Abstract
Ductular reaction (DR) is characterized by the proliferation of reactive bile ducts induced by liver injuries. DR is pathologically recognized as bile duct hyperplasia and is commonly observed in biliary disorders. It can also be identified in various liver disorders including non-alcoholic fatty liver disease. DR is associated with liver fibrosis and damage, and the extent of DR parallels to patient mortality. DR raises scientific interests because it is associated with transdifferentiation of liver cells and may play an important role in hepatic regeneration. The origin of active cells during DR can be cholangiocytes, hepatocytes, or hepatic progenitor cells, and associated signaling pathways could differ depending on the specific liver injury or animal models used in the study. Although further studies are needed to elucidate detailed mechanisms and the functional roles in liver diseases, DR can be a therapeutic target to inhibit liver fibrosis and to promote liver regeneration. This review summarizes previous studies of DR identified in patients and animal models as well as currently understood mechanisms of DR.
Introduction
The term ductular reaction (DR) is defined as “a reaction of ductular phenotype, possibly but not necessarily of ductular origin”, according to nomenclature that Roskams et al. introduced (1). DR is histologically observed in liver specimen and pathologically recognized as bile duct proliferation or hyperplasia and is commonly identified in biliary disorders such as primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC) and biliary atresia (BA). DR is not described; however, as ductular proliferation because this phenomenon encompasses a reaction associated with other liver tissues or cells, such as stroma, inflammatory cells, and infiltrated cells in the liver including bone marrow-derived macrophages (1). DR is often associated not only with bile duct proliferation but also with other reactions in the liver, such as inflammatory cell infiltration in portal areas (2). Hepatic progenitor cells (HPCs) are activated in chronic liver injury, and the HPC niche that consists of macrophages, myofibroblasts, and matrix develops and is associated with liver fibrogenesis during liver injury (3). Bile ductules are consistently accompanied by microvessels, and the number of ductules corresponds to the density of accompanying microvessels indicating a close relationship between DR and microvessels as well as angiogenesis (4). Therefore, even when DR is identified in various liver conditions as bile duct hyperplasia, the mechanisms, associated cells/tissues, and reactions in DR may differ depending on the pathology. Although this review summarizes currently understood mechanisms and pathways of DR that is identified histologically as bile duct hyperplasia, it should be noted that other liver cells, tissues, or niches are also involved in DR and could play a key role for the pathophysiology of liver diseases associated with DR.
Types of DR in liver diseases
DR is described as cholangiocyte or HPC proliferation depending on the pathology. However, there is inconsistency in the definition of DR in previous studies because identification methods for HPCs are not conclusively established. In DR studies, EpCAM and SOX9 have often been proposed as HPC markers and CK7 or CK19 have been used to identify cholangiocytes. Cholangiocytes; however, also express EpCAM and SOX9 and hence, these markers cannot identify HPC proliferation exclusively. Sackett et al. have identified Foxl1 as an HPC marker and demonstrated that the subpopulation of Foxl1+ HPCs is rare in normal livers but dramatically increased in disease conditions caused for example by 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet (5). TROP2 and Lgr5 have also been identified as markers of HPCs that proliferate during DDC-induced liver injury (6, 7). Other suggested markers for HPCs include OV6, A6, CD24, CD133, NGA2, and CXCR4 (8–10). Figure 1 shows suggested locations of cholangiocytes, HPCs, and hepatocytes as well as selected markers proposed to identify these cells during liver injury.
Figure 1. Location of cholangiocytes, HPCs, and hepatocytes in the liver.
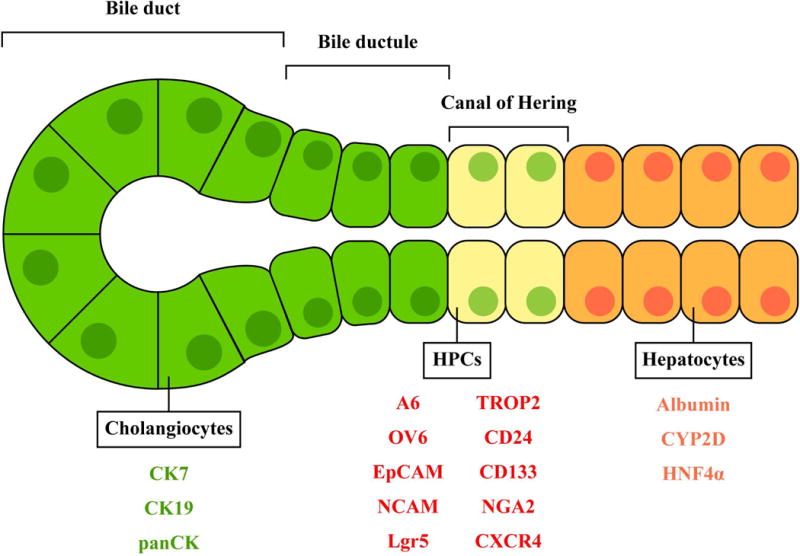
Identified markers for these cells are shown in green, red, and orange, respectively.
Self-proliferation of cholangiocytes
Cholangiocytes are heterogeneous in protein expression as well as proliferative functions between small and large cholangiocytes (11). DR (elevated CK19+ cells) can be identified in the liver of rodents with bile duct ligation (BDL) as large but not small cholangiocytes proliferate during BDL-induced liver injury (12). Acute carbon tetrachloride (CCl4) administration damages large but not small cholangiocytes in rats, and small cholangiocytes de novo proliferate showing DR (expanded CK19+ cells) to compensate for the loss of large cholangiocyte mass in this model (13). As both small and large cholangiocytes express CK7 and CK19, it is not feasible to distinguish two subsets of cholangiocytes histologically using these markers. Three-dimensional tracking of labeled cholangiocytes revealed that cholangiocytes were heterogeneous in proliferative capacity during thioacetamide (TAA)-induced liver injury in vivo; some of these proliferative cholangiocytes were not clustered but scattered in biliary tree (14). It was not defined whether these proliferative biliary cells were small or large cholangiocytes or HPCs.
Bile duct regeneration driven by hepatocytes
DR during biliary injury is driven not only by cholangiocyte self-proliferation but also hepatocyte transdifferentiation (15–17). Michalopoulos et al. isolated hepatocytes from dipeptidyl peptidase (DPP) IV+ rats and transplanted them into DPPIV- rats to generate chimera livers in vivo (18). In this model, 47.5% of cholangiocytes were DPPIV+ after cholangiocyte damage caused by BDL plus pretreatment with 4,4’-methylenedianiline (DAPM), suggesting that hepatocytes transdifferentiate into cholangiocytes during severe biliary damage (19). Another study also transplanted hepatocytes isolated from DPPIV+ rats into DPPIV− rats, and found that some regenerated cholangiocytes were CK19+ as well as DPPIV+ after BDL, suggesting that those cholangiocytes were hepatocyte-derived (20). Biliary damage caused by BDL or DDC diet induced morphological changes as well as OPN and SOX9 expression in hepatocytes, leading to transdifferentiation into cholangiocytes (21). Tarlow et al. transplanted purified fluorescently marked hepatocytes into mice to trace these cells in vivo (22). In this model, hepatocyte-derived biliary cells were observed after 6-week DDC diet. This study demonstrates that hepatocytes transdifferentiate directly into biliary phenotypes in vivo, and gene expression profiles of hepatocyte-derived biliary cells analyzed by RNA-seq are different from those of cholangiocytes. Font-Burgada et al. identified hybrid hepatocytes at canal of Hering that expressed SOX9 as well as HNF4α (23). These hybrid hepatocytes showed robust proliferation and contributed to hepatocyte regeneration during acute liver injury caused by CCl4, and they also transdifferentiated into cholangiocytes during cholestatic liver injury caused by 3-week DDC diet. These findings suggest that hybrid hepatocytes may be HPCs or at least plastic enough to differentiate into hepatocytes or cholangiocytes depending on the specific liver injury. It is not fully understood; however, whether hepatocytes and/or hybrid hepatocytes transdifferentiate into identical cholangiocytes or biliary-like cells with different gene expression profiles from cholangiocytes in all liver injuries. Although these previous studies indicate that hepatocytes transdifferentiate into biliary cells, further studies are required to prove if this transdifferentiation may occur in human diseases.
Hepatocyte regeneration driven by cholangiocytes and/or HPCs
Studies using liver specimen of patients with cirrhosis have identified typical cholangiocytes that express CK19 and hepatocytes expressing HepPar1, but have found that there are transitional cells between cholangiocytes and hepatocytes expressing different markers such as EpCAM and NCAM. In addition, the expression level of markers in these transitional cells varies depending on the maturation of liver formation (24, 25). It is not fully elucidated to date whether cholangiocytes and/or HPCs can transdifferentiate into hepatocytes during liver regeneration. Specific lineage labeling of the biliary compartment has demonstrated that proliferative cells during liver injuries are from the biliary compartment, but these cells do not contribute to hepatocyte regeneration (26). Other studies have shown that regenerated hepatocytes in chronic liver injury are neither cholangiocyte- nor stem cell-derived but a result of self-duplication of hepatocytes (27, 28). Despite controversies, accumulating evidence suggests that the origin of regenerated hepatocytes depends on the selected animal models and experimental conditions. In certain conditions with severe hepatocyte damage, cholangiocytes can transdifferentiate into hepatocytes through HPCs. Lu et al. have demonstrated that induction of hepatocyte damage (>98% hepatocyte loss) by Ah-Cre-mediated deletion of Mdm2, which causes p53-mediated senescence and apoptosis in hepatocytes, induces DR (increased numbers of panCK+ cells) and activation of HPCs with biliary origin in mice (29). This study showed that transplantation of biliary HPCs into other mice with severe hepatocyte loss facilitated liver regeneration by differentiation into hepatocytes. Another study has demonstrated that cholangiocytes change their morphological phenotypes, proliferate, and express SOX-9b followed by transdifferentiation into hepatocytes by expressing hepatocyte-specific proteins during bacterial nitroreductase-mediated hepatocyte ablation in zebrafish (30). Raven et al. induced liver damage by TAA, DDC diet, or methionine- and choline-deficient (MCD) diet, and also inhibited hepatocyte growth factor signaling and regeneration by AAV8-TBG-Cre-mediated β1-integrin ablation in mice (31). In this model, DR (elevated CK19+ cells) was observed in β1-integrin-deleted mice, and cholangiocytes became invasive showing atypical morphology. This study showed that regenerated hepatocytes after the recovery period were not hepatocyte- but rather cholangiocyte-derived. Hepatocytes regenerated from cholangiocytes were located adjacent to CK19+SOX9+ cells; the number of those biliary-originated hepatocytes decreased with distance from CK19+ cells. Lineage tracing studies have demonstrated that biliary cells (cholangiocytes and/or HPCs) do not contribute to liver regeneration during liver injury induced by partial hepatectomy (PHx) or CCl4, proliferate but do not transdifferentiate into hepatocytes during DDC diet-induced injury, and proliferate and transdifferentiate into hepatocytes during choline-deficient-ethionine-supplemented (CDE) diet (32, 33). Although it is still not fully elucidated whether cholangiocytes transdifferentiate into hepatocytes directly or via HPCs, these findings suggest that cholangiocytes and/or HPCs transdifferentiate into hepatocytes to restore parenchymal functions in certain liver conditions. Figure 2 summarizes types of DR.
Figure 2. Types of DR.
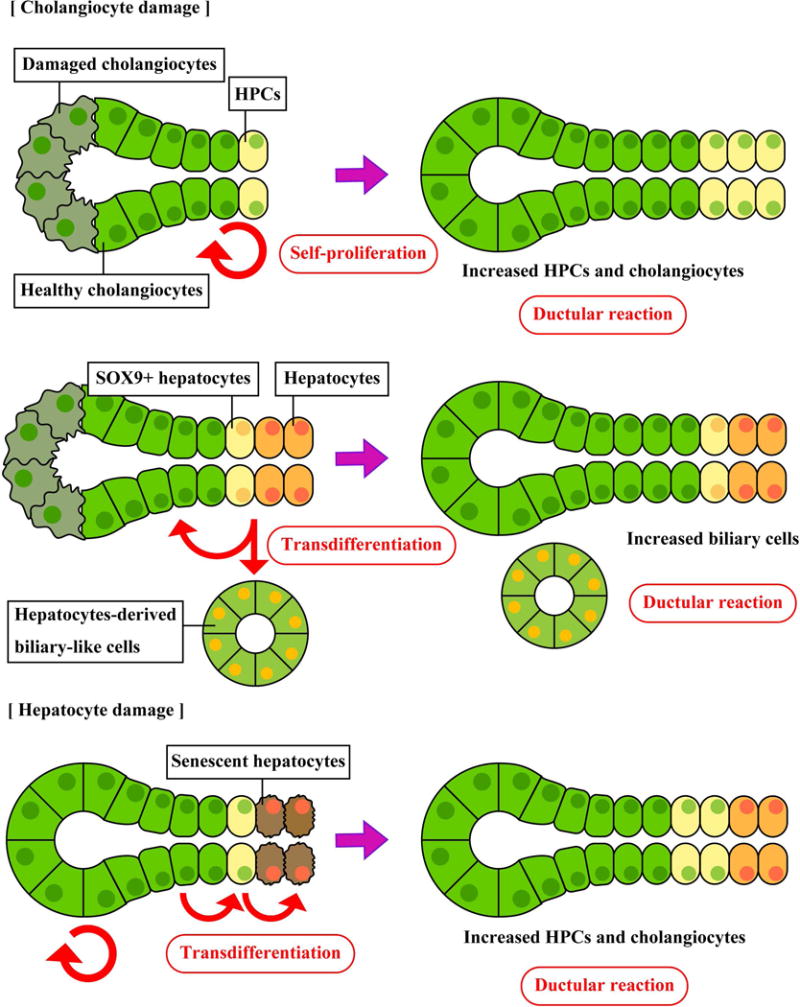
During cholangiocyte damage, cholangiocytes and/or HPCs proliferate to compensate biliary cell population and functions. Hepatocytes transdifferentiate into cholangiocytes and/or biliary-like cells that have different gene expression profiles. During hepatocyte damage, cholangiocytes start proliferation and transdifferentiate into hepatocytes via HPCs. The types of DR and the origin of active cells vary depending on liver injuries or experimental conditions.
DR in liver diseases
Table 1 shows selected previous studies for DR identified in patients with various liver diseases. As there are no gold standard methods to identify specific cholangiocytes or HPCs and the origin of these cells conclusively, types of DR in these studies are unknown.
Table 1.
Selected studies that identify DR in human patients
| Disease | Cells detected | Markers used |
|---|---|---|
| Primary biliary cholangitis | Cholangiocytes/HPCs | CK19, EpCAM, OV6 (34) |
| Primary sclerosing cholangitis | Cholangiocytes/HPCs | CK19, EpCAM, OV6 (34) |
| Biliary atresia | Cholangiocytes | CK7 (35) |
| Alcoholic hepatitis | Cholangiocytes/HPCs | CK7, EpCAM (36) |
| Alcoholic hepatitis | Cholangiocytes | CK7, CK19 (37) |
| Non-alcoholic steatohepatitis | Cholangiocytes | CK7 (2) |
| Hepatitis B virus-positive liver cirrhosis | Cholangiocytes | CK19 (38) |
| Hepatitis C virus-positive liver cirrhosis | Cholangiocytes | CK19 (38) |
| Hepatitis C virus recurrence | Cholangiocytes | CK7 (39) |
| Hepatitis C virus recurrence | Cholangiocytes | CK7 (40) |
| Hepatitis B virus-related hepatocellular carcinoma | Cholangiocytes | CK19 (41) |
| hepatocellular carcinoma after hepatectomy | Cholangiocytes | CK7 (42) |
| hepatocellular carcinoma | Cholangiocytes | CK7 (43) |
Cholestatic liver diseases
DR is often observed in patients with cholestatic liver diseases such as PBC, PSC and BA. Liver specimen from PBC or PSC patients showed extensive DR (expanded CK19+, EpCAM+, and OV6+ cells) compared to healthy individuals (34). DR (expanded CK7+ cells) was also identified from liver sections of BA patients (35).
Alcoholic and non-alcoholic liver diseases
Heavy alcohol consumption induces acute or chronic alcoholic liver disease (ALD) including liver steatosis, inflammation, and fibrosis. Alcoholic hepatitis (AH) is the severe condition of ALD characterized by severe liver inflammation and fibrosis. Liver specimen from patients with AH showed DR (elevated expression of CK7 or EpCAM) compared to normal livers; the expression levels of CK7 correlated with 90-day survival rates of the patients indicating association between grades of DR and mortality (36). Dubuquoy et al. have also identified DR (elevated CK7+ or CK19+ cells) in AH patients, and have found that proliferation of hepatocytes is not elevated indicating cholangiocyte- or HPC-derived DR (37).
Non-alcoholic fatty liver disease (NAFLD) shows similar liver steatosis to ALD without alcohol consumption. NAFLD can progressively lead to non-alcoholic steatohepatitis (NASH). Liver specimen of patients with NASH showed DR (expanded keratin-expressed area) (2). In addition, NASH patients at higher stages of liver fibrosis showed higher grades of DR indicating a correlation between DR and disease progression of NASH (2).
Chronic viral hepatitis
DR (elevated CK19+ cells) was observed in patients with Hepatitis B virus (HBV)- or Hepatitis C virus (HCV)-positive liver cirrhosis (38). Increased numbers of HPCs were also observed in liver sections suggesting an association between DR and liver cirrhosis caused by viral infection (38). Prakoso et al. have identified DR (expanded CK7+ area) in patients with HCV recurrence compared to healthy individuals; patients with higher liver fibrosis stages show higher DR (39). In another study, DR (CK7+ area) was observed in patients with HCV recurrence after liver transplantation, and patients with more severe liver conditions such as cirrhosis or cholestatic hepatitis showed higher DR compared to patients with slow progression of HCV recurrence (40).
Hepatocellular carcinoma
A study using 120 patients with HBV-related hepatocellular carcinoma (HCC) has demonstrated that patients with higher grades of DR (CK19+ area) at peritumoral regions show higher inflammation grades or liver fibrosis stages (41). Another study using patients with PHx and post-operative HCC has shown that PCNA labeling index of DR is correlated with inflammation grades and fibrosis stages (42). Park et al. analyzed patterns of DR (CK7+ cells) in HCC patients and demonstrated that high grades of DR were observed in noninvasive HCC but low or no DR was identified in highly invasive HCC suggesting the possible feature of DR as a tool to distinguish invasive and noninvasive HCC (43).
Association of DR with liver fibrosis and senescence
Studies using human liver specimen demonstrate that DR is closely related to liver fibrosis caused by various factors including alcoholic or non-alcoholic fatty liver, or viral infection. Although hepatic stellate cells (HSCs) and portal fibroblasts are the major cells contributing to fibrogenesis during liver damage (44), inhibition of cholangiocyte proliferation and activation by blocking signaling pathways such as the secretin/secretin receptor axis attenuated liver fibrosis as well as HSC proliferation and fibrogenesis via decreased TGF-β1 signaling, indicating a close relationship between cholangiocyte activation and HSC fibrogenesis (45). HSC activation is associated with HPC proliferation and liver regeneration via production of hepatocyte growth factor (HGF) (46, 47). Another study has demonstrated that IL-13 induces proliferation of cholangiocytes as well as activation and fibrogenesis in fibroblasts independently (48). Inhibition of IL-13 attenuated liver fibrosis in vivo, showing the key role of IL-13 signaling in liver fibrosis (49). These findings indicate that liver fibrosis often accompanies DR because of close relationship between HSCs and biliary cells, and DR can occur not only in biliary disorders but also in various liver injuries regulated by cytokines such as IL-13. This implicates the importance of DR and its grade as a sign of liver conditions and fibrosis during liver injury.
Although transdifferentiation of cholangiocytes into hepatocytes is still controversial, hepatocyte senescence caused by Mdm2 deletion induced biliary transdifferentiation into hepatocytes, indicating association between cellular senescence and differentiation of biliary cells (29). Ikeda et al. analyzed liver specimen from patients with HBV- or HCV-induced hepatitis and found that the expression levels of p21 in hepatocytes correlated to the degree of DR (CK7+ or CK19+ cells) showing an association between hepatocyte senescence and biliary proliferation (50). Another study identified DR (CK7+ cells) in patients with hereditary hemochromatosis and showed the association of DR with hepatocyte senescence, portal inflammation, and fibrosis stages (51). Alcohol consumption increases cellular senescence in hepatocytes, and it is associated with liver fibrosis via miR-34a expression (52). Clouston et al. have shown strong correlation between DR and HPC expansion as well as association between hepatocyte senescence and HPC proliferation in HCV positive patients (53). Although further studies are required, these studies suggest that cellular senescence in hepatocytes may drive DR and fibrogenesis by inducing biliary proliferation and/or transdifferentiation.
Pathways of DR-associated transdifferentiation
Notch signaling
Decoy oligodeoxynucleotide inhibition of RBP-jκ, which is a downstream target of Notch receptors, attenuated DDC-induced liver fibrosis in mice suggesting association between Notch signaling and liver fibrogenesis (54). Lu et al. have demonstrated that overexpression of RBP-Jκ in isolated murine HPCs induces HPC proliferation and elevated expression of CK7 and CK19 in HPCs, and inhibition of Notch signaling by DAPT (indirect Notch signaling blocker) attenuates expression of those cytokeratin proteins in vitro (55). BDL-induced DR (expanded OV6+ or CK19+ cells) showing co-localization of OV6/CK19 and SOX9/CK19, and expression levels of Notch receptors and their ligands were also elevated by BDL in rats (56). DAPT treatments attenuated BDL-induced liver fibrosis in vivo, and sodium butyrate-induced HPC differentiation into biliary phenotypes was inhibited by DAPT using WB-F344 cell line (56). AAV8-TBG-Cre-mediated overexpression of Notch1 in hepatocytes elevates expression of OPN and SOX9 in hepatocytes in vivo (21). These findings suggest that Notch signaling is associated with transdifferentiation of HPCs and/or hepatocytes into biliary phenotypes leading to DR followed by liver fibrosis.
Hippo/YAP signaling
High expression of YAP, which is an effector of Hippo signaling was observed in bile ducts of BA patients compared to non-BA patients (57). Elevated YAP expression in bile ducts was also identified in PSC and PBC patients; YAP−/− mice showed attenuated bile duct hyperplasia during BDL indicating association between DR and Hippo/YAP signaling (58). Yimlamai et al. have demonstrated that hepatocyte-specific YAP expression induces elevated expression of HPC and biliary markers including CK19, SOX9, and A6, and have also shown that Notch signaling is a functional target of YAP in vivo (59). These findings suggest that Hippo/YAP signaling is associated with DR and hepatocyte transdifferentiation into cholangiocytes via activation of Notch signaling.
Wnt/β-catenin signaling
DDC diet increases the number of A6+ cells in the mouse liver and β-catenin is co-localized with A6 (60). It has also been shown that A6+ cell numbers are decreased during DDC diet in β-catenin knockout mice, indicating association of β-catenin with DR during biliary damage (60). Wntless is a protein required for transportation and secretion of Wnt proteins and knocking out of Wntless causes insufficient secretion of Wnt proteins and inactivation of Wnt/β-catenin signaling. Okabe et al. have demonstrated that mice with liver-specific Wntless knockout show less DR (A6+ and CK19+ cells) and liver fibrosis during DDC diet (61). Lyz2-Cre-mediated specific deletion of Wntless in liver macrophages caused impaired liver regeneration and less cell proliferation in the liver after PHx (62). Effects of Wntless deletion are controversial; however, Irvine et al. have demonstrated that Lyz2-Cre-mediated Wntless deletion in liver macrophages exacerbates DR (expanded wide spectrum cytokeratin positive cells) and liver fibrosis caused by TAA in mice (63). Boulter et al. have explored the fate of HPCs during liver injury depending on Notch or Wnt/β-catenin signaling using CDE diet as a hepatocyte regeneration model and DDC diet as a cholangiocyte regeneration model in mice (64). In this model, Notch signaling was reduced and Wnt/β-catenin signaling was maintained leading to hepatocyte regeneration during CDE diet, and Notch signaling was upregulated and Wnt/β-catenin signaling was downregulated leading to cholangiocyte regeneration during DDC diet. The authors have also demonstrated that Wnt/β-catenin signaling drives cholangiocarcinoma growth (65). It is not fully understood whether Wnt/β-catenin signaling is required for both or either hepatocyte and cholangiocyte regeneration. Further studies are needed to elucidate functional roles of Wnt/β-catenin signaling in various liver injuries.
HGF/c-Met signaling
Human recombinant HGF induced liver regeneration in rats with PHx (66). Mx1-Cre-mediated HGF receptor c-Met deletion in the liver decreased DR (CK19+ cells) and cell proliferation in the liver during DDC diet (46). Alb-Cre-mediated c-Met deletion in hepatocytes also decreased numbers of A6+ HPCs in the liver compared to control (46). Treatments of hepatocytes with HGF induced hepatocyte transdifferentiation into biliary phenotypes in vitro (67). Inhibition of phosphatidylinositol 3-kinase, a downstream target of HGF/c-Met signaling, inhibited this transdifferentiation from hepatocytes into cholangiocytes (67). These findings suggest that HGF/c-Met signaling is associated with hepatocyte transdifferentiation into cholangiocytes.
TWEAK/Fn14 pathway
A study has shown that CDE diet induces elevated Fn14 expression in the liver and Fn14 is co-localized with panCK (68). This study has also demonstrated that Fn14 knockout mice show less DR (A6+ or CK19+ cell counts) during CDE diet compared to wild-type, and treatments of TWEAK, which is a Fn14 ligand, induce proliferation of the HPC line, BMOL cells suggesting an association between TWEAK/Fn14 pathway and DR. Administration of recombinant TWEAK induced DR (expanded panCK+ cells) in mice (69). Lu et al. have demonstrated that Fn14 knockout mice show less DR (panCK+ cells) after severe hepatocyte damage caused by Ah-Cre-mediated deletion of Mdm2 (29). Administration of recombinant TWEAK induced panCK+ cell expansion in Mdm2-deleted mice (29). Although it is unclear whether TWEAK/Fn14 pathway is required for transdifferentiation, these findings suggest that this pathway is associated with HPC and/or cholangiocyte proliferation leading to DR. Figure 3 summarizes signaling pathways associated with transdifferentiation leading to DR.
Figure 3.
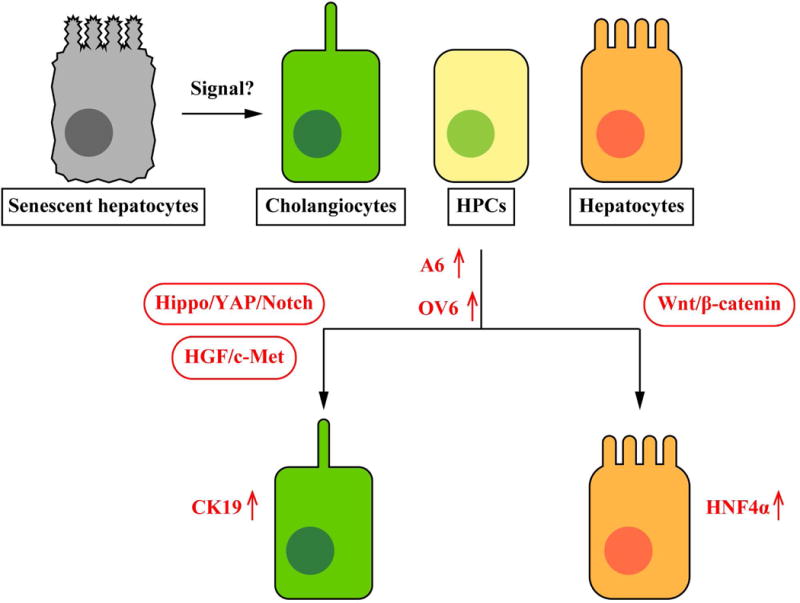
Pathways of DR-associated transdifferentiation. Hippo/YAP/Notch signaling as well as HGF/c-Met signaling are associated with transdifferentiation into cholangiocytes, and Wnt/β-catenin signaling is associated with transdifferentiation into hepatocytes. Expression levels of stem cell or oval cell markers such as A6 and OV6 are elevated during this process. Although TWEAK/Fn14 signaling induces HPC and/or cholangiocyte proliferation, it is unclear whether this signaling is required for transdifferentiation of liver cells. It is also unclear whether hepatocytes and/or cholangiocytes transdifferentiate directly or via HPCs. Previous studies indicate senescent hepatocytes may trigger transdifferentiation leading to DR and liver regeneration.
Conclusions and perspectives
Current studies have demonstrated that DR is identified in various liver diseases, and types and mechanisms of DR may differ depending on liver injuries or animal models. Studies of DR are confusing depending on experimental conditions because of the complexity of DR. DR plays a key role in pathogenesis of various liver diseases and is associated with disease conditions such as liver fibrosis stages and mortality. DR is also an important factor for liver regeneration during both hepatocyte and cholangiocyte damage.
DR and cholangiocyte activation/proliferation can be a target for therapies of liver diseases. For example, we have demonstrated that melatonin administration or complete dark environment for BDL rats or Mdr2−/− mice decrease cholangiocyte proliferation and bile duct mass as well as liver fibrosis via activation of MT1 melatonin receptor in cholangiocytes (70–72). It is still unclear; however, whether DR promotes liver fibrosis via elevated bile duct mass and cholangiocyte activation, or DR protects the liver during injury by enhancing liver regeneration.
Acknowledgments
The authors gratefully acknowledge Thao Giang (Texas A&M College of Medicine, Temple TX) for her assistance in proofreading of the manuscript.
Portions of this work was supported in part by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Baylor Scott & White, a VA Research Career Scientist Award, a VA Merit Award (5I01BX000574) to Dr. Alpini, a VA Merit Award (5I01BX002192) to Dr. Glaser, a VA Merit Award (1I01BX001724) to Dr. Meng, a VA Merit Award (1I01BX003031) to Dr. Francis from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, and the NIH grants DK115184, DK058411, DK076898, DK107310, DK062975, DK110035, and DK108959 to Drs. Alpini, Meng, Glaser, and Francis. This material is the result of work supported by resources at the Central Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Abbreviations
- AH
alcoholic hepatitis
- ALD
alcoholic liver disease
- BA
biliary atresia
- BDL
bile duct ligation
- CCl4
carbon tetrachloride
- CDE
choline-deficient-ethionine-supplemented
- DAPM
4,4’-methylenedianiline
- DDC
3,5-deithoxycarbonyl-1,4-dihydrocollidine
- DPP
dipeptidyl peptidase
- DR
ductular reaction
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HGF
hepatocyte growth factor
- HPCs
hepatic progenitor cells
- HSCs
hepatic stellate cells
- MCD
methionine- and choline-deficient
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- PBC
primary biliary cholangitis
- PHx
partial hepatectomy
- PSC
primary sclerosing cholangitis
- TAA
thioacetamide
Footnotes
The authors have no conflict of interest to declare.
References
- 1.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 2.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzini S, Bird TG, Boulter L, Bellamy C, Samuel K, Aucott R, Clayton E, et al. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut. 2010;59:645–654. doi: 10.1136/gut.2009.182345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouw AS, van den Heuvel MC, Boot M, Slooff MJ, Poppema S, de Jong KP. Dynamics of the vascular profile of the finer branches of the biliary tree in normal and diseased human livers. J Hepatol. 2006;45:393–400. doi: 10.1016/j.jhep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, Kaestner KH, et al. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology. 2009;49:920–929. doi: 10.1002/hep.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka M, Miyajima A. Identification and isolation of adult liver stem/progenitor cells. Methods Mol Biol. 2012;826:25–32. doi: 10.1007/978-1-61779-468-1_3. [DOI] [PubMed] [Google Scholar]
- 7.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 9.Qiu Q, Hernandez JC, Dean AM, Rao PH, Darlington GJ. CD24-positive cells from normal adult mouse liver are hepatocyte progenitor cells. Stem Cells Dev. 2011;20:2177–2188. doi: 10.1089/scd.2010.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Siegel CT, Shuai L, Lai J, Zeng L, Zhang Y, Lai X, et al. Repair of liver mediated by adult mouse liver neuro-glia antigen 2-positive progenitor cell transplantation in a mouse model of cirrhosis. Sci Rep. 2016;6:21783. doi: 10.1038/srep21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaser SS, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, Francis HL, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol. 1998;274:G767–775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 13.LeSage GD, Benedetti A, Glaser S, Marucci L, Tretjak Z, Caligiuri A, Rodgers R, et al. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology. 1999;29:307–319. doi: 10.1002/hep.510290242. [DOI] [PubMed] [Google Scholar]
- 14.Kamimoto K, Kaneko K, Kok CY, Okada H, Miyajima A, Itoh T. Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. Elife. 2016;5 doi: 10.7554/eLife.15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011;458:251–259. doi: 10.1007/s00428-011-1048-3. [DOI] [PubMed] [Google Scholar]
- 16.Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. II. Ontogenic liver growth in childhood. Virchows Arch. 2011;458:261–270. doi: 10.1007/s00428-011-1049-2. [DOI] [PubMed] [Google Scholar]
- 17.Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. III. Implications for liver pathology. Virchows Arch. 2011;458:271–279. doi: 10.1007/s00428-011-1050-9. [DOI] [PubMed] [Google Scholar]
- 18.Michalopoulos GK, Bowen WC, Mule K, Lopez-Talavera JC, Mars W. Hepatocytes undergo phenotypic transformation to biliary epithelium in organoid cultures. Hepatology. 2002;36:278–283. doi: 10.1053/jhep.2002.34858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yovchev MI, Locker J, Oertel M. Biliary fibrosis drives liver repopulation and phenotype transition of transplanted hepatocytes. J Hepatol. 2016;64:1348–1357. doi: 10.1016/j.jhep.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Font-Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, Taniguchi K, et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell. 2015;162:766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H, Rogler LE, Teperman L, Morgan G, Rogler CE. Identification of hepatocytic and bile ductular cell lineages and candidate stem cells in bipolar ductular reactions in cirrhotic human liver. Hepatology. 2007;45:716–724. doi: 10.1002/hep.21557. [DOI] [PubMed] [Google Scholar]
- 25.Stueck AE, Wanless IR. Hepatocyte buds derived from progenitor cells repopulate regions of parenchymal extinction in human cirrhosis. Hepatology. 2015;61:1696–1707. doi: 10.1002/hep.27706. [DOI] [PubMed] [Google Scholar]
- 26.Jors S, Jeliazkova P, Ringelhan M, Thalhammer J, Durl S, Ferrer J, Sander M, et al. Lineage fate of ductular reactions in liver injury and carcinogenesis. J Clin Invest. 2015;125:2445–2457. doi: 10.1172/JCI78585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaub JR, Malato Y, Gormond C, Willenbring H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014;8:933–939. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, Pikarsky E, et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, Guest RV, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He J, Lu H, Zou Q, Luo L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology. 2014;146:789–800 e788. doi: 10.1053/j.gastro.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Raven A, Lu WY, Man TY, Ferreira-Gonzalez S, O’Duibhir E, Dwyer BJ, Thomson JP, et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Espanol-Suner R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, Jacquemin P, et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575 e1567. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigo-Torres D, Affo S, Coll M, Morales-Ibanez O, Millan C, Blaya D, Alvarez-Guaita A, et al. The biliary epithelium gives rise to liver progenitor cells. Hepatology. 2014;60:1367–1377. doi: 10.1002/hep.27078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crosby HA, Hubscher S, Fabris L, Joplin R, Sell S, Kelly D, Strain AJ. Immunolocalization of putative human liver progenitor cells in livers from patients with end-stage primary biliary cirrhosis and sclerosing cholangitis using the monoclonal antibody OV-6. Am J Pathol. 1998;152:771–779. [PMC free article] [PubMed] [Google Scholar]
- 35.Kinugasa Y, Nakashima Y, Matsuo S, Shono K, Suita S, Sueishi K. Bile ductular proliferation as a prognostic factor in biliary atresia: an immunohistochemical assessment. J Pediatr Surg. 1999;34:1715–1720. doi: 10.1016/s0022-3468(99)90652-8. [DOI] [PubMed] [Google Scholar]
- 36.Sancho-Bru P, Altamirano J, Rodrigo-Torres D, Coll M, Millan C, Jose Lozano J, Miquel R, et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology. 2012;55:1931–1941. doi: 10.1002/hep.25614. [DOI] [PubMed] [Google Scholar]
- 37.Dubuquoy L, Louvet A, Lassailly G, Truant S, Boleslawski E, Artru F, Maggiotto F, et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015;64:1949–1960. doi: 10.1136/gutjnl-2014-308410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun C, Jin XL, Xiao JC. Oval cells in hepatitis B virus-positive and hepatitis C virus-positive liver cirrhosis: histological and ultrastructural study. Histopathology. 2006;48:546–555. doi: 10.1111/j.1365-2559.2006.02372.x. [DOI] [PubMed] [Google Scholar]
- 39.Prakoso E, Tirnitz-Parker JE, Clouston AD, Kayali Z, Lee A, Gan EK, Ramm GA, et al. Analysis of the intrahepatic ductular reaction and progenitor cell responses in hepatitis C virus recurrence after liver transplantation. Liver Transpl. 2014;20:1508–1519. doi: 10.1002/lt.24007. [DOI] [PubMed] [Google Scholar]
- 40.Sclair SN, Fiel MI, Wu HS, Doucette J, Aloman C, Schiano TD. Increased hepatic progenitor cell response and ductular reaction in patients with severe recurrent HCV post-liver transplantation. Clin Transplant. 2016;30:722–730. doi: 10.1111/ctr.12740. [DOI] [PubMed] [Google Scholar]
- 41.Cai X, Li F, Zhang Q, Xu M, Qu Y, Wan X, Gao C, et al. Peritumoral ductular reaction is related to nuclear translocation of β-catenin in hepatocellular carcinoma. Biomed Pharmacother. 2015;76:11–16. doi: 10.1016/j.biopha.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 42.Ye F, Jing YY, Guo SW, Yu GF, Fan QM, Qu FF, Gao L, et al. Proliferative ductular reactions correlate with hepatic progenitor cell and predict recurrence in HCC patients after curative resection. Cell Biosci. 2014;4:50. doi: 10.1186/2045-3701-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park YN, Kojiro M, Di Tommaso L, Dhillon AP, Kondo F, Nakano M, Sakamoto M, et al. Ductular reaction is helpful in defining early stromal invasion, small hepatocellular carcinomas, and dysplastic nodules. Cancer. 2007;109:915–923. doi: 10.1002/cncr.22460. [DOI] [PubMed] [Google Scholar]
- 44.Perepelyuk M, Terajima M, Wang AY, Georges PC, Janmey PA, Yamauchi M, Wells RG. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G605–614. doi: 10.1152/ajpgi.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu N, Meng F, Invernizzi P, Bernuzzi F, Venter J, Standeford H, Onori P, et al. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-β1 biliary secretion in mice. Hepatology. 2016;64:865–879. doi: 10.1002/hep.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishikawa T, Factor VM, Marquardt JU, Raggi C, Seo D, Kitade M, Conner EA, et al. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology. 2012;55:1215–1226. doi: 10.1002/hep.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pintilie DG, Shupe TD, Oh SH, Salganik SV, Darwiche H, Petersen BE. Hepatic stellate cells’ involvement in progenitor-mediated liver regeneration. Lab Invest. 2010;90:1199–1208. doi: 10.1038/labinvest.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gieseck RL, 3rd, Ramalingam TR, Hart KM, Vannella KM, Cantu DA, Lu WY, Ferreira-Gonzalez S, et al. Interleukin-13 activates distinct cellular pathways leading to ductular reaction, steatosis, and fibrosis. Immunity. 2016;45:145–158. doi: 10.1016/j.immuni.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikeda H, Sasaki M, Sato Y, Harada K, Zen Y, Mitsui T, Nakanuma Y. Bile ductular cell reaction with senescent hepatocytes in chronic viral hepatitis is lost during hepatocarcinogenesis. Pathol Int. 2009;59:471–478. doi: 10.1111/j.1440-1827.2009.02395.x. [DOI] [PubMed] [Google Scholar]
- 51.Wood MJ, Gadd VL, Powell LW, Ramm GA, Clouston AD. Ductular reaction in hereditary hemochromatosis: the link between hepatocyte senescence and fibrosis progression. Hepatology. 2014;59:848–857. doi: 10.1002/hep.26706. [DOI] [PubMed] [Google Scholar]
- 52.Wan Y, McDaniel K, Wu N, Ramos-Lorenzo S, Glaser T, Venter J, Francis H, et al. Regulation of cellular senescence by miR-34a in alcoholic liver injury. Am J Pathol. 2017;187:2788–2798. doi: 10.1016/j.ajpath.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–818. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 54.Lee SJ, Kim KH, Pak SC, Kang YN, Yoon GS, Park KK. Notch signaling affects biliary fibrosis via transcriptional regulation of RBP-jκ in an animal model of chronic liver disease. Int J Clin Exp Pathol. 2015;8:12688–12697. [PMC free article] [PubMed] [Google Scholar]
- 55.Lu J, Zhou Y, Hu T, Zhang H, Shen M, Cheng P, Dai W, et al. Notch signaling coordinates progenitor cell-mediated biliary regeneration following partial hepatectomy. Sci Rep. 2016;6:22754. doi: 10.1038/srep22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Du G, Xu Y, Li X, Fan W, Chen J, Liu C, et al. Inhibition of notch signaling pathway prevents cholestatic liver fibrosis by decreasing the differentiation of hepatic progenitor cells into cholangiocytes. Lab Invest. 2016;96:350–360. doi: 10.1038/labinvest.2015.149. [DOI] [PubMed] [Google Scholar]
- 57.Gurda GT, Zhu Q, Bai H, Pan D, Schwarz KB, Anders RA. The use of Yes-associated protein expression in the diagnosis of persistent neonatal cholestatic liver disease. Hum Pathol. 2014;45:1057–1064. doi: 10.1016/j.humpath.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, Nayar SK, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/β-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 61.Okabe H, Yang J, Sylakowski K, Yovchev M, Miyagawa Y, Nagarajan S, Chikina M, et al. Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice. Hepatology. 2016;64:1652–1666. doi: 10.1002/hep.28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, et al. β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 2014;60:964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Irvine KM, Clouston AD, Gadd VL, Miller GC, Wong WY, Melino M, Maradana MR, et al. Deletion of Wntless in myeloid cells exacerbates liver fibrosis and the ductular reaction in chronic liver injury. Fibrogenesis Tissue Repair. 2015;8:19. doi: 10.1186/s13069-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, Ridgway RA, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125:1269–1285. doi: 10.1172/JCI76452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishii T, Sato M, Sudo K, Suzuki M, Nakai H, Hishida T, Niwa T, et al. Hepatocyte growth factor stimulates liver regeneration and elevates blood protein level in normal and partially hepatectomized rats. J Biochem. 1995;117:1105–1112. doi: 10.1093/oxfordjournals.jbchem.a124814. [DOI] [PubMed] [Google Scholar]
- 67.Limaye PB, Bowen WC, Orr AV, Luo J, Tseng GC, Michalopoulos GK. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2008;47:1702–1713. doi: 10.1002/hep.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tirnitz-Parker JE, Viebahn CS, Jakubowski A, Klopcic BR, Olynyk JK, Yeoh GC, Knight B. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology. 2010;52:291–302. doi: 10.1002/hep.23663. [DOI] [PubMed] [Google Scholar]
- 69.Bird TG, Lu WY, Boulter L, Gordon-Keylock S, Ridgway RA, Williams MJ, Taube J, et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci USA. 2013;110:6542–6547. doi: 10.1073/pnas.1302168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Renzi A, DeMorrow S, Onori P, Carpino G, Mancinelli R, Meng F, Venter J, et al. Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology. 2013;57:1130–1141. doi: 10.1002/hep.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu N, Meng F, Zhou T, Han Y, Kennedy L, Venter J, Francis H, et al. Prolonged darkness reduces liver fibrosis in a mouse model of primary sclerosing cholangitis by miR-200b down-regulation. FASEB J. 2017;31:4305–4324. doi: 10.1096/fj.201700097R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Renzi A, Glaser S, Demorrow S, Mancinelli R, Meng F, Franchitto A, Venter J, et al. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol. 2011;301:G634–643. doi: 10.1152/ajpgi.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


