Abstract
Aims:
To determine the association between 20-year trajectories in insulin resistance (IR) since young adulthood and appendicular lean mass (ALM) at middle-age in adults without diabetes.
Methods:
A prospective cohort study was designed among young and middle-aged US men (n=925) and women (n=1,193). Fasting serum glucose and insulin were measured five times in 1985–2005. IR was determined using the homeostasis model assessment (HOMA). ALM was measured in 2005 and ALM adjusted for BMI (ALM/BMI) was the outcome. Sex-specific analyses were performed using latent class models and multivariable linear regressions.
Results:
Three HOMA-IR trajectories were identified. Compared to the low-stable group, the adjusted ALM/BMI difference was −0.041 (95% CI: −0.060 to −0.022) and −0.114 (−0.141 to −0.086) in men, and −0.052 (−0.065 to −0.039) and −0.043 (−0.063 to −0.023) in women, respectively, for the medium-increase and high-increase group. Further adjusting for the treadmill test duration attenuated these estimates to −0.022 (−0.040 to −0.004) and −0.061 (−0.089 to −0.034) in men and −0.026 (−0.038 to −0.014) and −0.007 (−0.026 to 0.012) in women.
Conclusions:
Compared to the low-stable insulin resistance trajectory between early and middle adulthood, the high-increase insulin resistance trajectory was associated with lower ALM/BMI middle-aged men, but not women, without diabetes, after taking into account cardiorespiratory fitness.
Keywords: Insulin resistance, Appendicular lean mass, Diabetes, Sarcopenia, Early prevention
1. Introduction
Sarcopenia, an age-associated decline in lean mass, muscle strength, and physical performance, is a common health problem in older adults (i.e., 65 years or older).1–3 Sarcopenia has been associated with a range of adverse consequences including fall, hospitalization, and mortality.4,5 Currently, there is no cure for age- and disease-related lean mass loss.6 Lean mass acquired in early life, together with age- and disease-related loss, largely determine lean mass among older adults.6–8 Therefore, identifying factors associated with lean mass loss and preservation in early and middle adulthood may play a critical role in improving the quality of life and reducing health and economic burden resulting from sarcopenia in later life.9
Elevated insulin resistance and hyperglycemia are associated with accelerated loss of lean mass and/or function in older adults with or without diabetes (mean age of 60 years or older); most of these studies are cross-sectional.10–13 However, our understanding of the association between insulin resistance and lean mass is limited in young and middle-aged adults without diabetes, even though lean mass begins to decline at a rate of roughly 1% annually starting at 30 years of age.14 Given the aforementioned evidence from older populations, we hypothesize that an increasing trajectory of insulin resistance over time between early and middle adulthood is associated with lower appendicular lean mass, as compared to a relatively stable insulin resistance trajectory, in middle-aged adults without diabetes. To test this hypothesis, we used 20 years of follow-up data from the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
2. Participants and Methods
2.1. Study population
The CARDIA study is an ongoing multicenter longitudinal cohort study that enrolled 5,115 black and white men and women aged 18–30 years in 1985–86 from four field centers: Birmingham, AL; Oakland, CA; Chicago, IL; and Minneapolis, MN. Participants were followed and received extensive examination 2, 5, 7, 10, 15, 20, 25, and 30 years after enrollment. The details of the CARDIA study design can be found elsewhere.15 The current study used data from the ancillary CARDIA Fitness Study in which 2,704 participants underwent dual-energy X-ray absorptiometry (DXA) for assessment of body composition at year 20. Thus, only data between year 0 and 20 were included for current analyses. Participants with either type 1 or type 2 diabetes mellitus (n=236) at any exam between year 0 and 20 were excluded because i) diabetes is a known strong risk factor for muscle loss;6 ii) glucose levels of participants with diabetes on glucose-lowering medication do not reflect natural glucose homeostasis and cannot be compared to glucose levels in those with self-management only. Diabetes was defined as having fasting blood glucose ≥7 mmol/L (126 mg/dL), or 2-hour glucose ≥11.1 mmol/L (200 mg/dL) from the 75-gram glucose tolerance test, or HbA1C ≥ 6.5% (48 mmol/mol), or use of glucose-lowering medications. We further excluded one participant who self-reported transgender status. Glucose and insulin levels at a visit where participants were pregnant or fasted <8 hours before examination were set to missing. Participants with less than three measurements of fasting insulin and glucose (n=240) or missing data for the treadmill test (n=109) were excluded. Participants provided consent at each exam and institutional review boards at each field center approved the study protocols.
2.2. Data collection
All data were collected according to standardized protocols across all exams. For anthropometric assessment, participants were in light clothes and without shoes. For laboratory assays, participants were asked to fast overnight for at least 8 hours and not to smoke or perform heavy exercise prior to each examination.
2.3. Anthropometric assessment
Body weight was measured by a balance-beam scale and height was measured using a vertically mounted metal centimeter ruler and carpenter’s square. Body Mass Index (BMI) was calculated as weight (kg) divided by height in square meters (m2). Fat mass, bone mineral content, and non-bone non-fat mass were quantified by DXA ((Hologic QDR 4500W, Delphi 11.2, Discovery XP 12.1, Discovery XP 2002; Hologic, Bedford, MA), and separated into trunk and appendicular components. Quality control and calibration processes for these DXA machines have been previously described.16 Appendicular lean mass (ALM) was computed as the sum of non-fat, non-bone mass for both arms and legs. Percent total body fat was also determined. We focused on ALM rather than total body lean mass, because ALM is included in the operational criteria to define sarcopenia by several groups including the Foundation of the National Institutes of Health Sarcopenia Project (FNIHSP),1 the International Working Group (IWG),2 and the European Working Group for Sarcopenia in Older Persons (EWGSOP).3 The correlation between ALM and total body lean mass of our study sample was 0.97. We used ALM adjusted for BMI (ALM/BMI) as the study outcome, as recommended by the FNIHSP.1 This definition is more conservative than ALM adjusted for height squared, which is the recommendation of the IWG and EWGSOP.17 Further, in a pooled analysis of six different cohort studies, a consistent association between low lean mass and incident mobility impairment is seen in men and women when ALM/BMI is used as the exposure.18
2.4. Laboratory measurements, dietary assessment, and questionnaires
Fasting serum blood glucose level and fasting insulin level were measured at years 0, 7, 10, 15, and 20. The insulin resistance index, homeostasis model assessment-estimated insulin resistance (HOMA-IR), was calculated as follows: (fasting insulin in μU/mL * fasting glucose in mmol/L) divided by 22.5.19 Estimated glomerular filtration rate (eGFR) was calculated using serum creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation.20 eGFR was available at years 0, 10, 15, and 20. High sensitivity C-reactive protein (hs-CRP) was measured using high-sensitivity nephelometry-based methods (BNII nephelometer, Dade Behring, Eschborn, Germany) at years 7, 15, and 20.
Dietary assessment was performed using the CARDIA 28-day Diet History (approximately 700 items) at years 0, 7, and 20. The reliability and validity of the Diet History has been previously reported.21 Dietary intake data were processed with the Nutrition Data Systems for Research software program (Nutrition Coordinating Center, University of Minnesota). Diet quality was quantified according to alternate healthy eating index 2010 (aHEI-2010).
Self-reported age, sex, race, education, smoking, alcohol drinking, and menopause status were collected using standardized questionnaires. Physical activity data were collected using the CARDIA Physical Activity History which queries the frequency, duration, and intensity of 13 different activities during the previous year. A continuous total physical activity intensity score was derived.22
A graded treadmill exercise testing using a modified Balke protocol was conducted at years 0, 7, and 20 to assess maximal symptom-limited performance for CARDIA participants who completed up to nine 2-min exercise stages of progressively increasing difficulty. The details of the treadmill test protocol have been described elsewhere.23 We excluded the year 7 test due to a protocol violation at Minneapolis site (n=1,139), because Minneapolis participants may be allowed to hold onto the treadmill trailing, leading to longer treadmill test duration.24 Duration of the treadmill test was used to assess cardiorespiratory fitness.
2.5. Statistical Analysis
We studied the association between HOMA-IR trajectories over 20 years as the exposure and ALM/BMI measured at year 20 as the outcome. Because of the marked differences in ALM between men and women, all analyses were stratified by sex. Demographic, anthropometric, and clinical characteristics at year 20 were described according to sex-specific quartiles of ALM/BMI.
The primary exposure was determined with latent class models implemented with SAS Proc Traj to identify discrete groups who shared similar underlying trajectories in HOMA-IR.25 The optimal number of trajectory groups was determined by Bayesian Information Criterion26 and ensuring that group size did not fall below 5% of the sample participants. The posterior predicted probability for each participant of being a member in each of the trajectory groups was calculated. Participants were assigned to the group with the highest posterior predicted probability. The names of groups were determined by the baseline HOMA-IR level (e.g., low, medium, high) and the following 20-year trajectory (e.g., increase, decrease or stable determined by the slope). These terms were selected based on qualitative rather than quantitative assessment, which is in line with the current practice for trajectory analysis.26 Due to the skewed distribution of HOMA-IR, it was natural log-transformed for trajectory analyses. It was then back-transformed to geometric means when plotting the trajectory groups for visualization.
With identified trajectories as the exposure, linear regression was used to determine the association between antecedent distinct HOMA-IR trajectories and current ALM/BMI (in continuous form) at year 20. We tested the hypothesis in the fully adjusted model (i.e., Model 3 below) that an increasing trajectory in insulin resistance, as compared to a relatively stable insulin resistance trajectory, between early and middle adulthood was associated with lower ALM/BMI in middle-aged adults without diabetes, independent of demographic, lifestyle, clinical factors, and cardiorespiratory fitness. The models were sequentially adjusted for age, race (black and white), field center, highest education completed (Model 1), cumulative smoking pack-years, cumulative number of alcohol drinks, cumulative physical activity score, cumulative total calorie (kcal), cumulative protein intake (gram), cumulative fiber intake (gram), cumulative aHEI score, and cumulative eGFR between year 0 and year 20, and average hs-CRP level measured at years 7, 15 and 20; for women, time-varying menopausal status (yes/no) was also included (Model 2); however this question was not asked before year 7 due to their young age. eGFR was added as a covariate, because determining insulin resistance trajectories as an independent predictor of ALM/BMI was our primary objective. eGFR can be either a confounder or a mediator in that prevalence of sarcopenia increased in people with early stage chronic kidney disease27 and eGFR might have a bidirectional association with insulin resistance.28 The final Model 3 further adjusted for cardiorespiratory fitness at year 0 and the change in cardiorespiratory fitness between year 0 and 20. Cardiorespiratory fitness has been associated with insulin resistance29 and body composition30 and thus is qualified as a confounder for the association between insulin resistance trajectories and ALM/BMI. We assumed that the value of covariates remained unchanged until the next available value when computing cumulative covariates.
Two secondary analyses were performed. First, we stratified analyses by dichotomizing the study sample at the highest sex-specific quartile of percent total body fat, an approach previously used to categorize body fat in the literature.31 Thus in the current study, men with percent total body fat ≥26.5% and women with percent total body fat ≥40.3% were classified as obese. This stratification analysis by obesity was performed because co-existence of obesity and low muscle mass and function (termed “sarcopenic obesity”) might synergistically influence risk of developing adverse health outcomes.32 Second, we evaluated the associations of 20-year fasting glucose and insulin trajectories with ALM/BMI to determine the influence of the two component measures of HOMA-IR on ALM/BMI.
A sensitivity analysis with the median imputation for missing data for the treadmill test was conducted to evaluate the robustness of the results. All analyses were performed using SAS version 9.4. Two sided P value <0.05 was considered statistically significant.
3. Results
Of the 925 men and 1,193 women included for analyses at year 20, the mean (SD) of ALM/BMI was 0.89 (0.08) in the lowest quartile and 1.26 (0.08) in the highest quartile in men, while women had a mean ALM/BMI of 0.59 (0.05) in the lowest quartile and 0.89 (0.07) in the highest quartile (Table 1). In both men and women, participants with higher ALM/BMI had lower HOMA-IR, lower BMI, and lower percent total body fat, were more physically active, and had longer duration of the treadmill test, lower eGFR, lower hs-CPR, and consumed more fiber and energy (P <0.05). Among men, those with higher ALM/BMI were more likely to be black and slightly younger (P <0.05). Among women, those with higher ALM/BMI had slightly more years of education, were more likely to drink alcohol, consumed more protein and a higher quality diet, and were less likely to have gone through menopause (P<0.05).
Table 1.
Characteristics by quartiles of ALM/BMI in men and women at year 20
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P | |
|---|---|---|---|---|---|
| Men | |||||
| N | 232 | 230 | 232 | 231 | |
| ALM/BMI | 0.89 ± 0.08 | 1.03 ± 0.02 | 1.11 ± 0.03 | 1.26 ± 0.08 | <0.0001 |
| HOMA-IR3 | 3.3 ± 2.5 | 2.9 ± 2.1 | 2.6 ± 1.7 | 2.4 ± 1.4 | 0.0001 |
| BMI, kg/m2 | 30.8 ± 8.5 | 28.0 ± 3.5 | 27.4 ± 4.0 | 25.6 ± 3.6 | 0.0001 |
| Percent total body fat, % | 27.9 ± 5.1 | 23.4 ± 4.5 | 21.8 ± 4.5 | 18.0 ± 4.8 | <0.0001 |
| Age, years | 45.6 ± 3.5 | 44.9 ± 3.5 | 45.2 ± 3.4 | 44.8 ± 3.4 | 0.04 |
| Black | 18.5 | 36.1 | 43.5 | 48.5 | 0.0001 |
| Highest education, years | 15.9 ± 2.8 | 15.5 ± 2.6 | 15.7 ± 2.6 | 15.6 ± 2.5 | 0.3 |
| Current smoker, yes | 19.8 | 18.7 | 18.1 | 17.8 | 0.9 |
| Current alcohol drinker, yes | 80.6 | 81.3 | 78.0 | 80.5 | 0.8 |
| Physical activity scorea | 293 ± 307 | 357 ± 360 | 413 ± 458 | 453 ± 391 | 0.0001 |
| Duration of the treadmill test, min | 7.8 ± 2.0 | 8.8 ± 1.9 | 9.1 ± 2.1 | 9.9 ± 2.3 | <0.0001 |
| eGFRa, mL/min per 1.73 m2 | 99.9 ± 17.7 | 93.1 ± 21.1 | 93.1 ± 23.0 | 93.1 ± 22.8 | 0.007 |
| hs-CRPa, mg/L | 1.14 ± 1.41 | 0.95 ± 1.30 | 0.72 ± 1.01 | 0.52 ± 0.77 | <0.0001 |
| Total calories, kcal | 2697 ± 1191 | 2637 ± 1205 | 2954 ± 1757 | 3063 ± 1616 | 0.008 |
| Protein intake, gram | 100.4 ± 45.4 | 100.5 ± 46.0 | 109.1 ± 56.3 | 110.6 ± 53.6 | 0.07 |
| Fiber, gram | 22.1 ± 9.9 | 22.4 ± 11.8 | 25.1 ± 14.1 | 25.6 ± 13.2 | 0.005 |
| Alternate HEI 2010 scoreb | 51.4 ± 11.8 | 52.7 ± 11.8 | 53.1 ± 12.8 | 54.3 ± 11.7 | 0.1 |
| Women | |||||
| N | 299 | 298 | 297 | 299 | |
| ALM/BMI | 0.59 ± 0.05 | 0.69 ± 0.02 | 0.77 ± 0.02 | 0.89 ± 0.07 | <0.0001 |
| HOMA-IRa | 3.2 ± 2.1 | 2.6 ± 1.8 | 2.4 ± 1.7 | 2.0 ± 1.2 | <0.0001 |
| BMI, kg/m2 | 33.7 ± 7.1 | 29.2 ± 6.1 | 26.7 ± 5.3 | 23.9 ± 4.2 | 0.0001 |
| Percent total body fat, % | 41.8 ± 5.1 | 36.6 ± 5.2 | 32.8 ± 5.7 | 27.9 ± 5.7 | <0.0001 |
| Age, years | 45.6 ± 3.6 | 45.2 ± 3.7 | 45.1 ± 3.6 | 44.9 ± 3.6 | 0.09 |
| Black | 45.2 | 45.3 | 37.4 | 41.5 | 0.2 |
| Highest education, years | 15.2 ± 2.4 | 15.7 ± 2.5 | 15.9 ± 2.4 | 16.4 ± 2.4 | 0.0001 |
| Current smoker, yes | 17.1 | 16.4 | 16.5 | 11.7 | 0.2 |
| Current alcohol drinker, yes | 68.9 | 74.8 | 82.5 | 88.0 | <0.0001 |
| Physical activity scorea | 160 ± 212 | 222 ± 293 | 281 ± 326 | 316 ± 381 | <0.0001 |
| Duration of the treadmill test, min | 4.7 ± 1.6 | 5.9 ± 1.9 | 6.7 ± 2.2 | 8.0 ± 2.4 | 0.0001 |
| eGFRa, mL/min per 1.73 m2 | 101.4 ± 20.2 | 100.8 ± 23.1 | 91.9 ± 19.5 | 89.3 ± 23.5 | 0.0001 |
| hs-CRPa, mg/L | 2.85 ± 5.63 | 1.59 ± 3.50 | 0.85 ± 2.18 | 0.55 ± 0.99 | <0.0001 |
| Total calories, kcal | 1955 ± 920 | 2000 ± 844 | 2136 ± 961 | 2126 ± 930 | 0.048 |
| Protein intake, gram | 74.3 ± 34.8 | 75.4 ± 32.4 | 79.7 ± 35.2 | 82.7 ± 37.4 | 0.02 |
| Fiber, gram | 18.0 ± 10.1 | 19.8 ± 9.6 | 20.9 ± 11.0 | 23.2 ± 13.0 | 0.0001 |
| Alternate HEI 2010 scoreb | 52.8 ± 12.3 | 57.2 ± 13.9 | 57.3 ± 12.8 | 61.6 ± 13.0 | <0.0001 |
| Menopause | 28.4 | 23.5 | 17.5 | 13.7 | <0.0001 |
ALM, appendicular lean mass; hs-CRP, high sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; HEI, healthy eating index; HOMA-IR, homeostatic model assessment of insulin resistance.
Values were % or mean ± standard deviation or median ± interquartile range when specified by a.
P values were obtained from Chi-square test, ANOVA test, or Kruskal-Wallis test for not-normally distributed variables specified by a.
The range of alternate HEI 2010 score is 0–110. Higher score means higher diet quality.
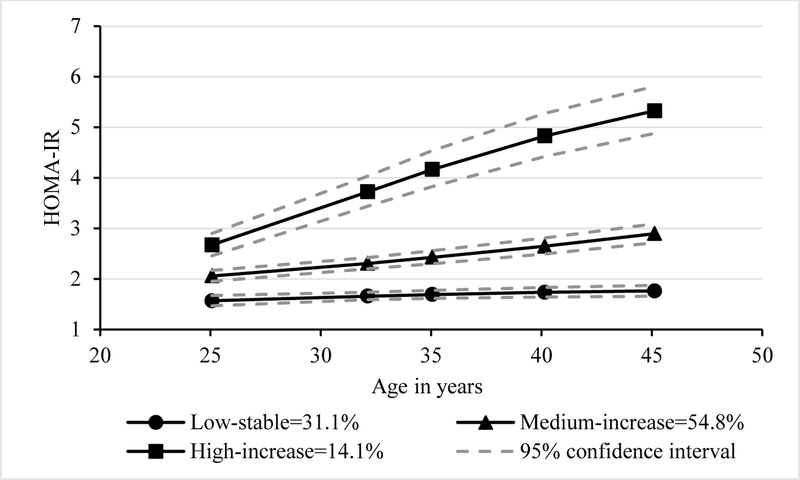
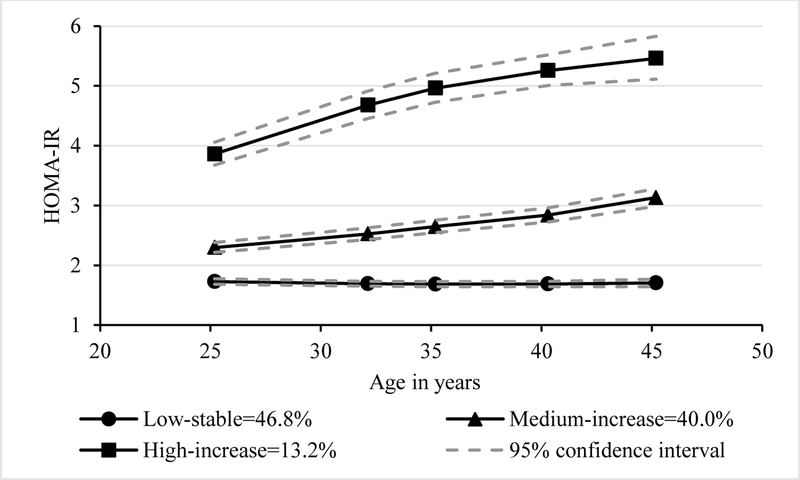
Three HOMA-IR trajectories were identified. In men, 31.1% belonged to the low-stable group, 54.8% medium-increase group, 14.1% high-increase group (Fig. 1A). In women, 46.8% belonged to the low-stable group, 40.0% medium-increase group, and 13.2% high-increase group (Fig. 1B).
Figure 1A.
Trajectory groups of insulin resistance in men
HOMA-IR, homeostatic model assessment of insulin resistance. The percentages in the figures represented proportions of participants in each group.
The mean ALM/BMI, mean treadmill test duration at year 0 and 20, and change in the treadmill test duration between year 0 and 20 differed by HOMA-IR trajectory groups similarly in men and women (Supplemental Table 1). In obese men and women (based on percent total body fat), we did not see a difference in the mean ALM/BMI across three trajectory groups (P≥0.2, Supplemental Table 2). Men or women in the high-increase trajectory had the lowest treadmill test duration at year 0 and 20 (P≤0.003, Supplemental Table 3). No difference by trajectory groups was found for the change in the treadmill test duration between year 0 and 20.
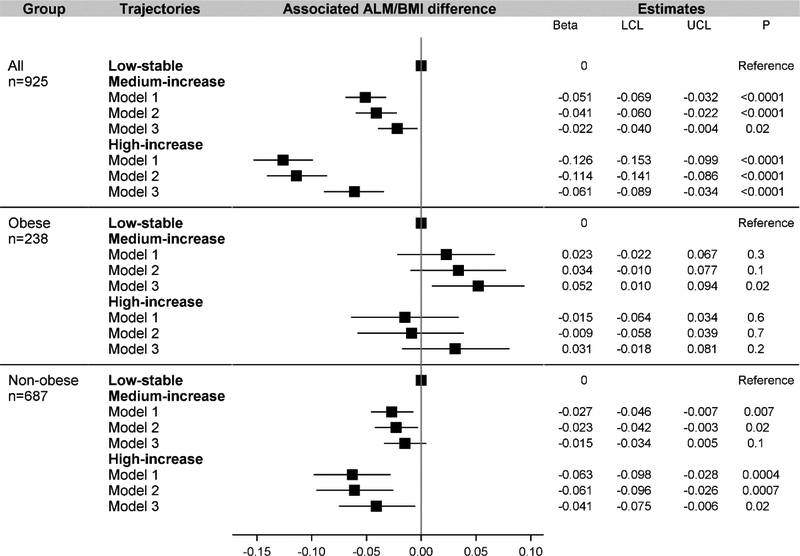
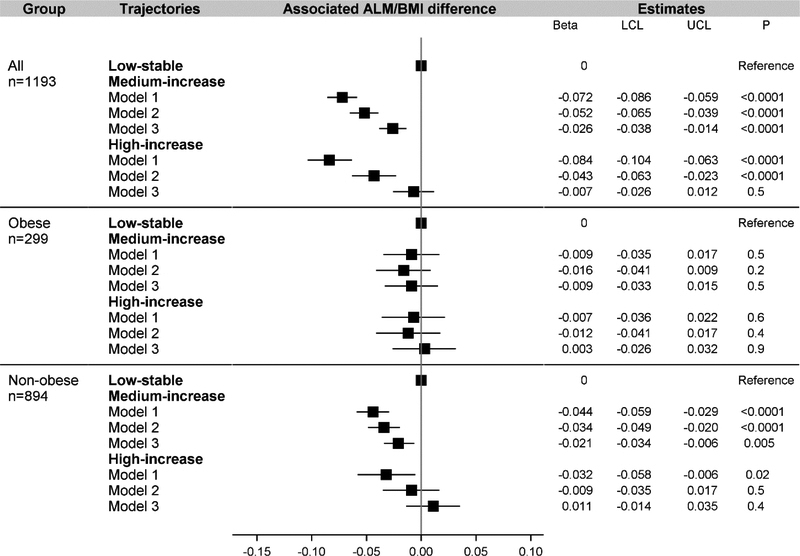
A graded association was found in men (Fig. 2) but not in women (Fig. 3) between HOMA-IR trajectories and ALM/BMI. In men, compared with the low-stable group in Model 2 where cardiorespiratory fitness was not adjusted, the associated difference in ALM/BMI was −0.041 (95% CI: −0.060 to −0.022) for the medium-increase group and −0.114 (−0.141 to −0.086) for the high-increase group. In women, compared with the low-stable group in Model 2, the associated difference for the medium-increase group (−0.052 [−0.065 to −0.039]) was similar to that for the high-increase group (−0.043 [−0.063 to −0.023]). After adjusting for cardiorespiratory fitness in Model 3, compared with the low-stable group in men, the estimate was −0.022 (−0.040 to −0.004) for the medium-increase group and −0.061 (−0.089 to −0.034) for the high-increase group. In women, the estimate was −0.026 (−0.038 to −0.014) for the medium-increase group and −0.007 (−0.026 to 0.012) for the high-increase group. Although attenuated, the inverse association largely persisted in non-obese men or women.
Figure 1B.
Trajectory groups of insulin resistance in women
HOMA-IR, homeostatic model assessment of insulin resistance. The percentages in the figures represented proportions of participants in each group.
Figure 2.
Association between trajectory groups of insulin resistance and ALM/BMI in men
ALM, appendicular lean mass; LCL, lower confidence limit; UCL, upper confidence limit. “Obese” was defined in men with percent total body fat ≥26.5%. Model 1 adjusted for age, race, center, education. Model 2 further adjusted for cumulative value of smoking pack years, alcohol consumption units, physical activity score, energy, protein intake, fiber intake, alternate Healthy Eating Index score, and eGFR between year 0 and year 20, and average hs-CRP between year 7 and 20. Model 3 further adjusted for the duration of the treadmill test at year 0 and change in the duration of the treadmill test between year 0 and year 20.
The sensitivity analyses with the median imputation for the missing data revealed robust results. Compared with the low-stable group in Model 3, in men, the associated difference in ALM/BMI was −0.021 (95% CI: −0.039 to −0.003) for the medium-increase group and −0.057 (−0.084 to −0.030) for the high-increase group. In women, the associated difference in ALM/BMI was −0.029 (95% CI: −0.041 to −0.017) for the medium-increase group and −0.011 (−0.029 to 0.008) for the high-increase group.
The three trajectories were similar between fasting insulin and HOMA-IR (Supplemental Fig. 1A and 1B). Also, the trajectories of fasting insulin showed similar associations with ALM/BMI in men (Supplemental Fig. 2) and in women (Supplemental Fig. 3) as the HOMA-IR trajectories. The graded association was also seen in men, but not women. Fasting glucose level in men and women increased over time for each trajectory group (Supplemental Fig. 4A and 4B). Fully adjusted Model 3 did not reveal a significant association between fasting glucose trajectories and ALM/BMI in men (Supplemental Fig. 5) and in women (Supplemental Fig. 6).
4. Discussion
Among CARDIA participants, a high-increase trajectory in insulin resistance over 20 years between early and middle adulthood was associated with lower ALM/BMI in middle-aged men without diabetes compared with a low-stable trajectory in insulin resistance, even after accounting for cardiorespiratory fitness. In women, this inverse insulin resistance-ALM/BMI association was no longer significant after adjusting for cardiorespiratory fitness. We observed a graded association (i.e., ALM/BMI was lower in each successively higher insulin resistance trajectory group, compared to the low stable group) in men, but not women. The inverse association persisted only in non-obese men and women.
Previous studies investigating the association of diabetes or related biomarkers (blood glucose, insulin, and insulin resistance) with lean mass did not adjust for cardiorespiratory fitness and thus may have overestimated the association.10–13 Our study suggests that adjusting for cardiorespiratory fitness considerably attenuated the inverse association between insulin resistance trajectories and ALM/BMI. This attenuation occurred after a number of covariates including physical activity were taken into consideration. Physical activity and cardiorespiratory fitness are two related, but also distinct measures.33 The former is usually self-reported in the literature and also in our study. Therefore, we could not rule out the impact of the imprecise self-reported physical activity data on the obtained association. The latter is an objective reflection of recent physical activity that also has a genetic component.33 Physical activity and cardiorespiratory fitness may have different implications in the age/disease related loss of lean mass that deserves future investigations, but is beyond the scope of this study.
Our analyses suggest that insulin resistance, rather than glucose levels, is an independent determinant of lean mass in young and middle aged adults without diabetes. In our study, the associations of fasting insulin and insulin resistance trajectories with ALM/BMI were more robust than the association between fasting glucose trajectories and ALM/BMI, particularly in men. It is possible that elevated insulin level and decreased insulin sensitivity in people with high glucose but still within the normal range may have already started to disrupt the balance between muscle protein synthesis and degradation.6,34 Insulin resistance is an important underlying mechanism for accelerated muscle loss.34 Elevated insulin resistance breaks the balance between muscle protein synthesis and degradation via various biological pathways including mitochondrial dysfunction, alteration of autophagy pathway, and stimulation of muscle protein degradation via the ubiquitin-proteasome proteolytic pathway.6 Decreased muscle mass is also accompanied by reduced mitochondria density and function and less surface area for insulin-mediated glucose uptake, which further exacerbates insulin resistance.6,10
A previous study reported a weaker and less clear graded association between 2-hour glucose and muscle strength in women than men.12 Similarly, in our study, the graded association between insulin resistance trajectories and ALM/BMI was only seen in men. The difference in body composition, fat distribution (on average, women have more peripheral fat and men have more visceral fat), sex hormones and adipokines may be relevant to this noted sex difference.35 Also, the sex difference in cardiorespiratory fitness levels has been noted in the published studies using either maximal oxygen uptake or treadmill test duration as the fitness measure36,37 and was also confirmed in our study (mean duration of the treadmill test was 2.6 minutes longer in men than women, P<0.0001). However, it remains unclear how these factors may impact sexual dimorphism in the association between insulin resistance and ALM/BMI. Further, it is unclear if the greater age-related decline in muscle mass over time in men than women,38 which creates greater variation over time in men, may contribute to this sexual dimorphism.
The inverse association between insulin resistance trajectories and ALM/BMI found in non-obese men and women (i.e., those without high percent total body fat) implies that even in individuals without obesity and diabetes, maintaining low insulin resistance over time may be beneficial for preserving lean mass. However, among obese individuals with high percent total body fat, we did not observe an association between insulin resistance trajectories and ALM/BMI. This might be explained by similar ALM/BMI values across the three insulin resistance trajectory groups in obese participants, which was not the case in non-obese participants. Potentially, losing fat mass may be more relevant for maintaining relative muscle mass in obese people than improving insulin sensitivity, although they are not independent from each other. There is evidence that the anabolic action of insulin and physical activity are both less effective at promoting muscle protein synthesis in people with increasing adiposity.39,40 Of note, due to the high correlation between body fat and BMI as part of the outcome, stratifying by percent total body fat may be an over-adjustment, because in individuals without high percent total body fat, the association between insulin resistance and ALM/BMI was greatly attenuated.
To the best of our knowledge, ours is the first study investigating the relationship between long-term trajectories in insulin resistance and ALM/BMI in young and middle-aged adults. We have added to the literature by characterizing insulin resistance over a 20-year period between early and middle adulthood where published data are lacking. Our study sample included a large number of black and white men and women within a well characterized cohort (i.e., CARDIA) which allowed for adjustment for a number of potential confounding variables such as cardiorespiratory fitness that has been previously omitted. Some limitations should be noted. First, we excluded participants who developed diabetes during follow-up. Thus, our findings may not be generalized to those who develop diabetes during young and middle adulthood. Second, missing data are generally inevitable in epidemiologic follow-up studies and may bias the estimates upward or downward. Additional analyses found that 9.3% of men and 10.1% of women did not have three or more measurements of fasting glucose and insulin required for trajectory analyses. Those individuals were more likely to be black, but they were not different compared to those with three or more measurements in terms of HOMA-IR, BMI, ALM, and percent total body fat (data not shown in the table). Approximately 5.2% of women and 4.4% of men had missing data for the treadmill test, but the median imputation analysis supported the robustness of the data. Third, although we adjusted for a number of covariates, residual confounding is possible. For example, an important covariate inflammatory marker hs-CRP was only available at years 7, 15, and 20, not in earlier years. Also, we made an arbitrary assumption for calculating cumulative confounders by assuming constant value between last and next available value. Fourth, we had one measure of body composition and thus a cross-sectional analysis of our outcome ALM/BMI. Temporal association and direction of the association cannot be inferred. Specifically, it was unknown if those participants with low ALM/BMI at year 20 may already have low ALM/BMI at year 0 regardless of their insulin resistance trajectories. Finally, the distribution of BMI within the obese category was constrained due to the table limits of DXA machines.
In conclusion, a high-increase insulin resistance trajectory, as compared to a low-stable insulin resistance trajectory, between early and middle adulthood was associated with lower ALM/BMI in middle-aged men, but not women, without diabetes, after accounting for cardiorespiratory fitness. Future research is needed to understand the differences by sex and obesity status in the insulin resistance and ALM/BMI association and to determine whether insulin resistance can predict change in ALM/BMI over time in young and middle-aged adults.
Supplementary Material
Figure 3.
Association between trajectory groups of insulin resistance and ALM/BMI in women
ALM, appendicular lean mass; LCL, lower confidence limit; UCL, upper confidence limit. “Obese” was defined in women with percent total body fat ≥40.3%. Model 1 adjusted for age, race, center, education. Model 2 further adjusted for cumulative value of smoking pack years, alcohol consumption units, physical activity score, energy, protein intake, fiber intake, alternate Healthy Eating Index score, and eGFR between year 0 and year 20, average hs-CRP between year 7 and 20, and time-varying menopausal status between year 7 and year 20. Model 3 further adjusted for the duration of the treadmill test at year 0 and change in the duration of the treadmill test between year 0 and year 20.
Acknowledgments
Funding information
This study was supported by the postdoctoral fellowship from the American Heart Association Strategically Focused Research Networks (14SFRN20480260). MPB was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health under Award Numbers T32HL069771 to conduct the current work. JBM was supported by K24 DK080140.
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
All authors reported no conflicts of interest.
References
- 1.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol (1985). 2003;95(4):1717–1727. [DOI] [PubMed] [Google Scholar]
- 5.Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS One. 2017;12(1):e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mobasheri A, Mendes AF. Physiology and pathophysiology of musculoskeletal aging: current research trends and future priorities. Front Physiol. 2013;4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melton LJ 3rd, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48(6):625–630. [PubMed] [Google Scholar]
- 9.Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014;72(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalyani RR, Metter EJ, Ramachandran R, Chia CW, Saudek CD, Ferrucci L. Glucose and Insulin Measurements From the Oral Glucose Tolerance Test and Relationship to Muscle Mass. J Gerontol a-Biol. 2012;67(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barzilay JI, Cotsonis GA, Walston J, et al. Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged >or=70 years. Diabetes Care. 2009;32(4):736–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care. 2005;28(10):2541–2542. [DOI] [PubMed] [Google Scholar]
- 13.Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38(1):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12(6):403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. [DOI] [PubMed] [Google Scholar]
- 16.Zhu N, Jacobs DR Jr., Sidney S, et al. Fat mass modifies the association of fat-free mass with symptom-limited treadmill duration in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2011;94(2):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dam TT, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69(5):584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69(5):576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K, Slattery M, Jacobs D Jr., et al. A study of the reliability and comparative validity of the cardia dietary history. Ethn Dis. 1994;4(1):15–27. [PubMed] [Google Scholar]
- 22.Jacobs DRJ, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulmon Rehab 1989;9:448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidney S, Haskell WL, Crow R, et al. Symptom-limited graded treadmill exercise testing in young adults in the CARDIA study. Med Sci Sports Exerc. 1992;24(2):177–183. [PubMed] [Google Scholar]
- 24.Sidney S, Sternfeld B, Haskell WL, Quesenberry CP, Crow RS, Thomas RJ. Seven-year change in graded exercise treadmill test performance in young adults in the CARDIA study. Med Sci Sport Exer. 1998;30(3):427–433. [DOI] [PubMed] [Google Scholar]
- 25.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Method Res. 2001;29(3):374–393. [Google Scholar]
- 26.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 27.Moon SJ, Kim TH, Yoon SY, Chung JH, Hwang HJ. Relationship between Stage of Chronic Kidney Disease and Sarcopenia in Korean Aged 40 Years and Older Using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008–2011. Plos One. 2015;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nashar K, Egan BM. Relationship between chronic kidney disease and metabolic syndrome: current perspectives. Diabetes Metab Syndr Obes. 2014;7:421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill JM. Physical activity, cardiorespiratory fitness and insulin resistance: a short update. Curr Opin Lipidol. 2007;18(1):47–52. [DOI] [PubMed] [Google Scholar]
- 30.Ross R, Katzmarzyk PT. Cardiorespiratory fitness is associated with diminished total and abdominal obesity independent of body mass index. Int J Obes Relat Metab Disord. 2003;27(2):204–210. [DOI] [PubMed] [Google Scholar]
- 31.Myint PK, Kwok CS, Luben RN, Wareham NJ, Khaw KT. Body fat percentage, body mass index and waist-to-hip ratio as predictors of mortality and cardiovascular disease. Heart. 2014;100(20):1613–1619. [DOI] [PubMed] [Google Scholar]
- 32.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122(7):743–752. [DOI] [PubMed] [Google Scholar]
- 34.Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229(2):R67–81. [DOI] [PubMed] [Google Scholar]
- 35.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6 Suppl 1:60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CY, Haskell WL, Farrell SW, et al. Cardiorespiratory Fitness Levels Among US Adults 20–49 Years of Age: Findings From the 1999–2004 National Health and Nutrition Examination Survey. American Journal of Epidemiology. 2010;171(4):426–435. [DOI] [PubMed] [Google Scholar]
- 37.Al-Mallah MH, Juraschek SP, Whelton S, et al. Sex Differences in Cardiorespiratory Fitness and All-Cause Mortality: The Henry Ford ExercIse Testing (FIT) Project. Mayo Clin Proc. 2016;91(6):755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visser M, Pahor M, Tylavsky F, et al. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol (1985). 2003;94(6):2368–2374. [DOI] [PubMed] [Google Scholar]
- 39.Guillet C, Delcourt I, Rance M, et al. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab. 2009;94(8):3044–3050. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson MI, Dobson JP, Greene NP, et al. Abnormal protein turnover and anabolic resistance to exercise in sarcopenic obesity. FASEB J. 2013;27(10):3905–3916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.