Abstract
Regulation of the intestinal mucus layer by goblet cells is important for preventing inflammation and controlling infection. IL-33, a cytokine upregulated in inflammatory bowel disease and helminth infection, induces intestinal goblet cells, but the mechanism remains unclear. Enteroids are three dimensional structures of primary small intestinal epithelial cells that contain all differentiated intestinal epithelial cell types. We developed an enteroid-immune cell co-culture model to determine the mechanism through which IL-33 affects intestinal goblet cell differentiation. We report that IL-33 does not directly induce goblet cell differentiation in murine enteroids; however, IL-13, a cytokine induced by IL-33, markedly induces goblet cells and gene expression consistent with goblet cell differentiation. When enteroids are co-cultured with CD90+ mesenteric lymph node cells from IL33-treated mice, IL-33 then induces IL-13 secretion by group 2 innate lymphoid cells and enteroid gene expression consistent with goblet cell differentiation. In co-cultures, IL-33-induced Muc2 expression is dependent on enteroid Il4ra expression demonstrating a requirement for IL-13 signaling in epithelial cells. In vivo, IL-33-induced intestinal goblet cell hyperplasia is dependent on IL-13. These studies demonstrate that IL-33 induces intestinal goblet cell differentiation not through direct action on epithelial cells, but indirectly through IL-13 production by goup 2 innate lymphoid cells.
Keywords: interleukin-33, epithelial cells, goblet cells, interleukin-13
INTRODUCTION
Goblet cells generate the first line of defense at the intestinal mucosa, primarily through secretion of the mucin MUC2, which generates the intestinal mucus layer (1). The mucus layer prevents the luminal contents, particularly intestinal microbiota, from coming in contact with intestinal epithelial cells. However, when the mucus becomes penetrable, this leads to colitis in mice and is associated with disease in ulcerative colitis patients (2, 3). In fact, rare variants in MUC2 were recently found to be associated with ulcerative colitis (4). Mucins are also upregulated during helminth infections and are important for their expulsion (5).
IL-33 is a member of the IL-1 family of cytokines that signals through the IL-33 receptor (IL-33R, also called ST2) (6). Many cells types express IL-33R, including immune cells, epithelial cells and stromal cells (7–10). Mucosal IL-33 is increased during helminth infection and colitis. IL-33 augments type 2 cytokine (IL-4, IL-5, IL-13) production from T cells and innate lymphoid cells (ILCs), which is important for helminth expulsion (11–13). Injection of supraphysiological levels of IL-33 induces goblet cells in the healthy mouse intestine (6). We and others have demonstrated that IL-33 and IL-33R are protective in murine models of ulcerative colitis, in part through preservation of goblet cells (14, 15). However, the mechanism through which IL-33 regulates intestinal goblet cells, whether direct or indirect, and the important secreted intermediaries, remains under debate (8, 14).
Enteroids are structures of primary small intestinal epithelium grown from isolated crypt stem cells that contain the full complement of differentiated intestinal epithelial cell types, including goblet cells (16). The application of enteroids to the study of the intestinal epithelium overcomes many of the limitations of conventional transformed cell lines, which may behave differently than primary cells and have limited capacity for differentiation. The refinement of techniques to co-culture enteroids with other cell types holds promise for advancing studies of the interaction between the intestinal epithelium and other cellular compartments, such as mucosal-associated immune cells (17, 18).
In this study we modeled intestinal immune-epithelial interactions by co-culturing murine enteroids with mesenteric lymph node (MLN) cells enriched for group 2 ILCs (ILC2s) to demonstrate that IL-33 induces epithelial goblet cell differentiation through stimulation of ILC2s to produce IL-13. IL-13, but not IL-33, directly induced goblet cell differentiation in enteroids cultured alone. IL-33 induction of goblet cell differentiation was dependent on the presence of ILC2-enriched MLN cells and enteroid IL-13 signaling in vitro, and on IL-13 in vivo.
MATERIALS AND METHODS
Mice and In Vivo Treatment
Il13–/– (Balb/C), Il4ra–/– (Balb/C) (Jackson Labs strain 003514), Il1rl1 (IL33-R)–/– (C57BL/6), Lgr5-EGFP-IRES-creERT2 (Jackson Lab strain 008875) and wild type (WT) C57BL/6 and Balb/C mice were bred at CCHMC and under specific pathogen free conditions and maintained on a standard laboratory chow diet in a half-day light cycle exposure and temperature-controlled environment. Male and female strain-matched mice were used and were age 6–12 weeks at the start of the experiments. The generation of the Il13–/– mice and Il1rl1–/– mice was previously described (19, 20). Mice were given phosphate-buffered saline or 0.4 ug rIL-33 daily i.p. for 4 days. The study was carried out following recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The CCHMC Institutional Animal Care and Use Committee approved the protocol.
Generation of murine enteroids and co-cultures
Mouse ileum was dissected and flushed with ice cold PBS. The ileum was opened longitudinally, cut into 1 cm pieces and incubated in 2 mM EDTA for 30 minutes at 4oC with rocking. The tissue was transferred into a new tube containing 5 mL Shaking buffer (PBS:43.3 mM Sucrose:54.9 mM Sorbitol) and shaken gently by hand for 2 minutes. Dispersed crypts were plated overnight in Matrigel (Corning, Corning, NY) with enteroid growth media (Advanced DMEM:F12, 2 mM GlutaMax, 10 mM HEPES, 100 U/mL penicillin, 100 ug/mL streptomycin, 1X N2 supplement, 1x B27 supplement (all from Invitrogen)) containing EGF (50ng/mL, Sigma, St. Louis, MO), 20% L-WRN conditioned media (L-WRN cells from ATCC, Manassas, VA). The following day, 100 ng/mL IL-33 (Peprotech, Rocky Hill, NJ) was added daily to the cultures for up to 5 days. Growth factors were replenished every other day. For co-cultures, enteroids were plated on hanging transwells (Corning). MLN cells from IL-33-treated or naïve mice (400,000 cells) were plated in the well and were stimulated with IL-2 (Peprotech), IL-7 (Peprotech) and anti-IFN-γ (Biolegend, San Diego, CA) with or without IL-33 for 4 days. WEHI-YH2 cells (kindly provided by Antony Burgess) (21) were plated to confluency in 24-well plates. Primary murine colon myofibroblasts (CMF) were generated using the methods previously described (22). In brief, mouse colons were treated with EDTA followed by digestion with collagenase and single cells were plated onto tissue culture plates for 3 hours before non-adherent cells were removed. Passage 2 was plated to confluency in 24-well plates for co-culture experiments. For each experiment, a separate mouse was used to generate enteroids, and each experiment was performed in triplicate.
Immunofluorescence
Enteroids were plated in a very thin layer of Matrigel or myofibroblasts were plated on IBIDI 4 well chamber slides. Enteroids were stimulated for 5 days with rIL-33 (100 ng/mL) or rIL-13 (10 ng/ml). Enteroids or myofibroblasts were fixed with 4% paraformaldehyde and permeabilized in PBS containing 0.1% Tween. Enteroids or myofibroblasts were stained using rabbit anti-ST2 (1:100, AB25877 from AbCam), chicken anti-GFP (1:1000 GFP-1010, Aves labs), rabbit anti-IL4r (1:100, PAS-38615, Invitrogen), rabbit anti-IL13ra1 (1:100 Pas-50989, Invitrogen) or rabbit anti-vimentin (1:100, ab45939, AbCam) followed by donkey anti-rabbit AF594 (1:200, Jackson Immuno) or donkey anti-Chicken AF488 (1:200, Jackson Immuno). Cells were also stained with FITC-UEA I (Vector Labs, Burlingame, CA), Phalloidin:AF647 (A22287, ThermoFisher) and nuclei were counterstained with Hoechst (1:1000, B2261, Sigma Aldrich). Enteroids were visualized using a Nikon A1 inverted confocal microscope. 5 um confocal optical sections were opened in NIS Viewer (Nikon), and nuclei and goblet cells were counted.
Histopathology
Mouse colon and jejunum sections were stained with periodic acid Schiff (PAS) and staining was quantified (3.14 ± 0.21 mm2 of distal colon and 1.67 ± 0.15 mm2 of jejunum) using a modified nuclear algorithm with Aperio Imagescope software (Buffalo Grove, IL) as previously described (15).
RNA Expression
RNA was isolated from tissue using the RNeasy Mini Kit (Qiagen, Valencia, CA) per the manufacturer’s instructions. RNA (100 ng) was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific, Waltham, MA). Real-time PCR was performed with TaqMan Gene Expression Assays (Life Technologies, Carlsbad, CA) for Spdef (Mm00600221_m1), Atoh1 (Mm00476035_s1), Muc2 (Mm00524818_m1), Areg (Mm01354339_m1), Il13 (Mm00434204_m1), Il1rl1 (Mm01233982_m1), Retnlb (Mm00445845_m1) and Gapdh (Mm99999915_g1). All reactions were performed on a StepOnePlus real-time PCR system (ThermoFisher Scientific). Relative mRNA levels were determined using the 2−ΔΔCT method with Gapdh as the reference.
Western Blot
Membranes were blocked with 5% nonfat dry milk in TBS-Tween (0.05%) for 1 hour and incubated overnight at 4°C with primary antibodies against Actin (Seven Hills Bioreagents, Cincinnati, OH) and Phospho-p44/42 MAPK (ERK1/2) (Cell Signaling, Danvers, MA). Membranes were incubated with HRP-linked secondary antibodies, anti-rabbit- or anti-mouse and visualized using ECL Prime and FujiFilm LAS-4000 Gel Documentation system (GE Healthcare, Piscataway, NJ). Band densitometry was determined using Image J (NIH), and the ratio of the intensity of Phospho-p44/42 MAPK to Actin was used to determine fold activation of signaling.
Flow cytometric analysis and cell sorting
For co-culture experiments, MLN cells from IL-33-treated mice were stained with PerCp-Cy5.5-conjugated CD90.2 (30-H12) and APC-Cy7-conjugated CD45 (30-F11, Biolegend, San Diego, CA) followed by cell sorting with a FACSAria II (BD Biosciences, San Jose, CA) For intracellular cytokine analyses, after restimulation with phorbol 12-myristate 13-acetate/ionomycin and treatment with Golgi blocker, LP and MLN cells were stained with biotinylated anti–T1/ST-2 (DJ8, MD Biosciences, Oakdale, MN) followed by Streptavidin-BV650, APC-conjugated anti-CD4 (GK1.5), FITC-conjugated anti-CD3 (17A2) and PerCp-Cy5.5-conjugated B220 (RA3–6B2), FCεRI (MAR-1), CD11c (N418), NK1.1 (PK136) and CD11b (M1/70) (Biolegend). Stained cells were fixed and permeabilized for intracellular cytokine staining by using PE-conjugated anti–IL-13 (eBio13A, eBioscience, Waltham, MA). MLN and LP cells were then analyzed with an LSR II (BD Biosciences, San Jose, CA).
Statistical Analysis
For all data from experiments with three or more groups, non-parametric Kruskal-Wallis test was performed followed by two-stage step-up method of Benjamini, Krieger, and Yekutieli for false discovery rate. Data from experiments with two groups was analyzed using the non-parametric Mann-Whitney test. Individual data points and medians are plotted on all graphs. The analysis was performed on Prism software (version 7.0b, GraphPad Software, La Jolla, CA).
RESULTS
IL-33 does not directly induce goblet cells in murine enteroids
We first tested whether IL-33 directly induces goblet cells in primary murine enteroids. Fluorescence microscopy demonstrated that IL-33R is expressed on murine enteroids derived from mouse ileal crypts (Fig. 1A). Both goblet cells, labeled by UEA-1 staining of mucins, and stem cells, labeled by anti-GFP in Lgr5-EGFP-IRES-creERT2 reporter miceLgr5, expressed IL-33R, as well as IL-4RA and IL-13RA1 (Fig 1A-C and Supplemental Fig. 1A-C). We then stimulated enteroids with rIL-33 (10 ng/mL, data or shown or 100 ng/ml) for up to 5 days to determine the effect of IL-33 on goblet cell differentiation. By real-time RT-PCR we did not detect alteration in the expression of the transcription factors Atoh1 and Spdef, which direct commitment to the secretory and goblet cell lineages, respectively, nor Muc2, the primary mucin produced by intestinal goblet cells, or Retnlb and Tff3, secretory products of goblet cells (Fig. 1D, data not shown for Tff3).
FIGURE 1.

IL-33 and IL-13 effects on goblet cell differentiation in murine enteroids. Immunofluorescence microscopy for (A) IL-33R (B) IL-4RA and (C) IL-13RA1 (red) in enteroids counterstained with Hoecht (blue), Phalloidin (pink) and UEA1 or LGR5 (green). Scale bars = 20 μM (D-E) Graphs of real-time RT-qPCR analysis of enteroids treated with (D) IL-33 (100 ng/mL) or (E) IL-13 (10 ng/mL). (F) Representative photomicrographs of UEA-1 FITC (green) immunofluorescence microscopy in enteroids treated for 4 days with PBS, IL-33 or IL-13. Enteroids are counterstained with DAPI (blue) and Phalloidin (pink) (G) Quantification of goblet cells. Scale bars = 20 μM. White arrows indicate goblet cells. 7–9 wells per condition pooled from 3 independent experiments; **P < 0.01, ***P < 0.001.
Since IL-33 induces IL-13 production by T cells and ILCs (6, 11), and others have shown that IL-13 induces intestinal goblet cell differentiation (19, 23), we tested whether IL-13 induces goblet cell differentiation in our primary murine enteroid cultures. As expected, IL-13 significantly induced expression of Atoh1, Spedf, Muc2 and Retnlb (Fig. 1E). However, Tff3 was not induced (data not shown). We performed confocal microscopy analysis of goblet cells using UEA1 staining, which confirmed that IL-13, but not IL-33, directly induced goblet cells in enteroids (Fig. 1F and G).
IL-33 induction of goblet cells is dependent on intestinal-associated CD45+CD90+ immune cells in vitro
Since we did not observe a direct effect of IL-33 on enteroid goblet cells, we hypothesized that IL-33 affects goblet cells indirectly through action on either myofibroblasts or lymphoid cells. We chose to examine both intestinal myofibroblasts and immune cells, since both have been shown to respond to IL-33 and can make mediators that could affect goblet cell differentiation and mucus production (6, 9, 12, 14, 24, 25). In order to examine the role of secreted factors from an intermediate cell type, we developed a co-culture system whereby murine enteroids were suspended on a semipermeable membrane over other cell types and both exposed to IL-33 (Fig. 2A). We first demonstrated that MLN cells, the colonic subepithelial myofibroblast cell line, WEHI-YH2 cells, and primary CMF all expressed Il1rl1 (Fig. 2B). Furthermore, immunofluorescence analysis demonstrated that CMF expressed IL-33R, and purity of this population was confirmed with vimentin staining (Fig. 2C). To determine whether myofibroblasts are required for IL-33 to affect epithelial goblet cells, we treated enteroids co-cultured with WEHI-YH2 cells or CMF with IL-33 for 4 days. We observed no change in enteroid Muc2 expression (Fig. 2D).
FIGURE 2.

IL-33 induced goblet cells in enteroids co-cultured with immune cells, but not those with myofibroblasts. (A) Diagram of in vitro model wherein enteroids in matrigel were suspended in transwells in co-culture over either CD90+ MLN cells from IL33-treated mice, WEHI-YH2 myofibroblast cells or primary CMF with or without IL-33 (100 ng/mL). (B) Il1rl1 expression in CMF, WEHI-YH2 and MLN cells. (C) Immunofluorescence analysis of IL-33R and vimentin in primary CMF counterstained with Hoecht. Scale bars = 25 μM Atoh1, Spdef and Muc2 expression in enteroids co-cultured with (D) primary CMF or WEHI-YH2 cells, (E) WT CD90+ or (F) Il1rl1–/– CD90+ MLN cells was assessed by real time RT-qPCR. Data is graphed as medians. P < 0.001, n = 8–16 wells pooled from 3 or 4 independent experiments.
We then sought to determine whether intestinal-associated T cells or ILCs are required for IL-33 to affect enteroid goblet cells by treating enteroids co-cultured with CD45+CD90+ mesenteric lymph node (MLN) cells from IL-33-treated mice (IL-33 i.p.) with additional IL-33 in vitro (IL-33 i.p. + IL-33). IL-33 treatment in vitro significantly induced Atoh1, Spdef and Muc2 expression in enteroids co-cultured with MLN cells (Fig. 2E). Furthermore, increases in goblet cell markers were dependent on IL-33R expression on CD90+ MLN cells. Il1rl1–/– CD90+ MLN cells treated with IL-33 and co-cultured with enteroids did not induce Atoh1, Spdef or Muc2 expression compared to media alone (Fig. 2F). Collectively, these data show that IL-33R-expressing T cells and/or ILCs are required for IL-33 to induce epithelial goblet cells.
IL-13 produced by primarily ILC2s is required for IL-33-induced goblet cell differentiation in vitro
Since IL-33 induces IL-13 production by T cells and ILCs, and we observed IL-13 directly induces goblet cell differentiation in murine enteroids (Fig. 1 and 2), we tested the role of IL-13 signaling in the interaction between IL-33, intestinal immune cells, and epithelial cells. IL-33 significantly induced Il13 expression, as detected by real-time RT-PCR (Fig. 3A), and IL-13 protein production, as detected by ELISA (Fig. 3B), in MLN cells co-cultured with enteroids. IL-4 was undetectable at baseline, and IL-33 induced minimal IL-4 production (0.45 ± 0.26 pg/mL IL-4 compared to 4,297 ± 698 pg/mL IL-13).
FIGURE 3.

IL-33 induced IL-13 from ILC2s in MLN:enteroid co-cultures. CD90+ MLN cells from C57BL/6 mice were co-cultured with enteroids for 4 days with or without 100 ng/mL IL-33 and IL-13 was assessed. (A) Il13 real-time RTq-PCR (B) IL-13 ELISA. (C) Representative flow plots for CD4+ and CD4– IL-13+ cells from co-cultured MLN cells from C57BL/6 mice and expression of IL-33R and CD3 on CD4+ and CD4– IL-13+ cells. (D) Quantification of IL-13+ cells from (C). (E) Representative flow plots for CD4+ and CD4– IL-13+ cells from co-cultured MLN cells from Balb/C mice. (F) Quantification of IL-13+ cells from (E). *P < 0.05 ***P < 0.001. n = 6–9 wells pooled from 3 independent experiments.
In order to identify the MLN cells producing IL-13, flow cytometry analysis was performed for IL-13 and markers for T cells and ILC2s. All cells were negative for lineage markers B220, CD11b, NK1.1, FCεRI and CD11c (data not shown). Both CD4+ and CD4– cells expressed IL-13 (Fig. 3C). However, IL-33 treatment in vitro only induced IL-13 production in CD4– cells, and 94 ± 3.8% of the IL-13-expressing cells after IL-33 treatment were CD4– (Fig. 3D). Both CD4+ and CD4– IL-13-producing cells also expressed IL-33R, which in known to be expressed on Th2 cells and ILC2s (26) (Fig. 3C). As expected, CD4+IL-13+ cells also expressed CD3, while CD4–IL-13+ cells were CD3–, consistent with an ILC2 phenotype (Lineage–CD90+CD4–CD3–IL-33R+, Fig. 3C). We confirmed the finding from C57BL/6 mice in Balb/C mice, since Balb/C mice are known to be Th2-prone and were required for other experiments (Fig. 3E) (27). In Balb/C mice, we utilized mice without IL-33 i.p. (naïve) since IL-33 i.p. leads to high levels of IL-13 secretion in vitro even without additional IL-33 added in vitro (Fig. 4A). Similarly to C57BL/6 mice, Balb/C mice exhibited a large increase in ILC2s with IL-33 stimulation in vitro, but there was not a significant increase in IL-13+CD4+ cells from naïve to IL-33 i.p. or IL-33 i.p. + IL-33 (Fig. 3E).
FIGURE 4.

IL-33-exposed CD90+ MLN cells induce gene expression consistent with goblet cell differentiation in WT but not Il4ra–/– enteroids. CD90+ MLN cells were purified from naïve mice or mice given IL-33 (0.4 ug) i.p. daily for 4 days. (A) IL-13 and IL-4 secretion in co-cultures was determined by ELISA, ND, not detected. MLN cells were co-cultured with (B) WT or (C) Il4ra–/– enteroids with 100 ng/ml IL-33 added in vitro to some cultures, and enteroid mRNA expression was assessed by real time RT-qPCR *P < 0.05, ***P < 0.001; n = 9 wells pooled from 3 independent experiments
To confirm that epithelial cell-intrinsic IL-13 signaling is required for IL-33 induction of Muc2 expression, we generated enteroids from Il4ra–/– Balb/C mice (IL-4Rα is a component of the IL13 receptor-α−1 heterodimer). CD45+CD90+ MLN cells were isolated from naïve or IL-33 i.p. mice, cocultured with enteroids derived from WT or Il4ra–/– mice, and, in some conditions, stimulated with additional rIL-33. MLN cells from IL-33 i.p. mice secreted IL-13 in vitro (5.9 ± 1.5 ng/mL in the media from IL-33 i.p. mice versus undetectable in that of naïve mice), which was further augmented by the addition of IL-33 to the culture media (45.7±13.6 ng/mL, Fig. 4A). Comparatively very small amounts of IL-4 were secreted by the MLN cells from the IL-33 i.p. mice (0.006±0.001 ng/mL), and this was not increased with additional IL-33 in vitro (Fig. 4A).
Muc2 and Atoh1 expression were significantly increased in WT enteroids co-cultured with MLN cells from IL-33 i.p. mice both with and without IL-33 in culture (Fig. 4B). There were numerical but not statistically significant increases in Spedf in co-cultures with MLN cells from IL-33 i.p. mice both with and without IL-33 in culture. In contrast, no increase in Atoh1, Spdef or Muc2 was observed in Il4ra–/– enteroids co-cultured with MLN cells from IL-33 i.p. mice with or without IL-33 (Fig. 4C). These studies indicate that goblet cell differentiation induced by IL-33 in enteroids is dependent on IL-13 signaling in the enteroids.
MLN cells cultured with IL-33 also expressed increased levels of mRNA for the epidermal growth factor ligand amphiregulin (Areg) (Fig. 5A), which has been shown to be important for maintaining goblet cells during DSS-induced colitis (14). While amphiregulin (50 ng/mL) did induce phopho-ERK 1/2 in enteroids (Fig. 5B), it did not alter Atoh1, Spdef or Muc2 expression (Fig. 5C), further supporting that IL-13 secreted predominantly from ILC2s is primarily responsible for the effect of IL-33 on intestinal goblet cells.
FIGURE 5.
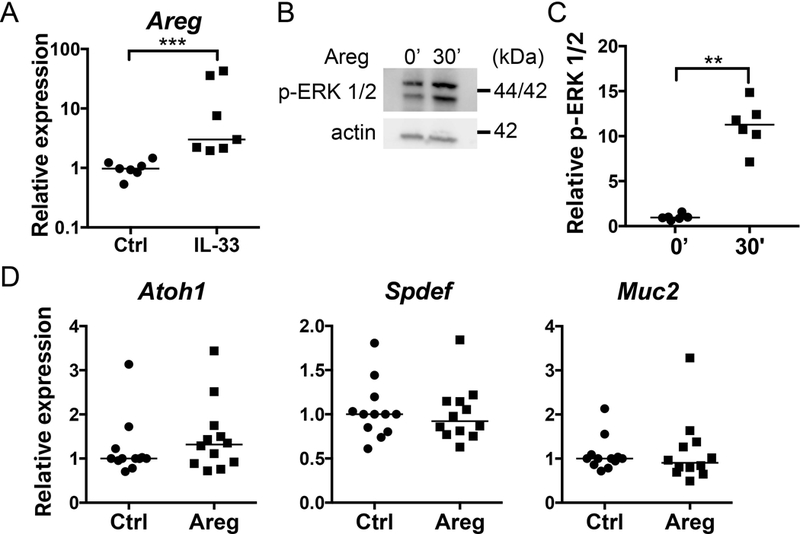
Areg does not induce gene expression consistent with goblet cell differentiation in enteroids. (A) Areg expression in CD90+ MLN co-cultured with enteroids with or without IL-33 (100 ng/mL) for 4 days. (B) Representative western blot and (C) quantification of ERK 1/2 activation following Areg stimulation for 30 minutes. (D) Real-time RTq-PCR analysis of enteroids stimulated with Areg (100 ng/mL) for 4 days. ***P < 0.001 n = 9 wells pooled from 3 independent experiments.
Induction of goblet cells by IL-33 in vivo is dependent on IL-13
Although is known that IL-13 induces goblet cell hyperplasia in vivo (19) and that IL-33 potentiates IL-13 secretion by T cells and ILCs, it is not known whether IL-13 is required for IL-33 to induce goblet cell hyperplasia in vivo. We administered IL-33 i.p. for 4 days to WT and Il13–/– mice and sacrificed mice to examine intestinal goblet cells. In the colon, IL-33 significantly increased the number of PAS+ goblet cells in WT mice but had no effect on goblet cell number in Il13–/– mice (Fig. 6A and 6B). In line with this finding, colon mucosal Atoh1, Spdef, and Muc2 expression were increased in IL-33-treated WT mice, but not in Il13–/– mice (Fig. 6B).
FIGURE 6.

IL-33-induced intestinal goblet cell hyperplasia is dependent on IL-13 in vivo. Representative photomicrographs of PAS-stained goblet cells in (A) colon and (C) small intestine. Quantification of PAS staining using a modified nuclear algorithm and real time RT-qPCR analysis of tissue RNA from the (B) colon and (D) small intestine. Real time RT-qPCR for Il13 and Areg from the (E) colon and (F) small intestine. *P < 0.05, **P < 0.01, ***P < 0.001, n = 6–10 mice per group across 3 independent experiments.
We observed similar effects in the small intestine, with IL-33 inducing increased goblet cells in WT but not Il13–/– mice (Fig. 6C and 6D). There were also numerical increases in mean small intestinal mucosal Atoh1, Spdef, and Muc2 expression with IL-33 treatment in WT but not Il13–/– mice in vivo. These differences did not reach statistical significance, likely due to high variability in the expression of these mRNAs in the small intestine (Fig. 6D). Interestingly, there was some signal, albeit inconsistent, for increased goblet cell markers in Il13–/– mice at baseline compared to WT, which decreased with IL-33 treatment. These numerical differences were only statistically significant in the cases of increased small intestinal Spdef expression in Il13–/– mice compared to WT mice, and decreased Atoh1 expression in IL-33-treated compared to untreated Il13–/– mice.
Since others have demonstrated that Areg is required for IL-33-induction of goblet cells during inflammation, we examined both Il13 and Areg expression in the large and small intestine following IL-33 i.p. As expected, Il13 was significantly increased in both the colon and the jejunum (Fig. 6E and 6F). However, there was no increase in Areg in the large or small intestine with IL-33 i.p. compared to control (Fig. 6E and 6F).
DISCUSSION
We have applied an enteroid-immune cell co-culture system as a model of epithelial-immune cross-talk in the intestine to demonstrate that IL-33 induces goblet cell differentiation indirectly by stimulating primarily ILCs to produce IL-13, rather than through direct action on epithelial cells. Although intestinal epithelial cells express the IL-33R and activate ERK 1/2 in response to IL-33, IL-33 did not directly induce goblet cell differentiation, while IL-13 did. In co-cultures with CD90+ MLN cells from IL-33-treated mice, IL-33 induced Muc2 and Atoh1 expression, indicating increased goblet cell differentiation. CD3–CD90+ST2+ ILC2s from MLN of IL-33-treated mice produce large amounts of IL-13 in co-cultures. The IL-33-induced Muc2 response in vitro was dependent on enteroid Il4ra expression. Furthermore, IL-33 induction of goblet cells in mice in the large and small intestine was dependent on IL-13.
Large doses of IL-33 administered to mice leads to goblet cell hyperplasia, both in the lungs and the intestines (6), but the mechanisms by which IL-33 induces goblet cells in the intestines have not been fully delineated. IL-33 increases production of the Th2 cytokine IL-13 both in vitro and in vivo in T cells and ILC2s (6, 11, 12), and IL-13 is able to induce intestinal and lung goblet cells (19, 23, 28, 29). We now tie together this circuit by demonstrating the dependence of IL-33-induced goblet cell differentiation on primarily IL-13 producing ILC2s and epithelial IL-13 signaling in vitro and IL-13 in vivo.
Since IL-33-treated mice have goblet cell hyperplasia, for co-cultures with immune cells, we used MLN from IL-33-treated mice as a relevant source of immune cells for the intestine, as other studies have previously done (14). Since IL-33 is known to induce a type 2 immune response in both T cells and innate lymphoid cells (6, 11, 12), we isolated CD90+ cells from the MLN to capture both cell types. When stimulated further with IL-33 in vitro, CD90+ MLN cells made substantial amounts of IL-13. Importantly, in Trichuris muris (13)and N. brasiliensis (12) infections, nanogram amounts of IL-13 are produced, which is what we saw following IL-33 stimulation of MLN cells in vitro. Although both ILCs and T helper cells produced IL-13, over 90% of the IL-13-producing cells were ILC2s. This is consistent with the findings of others that IL-33 treatment leads to accumulation of ILC2s in the MLN (14). Furthermore, in the setting of Nippostrongylus brasiliensis infection, ILC2s are the predominant cell type producing IL-13 and T cells are not required for IL-13 production to be induced (12, 30). However, a limitation of this study is that in an unchallenged mouse, the majority of T cells in the MLN are naïve, and there are only a minority of IL-33R-expressing Th2 cells able to respond to IL-33 (31). We now show that IL-33-exposed MLN cells can increase goblet cell differentiation in murine enteroids in vitro. Furthermore, we used Il4ra–/– enteroids to show that this effect is dependent on epithelial intrinsic IL-13 signaling.
This study is one of a small number of emerging reports of intestinal epithelial organoids co-cultured with other cell types as a more complex model of the intestinal mucosa. Intestinal subepithelial myofibroblasts support the growth of enteroids and colon organoids (colonoids) when co-cultured together (21, 32, 33). Macrophages co-cultured on the basolateral surface of enteroid monolayers enhance epithelial barrier function and maturation, and phagocytose apical bacteria (17). Co-cultures of T lymphocytes and enteroids have also been reported. One group co-cultured group 3 ILCs (ILC3s) from Il22-deficient mice in matrigel with enteroids to demonstrate that ILC3 augmentation of enteroid growth is IL-22-dependent (18). Our approach differed in that enteroids in matrigel were on a standing semipermeable insert so that ILC2-enriched lymphoid cells were physically separated from the enteroids. This approach further supported that an ILC2 secreted factor rather than direct intercellular interactions were responsible for enteroid goblet cell differentiation in our system.
Several studies have begun to dissect the role of IL-33 in regulating intestinal secretory cell differentiation, including goblet cells (8, 34). With the discovery of IL-33, it was demonstrated that IL-13 is required for IL-33-induced goblet cell hyperplasia in the lung in vivo (6). A more recent study demonstrated a positive feedback circuit in vivo whereby intestinal tuft cells produce IL-25, which potentiates IL-13 production by ILC2s, which then stimulates the further differentiation of epithelial tuft cells (34). The same study showed that IL-33 similarly induces tuft cells through ILC2 IL-13 production. Tuft cells branch off from a common secretory progenitor cell as goblet cells and require Atoh1, but not Spdef (35). We now build on these findings by demonstrating a the requirement of ILC2s and IL-13 for induction of goblet cell differentiation by IL-33 using an in vitro model of epithelial-immune crosstalk.
Interestingly, we show that in IL-13-deficient mice, there is a signal for increased goblet cell markers in mouse intestine, which decreased following IL-33 treatment. These differences were, for the most part, not statistically significant. It is possible that at baseline, in the chronic absence of IL-13, other cytokines known to induce goblet cells, such as IL-22 could be increased (36). Furthermore, without the strong induction of IL-13 by IL-33 in IL-13-deficient mice, other cytokines induced by IL-33, such as IFN-gamma (37), which is known to decrease goblet cells, may take the lead in regulating goblet cells (38, 39).
In contrast to our findings, others have proposed that IL-33 directly induces goblet cell differentiation in the intestinal epithelium. One group of investigators similarly treated murine enteroids with IL-33 and observed marked increases in Paneth cell numbers and the Paneth cell marker Ang4, with more modest increases in goblet cells and Muc2 expression (8). They went on to show that the induction of Paneth cell differentiation was independent of enteroid Il4ra expression. Using the same IL-33 concentration and exposure duration, we did not observe any effect of IL-33 alone on enteroid goblet cell numbers, the secretory lineage differentiation marker Atoh1, or the goblet cell differentiation markers Spdef and Muc2 across multiple experiments. Only with the addition of MLN cells enriched for IL-13 producing ILC2s did we observe effects of IL-33 on enteroid goblet cell numbers and marker expression, leading us to conclude that IL-33 primarily acts on ILCs to indirectly induce goblet cell differentiation. IL-33 may directly activate other important pathways in intestinal epithelial cells. Recently is has been demonstrated that IL-33 acts directly on the Caco2 intestinal epithelial cell line to increase proliferation through upregulation of miR-320a (40), and further studies are warranted to explore direct effects of IL-33 in intestinal epithelial cells.
Goblet cell depletion is a pathologic hallmark of inflammatory bowel disease. We and others previously demonstrated that in the setting of colitis IL-33 and IL-33R reduce histopathologic severity and preserve goblet cells (14, 15). Here, we show that in unchallenged mice and primary intestinal epithelial cells, IL-33 induces goblet cell differentiation through IL-13 produced mainly by ILC2s. Others have shown that in the setting of epithelial injury and acute colitis induced by dextran sodium sulfate, IL-33 protection and preservation of goblet cells is dependent on ILC-intrinsic Areg (14). Although we corroborate that ILC2s in culture expressed Areg, direct stimulation of enteroids with Areg did not increase goblet cells, despite activation of signaling. We found that IL-33 robustly increased intestinal mucosal Il13 expression, but did not affect Areg expression in vivo and that IL-33 induced goblet cell hyperplasia was IL-13-dependent. Together these studies indicate that although Areg is important for epithelial repair and preservation of goblet cells during colitis, IL-13, and not Areg, induced by IL-33 directly stimulates goblet cell differentiation in unchallenged primary epithelium.
IL-33 induction of goblet cells through promoting ILC2s and IL-13 expression may be important for immune regulation in multiple settings. IL-33 expression is increased in the colon of patients with ulcerative colitis as well as in various mouse models of colitis and parasitic infection (13, 15, 41–44). We acknowledge that the levels of IL-33 we used in vitro and in vivo were higher than reported serum levels in humans during health or disease; however, it is difficult to ascertain the levels of IL-33 that cells are exposed to in the tissue microenvironment. IL-13 is required for expulsion of N. brasiliensis and H. polygyrus adult parasites from the intestinal lumen by inducing intestinal epithelial cells to differentiate into goblet cells that secrete resistin-like molecule (RELM) β (28). IL-33 induction of IL-13-producing ILC2s is important for worm expulsion during N. brasiliensis infection (12). Loss of mucus leads to colitis in mice and goblet cell depletion is associated with disease in human ulcerative colitis and necrotizing enterocolitis (2, 3, 45). We have previously shown that IL-33 limits goblet cell depletion during oxazolone colitis (15). Furthermore, we have also reported that increased mucosal ll13 expression at diagnosis is associated with superior outcomes in pediatric ulcerative colitis patients (46). Recently, ILC2s have been shown to help promote resolution of inflammation in a mouse model of arthritis (47). IL-33-induction of ILC2s, IL-13 and goblet cells is an important protective mechanism in the intestine and could be targeted for future therapeutics to promote healing following inflammation.
In conclusion, our data in a relevant primary murine enteroid culture system demonstrate that IL-33 indirectly induces goblet cell differentiation through IL-13 produced predominantly by ILC2s. Furthermore, our study demonstrates that IL-13 is required for IL-33-induced goblet cell hyperplasia in vivo in mice. We have successfully developed an enteroid-immune cell co-culture system, which can be leveraged to assess other roles for immune cells in epithelial cell functions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. Andrew N. J. McKenzie (MRC Laboratory of Molecular Biology, Cambridge, United Kingdom) for providing Il1rl1–/– mice and Dr. Antony Burgess (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) for providing the WEHI-YH2 cells. The authors also thank Dr. Jorge Bezerra (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH) for providing recombinant mouse IL-33.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under awards K23DK094832, R03DK110487 and R01DK117119 to Michael J. Rosen, R01DK114123 to Theresa Alenghat and P30DK078392 for the Gene Analysis and Integrated Morphology Cores of the Digestive Disease Research Core Center in Cincinnati. This work was also supported by a Crohn’s & Colitis Foundation Research Fellowship Award to Amanda Waddell and funding from Cure for IBD.
Abbreviations used in this manuscript:
- CMF
colon myofibroblast
- ILC
innate lymphoid cell
- ILC2
group 2 innate lymphoid cell
- MLN
mesenteric lymph node
- WT
wild-type
Footnotes
DISCLOSURE
The authors have no conflicts of interest to disclose.
Conflict of Interest: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Pelaseyed T, Bergstrom JH, Gustafsson JK, Ermund A, Birchenough GM, Schutte A, van der Post S, Svensson F, Rodriguez-Pineiro AM, Nystrom EE, Wising C, Johansson ME, and Hansson GC. 2014. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 260: 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson ME, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjovall H, and Hansson GC. 2014. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, and Vallance BA. 2010. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 6: e1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visschedijk MC, Alberts R, Mucha S, Deelen P, de Jong DJ, Pierik M, Spekhorst LM, Imhann F, van der Meulen-de Jong AE, van der Woude CJ, van Bodegraven AA, Oldenburg B, Lowenberg M, Dijkstra G, Ellinghaus D, Schreiber S, Wijmenga C, C. Initiative on, Colitis, Parelsnoer I, Rivas MA, Franke A, van Diemen CC, and Weersma RK. 2016. Pooled Resequencing of 122 Ulcerative Colitis Genes in a Large Dutch Cohort Suggests Population-Specific Associations of Rare Variants in MUC2. PloS one 11: e0159609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artis D, and Grencis RK. 2008. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal immunology 1: 252–264. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, and Kastelein RA. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23: 479–490. [DOI] [PubMed] [Google Scholar]
- 7.Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, and Turnquist HR. 2014. IL-33 is an unconventional Alarmin that stimulates IL-2 secretion by dendritic cells to selectively expand IL-33R/ST2+ regulatory T cells. Journal of immunology 193: 4010–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahapatro M, Foersch S, Hefele M, He GW, Giner-Ventura E, McHedlidze T, Kindermann M, Vetrano S, Danese S, Gunther C, Neurath MF, Wirtz S, and Becker C. 2016. Programming of Intestinal Epithelial Differentiation by IL-33 Derived from Pericryptal Fibroblasts in Response to Systemic Infection. Cell Rep 15: 1743–1756. [DOI] [PubMed] [Google Scholar]
- 9.Maywald RL, Doerner SK, Pastorelli L, De Salvo C, Benton SM, Dawson EP, Lanza DG, Berger NA, Markowitz SD, Lenz HJ, Nadeau JH, Pizarro TT, and Heaney JD. 2015. IL-33 activates tumor stroma to promote intestinal polyposis. Proceedings of the National Academy of Sciences of the United States of America 112: E2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, and Artis D. 2011. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. In Nature immunology. 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, and Locksley RM. 2010. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America 107: 11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, and McKenzie AN. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464: 1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphreys NE, Xu D, Hepworth MR, Liew FY, and Grencis RK. 2008. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. Journal of immunology 180: 2443–2449. [DOI] [PubMed] [Google Scholar]
- 14.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, and Artis D. 2015. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proceedings of the National Academy of Sciences of the United States of America 112: 10762–10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waddell A, Vallance JE, Moore PD, Hummel AT, Wu D, Shanmukhappa SK, Fei L, Washington MK, Minar P, Coburn LA, Nakae S, Wilson KT, Denson LA, Hogan SP, and Rosen MJ. 2015. IL-33 Signaling Protects from Murine Oxazolone Colitis by Supporting Intestinal Epithelial Function. Inflammatory bowel diseases 21: 2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, and Clevers H. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265. [DOI] [PubMed] [Google Scholar]
- 17.Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, and Zachos NC. 2017. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep 7: 45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, Ivanov JA, Fu YY, Takashima S, Hua G, Martin ML, O’Rourke KP, Lo YH, Mokry M, Romera-Hernandez M, Cupedo T, Dow L, Nieuwenhuis EE, Shroyer NF, Liu C, Kolesnick R, van den Brink MRM, and Hanash AM. 2015. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528: 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenzie GJ, Bancroft A, Grencis RK, and McKenzie AN. 1998. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol 8: 339–342. [DOI] [PubMed] [Google Scholar]
- 20.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, and McKenzie AN. 2000. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. The Journal of experimental medicine 191: 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirokawa Y, Yip KH, Tan CW, and Burgess AW. 2014. Colonic myofibroblast cell line stimulates colonoid formation. American journal of physiology. Gastrointestinal and liver physiology 306: G547–556. [DOI] [PubMed] [Google Scholar]
- 22.Khalil H, Nie W, Edwards RA, and Yoo J. 2013. Isolation of primary myofibroblasts from mouse and human colon tissue. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, and Jay P. 2016. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwakiri D, and Podolsky DK. 2001. Keratinocyte growth factor promotes goblet cell differentiation through regulation of goblet cell silencer inhibitor. Gastroenterology 120: 1372–1380. [DOI] [PubMed] [Google Scholar]
- 25.Willemsen LE, Koetsier MA, van Deventer SJ, and van Tol EA. 2003. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 52: 1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, and Kamradt T. 1998. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proceedings of the National Academy of Sciences of the United States of America 95: 6930–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, and Locksley RM. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. The Journal of experimental medicine 169: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF Jr., Rothenberg ME, and Finkelman FD. 2009. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. The Journal of experimental medicine 206: 2947–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanoh S, Tanabe T, and Rubin BK. 2011. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Exp Allergy 41: 1747–1756. [DOI] [PubMed] [Google Scholar]
- 30.Hung LY, Lewkowich IP, Dawson LA, Downey J, Yang Y, Smith DE, and Herbert DR. 2013. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proceedings of the National Academy of Sciences of the United States of America 110: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peine M, Marek RM, and Lohning M. 2016. IL-33 in T Cell Differentiation, Function, and Immune Homeostasis. Trends Immunol 37: 321–333. [DOI] [PubMed] [Google Scholar]
- 32.Lahar N, Lei NY, Wang J, Jabaji Z, Tung SC, Joshi V, Lewis M, Stelzner M, Martin MG, and Dunn JC. 2011. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PloS one 6: e26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei NY, Jabaji Z, Wang J, Joshi VS, Brinkley GJ, Khalil H, Wang F, Jaroszewicz A, Pellegrini M, Li L, Lewis M, Stelzner M, Dunn JC, and Martin MG. 2014. Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PloS one 9: e84651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Moltke J, Ji M, Liang HE, and Locksley RM. 2016. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, and Jay P. 2011. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. The Journal of cell biology 192: 767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner JE, Stockinger B, and Helmby H. 2013. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog 9: e1003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, and Smith DE. 2008. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. International immunology 20: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 38.Chan JM, Bhinder G, Sham HP, Ryz N, Huang T, Bergstrom KS, and Vallance BA. 2013. CD4+ T cells drive goblet cell depletion during Citrobacter rodentium infection. Infect Immun 81: 4649–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Songhet P, Barthel M, Stecher B, Muller AJ, Kremer M, Hansson GC, and Hardt WD. 2011. Stromal IFN-gammaR-signaling modulates goblet cell function during Salmonella Typhimurium infection. PloS one 6: e22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopetuso LR, De Salvo C, Pastorelli L, Rana N, Senkfor HN, Petito V, Di Martino L, Scaldaferri F, Gasbarrini A, Cominelli F, Abbott DW, Goodman WA, and Pizarro TT. 2018. IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo DH, Che X, Kwak MS, Kim S, Kim JH, Ma HW, Kim DH, Kim TI, Kim WH, Kim SW, and Cheon JH. 2017. Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci Rep 7: 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, Saito Y, Fujiyama Y, and Andoh A. 2010. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. Journal of gastroenterology 45: 999–1007. [DOI] [PubMed] [Google Scholar]
- 43.Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, Fiocchi C, Vecchi M, and Pizarro TT. 2010. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proceedings of the National Academy of Sciences of the United States of America 107: 8017–8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BM, Lohning M, Belkaid Y, Fallon PG, and Powrie F. 2014. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513: 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodzic Z, Bolock AM, and Good M. 2017. The Role of Mucosal Immunity in the Pathogenesis of Necrotizing Enterocolitis. Front Pediatr 5: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen MJ, Karns R, Vallance JE, Bezold R, Waddell A, Collins MH, Haberman Y, Minar P, Baldassano RN, Hyams JS, Baker SS, Kellermayer R, Noe JD, Griffiths AM, Rosh JR, Crandall WV, Heyman MB, Mack DR, Kappelman MD, Markowitz J, Moulton DE, Leleiko NS, Walters TD, Kugathasan S, Wilson KT, Hogan SP, and Denson LA. 2017. Mucosal Expression of Type 2 and Type 17 Immune Response Genes Distinguishes Ulcerative Colitis From Colon-only Crohn’s Disease in Treatment-naive Pediatric Patients. Gastroenterology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, Lin NY, Dietel K, Bozec A, Herrmann M, Kaplan MH, Weigmann B, Zaiss MM, Fearon U, Veale DJ, Canete JD, Distler O, Rivellese F, Pitzalis C, Neurath MF, McKenzie ANJ, Wirtz S, Schett G, Distler JHW, and Ramming A. 2017. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nature medicine 23: 938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


