for reimbursement policies.
Level 2, Neighborhood Context, focuses primarily on neighborhood designation as MUA/MUP, which carries with it capital support for implementing federally qualified health centers (FQHC), prospective payment for patient care, discounted drug pricing, free vaccines, and assistance in recruiting and retaining primary care providers. It is necessary to apply for such a designation and proposals from a particular area must demonstrate significant community participation. Failure to receive designation limits the development of community health centers and requires patient travel for health care services at accredited facilities outside their neighborhood.6, 13, 14 Further details on designation criteria a discussed below.
At Level 3, Patient Resources and Reimbursement Criteria, refers to patient resources and policies that affect patient control and access to resources and service reimbursement for mammography and follow-up care. Socio-demographic factors, including age, race/ethnicity, education, household income and insurance status, affect the level of control the patient has over access to mammography and necessary diagnostic work-up following an abnormal mammogram. Insurance is a primary determinant of access to guideline concordant care.4, 5, 14 Reimbursement policies differ across Medicare, Medicaid and private insurance. Moreover, if the patient’s insurance provider network does not include a highly accredited provider (e.g. BICOE accredited facility) in the patient’s network, the primary care provider may not know of or consider such referrals. Other relevant resources include having a regular source of primary care, a history of regular screening mammography and knowledge and eligibility for programs IBCCP or charities.
Level 4, Facility Characteristics, considers the influence of policies governing proximal factors influencing how the tumor is first detected: these include level of facility accreditation, and DSH designation of facilities, a marker for low resources.5 Accreditation as a BICOE by the ACR indicates the screening program exceeds the national standard for mammography screening and follow-up for diagnosis.5 In Chicago, at the time of this research, 11 full service or academic facilities had BICOE designation. During this period, many patients received mammograms and diagnostic follow-up at unaccredited facilities.4, 5
We hypothesized that access to accredited sources of screening and follow-up was an important deterrent of later stage breast cancer diagnosis but that such access depended on local practice regarding referral. For example, if a primary care provider (PCP) made a referral for mammography or follow-up diagnosis to a facility that was able to provide follow-up diagnostic imaging then the referral will be more likely to lead to timely diagnosis.6, 20, 21 However, if the PCP referral was to a local DSH hospital that was unable to provide the recommended diagnostic follow-up, diagnostic resolution might require one or more additional referrals resulting in additional delays.4-6
Materials and Methods
Sample
Between 2005 and 2008, with the assistance of the Illinois State Cancer Registry, the Breast Cancer Care in Chicago study (BCCC) identified 1754 newly diagnosed breast cancer patients, using rapid case ascertainment from 54 hospitals located in and adjacent to Chicago. These patients were invited to participate in a survey designed to track their experience from initial discovery of a lesion through diagnosis. Eligible patients were female, diagnosed between ages 30–79, and Chicago residents when diagnosed. Nine hundred eighty nine patients, including 411 (42%) NHBlack patients, 397 (40%) NHWhite patients, and 181 (18%) Hispanic patients, completed a 90 minute computer-assisted personal interview representing a 56.4% response rate. A set of post stratification weights brought the sample back to approximately the distribution of the initial population of 1754 patients. From medical record data on 857 consenting patients, we obtained information on pathologic stage at diagnosis, tumor characteristics, and information on date of diagnosis.22 We obtained verbal consent at the time the patient was recruited and written consent at the time of the face to face interview. The Institutional Review Boards of the University of Illinois at Chicago (UIC) and the State of Illinois Department of Public Health approved the BCCC study.
Measures
Stage at diagnosis, was determined from the surgical pathology report abstracted from the patient’s medical record and assigned using the American Joint Commission on Cancer staging system.22 Stage was dichotomized at the point where breast cancer patients are most likely to have the best outcome from early detection and guideline concordant diagnostic follow-up. Stage 0 is in situ or cancer cells that have not invaded into breast tissue, stage 1 consists of smaller tumors that are confined to the breast. Stages 2–4 define larger tumors and those with regional or distant spread to lymph nodes and adjacent or distant organs. Stages 0,1 were coded as early stage and 2, 3, and 4 are coded as later stage in this analysis.23
Neighborhood Context
Index of Concentrated Disadvantage, based on data from the 2010 census, is a census tract level measure based on the sum of the proportion of residents in each census tract below the poverty line, unemployed, female-heads of households, under age 18 years.24-26
Medically Underserved Area Designation.
All census tracts in Chicago are clustered into 77 community areas. For each area eligibility for designation as MUA/MUP depends on a score between 0 and 62.5 on the Index of Medical Underservice (IMU), composed of four elements: ratio of primary care providers per 1,000 population, the infant mortality rate, percent of population with incomes below the poverty rate and percent over age 65. In Chicago between 6/04/1984 and 8/28/2008, 39 had IMU scores from 36.7 to 61.8 and designated MUA/MUPs Seven other eligible communities, scored from 48.6–61.7, were never designated.27 At the time of this study, the rate of poverty in eligible but never designated areas was 26% compared to 23% and 13% in designated and not-eligible areas, respectively. There were 195 safety net clinics in the designated areas compared to 15 in the eligible but never designated areas.27
At their initial contact with the project, patients provided their home address from which we assigned patient’s residence in areas that were either designated, eligible but never designated, or not-eligible for MUA/MUP designation. The patient’s address also was used to calculate driving time as the measure of distance to the facility.
Patient Resources and Health Care Access
The BCCC assessed past cancer screening behavior by whether the patient received a mammogram within 2 years. Whether the patient self-reported that she had a regular doctor was also obtained in the interview. Insurance was included as a component of patient resources and coded as private (no Medicaid or Medicare), Medicare (solely or with a private supplement), Medicaid (regardless of other insurance), or no insurance. This classification is mutually exclusive and exhaustive. A count of comorbidities ranging from zero to six was also obtained during the BCCC interview as were race, ethnicity, patients’ date of birth, years of schooling and family income. Age and education were measured in years; family income was measured categorically and recoded to midpoints in thousands of dollars.
Policy and Facility Characteristics
Accreditation of the facility of medical presentation is defined as accredited by the American College of Radiology as a Breast Imaging Center of Excellence (BICOE).18 In Chicago 11 mammography facilities were designated as BICOE. Twenty-three percent (N = 26) of the facilities including nonhospital sites and public health facilities included in this analysis were designated as disproportionate share facilities (DSH) by the state of Illinois. The BCCC ascertained the number of referrals involved in the diagnosis and dichotomized it as one or more than one facility visited. Diagnostic delay was defined as more than 60 days between self-reported date of first medical presentation and the date of a definitive diagnosis/biopsy. Mode of detection was based on the patients’ responses to a series of questions at interview and defined as symptomatic (lump, pain, clinical breast exam, etc.) versus screen detection (asymptomatic, routine radiologic screening).
Descriptive Statistics
Table 1 shows raw sample sizes, unweighted means or percentages and standard deviations for all variables by race/ethnicity prior to missing data imputation. Results for dichotomous variables are reported as percentages. As noted, previously, eighteen percent of the respondents were of Hispanic origin. The remainder were split about equally between NHWhite and NHBlack, about 40 percent each. Thirty five percent of NHWhite patients had later stage breast cancer (stage 2–4 vs. 0, 1) compared with 47% of NHBLack and 53% of Hispanic patients.
Table 1:
Descriptive Statistics by Race-Ethnicity
| Total | White | Black | Hispanic | ||
|---|---|---|---|---|---|
| Variable | N 989 | 397 | 411 | 181 | p valuec |
| Stage at diagnosis (N=857)ae | 0.003 | ||||
| 0 | 200 (23%) | 93 (27%) | 81 (23%) | 26 (16%) | |
| 1 | 289 (34%) | 131 (38%) | 109 (31%) | 49 (30%) | |
| 2 | 255 (30%) | 89 (26%) | 108 (31%) | 58 (36%) | |
| 3 | 97 (11%) | 28 (8%) | 46 (13%) | 23 (14%) | |
| 4 | 16 (2%) | 2 (1%) | 9 (3%) | 5 (3%) | |
| −0.28 | |||||
| Concentrated disadvantaged | −0.00 (3.02) | −2.13 (1.34) | 2.18 (3.05) | −0.28 (1.99) | <0.001 |
| MUA statuse | <.001 | ||||
| Non-eligible | 487 (49%) | 281 (71%) | 125 (30%) | 81 (45%) | |
| Designated MUA | 399 (40%) | 114 (29%) | 189 (46%) | 96 (53%) | |
| Eligible non-designated | 103 (10%) | 2 (1%) | 97 (24%) | 4 (2%) | |
| Drive time to mammography (mins) N=887ad | 17.6 (12.7) | 17.2 (13.8) | 18.34(12.1) | 16.6 (11.0) | 0.253 |
| Age (years)d | 56.0 (11.3) | 55.9 (11.2) | 56.9 (11.2) | 54.3 (11.6) | 0.043 |
| Education (years)d | 13.1 (3.1) | 14.6 (2.4) | 12.9 (2.2) | 10.4 (4.0) | <0.001 |
| Family income (000’s) N=952)ad | 60.6 (58.0) | 93.0 (66.3) | 39.5 (39.5) | 38.9 (39.2) | <0.001 |
| Regular Drb | 854 (86%) | 354 (89%) | 355 (86%) | 145 (80%) | 0.013 |
| Comorbidities (number)d | 0.71 (1.03) | 0.52 (0.89) | 0.90 (1.09) | 0.69 (1.09) | <0.001 |
| Insurance coveragee | <.001 | ||||
| Private | 524 (53%) | 282 (71%) | 166 (40%) | 76 (42%) | |
| Medicare | 205 (21%) | 83 (21%) | 94 (23%) | 28 (15%) | |
| Medicaid | 140 (14%) | 13 (3%) | 94 (23%) | 33 (18%) | |
| None | 120 (12%) | 19 (5%) | 57 (14%) | 44 (24%) | |
| Mammogram within past two yearsb | 630 (64%) | 259 (65%) | 261 (64%) | 110 (61%) | 0.585 |
| Disprortionate share facilityb | 279 (28%) | 42 (11%) | 152 (37%) | 85 (47%) | <0.001 |
| BICOE accredited facilityb | 600 (61%) | 321 (81%) | 190 (46%) | 89 (49%) | <0.001 |
| Visited more than one facility for dxb | 335 (34%) | 103 (26%) | 147 (36%) | 85 (47%) | <0.001 |
| Dx delayed more than 60 days (N=856)ab | 199 (22%) | 45 (12%) | 100 (27%) | 54 (32%) | <0.001 |
| Radiological dxb | 507 (51%) | 236 (59%) | 195 (47%) | 76 (42%) | <0.001 |
Reduced N due to missing data
Dichotomous variable (N, %)
Chi-square tests on categorical variables, ANOVA on continuous
Continuous variable (Mean, SD)
Categorical variable (N, %)
About half of the patients lived in areas not-eligible for designation as MUA/MUP. NHWhite patients were considerably less likely than NHBlack and Hispanic patients to live in designated MUA/MUP areas (29% vs. 46% and 53%, respectively) or to live in eligible but undesignated areas (0.5% vs. 24% and 2%, respectively).
Nearly all patients (86%) reported having a regular doctor. About 40% had either Medicare or Medicaid, 70% reported private insurance and 12% were uninsured. NHWhite patients were more likely than NHBlack and Hispanic patients to be covered by private insurance (54% and 41% respectively); Hispanic patients were most likely to be uninsured.
Compared with NHWhite patients (11%), NHBlack and Hispanic patients were more likely to be diagnosed at a DSH facility (37% and 47% vs. 11%, respectively), to be referred to more than one facility, (36% and 47% vs.26%) to experience a diagnostic delay in excess of 60 days (27% and 32% vs. 12%) and less likely to be diagnosed at a BICOE facility (46% and 49% vs. 81%). Finally, NHWhite patients were more likely than NHBlack or Hispanic patients to have their breast cancer initially detected through screening (59% vs. 47% and 42%, respectively).
Missing Data Imputation
As shown in Table 1, there was a considerable amount of missing data on stage at diagnosis (13%), drive-time to mammography facilities (10%), diagnostic delay (8%) and household income (4%), resulting in 713 of 989 patients with complete data on all variables. To account for missing data we used multiple imputation methods as implemented in Stata version 1428, 29 We used a “fully conditional” approach to impute 50 datasets28, using a separate statistical model appropriate for each variable with missing data. The dichotomous variables, later stage and diagnostic delay, were modeled using logistic regression. Income and drive time to mammography, continuous variables having skewed distributions, were modeled using predictive mean matching. The predictor variables in the imputation models consisted of all analysis variables with complete data plus two “auxiliary variables,” whether the respondent survived for five years following diagnosis and an initial estimate of stage at diagnosis, obtained during initial case finding prior to definitive surgery. Table 2 summarizes the imputation process. After imputation and after eliminating a few cases with scattered missing data, our final analytic weighted sample contained 977 cases.
Table 2:
Imputation Results
| Observations | ||||
|---|---|---|---|---|
| Variable | Complete | Incomplete | Imputed | Total |
| Later stage diagnosis | 852 | 125 | 120 | 977 |
| Diagnosis took > 60 days | 902 | 75 | 71 | 977 |
| Drive time to mammography | 883 | 94 | 91 | 977 |
| Family Income | 849 | 28 | 27 | 977 |
Results
Bivariate Associations
Table 3 shows bivariate associations between each of the independent variables and later stage diagnosis, again prior to missing data imputation. Although the strength of association varies as measured by the odds ratio from logistic regression, almost all variables are statistically significant. The exceptions are drive time to mammography, family income, comorbidity count, and diagnostic delay. However, all neighborhood context and institutional variables were significantly associated with later stage. Given the complex pattern of associations among these variables we retained all of them in the multivariate models reported below. We then estimated a sequence of multivariate logistic regression models for later stage, with variables entered into the model in the order corresponding to the multilevel theoretical approach described earlier and in Figure 1.
Table 3:
Bivariate Associations with Late Stage Diagnosis
| Variable | OR | p | LCI | UCI | |
|---|---|---|---|---|---|
| Race Ethnicity | |||||
| NHBlack | 1.560 | 0.004 | 1.151 | 2.122 | a |
| Hispanic | 1.941 | 0.001 | 1.313 | 2.871 | a |
| NHWhite (ref) | 1.000 | ||||
| Concentrated disadvantage | 1.072 | 0.004 | 1.022 | 1.124 | |
| MUA Status | |||||
| Designated MUA | 1.368 | 0.036 | 1.021 | 1.833 | a |
| Eligible, non-designated | 1.914 | 0.008 | 1.181 | 3.101 | a |
| Non-eligible (ref) | 1 | ||||
| Drive time to mammography (minutes) | 0.996 | 0.499 | 0.985 | 1.007 | |
| Age | 0.972 | 0.000 | 0.960 | 0.985 | |
| Education (Years) | 0.955 | 0.051 | 0.913 | 1.000 | |
| Family Income (000’s) | 0.999 | 0.662 | 0.997 | 1.002 | |
| Regular Doctor | 0.540 | 0.003 | 0.361 | 0.808 | |
| Comorbidities (count) | 0.881 | 0.154 | 0.780 | 1.040 | |
| Insurance coverage | |||||
| Medicare | 0.673 | 0.031 | 0.470 | 0.965 | a |
| Medicaid | 1.404 | 0.101 | 0.936 | 2.106 | a |
| No insurance | 1.377 | 0.139 | 0.901 | 2.104 | a |
| Private (ref) | 1.000 | 0.029 | 0.527 | 0.996 | |
| Mammogram within two years | 0.484 | 0.000 | 0.363 | 0.645 | |
| Disproportionate share facility | 1.403 | 0.030 | 1.033 | 1.907 | |
| BICCOE accredited facility | 0.564 | 0.000 | 0.425 | 0.749 | |
| Visited more than one facility for dx | 2.661 | 0.000 | 1.978 | 3.579 | |
| Diagnosis delayed more than 60 days | 0.948 | 0.767 | 0.665 | 1.352 | |
| Radiologic dx | 0.128 | 0.000 | 0.092 | 0.176 | |
Contrast to reference category
In order to deal with the collinear relationship between race-ethnicity and MUA/MUP status, we created indicator variables corresponding to the cells of the cross classification of MUA/MUP status and race-ethnicity. Two strata corresponding to NHWhites and Hispanics residing in MUA/MUP eligible but not designated areas had very small sample sizes (2 and 4 respectively). These six cases were dropped from further analysis.
Results are shown in Table 4. Model 1 shows unadjusted odds ratios for later stage diagnosis among the groups jointly defined by MUA/MUP status and race-ethnicity, compared to NHWhites in non-eligible areas (referent). For example, the odds of a later stage diagnosis for NHBLacks living in eligible not designated areas are 2.31 times as high as the reference group. For NHBlacks living in MUA/MIUP designated areas the odds ratio is 1.65. Hispanic odds are 2.24 for residents in not-eligible areas and 2.94 for those in designated areas relative to the reference group.
Table 4:
Multivariate Logistic Regression Modelsa
| Model 1: Race-Ethnicity | Model 2: Neighborhood | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | OR | p | LCI | UCI | OR | p | LCI | UCI |
| Race/Ethnicy/MUA | ||||||||
| NHWhite designated MUA | 1.609 | 0.055 | 0.990 | 2.615 | 1.551 | 0.076 | 0.955 | 2.519 |
| NHBlack non-eligible | 1.609 | 0.046 | 1.008 | 2.570 | 1.357 | 0.258 | 0.799 | 2.302 |
| NHBlack designated MUA | 1.650 | 0.016 | 1.096 | 2.485 | 1.410 | 0.177 | 0.856 | 2.321 |
| NHBlack eligible non-designated | 2.431 | 0.001 | 1.450 | 4.077 | 2.004 | 0.025 | 1.090 | 3.684 |
| Hispanic non-eligible | 2.238 | 0.005 | 1.280 | 3.913 | 2.109 | 0.010 | 1.199 | 3.710 |
| Hispanic designated MUA | 2.294 | 0.001 | 1.390 | 3.787 | 2.049 | 0.008 | 1.211 | 3.468 |
| NHWhite non-eligible (ref) | 1.000 | 1.000 | ||||||
| Concentrated disadvantage | 1.043 | 0.186 | 0.980 | 1.111 | ||||
| Drive time to mammography | 0.994 | 0.378 | 0.982 | 1.007 | ||||
| Model 3: Patient | Model 4: Organizational | |||||||
| Variable | OR | p | LCI | UCI | OR | p | LCI | UCI |
| Race/Ethnicy/MUA | ||||||||
| NHWhite designated MUA | 1.571 | 0.080 | 0.947 | 2.604 | 1.326 | 0.327 | 0.754 | 2.330 |
| NH Black non-eligible | 1.622 | 0.090 | 0.928 | 2.836 | 1.273 | 0.470 | 0.661 | 2.454 |
| NHBlack designated MUA | 1.462 | 0.157 | 0.864 | 2.472 | 1.081 | 0.803 | 0.587 | 1.991 |
| NHBlack eligible non-designated | 2.528 | 0.005 | 1.324 | 4.829 | 1.865 | 0.092 | 0.904 | 3.848 |
| Hispanic non-eligible | 2.082 | 0.019 | 1.126 | 3.850 | 1.520 | 0.243 | 0.752 | 3.071 |
| Hispanic designated MUA | 1.847 | 0.052 | 0.994 | 3.432 | 1.321 | 0.448 | 0.643 | 2.711 |
| NHWhite non-eligible (ref) | 1.000 | 1.000 | ||||||
| Concentrated disadvantage | 1.037 | 0.292 | 0.969 | 1.110 | 1.036 | 0.384 | 0.957 | 1.121 |
| Drive time to mammography | 0.991 | 0.180 | 0.979 | 1.004 | 0.988 | 0.100 | 0.973 | 1.002 |
| Age | 0.978 | 0.016 | 0.961 | 0.996 | 0.996 | 0.704 | 0.976 | 1.017 |
| Education | 0.971 | 0.359 | 0.912 | 1.034 | 0.968 | 0.375 | 0.901 | 1.040 |
| Family income | 1.002 | 0.143 | 0.999 | 1.006 | 1.004 | 0.037 | 1.000 | 1.007 |
| Regular doctor | 0.754 | 0.225 | 0.478 | 1.190 | 0.954 | 0.852 | 0.578 | 1.573 |
| Comorbidities | 0.940 | 0.453 | 0.799 | 1.105 | 0.987 | 0.893 | 0.814 | 1.196 |
| Insurance coverage | ||||||||
| Medicare | 1.023 | 0.925 | 0.639 | 1.638 | 1.019 | 0.946 | 0.594 | 1.747 |
| Medicaid | 1.249 | 0.380 | 0.760 | 2.052 | 1.224 | 0.478 | 0.700 | 2.141 |
| No Insurance | 0.933 | 0.793 | 0.558 | 1.561 | 0.903 | 0.729 | 0.506 | 1.611 |
| Private (ref) | 1.000 | 1.000 | ||||||
| Mammogram within two years | 0.645 | 0.010 | 0.462 | 0.901 | 0.885 | 0.509 | 0.616 | 1.272 |
| Disproportionate share facility | 1.018 | 0.933 | 0.677 | 1.531 | ||||
| BICOE accredited facility | 0.693 | 0.051 | 0.480 | 1.001 | ||||
| Visited more than one facility for dx | 0.939 | 0.760 | 0.628 | 1.404 | ||||
| Dx delayed > 60 days | 0.784 | 0.290 | 0.500 | 1.230 | ||||
| Radiologic dx | 0.132 | 0.000 | 0.089 | 0.196 | ||||
Results are based on multiple imputation of weighted data
After adjustment for concentrated disadvantage and drive time to mammography, (Model 2) all of the odds ratios for the race/ethnicity-MUA disparity in later stage were modestly attenuated but most remained significantly elevated compared to the referent (NHWhite patients residing in ineligible areas). The exceptions were comparison of NHBlacks residing eligible but not designated area and all Hispanics regardless of residence.
After controlling for patient resources in Model 3 none of the race/ethnicity-MUA disparity odds ratios for later stage were materially changed and all remained significant or marginally so. In particular, the disparity odds ratios for NHBlacks living in eligible not designated areas remained substantially elevated (OR=2.53). The disparity odds ratios for Hispanics, regardless of residence area also remained substantially elevated. Patients who received a mammogram in the previous two years had a one third lower odds of a late stage diagnosis (OR .645).
Finally, after controlling additionally for facility variables (including accreditation, DSH designation, diagnostic delay, whether more than one facility was visited in the diagnostic process) and mode of detection in Model 4, the disparity odds ratios for all race/ethnicity-MUA groups compared to the referent were substantially diminished and only the odds ratio for NHBlacks living in eligible not designated areas remained marginally elevated. Detection through screening was strongly inversely associated with later stage diagnosis (OR 0.13). In addition, diagnosis at a BICOE-accredited facility was marginally and inversely associated with later stage diagnosis (OR .693).
DISCUSSION
The focus of this study is the effect of various levels of policy on disparity between NHWhite female breast cancer patients and NHBlack and Hispanic breast cancer patient on later stage of diagnosis of breast cancer. After adjustment for policies affecting neighborhood context, mode of detection and facility accreditation/resources, there was no significant disparity in later stage breast cancer diagnosis between NHBlack or Hispanic patients compared to NHWhite patients. The results suggest that racial/ethnic differences in mode of detection and facility accreditation/resources account for most of the disparity in stage at diagnosis.
Comparison of patients in MUA/MUP designated areas with areas eligible but never designated provides another dimension to existing literature on how community organization may affect service delivery and potentially health outcomes like later stage at diagnosis.20, 26, 27, 30–33 But designation is primarily related to NHBlack and NHWhite disparity and in the end, the primary issues related to disparity in later stage at diagnosis appear to be access to mammography and follow-up at accredited facilities.
Although the relationship between race/ethnicity and MUA/MUP status is complex, by creating a set of indicator variables to represent the joint relationship we were able to determine that after controlling on neighborhood characteristics in Model 2, first, regardless of where they live, Hispanics suffered a disparity relative to NHWhites who live in ineligible areas and second, NHBlacks living in eligible but undesignated areas also were at a disadvantage compared to the reference group. This pattern persisted in Model 3. However, the disparities were eliminated after controlling on level four variables (organizational characteristics) in Model 4.
Limitations
There are aspects of this study that limit its generalizability. First, the data were cross-sectional and the experiences were self-reported. Although medical record data confirmed some dates such as when screening and diagnostic follow-up took place, we had no information on the systemic aspects of the process, particularly the patterns of interaction between patients, primary care providers and specialists.
Second, patients were interviewed between 2005 and 2008, prior to the introduction of the Affordable Care Act. At that time the disparity in breast cancer mortality between NHBlack and NHWhite patients in Chicago was 68% higher than the national disparity.11 Recent data indicate that the Chicago mortality disparity is equal to national disparity level 48%.11, 12, 15 This significant change probably reflects increased insurance coverage due to the Affordable Care Act (ACA) and local efforts in patient navigation to improve screening and follow-up at DSH hospitals and the implementation of state-wide quality assurance program supported with state funding.34
Third, access to primary care in the eligible, but never designated areas, was more limited than in the designated areas and the literature contains several studies documenting the lack of integration of the primary care practices with specialty care providers, more generally.21, 22, 35, 36
Finally, while modest, the study response rate of 54% is in line with response rates found in current surveys. Registrars who collected data for the Illinois State Cancer Registry identified eligible patients and provided information on diagnosing facility and patient age and race ethnicity for those whom they could not contact, enabling us to create post stratification weights that brought the distribution of our analysis sample closer to the total population of eligible breast cancer patients during the study period.
However our findings are consistent with findings from other research about the experience of disadvantaged women in obtaining a mammogram and where necessary follow-up diagnosis of an abnormality conducted during our study period. Those results showed that women were more likely to be screened at lower resourced and non-accredited facilities,4, 5 to experience delays in care6 and less likely to be referred to comprehensive care centers for follow-up. 4, 5
Recommendations for Further Research
Optimal design of multilevel interventions addressing disparities in later stage breast cancer diagnosis should focus on the health care system and would benefit from enhanced understanding of pathways to detection and diagnosis available to patients in medically underserved communities and potential incentives for improvements that enhance the process. Future studies should track patients through the process from initial receipt of an abnormal screening mammogram result through diagnostic resolution and collect detailed data on patient and provider interaction and the resulting barriers related to travel burden, insurance networks of care, copays and deductibles, inability to obtain timely appointments, missed appointments by the patient; primary care requirements for return visits for new orders rather than providing a global order for follow-up, unnecessary repetition of diagnostic procedures, and other factors.
Multilevel approaches to studying health disparities are becoming the research standard for understanding and addressing health disparities7–9 It is worth noting that multilevel approaches to policy are already an important tool in public health, as demonstrated by the successful policy outcomes in tobacco use and other areas such as energy balance behavior and HPV vaccination. This and other studies4, 11, 13, 22, 26, 31, 32 conducted in Chicago strongly support a policy focus including exploration of policies and incentives to increase referrals to BICOE facilities and to support facilities in obtaining BICOE designation. Navigation research studies could be developed to study how to best increase referrals to BICOE facilities and overcome systematic barriers to accessing them.8, 34 New research should also address insurance and the role of primary care including mergers of PCP clinic systems with specialty care providers and other attempts to integrate primary care in safety net clinics into specialty care practice. Our study did not allow actual assessment of the primary care role in the process in Chicago.
Acknowledgements
The Breast Cancer Care in Chicago Study, which obtained the data in this paper, gratefully acknowledges the active support of the Illinois State Cancer Registry staff in timely identifying the breast cancer patients through rapid case ascertainment and informing them of the project. We also relied heavily on the University of Illinois Survey Research Laboratory for recruiting, interviewing and preparing the interview data for analysis.
Grant Support
1P50 CA106743–01-5. UIC Center for Population Health and Health Disparities. R. Warnecke PI 07/01/03–6/30/06.
1P50 CA106743–07-8 Center for Population Health and Health Disparities, R. Warnecke PI, NIH/NCI 9/30/03–8/31/08 ($7.2M) No-Cost Extension and Supplement through 8/31/09.
1P50CA10743–06S1 (OBSSR funded) Safety Net Care in Chicago. R. Warnecke (Contact Co-PI)
2P50 CA106743–10-15.UIC Center for Population Health and Health Disparities. R. Warnecke, Co-PI (Contact) E. Calhoun Co-PI. 07/01/10–6/30/2016
2P50 CA1067–10S. Supplement Center for Population Health and Health Disparities. R. Warnecke PI 07/01/16–6/30/17.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest.
References
- 1.Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst. 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maskarinec G, Sen C, Koga K, Conroy SM. Ethnic differences in breast cancer survival: status and determinants. Womens Health (Lond). 2011;7: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Keefe EB, Meltzer JP, Bethea TN. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front Public Health. 2015;3: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauscher GH, Allgood KL, Whitman S, Conant E. Disparities in screening mammography services by race/ethnicity and health insurance. J Womens Health (Larchmt). 2012;21: 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauscher GH, Murphy AM, Orsi JM, Dupuy DM, Grabler PM, Weldon CB. Beyond the mammography quality standards act: measuring the quality of breast cancer screening programs. AJR Am J Roentgenol. 2014;202: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina Y, Silva A, Rauscher GH. Racial/Ethnic Disparities in Time to a Breast Cancer Diagnosis: The Mediating Effects of Health Care Facility Factors. Med Care. 2015;53: 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98: 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paskett E, Thompson B, Ammerman AS, Ortega AN, Marsteller J, Richardson D. Multilevel Interventions To Address Health Disparities Show Promise In Improving Population Health. Health Aff (Millwood). 2016;35: 1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croyle RT, Srinivasan S. Informing Future Population Health Interventions. Cancer Epidemiol Biomarkers Prev. 2016;25: 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iceland JD, Weinberg H, Steinmetz E. Racial and Ethnic Segregation in the United States: 1980–2000. Washington, D.C.: U. S. Bureau of the Census, 2002. [Google Scholar]
- 11.Hunt BR, Whitman S, Hurlbert MS. Increasing Black:White disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 2014;38: 118–123. [DOI] [PubMed] [Google Scholar]
- 12.Sighoko D, Murphy AM, Irizarry B, Rauscher G, Ferrans C, Ansell D. Changes in the racial disparity in breast cancer mortality in the ten US cities with the largest African American populations from 1999 to 2013: The reduction in breast cancer mortality disparity in Chicago. Cancer Causes Control. 2017;28: 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patrick G, Bisgaier J, Hasham I, Navarra T, Hickner J. Specialty care referral patterns for the underserved: a study of community health centers on the South Side of Chicago. J Health Care Poor Underserved. 2011;22: 1302–1314. [DOI] [PubMed] [Google Scholar]
- 14.Rauscher GH, Conant EF, Kahn JA, Berbaum ML. Mammogram image quality as a potential contributer to disparities in breast cancer stage at diagnosis: an observational study. BMC Cancer. 2012;13: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschman J, Whitman S, Ansell D. The black:white disparity in breast cancer mortality: the example of Chicago. Cancer Causes Control. 2007;18: 323–333. [DOI] [PubMed] [Google Scholar]
- 16.Zapka J, Taplin SH, Ganz P, Grunfeld E, Sterba K. Multilevel factors affecting quality: examples from the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: Understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Radiology. Breast Imaging Center of Excellence (BICOE). Available from URL: https://www.acr.org/quality-safety/accreditation/bicoe [accessed 2/21/17.
- 19.Central Management Services. Disproportionate Share Hospital (DSH). Available from URL: https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/dsh.html [accessed 2/20/17, 2017].
- 20.Chen AH, Rittenhouse D. The un-managed system of Medicare referrals. J Gen Intern Med. 2012;27: 487–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brazda A, Estroff J, Euhus D, et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol. 2010;17 Suppl 3: 291–296. [DOI] [PubMed] [Google Scholar]
- 22.Mortel M, Rauscher GH, Murphy AM, Hoskins K, Warnecke RB. Racial and Ethnic Disparity in Symptomatic Breast Cancer Awareness despite a Recent Screen: The Role of Tumor Biology and Mammography Facility Characteristics. Cancer Epidemiol Biomarkers Prev. 2015;24: 1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer AJCo. Cancer Staging References. Available from URL: https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx [accessed 3/27/17, 2017].
- 24.Browning CR, Cagney KA. Neighborhood structural disadvantage, collective efficacy, and self-rated physical health in an urban setting. J Health Soc Behav. 2002;43: 383–399. [PubMed] [Google Scholar]
- 25.Tarlov E, Zenk SN, Campbell RT, Warnecke RB, Block R. Characteristics of mammography facility locations and stage of breast cancer at diagnosis in Chicago. J Urban Health. 2009;86: 196–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett RE, Cho YI, Weaver KE, et al. Neighborhood change and distant metastasis at diagnosis of breast cancer. Ann Epidemiol. 2008;18: 43–47. [DOI] [PubMed] [Google Scholar]
- 27.Lopes PM. State-wide Rational Service Areas for Primary Care Services: Lessons from Six States. In: Administration HRaS, editor. Washington: 2000. [Google Scholar]
- 28.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30: 377–399. [DOI] [PubMed] [Google Scholar]
- 29.Enders CK. Applied Missing Data Analysis. New York: Guildford Press, 2010. [Google Scholar]
- 30.Fowler BA. Neighborhood–level influences on delays in diagnostic follow-up from mammography screening in African-American women: a systematic review. Journal of Women’s Health. 2014;3: 151–159. [Google Scholar]
- 31.Paskett ED, Dudley D, Young GS, et al. Impact of Patient Navigation Interventions on Timely Diagnostic Follow Up for Abnormal Cervical Screening. J Womens Health (Larchmt). 2016;25: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plascak JJ, Llanos AA, Pennell ML, Weier RC, Paskett ED. Neighborhood factors associated with time to resolution following an abnormal breast or cervical cancer screening test. Cancer Epidemiol Biomarkers Prev. 2014;23: 2819–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell RT, Li X, Dolecek TA, Barrett RE, Weaver KE, Warnecke RB. Economic, racial and ethnic disparities in breast cancer in the US: towards a more comprehensive model. Health Place. 2009;15: 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Battaglia TA, Bak SM, Heeren T, et al. Boston Patient Navigation Research Program: the impact of navigation on time to diagnostic resolution after abnormal cancer screening. Cancer Epidemiol Biomarkers Prev. 2012;21: 1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reschovsky JD, O’Malley AS. Do primary care physicians treating minority patients report problems delivering high-quality care? Health Aff (Millwood). 2008;27: w222–231. [DOI] [PubMed] [Google Scholar]
- 36.Hurley RE, Pham HH, Claxton G. A widening rift in access and quality: growing evidence of economic disparities. Health Aff (Millwood). 2005;Suppl Web Exclusives: W5–566–576. [DOI] [PubMed] [Google Scholar]